Abstract
Context: Patients with adrenal and extra-adrenal abdominal paraganglioma (PGL) almost invariably have increased plasma and urine concentrations of metanephrines, the O-methylated metabolites of catecholamines. We report four cases of biochemically silent abdominal PGL, in which metanephrines were normal despite extensive disease.
Objective: Our objective was to identify the mechanism underlying the lack of catecholamine hypersecretion and metabolism to metanephrines in biochemically silent PGL.
Design: This is a descriptive study.
Setting: The study was performed at a referral center.
Patients: One index case and three additional patients with large abdominal PGL and metastases but with the lack of evidence of catecholamine production, six patients with metastatic catecholamine-producing PGL and a mutation of the succinate dehydrogenase subunit B (SDHB) gene, and 136 random patients with catecholamine-producing PGL were included in the study.
Main Outcome Measures: Plasma, urine, and tumor tissue concentrations of catecholamines and metabolites were calculated with electron microscopy and tyrosine hydroxylase immunohistochemistry.
Results: All four patients with biochemically silent PGL had an underlying SDHB mutation. In the index case, the tumor tissue concentration of catecholamines (1.8 nmol/g) was less than 0.01% that of the median (20,410 nmol/g) for the 136 patients with catecholamine-producing tumors. Electron microscopy showed the presence of normal secretory granules in all four biochemically silent PGLs. Tyrosine hydroxylase immunoreactivity was negligible in the four biochemically silent PGLs but abundant in catecholamine-producing PGLs.
Conclusions: Patients with SDHB mutations may present with biochemically silent abdominal PGLs due to defective catecholamine synthesis resulting from the absence of tyrosine hydroxylase. Screening for tumors in patients with SDHB mutations should not be limited to biochemical tests of catecholamine excess.
Succinate dehydrogenase subunit B-related paragangliomas may be biochemically silent as a result of defective catecholamine synthesis due to absence of tyrosine hydroxylase.
Paragangliomas (PGLs) derive from either sympathetic tissue in adrenal and extra-adrenal locations, or from parasympathetic tissue of the head and neck (1). Adrenal PGLs are usually referred to as pheochromocytomas. Most patients with adrenal and extra-adrenal abdominal PGLs have increased plasma and urine concentrations of catecholamines (dopamine, norepinephrine, and epinephrine). In about 8–9% of patients with sporadic PGL and 21–31% with hereditary PGL, plasma concentrations and urinary outputs of catecholamines are normal (2). Nevertheless, such patients invariably have elevated plasma concentrations of the metanephrines, normetanephrine, and metanephrine. These O-methylated metabolites of norepinephrine and epinephrine are produced continuously within tumor cells and independently of catecholamine release, which can be variable or negligible, even in patients with large tumors (3). Exceptions in which plasma metanephrines can be normal include patients with very small tumors (<1 cm) that do not synthesize and metabolize sufficient amounts of catecholamines to produce positive test results (4). Other rare exceptions include patients with PGLs that only produce dopamine and which may be detected by increases in plasma methoxytyramine, the O-methylated metabolite of dopamine (5,6). In contrast to PGLs derived from sympathetic tissue, the vast majority of head and neck PGLs do not produce significant amounts of catecholamines (7).
Although there have been several reports of patients with abdominal PGLs of considerable size, yet normal plasma and urine catecholamine concentrations (8,9,10,11,12,13), it is unclear whether the normal catecholamines in these patients reflect defective secretion or an absence of releasable stores due to defects in synthesis or storage. Here, we report data from four patients with abdominal PGLs due to mutations of the gene for succinate dehydrogenase B (SDHB), all with large tumors or extensive metastatic disease and all without evidence of catecholamine hypersecretion. More importantly, all patients had normal plasma concentrations and urinary outputs of metanephrines, tumor biomarkers commonly recognized to provide positive test results in all but the smallest of tumors. To identify the defect underlying the lack of catecholamine hypersecretion and metabolism to metanephrines, we examined the biochemical, ultrastructural, and functional properties of the tumors in these patients. Data were compared with those from another group of patients with PGLs due to SDHB mutations, all of which showed consistent evidence of catecholamine production and metabolism.
