Abstract
Background: Exogenous administration of glucagon-like peptide (GLP)-1 improves glucose tolerance by stimulation of insulin secretion, inhibition of glucagon secretion, and delay of gastric emptying. It is not known which of these effects is involved in the action of endogenous GLP-1 to control blood glucose. To determine the role of endogenous GLP-1 on islet cell function and gastric emptying independent of variable glycemia, we clamped blood glucose before and during glucose ingestion with and without GLP-1 receptor blockade with exendin-[9–39] (Ex-9).
Methods: There were 10 healthy subjects that participated in two experiments each, one a control and one with infusion of 750 pm/kg · min Ex-9. Subjects consumed 75 g oral glucose solution mixed with d-xylose and 13C-glucose while their blood glucose levels were held fixed at approximately 8.9 mmol/liter.
Results: Plasma insulin levels during hyperglycemia alone were similar in the two studies (control, 282.5 ± 42 vs. Ex-9, 263.8 ± 59 pmol/liter) but were reduced by approximately 30% by Ex-9 after glucose ingestion (control, 1154 ± 203 vs. Ex-9, 835 ± 120 pmol/liter; P < 0.05). Blocking the action of endogenous GLP-1 caused an approximate 80% increase in postprandial glucagon concentrations. The appearance of ingested d-xylose in the blood was not affected by Ex-9, suggesting that postprandial secretion of GLP-1 has only minimal effects on gastric emptying of oral glucose.
Conclusions: These findings indicate that GLP-1 is an incretin in healthy humans at modestly supraphysiological blood glucose levels. The primary effect of GLP-1 to regulate oral glucose tolerance is mediated by effects on islet hormones and not on gastric emptying.
In healthy humans, endogenous glucagon-like peptide-1 regulates glucose tolerance by increasing insulin release and inhibiting glucagon secretion, but not by affecting gastric emptying.
Glucagon-like peptide 1 (GLP-1) is a gastrointestinal (GI) hormone secreted in response to nutrient intake that has an essential role in normal glucose tolerance (1). GLP-1 is a potent insulin secretagogue thought to be a major component of the incretin effect (2). In addition to stimulating insulin release, iv administration of synthetic GLP-1 at supraphysiological doses inhibits glucagon secretion (3), delays gastric emptying (4), and reduces hepatic glucose production (5). This broad array of actions has led to the development of drugs to treat diabetes that are based on GLP-1 signaling (6).
Understanding the role of endogenous GLP-1 in metabolic physiology has been greatly enhanced by the availability of a potent GLP-1 receptor (GLP-1r) antagonist, exendin-[9–39] (Ex-9). In nonhuman primates and in healthy human subjects, administration of Ex-9 caused a 30–35% increase in postprandial glucose during an oral glucose tolerance test (OGTT) (7,8). In these studies plasma insulin levels were actually greater during GLP-1r blockade, partly because of increased glycemia. Thus, administration of Ex-9 during an OGTT demonstrates that GLP-1 signaling is necessary for glucose tolerance but does not permit an assessment of the insulinotropic effect of GLP-1 because of varying levels of glycemia. More recently, Schirra et al. (9) compared the incretin effect in healthy subjects by measuring insulin secretion during intraduodenal (ID) and isoglycemic glucose infusions with and without Ex-9. These studies demonstrated that after intestinal glucose absorption, Ex-9 reduces β-cell secretion, disinhibits glucagon release, and attenuates antral motility, suggesting that endogenous GLP-1 acts as both an incretin and enterogastrone.
Previous studies using Ex-9 in humans did not control for the differences in plasma glucose when assessing insulin secretion during GLP-1r blockade, and although Schirra et al. (9) demonstrated clear effects of Ex-9 on GI motility and contraction, they did not directly measure gastric emptying. In the study described below, we determined the contribution of GLP-1 to postprandial insulin release in healthy humans using Ex-9, but in the presence of clamped blood glucose levels, to compare β-cell function in a controlled setting. We also estimated gastric emptying using markers for intestinal transit to assess the relative roles of incretin and motility regulation by GLP-1 after eating.
Subjects and Methods
Subjects
There were 11 healthy men and women (six females and five males), 27–45 yr of age, that participated in two series of experiments. The subjects ranged in body mass index from 20–31 kg/m2, had no family history of type 2 diabetes mellitus, were free of any chronic medical conditions, and received no medications. The studies were approved by the institutional review board of the University of Cincinnati, and all participants provided written informed consent before the studies.