Patients and Methods
Patients
Our index case no. S1, was a patient with a mutation of the SDHB gene and a large abdominal PGL with additional metastatic disease but the lack of evidence of catecholamine hypersecretion. We subsequently studied three additional patients (nos. S2–4) with malignant PGL and lack of catecholamine hypersecretion. All patients were included in a previous report on SDHB-related PGL (11), in which the lack of catecholamine hypersecretion was documented but in which the mechanism was not investigated or identified. Six patients (nos. C1–6) with metastatic catecholamine-producing SDHB-associated PGLs were included in the study for comparison. Individual patient characteristics are given in Table 1.
Table 1.
Patient characteristics
| Patient no. | Sex, age at diagnosis (yr) | SDHB mutation | Primary tumor location | Primary tumor size (cm) | Metastases location | Time until metastases (yr) | Symptoms at initial diagnosis |
|---|---|---|---|---|---|---|---|
| S1 | M, 38 | C196Y | L retroperitoneal | 15 × 10 × 7 | lu, b | 0 | Pain |
| S2 | M, 39 | R46Q | L extra-adrenal abd | 15 × 11.7 × 7.5 | ln, me, b | 0 | Pain |
| S3 | M, 60 | W200C | Aortic bifurcation | 3 × 1 × 2 | ln, lu, b | 0.2 | Pain, DVT, hematuria |
| S4 | M, 29 | G96D | L pararenal | 18 × 16 × 8.5 | b | 1.7 | Pain, fatigue |
| C1 | F, 37 | V140F | Pelvic | 15 × 9 × 6.2 | ln, li | 0.8 | None, incidentaloma |
| C2 | F, 24 | I127S | L juxta-adrenal | 3.5 × 2.8 × 3.8 | b | 3.5 | CA sympt, pain |
| C3 | F, 45 | V140F | R paraaortic abd | 5.5 × 4 × 3.5 | ln | 0 | CA sympt, dizziness |
| C4 | M, 10 | R46X | L para-adrenal | 3 × 3 × 2 | ln, lu, b, me | 0 | CA sympt, fatigue |
| C5 | F, 34 | R115X | Bladder | 2.7 × 2 × 1.5 | ln, lu | 2.5 | CA sympt |
| C6 | M, 35 | IVS3-1G>C | L pararenal | 14.5 × 8 × 7.5 | ln, li, b | 0.5 | CA sympt |
b, Bone; CA sympt, symptoms related to catecholamines excess; DVT, deep venous thrombosis; F, female; L, left; li, liver; ln, abdominal lymph node; lu, lung; M, male; me, mediastinum; R, right.
In a previous study, we showed a strong positive relationship between tumor size and summed plasma concentrations of metanephrine and normetanephrine (4). The correlations between tumor volume, catecholamine content, and summed plasma concentrations of normetanephrine and metanephrine in the 136 patients with catecholamine-producing PGLs from the previous report (4) were used as a comparison for our index case (no. S1). Samples of frozen tissue from primary tumors were not available from the three other patients (nos. S2–4) for this part of the study because these patients underwent surgery outside the National Institutes of Health (NIH), before referral to our center. The protocol for this study was approved by the Institutional Review Board of the National Institutes of Child Health and Human Development at the NIH. All patients provided written informed consent.
Plasma, urine, and tissue catecholamines and metabolites
Blood samples were collected into heparin-containing tubes by use of a forearm venous cannula, with patients supine for at least 20 min before sampling. Samples were collected on ice, and plasma was separated and stored at −80 C before analysis according to recommended procedures (14). Plasma was assayed by HPLC for concentrations of dopamine, norepinephrine, epinephrine, and their free O-methylated metabolites methoxytyramine, normetanephrine, and metanephrine as described previously (15,16). The 24-h urinary outputs of catecholamines and deconjugated (free plus conjugated) fractionated metanephrines were measured by liquid chromatography with electrochemical detection or tandem mass spectroscopy under a contract between the NIH Clinical Center and an outside commercial laboratory (Mayo Medical Laboratories, Rochester, MN).
Concentrations of dopamine, norepinephrine, and epinephrine in samples of frozen tumor tissue were quantified by liquid chromatography with electrochemical detection (4). Samples were obtained within 90 min of surgical removal of PGL, weighed, frozen, and homogenized in five to 10 volumes of 0.4 m perchloric acid containing 0.5 mm EDTA. Homogenates were centrifuged and supernatants collected for catecholamine determinations. Total tumor catecholamine content was calculated by multiplying tumor tissue concentration of catecholamines (epinephrine + norepinephrine) by tumor volume. The relations between tumor volume, tumor catecholamine content, and summed plasma concentrations of metanephrine and normetanephrine were evaluated by log-linear regression.