Peptides
GLP 1 (protein synthesis core laboratory, University of Washington) and Ex-9 (Clinalfa, Merck Biosciences AG, Läufelfingen, Switzerland) were synthesized using standard solid state techniques and were purified to greater than 95% by HPLC, and the authenticity was verified by mass spectroscopy and amino acid sequencing. Peptides were stored in lyophilized form at −20 C. Batches of lyophilized peptide sufficient for 2–3 months of experiments were weighed, and solubilized in 0.25% human serum albumin and stored frozen at −20 C. Aliquots were tested for sterility and pyrogenicity through the Research Pharmacy at Cincinnati Children’s Hospital. The use of synthetic GLP-1 and Ex-9 are approved under the U.S. Food and Drug Administration Investigational New Drugs 32,977 and 65,135.
Experimental protocols
All volunteers were weight stable for the 3 months before the studies, and were instructed to consume greater than 200 g carbohydrates for 3 d before each visit and not to engage in vigorous physical activity. They were admitted to the General Clinical Research Center on separate occasions after an overnight fast. Experiments in each subject were separated by intervals of at least 1 wk. Intravenous catheters were placed in each forearm for withdrawal of blood and infusion of hormones and glucose; the arm used for blood sampling was continuously warmed throughout each experiment by using a heating pad to arterialize the venous blood.
Study 1. Blockade of GLP-stimulated insulin secretion with Ex-9
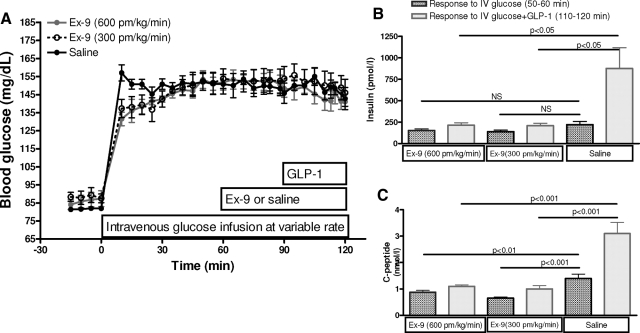
Four subjects participated in these studies. After removal of fasting blood samples in each experiment, a square wave of hyperglycemia was produced and maintained at a target blood glucose level of 8.3 mmol/liter as previously described (10). At 60 min, subjects received: 1) saline, or after priming dose of Ex-9; 2) Ex-9 at doses of 300 pm/kg · min; or 3) Ex-9 at doses of 600 pm/kg · min. At 90 min, GLP-1 at a rate of 0.5 pm/kg · min was infused for 30 min until the study was terminated (Fig. 1). Blood was sampled at 10-min intervals and stored on ice. Plasma was separated from blood cells within 60 min and stored at −80 C until assay.
Figure 1.
Antagonism of GLP-1 by Ex-9 in healthy humans. A, Blood glucose during the hyperglycemic clamps with GLP-1 infusion (0.5 pm/kg · min) alone (filled dark circles) or GLP-1 plus Ex-9 infusion at doses of 300 pm/kg · min (open circles) or 600 pm/kg · min (filled gray circle). In this paradigm, both doses of Ex-9 blocked the insulinotropic effect of GLP-1 comparing plasma insulin (B) and C-peptide (C) response to iv glucose infusion (dark bars) and iv glucose plus GLP-1 infusion (light bars) in three studies. Data presented as mean ± sem.
Study 2. Effect of GLP-1 on insulin secretion in response to oral glucose
There were 10 subjects that participated in this study, including three who had completed study 1. A modification of the iv-oral hyperglycemic clamp (11) was used to compare postprandial insulin release in response to regulatory factors such as GI hormones and neural stimuli compared with insulin release stimulated by an equal level of hyperglycemia alone. Subjects had preparation for studies as described in study 1, and at time zero, a glucose infusion was started to increase and maintain blood glucose at 8.9 mmol/liter. At 30 min, subjects received either: 1) an iv bolus of Ex-9 (7500 pm/kg) over 1 min, followed by a continuous infusion (750 pm/kg · min) for the remainder of the study; or 2) saline as a control; the order of the infusions was balanced so that half the subjects received first saline and half first Ex-9. Given the linear relationship between the dose of Ex-9 and its antagonist effect on the GLP-1r, and absence of any intolerance to infusions of the peptide in our first experiments, we adopted a higher dose of Ex-9 infusion for the blockade of endogenous GLP-1 than what was used in study 1 to increase the likelihood of a complete effect.
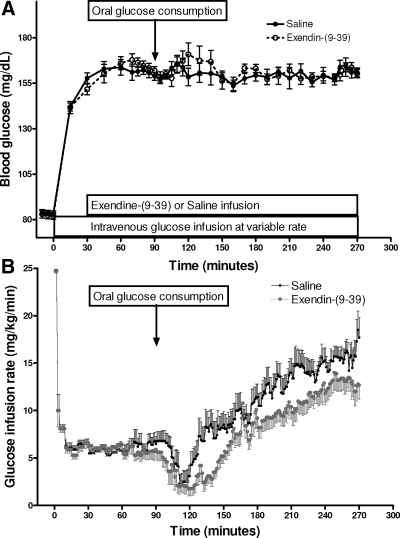
At 90 min, subjects ingested a 300-ml mixture of 75 g glucose with 15 g d-xylose, and 3 g 13C-glucose within 5 min (Fig. 2). The rate of iv glucose infusion was adjusted to maintain the blood glucose at the target rate throughout the study. Blood samples were drawn at −15, −10, 0, 15, 30, 45, 60, 70, 75, 80, 85, 90, 95,100, 105, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210, 220, 230, 240, 250, 255, 260, 265, and 270 min, stored on ice, and centrifuged within 60 min. Plasma was separated and stored at −80 C until assay.
Figure 2.
Blood glucose (A) and glucose infusion rate (B) during iv-oral glucose clamp studied with Ex-9 or saline infusion. Data presented as mean ± sem.
Assays
Blood samples were collected in tubes containing heparin for determinations of insulin, d-xylose, 13C-glucose, and glucose, and in tubes containing 50 mm EDTA plus 500 kallikrein inhibitory U/ml aprotinin for measurement of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), C peptide, and glucagon. Blood glucose concentrations were determined by the glucose oxidase method using a glucose analyzer (YSI 2300 STAT Plus; Yellow Springs Instruments, Yellow Springs, OH). Insulin concentrations were determined with a previously described RIA technique (12), and glucagon and C peptide were measured using commercial assay kits according to the manufacturer’s specifications (Linco Research, Inc., St. Charles, MO). Total GLP-1 was measured using an antibody directed against the COOH terminus of the peptide, recognizing both intact and metabolized GLP-1 (Linco Research). d-xylose was measured by colorimetric assay (13), and total GIP was measured by ELISA using a method that recognizes both intact and metabolized peptide (Linco Research). Plasma 13C-glucose enrichment was measured by gas chromatography-isotope ratio mass spectrometry and was expressed in atom percent excess as described before (14).
Calculations and analysis
Fasting concentrations of blood glucose and hormones were taken as the mean of the three samples drawn at the beginning of each day of study. The mean insulin levels from 70–90 min were used to calculate the insulin response to the clamped level of iv glucose, and the mean values from 95–270 min to calculate the insulin response to the clamp plus oral glucose.
Given that insulin concentrations increase steadily during a hyperglycemic clamp, we calculated anticipated insulin levels for the period of 95–270 min (ClampIns), had glucose not been ingested, using a formula previously derived (11) as:
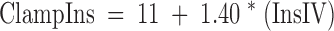 |
Where “InsIV” = mean insulin levels for 70–90 min during control studies.
The insulin response due to stimuli elicited by glucose ingestion (e.g. incretins, neural activation, portal glucose) was calculated for each individual in the control studies as:
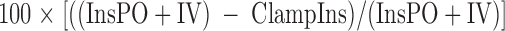 |
Where “InsPO+IV” = mean insulin levels for 95–270 min during the control studies.
The endogenous GLP-1 contribution to the enteral enhancement of insulin release (GLP-1 effect) was calculated as:
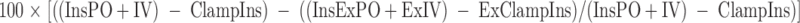 |
Where “InsExPO+ExIV” = mean insulin levels for 95–270 min during the Ex-9 studies, “ExClampIns” = mean insulin levels for 70–90 min during the Ex-9 studies.
Integrated values of 13C-glucose, d-xylose, glucagon, GLP-1, and GIP after oral glucose consumption were expressed as area under the response curve (AUC) over the level before glucose intake.
The parameters obtained from each subject in the two studies were compared using paired t-tests for data that followed a gaussian distribution and Wilcoxon rank sum test for data that were not normally distributed. Data are presented as the mean ± sem. Analyses were performed using Sigma Stat 3.0 (Richmond, CA) and SPSS 10.1(Chicago, IL).