Electron microscopy
Electron microscopy of PGL was performed to examine the presence and morphology of secretory granules, the intracellular storage units for catecholamines. Paraffin-embedded tissue samples of primary tumors from patient nos. S1–4, and C2 and 4 were deparaffinated in xylene and embedded in epoxy resin for electron microscopy. Ultrathin sections were double stained with uranyl acetate and lead citrate, and examined and photographed with a Phillips CM10 electron microscope (Phillips Electronic Instruments, Mahway, NJ).
Tyrosine hydroxylase immunohistochemistry
The enzyme tyrosine hydroxylase converts l-tyrosine to l-dihydroxyphenylalanine, which is the initial and rate-limiting step in catecholamine synthesis (17). Immunohistochemical staining for tyrosine hydroxylase was performed as previously described (18) on formalin-fixed paraffin-embedded sections of primary tumors, which were available in all patients, i.e. in nos. S1–4 and C1–6. Sections were preincubated for 1 h in a blocking solution consisting of 0.1 m PBS containing 10 mm Tris PBS (TPBS), 0.3%Triton X-100, 0.1% (wt/vol) sodium azide, and 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Sections were incubated with mouse monoclonal antibody to tyrosine hydroxylase (1:250; ImmunoStar, Inc., Hudson, WI) for 24 h at 4 C in a humidified chamber. Sections were washed (3 × 10 min in TPBS) and then incubated for 4 h at room temperature with secondary antibody (donkey antimouse Alexa Fluor 488, 1:500; Molecular Probes, Inc., Eugene, OR). Sections were washed (3 × 10 min in TPBS) and mounted in ProLong Gold Antifade (Molecular Probes). Rat adrenal sections, used as negative controls, were processed concurrently according to the same methods, with omission of the primary antibody. Sections were examined using a Zeiss LSM PASCAL 5 (Carl Zeiss MicroImaging, Inc., Thornwood, NY) equipped with Argon/2 458–514 to excite fluorescein isothiocyanate. Emission was collected through the fluorescein isothiocyanate filter (bandpass 505–530) using a 25× NeoFluor objective lens, with pinhole, detector gain, and laser power adjusted accordingly to acquire approximately 5-μm thick sections with few saturated pixels. Images were acquired with LSM 5 Image Browser software (Carl Zeiss MicroImaging). Although overall contrast was adjusted, no other modifications were made. Staining was described as negligible, moderate, or intense, and the distribution pattern of immunoreactive cells was noted.
Results
Case histories of patients with biochemically silent PGL
No. S1 (index case)
At age 38 yr, this male patient underwent a workup of left-sided abdominal pain, including computed tomography (CT) of the abdomen. This revealed a 15-cm left retroperitoneal mass and a small lesion of the right lower lung lobe. The lung lesion was removed by wedge excision, and identified as a metastatic lesion of a PGL. He lacked a history of signs and symptoms classically related to catecholamine excess; more specifically, there was no hypertension or reports of palpitations, headache, and diaphoresis. He was then referred to the NIH where a retroperitoneal PGL was resected along with the left kidney and spleen. Plasma concentrations and urine output of catecholamines and metanephrines were normal before and after surgery (Table 2). However, plasma levels of chromogranin A were elevated and remained elevated at 60% above the upper reference limit (URL) after surgery. An additional left pulmonary metastasis was removed. One year later, anatomical imaging and [123]-metaiodobenzylguanidine (MIBG) scintigraphy showed additional metastases in the left iliac bone and the second thoracic vertebral body. The patient underwent lumbar vertebroplasty, [131]-MIBG treatment, and chemotherapy (cyclophosphamide, vincristine, dacarbazine) resulting in stable disease. An underlying C196Y SDHB mutation was identified.
Table 2.
Biochemical phenotype
| Patient no. | Time since surgery (yr) | Plasma catecholamines and metabolites
|
Urine catecholamines and metabolites
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA (pg/ml) | MTY (pg/ml) | NE (pg/ml) | NMN (pg/ml) | E (pg/ml) | MN (pg/ml) | DA (μg/d) | NE (μg/d) | NMN (μg/d) | E (μg/d) | MN (μg/d) | VMA (mg/d) | ||
| S1 | 0.4 | 14 | 3 | 231 | 33 | 15 | 26 | 205 | 37 | 361 | 1.9 | 146 | 5.4 |
| S2 | 1.3 | 16 | NA | 261 | 48 | 38 | 56 | 402 | 70 | 338 | 23.0a | 250 | 5.3 |
| S3 | 1.1 | 18 | 6 | 544a | 71 | 21 | 29 | 240 | 47 | 394 | 5.9 | 161 | NA |
| S4 | 2.4 | 11 | 7 | 122 | 30 | 24 | 22 | 154 | 19 | 184 | 5.1 | 63 | 2.6 |
| C1 | 2.0 | 160 | 93 | 571 | 60 | 16 | 15 | 381 | 46 | 268 | 1.0 | 123 | NA |
| C2 | 0.7 | 7 | 14 | 598 | 187 | 4 | 48 | 249 | 113 | 764 | 2.1 | 74 | NA |
| C3 | 0.2 | 13 | 4 | 1986 | 470 | 24 | 14 | 262 | 435 | 2736 | 6.7 | 128 | 10.8 |
| C4 | pre | 2077 | 297 | 10065 | 1462 | 11 | 10 | 1207 | 1496 | 7410 | 3.5 | 257 | 29.3 |
| C5 | 3.5 | 16 | 26 | 789 | 285 | 7 | 24 | 363 | 247 | 1610 | 1.0 | 111 | NA |
| C6 | 1.6 | 810 | 945 | 2649 | 675 | 11 | 17 | 457 | 64 | 692 | 5.0 | 85 | 6.7 |
| URL | 46 | 14 | 498 | 112 | 83 | 61 | 400 | 71 | 521 | 20.0 | 261 | 40 | |
Results presented as absolute values, or times URL. Values that exceed the URL are printed in bold. DA, Dopamine; E, epinephrine; MN, metanephrine; MTY, methoxytyramine; NA, not available; NE, norepinephrine; NMN, normetanephrine; pre, presurgical; VMA, vanillylmandelic acid.
One-time abnormal result; repeated testing consistently showed normal values, number obtained outside the NIH.
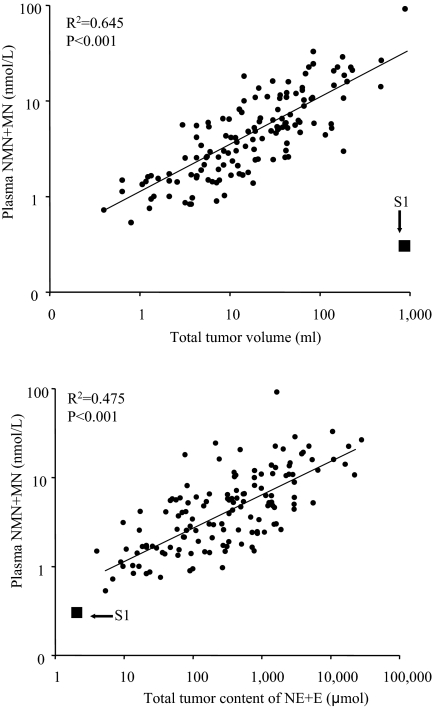
Concentrations of catecholamines in the primary retroperitoneal tumor were 0.9 nmol/g each for norepinephrine and epinephrine, and 0.03 nmol/g for dopamine. The tissue concentration of total catecholamines (1.8 nmol/g) of the tumor from this index patient was less than 0.01% that of the median (20,410 nmol/g) for the 136 patients with catecholamine-producing tumors and less than 0.4% the lowest concentration (521 nmol/g) for any single tumor. Consequently, the data point for this patient fell completely outside of the normal relationship between tumor volume and plasma concentrations of total metanephrines (Fig. 1, upper panel). However, after correction for differences in tissue catecholamine concentrations, the data point for the patient did fit in with the expected relationship of total tissue catecholamine contents with plasma concentrations of metanephrines (Fig. 1, lower panel). The findings of normal plasma metanephrines, despite large tumor volume in no. S1, representing a mismatch in the usual relationship between tumor volume and plasma metanephrines, could not be explored in the additional three cases (nos. S2–4) because they were operated outside the NIH, and no frozen tumor tissue was available for tissue catecholamine determinations.
Figure 1.