Results
Blockade of GLP-1-stimulated insulin secretion by Ex-9
Blood glucose was clamped at a mean concentration of 8.3 ± 0.2, 8.4 ± 0.3, and 8.3 ± 0.2 mmol/liter during control, low-dose Ex-9, and high-dose Ex-9 studies, respectively (Fig. 1A). The coefficient of variation (CV) of blood glucose in steady state (50–120 min) was 8.5% within, and 2% between, the three studies. Although fasting insulin and C peptide did not differ among the three studies, during the initial part of the hyperglycemic clamp (50–60 min), the values differed slightly, 219 ± 38, 138 ± 18, and 151 ± 18 pmol/liter in the control, low-dose Ex-9, and high-dose Ex-9 studies with corresponding concentrations of C peptide of 1.4 ± 0.2, 0.7 ± 0.0, and 0.9 ± 0.1 nmol/liter (P < 0.05 for the Ex-9 studies compared with saline). The individual CVs for insulin release in response to hyperglycemia ranged from 10–40% among the three tests, which is consistent with the day-to-day variability of glucose-stimulated insulin release secretion (15). Infusion of GLP-1 during the glucose clamp caused a substantial increase in plasma insulin and C peptide to mean levels of 876 ± 242 pmol/liter and 3.1 ± 0.4 nmol/liter from 110–120 min in the control study. In contrast, the response to GLP-1 was inhibited by both doses of Ex-9 with mean insulin levels of 208 ± 28 and 214 ± 28 pmol/liter, and C-peptide levels of 1.0 ± 0.1 and 1.1 ± 0.1 nmol/liter from 110–120 min with the 300 and 600 pmol/kg · min doses, respectively. The lower dose of Ex-9 blocked GLP-1 stimulated insulin and C peptide by 90 and 84% respectively, whereas the higher dose caused 92 and 86% suppression (Fig. 1, B and C), suggesting that the antagonistic effect of synthetic Ex-9 on the insulinotropic effects of supraphysiological doses of GLP-1 during moderate hyperglycemia is dose dependent.
Effect of endogenous GLP-1 on oral glucose-stimulated insulin secretion
Blood glucose levels were rapidly increased from fasting values of 4.6 ± 0.1 mmol/liter to hyperglycemic levels of 8.8 ± 0.1 and 8.7 ± 0.1 mmol/liter, with the glucose infusion and maintained for the duration of the studies with and without Ex-9, respectively (Fig. 2A). The glucose infusion rates to reach the glycemic targets did not differ in the control and Ex-9 studies, and the mean CV for the glucose concentrations during hyperglycemia with iv glucose (70–90 min) was 5.5% for the two studies in each subject. After glucose ingestion the glucose infusion was initially reduced sharply to compensate for the glucose influx from the gut, and increased within 50 and 60 min after glucose ingestion and reached the final infusion rates of 2.8 and 2.2-fold of the premeal infusions in the control and Ex-9 studies (Fig. 2B). The mean glucose infusion rate necessary to maintain the postprandial glycemic target was significantly less, by approximately 25%, during the Ex-9 compared with control (P < 0.05). The mean CV for glucose values between subjects was 3.3% during the 3-h period after oral glucose consumption.
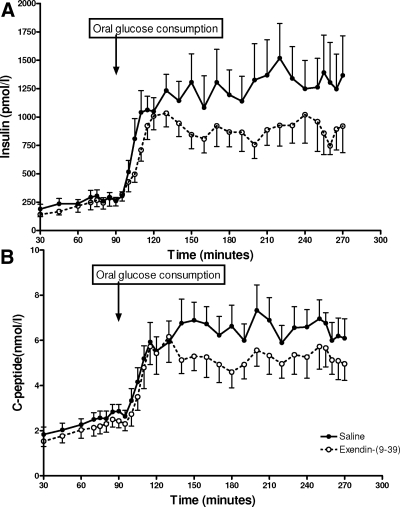
β-Cell secretion increased in response to iv glucose infusion during the control and Ex-9 studies, with no difference in insulin levels during hyperglycemia induced by iv glucose (70–90 min) in the control and Ex-9 studies (282.5 ± 42 and 263.8 ± 59 pmol/liter), but lower C-peptide levels during GLP-1r blockade (control, 2.7 ± 0.3 vs. Ex-9, 2.3 ± 0.3 nmol/liter; P < 0.05). After oral glucose ingestion (95–270 min), the mean insulin and C-peptide concentrations increased significantly in both the control and Ex-9 studies. However, Ex-9 treatment caused a significant reduction in postprandial plasma insulin (control, 1154 ± 203 vs. Ex-9, 835 ± 120 pmol/liter; P < 0.05) and C-peptide levels (control, 6 ± 0.7 vs. Ex-9, 4.9 ± 0.7 nmol/liter; P < 0.01) (Fig. 3, and Table 1).
Figure 3.
The insulin response to iv and oral glucose load during iv-oral glucose clamp with and without Ex-9 infusion shown as insulin (A) and C-peptide (B) values. Data presented as mean ± sem.
Table 1.