Relationship between tumor volume and plasma free metanephrines (MN) (upper panel), and between tumor catecholamine content and plasma free metanephrines (lower panel) in catecholamine-secreting PGLs. The arrows mark the data points for the biochemically silent tumor in patient no. S1. E, Epinephrine; NE, norepinephrine; NMN, normetanephrine.
Additional cases (nos. S2–4)
No. S2
From age 22 yr, this male patient experienced lower back pain after exercise. He sought medical attention after the pain became constant at age 26 yr. CT showed lytic lesions of the third and fourth lumbar vertebral bodies, a 15-cm necrotic mass in the caudal retroperitoneum, and multiple pathologically enlarged lymph nodes of the mediastinum. The retroperitoneal mass was removed, along with several regional lymph nodes. A PGL with local lymph node involvement and distant metastases was diagnosed. The patient’s history was negative for signs and symptoms of catecholamine excess. Despite the presence of metastases, plasma concentrations of catecholamines and their O-methylated metabolites were repeatedly normal, except for an isolated slight increase in epinephrine, which was not related to elevated metanephrine (Table 2). In addition, there was a one-time increase of urine dopamine just above the upper reference level, whereas plasma dopamine was normal. The plasma concentration of chromogranin A was elevated 2.9-fold above the upper limit of normal. The patient underwent six cycles of chemotherapy (cyclophosphamide, vincristine, dacarbazine) without response, and lumbar vertebroplasty. At age 29 yr, he had progressive disease, as shown by [123]-MIBG scintigraphy, and started [131]I-MIBG treatment. He is a carrier of an R46Q SDHB mutation.
No. S3
At age 58 yr, this patient developed pelvic pain, hematuria, weight loss, and deep venous thrombosis of the left leg. CT showed a 6-cm retroperitoneal mass adherent to the aortic bifurcation, with extension into the bony pelvis and the lumbosacral spine. During exploratory surgery, the mass was partially resected, and identified as a partially necrotic PGL with neuronal and lymphovascular invasion. The patient had no signs or symptoms of catecholamine excess. Subsequently, multiple vertebral and pulmonary metastases were found on CT and [123]-MIBG scintigraphy. He underwent cyber knife surgery of the remaining abdominal tumor, and external beam radiation therapy of vertebral lesions, resulting in partial pain relief. He was then referred to the NIH for further evaluation and treatment of metastatic PGL. Plasma concentrations and urine output of catecholamines and metanephrines were repeatedly normal, except for an isolated increase in plasma norepinephrine in the presence of a normal normetanephrine (Table 2). The plasma concentration of chromogranin A was elevated by 80% above the upper limit of normal. One year after the diagnosis, he underwent [131]I-MIBG treatment, resulting in further symptomatic relief. However, during the following 2 yr, he had gradually progressive disease. He is a carrier of a W200C SDHB mutation.
No. S4
This male patient presented at age 29 yr with an 8-month history of a left-sided varicocele and upper abdominal discomfort, which led to the discovery of an 18-cm mass near the left renal hilum on CT. From early childhood on, he had experienced recurrent anxiety attacks, accompanied by palpitations, headache, sweating, and occasional chest pain. He had a history of uncomplicated surgery of a left-sided cystic hydroma of the neck at age 23 yr. The retroperitoneal mass was resected, along with the left kidney and regional lymph nodes. The histopathology revealed an 18-cm PGL with vascular invasion, and tumor-negative lymph nodes. During follow-up at the NIH, 1.7 yr after surgery, multiple [123]-MIBG positive metastatic lesions throughout the skeleton were found. Plasma concentrations and urine output of catecholamines and O-methylated metabolites were consistently normal (Table 2). Plasma chromogranin A level was not determined. [131]I-MIBG treatment resulted in a partial response. He was found to have a G69D SDHB mutation.
Cases with catecholamine-secreting tumors
Plasma and urine findings in patients with SDHB-related catecholamine-secreting tumors were consistent with hypersecretion of norepinephrine and dopamine in patient nos. C1, 4, and 6, and norepinephrine alone in patient nos. C2, 3, and 5 (Table 2). As shown in Table 2, the metastases in no. C1 predominantly hypersecreted dopamine. However, before resection of the primary tumor at the referring institute, there were consistent elevations of plasma norepinephrine and normetanephrine, and urinary excretion of norepinephrine and normetanephrine (2.1- to 3.8-fold above the upper limits of normal).
Electron microscopy and tyrosine hydroxylase immunohistochemistry
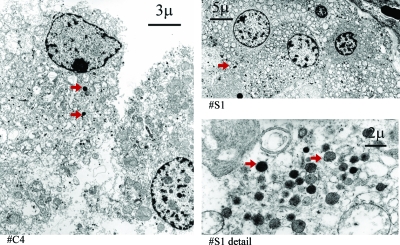
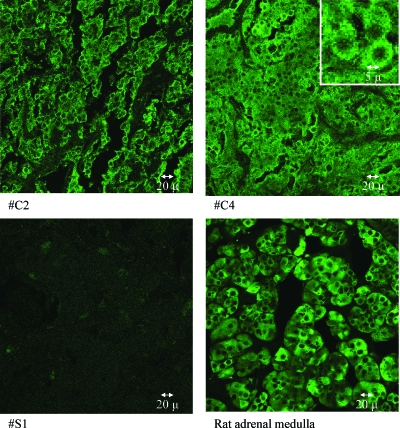
On electron microscopy, the catecholamine-secreting PGL and biochemically silent PGLs showed a similar cellular morphology (Fig. 2). This included the presence of electron-dense secretory granules in the cells of both tumor types. In the tumor of one patient (no. S1), the secretory granules appeared to be larger and less dense compared with the other tumors (Fig. 2). Tyrosine hydroxylase staining was intense in rat adrenal medulla and throughout all sections obtained from the PGLs that produced catecholamines from patient nos. C1–6 (Fig. 3). Staining was negligible in the tumors of all four patients (nos. S1–4) in whom there was no evidence of catecholamine hypersecretion.
Figure 2.
Electron microscopy of a catecholamine-secreting PGL (patient no. C4, left panel) and a biochemically silent PGL (patient no. S1, right panels). Red arrows indicate examples of secretory granules. Secretory granules are present in both the secreting and silent tumor.
Figure 3.
Tyrosine hydroxylase immunohistochemistry in two catecholamine-secreting PGLs (patient nos. C2 and C4, upper panels) in a biochemically silent PGL (patient no. S1, left-lower panel) and in rat adrenal medulla (right-lower panel). Tyrosine hydroxylase activity is abundant in the human and rat controls but absent in the silent tumor.
Discussion
Patients with catecholamine-producing PGLs but normal plasma concentrations and urinary outputs of catecholamines invariably do have elevated plasma and urine concentrations of metanephrines (3,4). However, plasma concentrations of metanephrines can be normal in patients with very small tumors or in those with rare PGLs that only produce dopamine (5,6,13). We now show that plasma concentrations and urinary outputs of metanephrines can also be normal in some cases of large metastatic SDHB-related PGLs. We found that the lack of catecholamine secretion and metabolism by these tumors results from a defect in the synthesis rather than in the storage or release of catecholamines, as indicated by the absence of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis (17), but the presence of storage vesicles, and the accumulation of MIBG. This report provides the first unequivocal evidence for the existence of such truly biochemically silent abdominal PGLs.
The lack of catecholamine hypersecretion poses a significant diagnostic challenge, as illustrated by the present and previously reported cases (8,9,10,12,13). Due to the absence of typical symptoms of catecholamine excess such as headache, palpitations, diaphoresis, and anxiety, the diagnosis of such tumors is usually delayed until an advanced stage of the disease. This delay may contribute to the development of malignant disease. Similar to PGLs that secrete only dopamine (5,6), patients with biochemically silent PGL present with advanced disease and atypical symptoms caused by tumor mass effects rather than catecholamine excess.
Biochemically silent abdominal PGLs appear to share features with parasympathetic head and neck PGLs, which invariably do not secrete or produce catecholamines (19). Similar to head and neck PGLs, one nonsecreting abdominal PGL was previously shown to be negative on Gomori’s stain for chromaffin tissue (12,20). However, the actual catecholamine content of tumor tissue was not measured in any of the previously reported, supposedly biochemically silent tumors (8,9,10,11,12,13). We now clearly show in our index patient that tumor tissue concentration of epinephrine, norepinephrine, and dopamine was negligible (<0.01%) compared with those of other patients with abdominal and adrenal PGLs. For the measurement of tumor catecholamines, frozen tissue specimens are required, which were not available for the additional three cases. However, these PGLs very likely also represented truly silent tumors because, despite their large size, plasma and urinary metanephrines were not increased, and there was a lack of staining for tyrosine hydroxylase.