Effect of oral glucose ingestion on β-cell hormonal response and GI peptides in studies with and without Ex-9
| Time (min) | Saline infusion | Ex-9 infusion |
|---|---|---|
| Blood glucose (mmol/liter) | ||
| Baseline | 4.6 ± 0.1 | 4.6 ± 0.1 |
| 70–90 | 8.9 ± 0.1 | 9 ± 0.1a |
| 95–270 | 8.7 ± 0.1 | 8.8 ± 0.1 |
| Insulin (pmol/liter) | ||
| Baseline | 60.1 ± 13.2 | 58.0 ± 13 |
| 70–90 | 282.5 ± 42 | 263.8 ± 59 |
| 95–270 | 1154.8 ± 203 | 835.7 ± 120a |
| C peptide (nmol/liter) | ||
| Baseline | 0.8 ± 0.2 | 0.8 ± 0.1 |
| 70–90 | 2.7 ± 0.3 | 2.3 ± 0.3a |
| 95–270 | 6 ± 0.7 | 4.9 ± 0.7b |
| Glucagon (pg/ml) | ||
| Baseline | 47.9 ± 3.6 | 53.0 ± 5.4 |
| 80–90 | 43.8 ± 4.5 | 47.2 ± 4.6 |
| AUC glucagon(pg/ml · min) | ||
| 95–270 | 221 ± 353 | 1657 ± 699a |
| Glucose infusion rate (mg/kg · min) | ||
| 70–90 | 6.4 ± 0.8 | 5.6 ± 0.7 |
| 95–270 | 10.6 ± 1.0 | 8.1 ± 0.9a |
| d-xylose (mmol/liter) | ||
| 95–120 | 0.34 ± 0.03 | 0.38 ± 0.02 |
| 95–270 | 0.61 ± 0.05 | 0.69 ± 0.03 |
| AUC 13C-glucose enrichment (atom %) | ||
| 95–120 | 19.2 ± 2.2 | 20.1 ± 2.6 |
| AUC GLP-1 (pmol/liter · min)c | ||
| 95–270 | 531 ± 137 | 1208 ± 246b |
| AUC GIP (nmol/liter · min)c | ||
| 95–270 | 19.3 ± 2 | 19.7 ± 2 |
The paired t-test or Wilcoxon rank test was used for comparison of individual values during the respective periods.
P < 0.05 vs. saline infusion.
P < 0.01 vs. saline infusion.
GIP and GLP-1 values were measured for nine and eight subjects, respectively.
The stimulation of insulin release by factors elicited by glucose ingestion accounted for 65 ± 3.5% of the postprandial insulin response (range 51–88%). The GLP-1 contribution to enteral enhancement of insulin release, calculated from the difference in the insulin response after glucose consumption in the control and Ex-9 studies, was 30.1 ± 6% with a remarkable interindividual variation (range 8–66%) (Table 2).
Table 2.
Individual calculations of the postprandial insulin enhancement induced by enteral stimuli (PIE-enteral) and the relative GLP-1 contribution to this response derived from plasma insulin levels in 10 healthy subjects
| Subject no. | BMI (kg/m2) | Age (yr) | PIE-enteral (%) | GLP-1 effect (%) |
|---|---|---|---|---|
| 1 | 20 | 29 | 60 | 8 |
| 2 | 22 | 26 | 51 | 12 |
| 3 | 23 | 39 | 55 | 13 |
| 4 | 24 | 34 | 69 | 14 |
| 5 | 22 | 26 | 59 | 25 |
| 6 | 19 | 38 | 61 | 29 |
| 7 | 20 | 45 | 79 | 50 |
| 8 | 30 | 34 | 88 | 53 |
| 9 | 25 | 27 | 64 | 58 |
| 10 | 31 | 34 | 63 | 66 |
| Mean ± sem | 23.9 ± 1.4 | 33.2 ± 1.8 | 64.8 ± 3.5 | 32.8 ± 6.8 |
BMI, Body mass index.
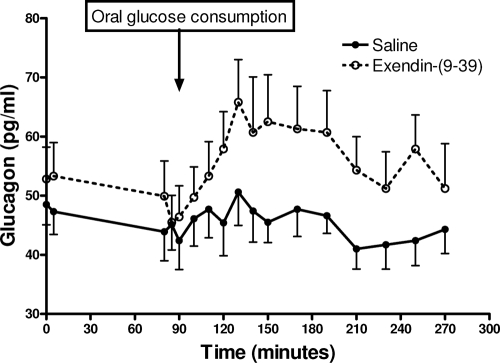
Fasting glucagon levels were 48 ± 4 and 53 ± 5 pg/ml during the control and Ex-9 studies, and decreased to 44 ± 5 and 47 ± 5 pg/ml during the first 90 min of the glucose clamp. There was no further change in plasma glucagon during the control study (mean of 45.5 ± 3.5 pg/ml from 95–270 min). Infusion of Ex-9 increased glucagon concentrations significantly after glucose intake compared with controls (Fig. 4). The glucagon AUC after oral glucose differed significantly between the two studies (Ex-9, 1657 ± 699 vs. control, 221 ± 3535 pg/ml · min; P < 0.05).
Figure 4.
Alteration in circulatory glucagon concentrations during iv-oral glucose clamp studied with and without Ex-9. Data prevented as mean ± sem.
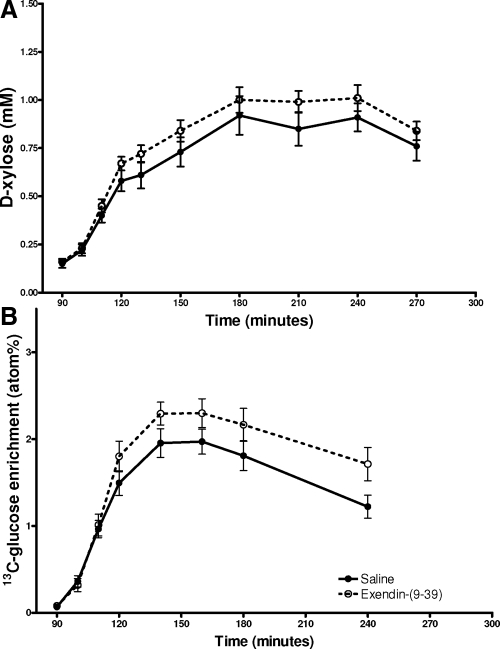
Plasma concentrations of d-xylose increased steadily after meal intake to a peak at 180–210 min of study (90–120 min after glucose ingestion). There was no difference in the plasma d-xylose values during the Ex-9 and control studies (Table 1 and Fig. 5A), indicating that the passage of the liquid meals from the stomach to the intestine was similar with and without blockade of GLP-1 action. Similarly, the initial increase in plasma 13C-glucose enrichment (90–120 min) was comparable in the two studies (AUCC-glucose13 19.2 ± 2 vs. 20.1 ± 2 atom percentage). The plasma 13C-glucose enrichment started to diverge between the two studies after 30 min, likely in response to the differences in plasma insulin with and without GLP-1r blockade (Fig. 5B). Comparing the average plasma concentrations of 13C-glucose and d-xylose in the first 30 min after oral glucose consumption showed a significant correlation in evaluating GI glucose appearance (ρ = 0.8; P < 0.01). Plasma GIP levels were comparable before meal ingestion in the two studies, and increased similarly in response to oral glucose intake with saline and Ex-9, suggesting that GLP-1r blockade has no effect on GIP secretion (Table 1). On the other hand, GLP-1 blockade significantly enhanced the GLP-1 AUC in response to food intake (P < 0.05; Table 1), whereas the baseline levels of GLP-1 were not different in studies with and without Ex-9 as previously reported (9).
Figure 5.
Changes in plasma d-xylose (A) and 13C-glucose (B) levels after oral glucose consumption studied with saline or Ex-9. Data presented as mean ± sem.
Discussion
In this study we sought to determine the role of endogenous GLP-1 on islet function and gastric emptying during the ingestion of a liquid glucose meal. Because the glycemic profile of an OGTT is significantly increased after GLP-1r blockade, interpretation of insulin responses is confounded. Therefore, we chose to use the iv-oral variant of the hyperglycemic clamp to maintain stable blood glucose levels before and after ingestion of the glucose load. In this setting, blocking GLP-1 action reduced postprandial insulin secretion by 30%, increased the glucagon response by 80%, but did not delay gastric emptying. These findings support the role of GLP-1 as an important regulator of postprandial glucose metabolism, and indicate that at least for liquid glucose ingestion, this effect is mediated primarily by changes in islet hormone concentrations.
In one of the first studies that used Ex-9 in humans, Edwards et al. (7) demonstrated that blocking the GLP-1r increased the postprandial glucose AUC by 35% after a liquid glucose meal. However, the overall insulin response between the two studies did not differ due to varying blood glucose levels. Thus, it was not possible to determine whether the increased glycemic excursion with Ex-9 was caused by disruption of the insulinotropic effects of GLP-1 or extra-islet effects, such as control of gastric emptying. Schirra et al. (9) studied the physiological effects of endogenous GLP-1 in healthy subjects during ID glucose administration with and without Ex-9. In their subjects glucose levels were 1 mmol/liter higher during the Ex-9 treatment, and insulin levels slightly lower confirming the importance of GLP-1 to control postprandial glucose in humans. On two subsequent occasions, the subjects were infused with glucose to match the glycemic levels of the Ex-9 and control ID studies, allowing calculation of the incretin effect by the classical method (2). In this experiment, GLP-1r blockade reduced the incretin effect, computed as the difference in insulin response between the ID glucose and matched isoglycemic studies, by approximately 50%. This is the most definitive demonstration that GLP-1 acts as an incretin in humans, is consistent with past estimates of the contribution of GLP-1 to postprandial insulin secretion based on exogenous infusion of GLP-1 (16,17), and suggests that factors besides GLP-1 contribute the remainder of the incretin effect after glucose intake.