Biochemically silent tumors may develop from subsets of undifferentiated cells originating from the primitive neural crest that lack the ability to produce catecholamines. Alternatively, tumor cells may specifically lose the machinery to produce catecholamines as part of tumor dedifferentiation; the loss of tyrosine hydroxylase may occur before the PGL is large enough to secrete significant amounts of catecholamines. The latter might explain why none of the present patients had a transient history of endocrine symptoms. In line with the possibility of tumor dedifferentiation, tyrosine hydroxylase immunoreactivity has been demonstrated elsewhere in three primary head and neck PGLs, but not in their metastatic lesions (21). Other than patients with small tumors (<1 cm) or with head and neck PGLs, the four reported here are the only patients in whom plasma concentrations of O-methylated metabolites have been normal in over 350 cases we have studied to date. Remarkably, all four patients had SDHB mutations. Whether the absence of catecholamine production and tyrosine hydroxylase is specifically linked to the SDHB-mutation remains to be elucidated. In most previously described cases of biochemically silent PGL, the underlying genotypes were not reported. We found that 10% of SDHB-related abdominal PGLs are biochemically silent (11). In the present study, secreting PGLs of the SDHB genotype served as controls. Control samples showed abundant tyrosine hydroxylase staining, a feature that is generally observed in the normal adrenal medulla and across sporadic and familial PGLs of different genotypes (18,22,23,24).
Chromaffin cells contain granules that mainly serve as storage units for catecholamines, which will target, dock, and fuse with the plasma membrane to allow stimulus-dependent secretion (25). We observed similar expression of granules in PGLs that both produced or did not produce catecholamines. The presence of such granules in the biochemically silent PGLs together with chromogranin A immunoreactivity indicates that despite the absence of tyrosine hydroxylase and the lack of catecholamine synthesis, the tumors did express many other components associated with the vesicular storage and secretory apparatus. Despite normal metanephrines, plasma concentrations of chromogranin A were consistently elevated in all three patients with biochemically silent PGLs in whom the analyte was measured. These findings indicate that as an alternative biochemical parameter, plasma chromogranin A may be particularly valuable in the setting of biochemically silent PGLs. The accumulation of the radiotracer MIBG, a catecholamine analog that is taken up by and stored in secretory granules, provides indirect evidence that the functional capacity of these granules is intact in biochemically silent PGLs.
In conclusion, we have established that the lack of catecholamine secretion in biochemically silent abdominal PGLs is not due to defects in the mechanisms of storage or secretion of catecholamines but, instead, reflects a defect in catecholamine synthesis resulting in a near complete absence of releasable stores. The defect is identified as an absence of tyrosine hydroxylase, the enzyme that catalyzes the initial and rate-limiting step in catecholamine biosynthesis. This phenotype appears to be associated with mutations of the SDHB gene, indicating that screening for tumors in identified carriers of that mutation should not be limited to biochemical tests of catecholamine excess (e.g. plasma or urinary fractionated metanephrines). Additional measurements of chromogranin A or even imaging studies seem warranted for excluding nonfunctional PGLs in such patients.
Footnotes
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Child Health and Human Development.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 7, 2008
Abbreviations: CT, Computed tomography; MIBG, metaiodobenzylguanidine; NIH, National Institutes of Health; PGL, paraganglioma; SDHB, succinate dehydrogenase B; TPBS, Tris PBS; URL, upper reference limit.