Our finding of an approximate 30% contribution of GLP-1 to the incretin effect is lower than what was reported in the study of Schirra et al. (9). Some of this difference may be attributable to intersubject variability and the relatively small sample sizes of these physiological experiments. However, whereas they compared the incretin effect at different glucose levels during experiments with Ex-9 or saline, we made our comparisons of Ex-9 and control at nearly identical glycemia. This approach has some advantage in that the effect of GLP-1 on insulin secretion is amplified at higher levels of glycemia (16). Therefore the assumption that the incretin effect, the relative β-cell response to enteral and isoglycemic stimuli, will be constant across different levels of glucose is open to question. Our results are robust to this potential confound because blood glucose is fixed during a glucose clamp, and suggest a slightly lower contribution of circulating GLP-1 to postprandial insulin secretion than what has been previously described. However, our findings are in general agreement with the data of Schirra et al. (9) in that they demonstrate that GLP-1 is an important physiological stimulus of insulin secretion in humans after glucose absorption.
The effect of glucose ingestion to stimulate insulin levels beyond those measured at a comparable level of hyperglycemia from iv glucose alone was approximately 65% in our control experiments, compatible with estimations of the incretin effect using the more typical 2-d method (9). Interestingly, we saw some effect of GLP-1r blockade to lower circulating insulin concentrations during stimulation with iv glucose alone when plasma GLP-1 was barely detectable, as previously observed (18). This suggests that the effects of endogenous GLP-1 may not be limited to endocrine actions alone.
Oral and iv glucose administration suppresses glucagon concentrations. However, the magnitude of suppression is more pronounced during an isoglycemic clamp than after oral glucose ingestion at similar glycemic levels (19), suggesting that factors regulating glucagon secretion are different in the fasting vs. fed state. Glucagon levels were mildly suppressed by iv glucose in the initial phase of our studies, and we were not able to detect any differences in plasma glucagon between the oral and iv glucose phases of the control experiment. However, postprandial glucagon levels were 80% higher as the result of GLP-1r blockade. Infusion of GLP-1 is known to decrease glucagon levels (20). Our result is in keeping with a previous report that attributed much of the decline in plasma glucagon after ID glucose administration to the effects of circulating GLP-1 (9).
d-xylose is minimally metabolized after absorption and has been used as an indirect method of assessing gastric transit similar to other small molecule markers such as acetaminophen (21). We also mixed 13C-glucose into our liquid glucose solution in eight subjects to compare the plasma d-xylose response with 13C-glucose enrichment. Because of the differences in insulin secretion and action in two studies, 13C-glucose appearance is not a good index of gastric emptying after the initial phase of absorption. However, the increase in 13C-glucose in the first 30 min after the meal correlated well with the increase in plasma d-xylose, suggesting that the two carbohydrates were absorbed at similar rates. Because there was no difference in the appearance of d-xylose with and without Ex-9, it appears that endogenous GLP-1 does not have a major impact on the emptying of a liquid glucose meal from the stomach. The sample size of 10 in our study was estimated to be sufficient to detect 20% difference in d-xylose appearance with the power of 70%. Thus, although we cannot exclude any effect of GLP-1 on gastric emptying, in our experimental model such an effect would be small.
Administration of exogenous GLP-1 has complex actions on gastric function (4,22) and has even been suggested to be the major mechanism by which GLP-1 controls postprandial glucose (23). It is generally agreed that at least at supraphysiological levels, GLP-1 delays gastric emptying by increasing antropyloric tone (9,24) and relaxing the gastric fundus to hold nutrients (25). Despite numerous studies in this area, there has been no previous assessment of the effects of endogenous GLP-1 on gastric emptying of an oral glucose load in humans. Our findings in this study, based on d-xylose appearance, are similar to what we reported previously in studies with Ex-9 in nonhuman primates (8), and are supported in this present data set by the identical enrichment of plasma with 13C-glucose in the first 30 min after glucose consumption. Although these findings are somewhat surprising in light of the substantial body of evidence supporting delayed gastric emptying as a major extrapancreatic action of GLP-1, we believe they identify a key distinction between physiological and pharmacological effects. Our results are also compatible with those from a recent study that showed administration of dipeptidyl peptidase-4 inhibitors, which enhance endogenous GLP-1 by 3-fold, did not alter meal appearance and gastric emptying in patients with type 2 diabetes (26). Nonetheless, it will be important to examine the effects of endogenous GLP-1 on gastric function during solid meal consumption to understand fully the role of this incretin in postprandial metabolism.
One interesting finding in this cohort of healthy subjects was that the calculated values of GLP-1 contribution to insulin secretion after oral glucose consumption were remarkably diverse (ranging from 8–66%). This was greater than the range of our calculated surrogate for the incretin effect (51–88%). This interindividual variability in the insulinotropic effect of endogenous GLP-1 could not be accounted for by between-study differences in blood glucose, gastric emptying, or plasma levels of GIP or GLP-1 for each individual. It is notable that we did not find any relationship within individuals of GLP-1 effects on insulin and glucagon responses, although this was not a primary objective of the study. Interindividual variability in GLP-1 secretion has been studied previously (27), but we are unaware of systematic comparisons of variability in GLP-1 action.