References
- DeLellis RA, Lloyd RV, Heitz PU, Eng C 2004 Pathology and genetics: World Health Organization classification of tumours of endocrine organs. Oxford, UK: Oxford University Press [Google Scholar]
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G 2002 Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287:1427–1434 [DOI] [PubMed] [Google Scholar]
- Crout JR, Sjoerdsma A 1964 Turnover and metabolism of catecholamines in patients with pheochromocytoma. J Clin Invest 43:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Goldstein DS, Mannelli M, Csako G, Walther MM, Brouwers FM, Pacak K 2005 Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin Chem 51:735–744 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, Adams KT, Pacak K 2005 Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab 90:2068–2075 [DOI] [PubMed] [Google Scholar]
- Proye C, Fossati P, Fontaine P, Lefebvre J, Decoulx M, Wemeau JL, Dewailly D, Rwamasirabo E, Cecat P 1986 Dopamine-secreting pheochromocytoma: an unrecognized entity? Classification of pheochromocytomas according to their type of secretion. Surgery 100:1154–1162 [PubMed] [Google Scholar]
- Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, Young Jr WF 2001 Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 86:5210–5216 [DOI] [PubMed] [Google Scholar]
- Bonnet S, Durand X, Baton O, Gimenez-Roqueplo AP, Baudin E, Visset J, Algayres JP, Baranger B 2006 [Malignant hereditary paraganglioma: problems raised by non-functional forms management]. Ann Chir 131:626–630 (French) [DOI] [PubMed] [Google Scholar]
- Holden A 1995 Non-functional malignant extra-adrenal retroperitoneal paraganglioma. Australas Radiol 39:392–395 [DOI] [PubMed] [Google Scholar]
- Louafy L, Lakhloufi A, Hamdaoui R, Chehab F, Khaiz D, Bouzidi A 2001 [Non-functional retroperitoneal paraganglioma]. Prog Urol 11:512–516 (French) [PubMed] [Google Scholar]
- Timmers HJ, Kozupa A, Eisenhofer G, Raygada M, Adams KT, Solis D, Lenders JW, Pacak K 2007 Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 92:779–786 [DOI] [PubMed] [Google Scholar]
- Olson JR, Abell MR 1969 Nonfunctional, nonchromaffin paragangliomas of the retroperitoneum. Cancer 23:1358–1367 [DOI] [PubMed] [Google Scholar]
- Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, Mann K, Petersenn S 2006 Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol 154:409–417 [DOI] [PubMed] [Google Scholar]
- Willemsen JJ, Sweep CG, Lenders JW, Ross HA 2003 Stability of plasma free metanephrines during collection and storage as assessed by an optimized HPLC method with electrochemical detection. Clin Chem 49:1951–1953 [DOI] [PubMed] [Google Scholar]
- Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ 1993 Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem 39:97–103 [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Stull R, Keiser HR, Sunderland T, Murphy DL, Kopin IJ 1986 Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem 32:2030–2033 [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S 1964 Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917 [PubMed] [Google Scholar]
- Huynh TT, Pacak K, Brouwers FM, Abu-Asab MS, Worrell RA, Walther MM, Elkahloun AG, Goldstein DS, Cleary S, Eisenhofer G 2005 Different expression of catecholamine transporters in phaeochromocytomas from patients with von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2. Eur J Endocrinol 153:551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CA, Vortmeyer AO, Zhuang Z, Brouwers FM, Pacak K 2002 New insights into the genetics of familial chromaffin cell tumors. Ann NY Acad Sci 970:11–28 [DOI] [PubMed] [Google Scholar]
- Hillarp N, Hokfelt B 1955 Histochemical demonstration of noradrenaline and adrenaline in the adrenal medulla. J Histochem Cytochem 3:1–5 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakashima S, Kumanishi T, Ikuta F 1987 Paragangliomas of the craniocervical region. An immunohistochemical study on tyrosine hydroxylase. Acta Neuropathol 73:227–232 [DOI] [PubMed] [Google Scholar]
- Brady S, Lechan RM, Schwaitzberg SD, Dayal Y, Ziar J, Tischler AS 1997 Composite pheochromocytoma/ganglioneuroma of the adrenal gland associated with multiple endocrine neoplasia 2A: case report with immunohistochemical analysis. Am J Surg Pathol 21:102–108 [DOI] [PubMed] [Google Scholar]
- Cleary S, Brouwers FM, Eisenhofer G, Pacak K, Christie DL, Lipski J, McNeil AR, Phillips JK 2005 Expression of the noradrenaline transporter and phenylethanolamine N-methyltransferase in normal human adrenal gland and phaeochromocytoma. Cell Tissue Res 322:443–453 [DOI] [PubMed] [Google Scholar]
- Meijer WG, Copray SC, Hollema H, Kema IP, Zwart N, Mantingh-Otter I, Links TP, Willemse PH, de Vries EG 2003 Catecholamine-synthesizing enzymes in carcinoid tumors and pheochromocytomas. Clin Chem 49:586–593 [DOI] [PubMed] [Google Scholar]
- Chou YY, Lee YS 1998 Ultrastructural and biochemical characterization of catecholamine release mechanisms in cultured human pheochromocytoma cells. Chin Med J (Engl) 111:1018–1024 [PubMed] [Google Scholar]