In summary, we have examined the effects of endogenous GLP-1 that mediate oral glucose tolerance. In a setting of fixed blood glucose, slightly above normal postprandial concentrations, blockade of the GLP-1r causes a reduction in the insulin response and increased plasma glucagon but does not affect gastric emptying. These findings distinguish between actions of GLP-1 that are physiological, like the regulation of islet hormone secretion, and others, such as gastric emptying, that may only pertain at pharmacological GLP-1 levels. Continued elucidation of the role of GLP-1 in normal physiology is important for understanding metabolic dysfunction in diabetes and has increased relevance with the advent of GLP-1 based therapies (6).
Acknowledgments
We thank Kay Ellis, Clinton Elfers, Ron Bitner, and the nursing staff from the Clinical Research Center of Cincinnati Children’s Hospital for their expert technical assistance.
Footnotes
This work was supported by National Institutes of Health Grants DK57900-05 (to D.A.D.) and M01-RR-08084 (Cincinnati Children’s Hospital General Clinical Research Center). T.P.V. is currently in the Department of Medicine at Mt. Sinai School of Medicine.
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 30, 2008
Abbreviations: AUC, Area under the response curve; CV, coefficient of variation; Ex-9, exendin-[9–39]; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP, glucagon-like peptide; GLP-1r, glucagon-like peptide 1 receptor; ID, intraduodenal; OGTT, oral glucose tolerance test.
References
- Kieffer TJ, Habener JF 1999 The glucagon-like peptides. Endocr Rev 20:876–913 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W 1986 Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 63:492–498 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA 1996 Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care 19:580–586 [DOI] [PubMed] [Google Scholar]
- Naslund E, Gutniak M, Skogar S, Rossner S, Hellstrom PM 1998 Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr 68:525–530 [DOI] [PubMed] [Google Scholar]
- Prigeon RL, Quddusi S, Paty B, D'Alessio DA 2003 Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:E701–E707 [DOI] [PubMed] [Google Scholar]
- Ahren B, Schmitz O 2004 GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 36:867–876 [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR 1999 Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 48:86–93 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Vogel R, Prigeon R, Laschansky E, Koerker D, Eng J, Ensinck JW 1996 Elimination of the action of glucagon-like peptide 1 causes an impairment of glucose tolerance after nutrient ingestion by healthy baboons. J Clin Invest 97:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B 2006 Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Andersen DK, Elahi D, Brown JC, Tobin JD, Andres R 1978 Oral glucose augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest 62:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA 2006 β-Cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab 91:185–191 [DOI] [PubMed] [Google Scholar]
- Eberts TJ, Sample RH, Glick MR, Ellis GH 1979 A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem 25:1440–1443 [PubMed] [Google Scholar]
- Tissot S, Normand S, Guilluy R, Pachiaudi C, Beylot M, Laville M, Cohen R, Mornex R, Riou JP 1990 Use of a new gas chromatograph isotope ratio mass spectrometer to trace exogenous 13C labelled glucose at a very low level of enrichment in man. Diabetologia 33:449–456 [DOI] [PubMed] [Google Scholar]
- Bardet S, Pasqual C, Maugendre D, Remy JP, Charbonnel B, Sai P 1989 Inter and intra individual variability of acute insulin response during intravenous glucose tolerance tests. Diabete Metab 15:224–232 [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Madsbad S, Holst JJ 2003 Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept 114:115–121 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W 1993 Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7–36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab 76:912–917 [DOI] [PubMed] [Google Scholar]
- Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M 1998 Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J Clin Invest 101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Deacon CF, Schmidt WE, Holst JJ, Nauck MA 2007 Suppression of glucagon secretion is lower after oral glucose administration than during intravenous glucose administration in human subjects. Diabetologia 50:806–813 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH 2002 Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87:1239–1246 [DOI] [PubMed] [Google Scholar]
- Medhus AW, Sandstad O, Bredesen J, Husebye E 2000 Stimulation of the small intestine by nutrients in relation to phase of the migrating motor complex. Scand J Gastroenterol 35:494–500 [DOI] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ 1993 Truncated GLP-1 (proglucagon 78–107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38:665–673 [DOI] [PubMed] [Google Scholar]
- Nauck MA 1999 Is glucagon-like peptide 1 an incretin hormone? Diabetologia 42:373–379 [DOI] [PubMed] [Google Scholar]
- Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B 1996 Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M 2002 Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 282:G424–G431 [DOI] [PubMed] [Google Scholar]
- Vella A, Bock G, Giesler PD, Burton DB, Serra DB, Saylan ML, Dunning BE, Foley JE, Rizza RA, Camilleri M 2007 Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 56:1475–1480 [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ 2001 Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723 [DOI] [PubMed] [Google Scholar]