Abstract
Despite the recent introduction of many improved immunosuppressive agents for use in transplantation, acute rejection affects up to 55% of lung transplant recipients within the first year after transplant. Acute lung allograft rejection is defined as perivascular or peribronchiolar mononuclear inflammation. Although histopathologic signs of rejection often resolve with treatment, the frequency and severity of acute rejections represent the most important risk factor for the subsequent development of bronchiolitis obliterans syndrome (BOS), a condition of progressive airflow obstruction that limits survival to only 50% at 5 years after lung transplantation. Recent evidence demonstrates that peribronchiolar mononuclear inflammation (also known as lymphocytic bronchiolitis) or even a single episode of minimal perivascular inflammation significantly increase the risk for BOS. We comprehensively review the clinical presentation, diagnosis, histopathologic features, and mechanisms of acute cellular lung rejection. In addition, we consider emerging evidence that humoral rejection occurs in lung transplantation, characterized by local complement activation or the presence of antibody to donor human leukocyte antigens (HLA). We discuss in detail methods for HLA antibody detection as well as the clinical relevance, the mechanisms, and the pathologic hallmarks of humoral injury. Treatment options for cellular rejection include high-dose methylprednisolone, antithymocyte globulin, or alemtuzumab. Treatment options for humoral rejection include intravenous immunoglobulin, plasmapheresis, or rituximab. A greater mechanistic understanding of cellular and humoral forms of rejection and their role in the pathogenesis of BOS is critical in developing therapies that extend long-term survival after lung transplantation.
Keywords: antibody formation, histocompatibility testing, transplant immunology, bronchiolitis obliterans, innate immunity
The lung is characterized by the highest rates of rejection among the commonly transplanted solid organs and disappointing long-term outcomes despite modern immunosuppressive regimens. As reported by the Registry of the International Society for Heart and Lung Transplantation (ISHLT), as many as 55% of lung transplant recipients are treated for acute allograft rejection in their first year after transplantation, and only 50% of lung recipients are alive 5 years after transplant (1). The increased susceptibility of the lung to injury, infection, and constant environmental exposure with local innate immune activation likely contribute to the high rates of rejection. Multiple studies have demonstrated that acute vascular (A-grade) or airway (B-grade) rejection are the main risk factors for bronchiolitis obliterans syndrome (BOS), a condition of progressive airflow obstruction that represents the most common cause of death beyond the first year after transplant (2).
Herein, we present the immunologic basis for acute lung allograft rejection, describing the clinical and pathologic features of acute cellular vascular rejection and acute airway rejection also known as lymphocytic bronchiolitis (Figure 1). In addition, we discuss our emerging understanding of the importance of humoral rejection in lung transplantation, including the use of highly sensitive solid phase technologies to detect anti-human leukocyte antigen (HLA) antibodies, which can be present in patients before transplant or develop de novo after transplantation. We highlight current strategies for the prevention and treatment of both cellular and humoral allograft rejection.
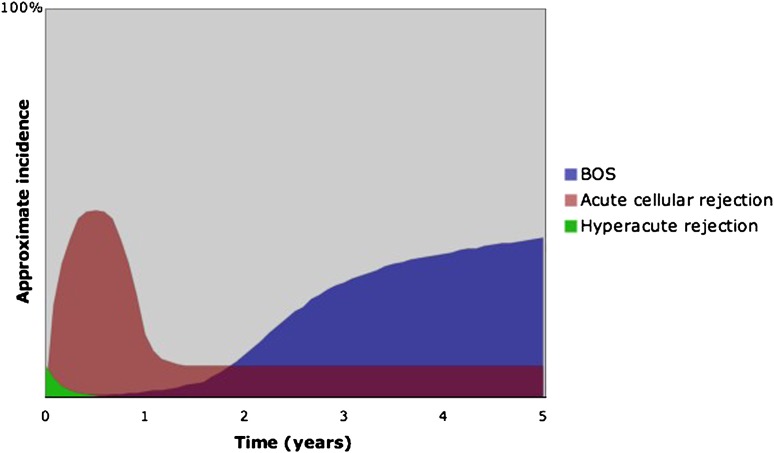
Figure 1.
Relative incidence of rejection by time post lung transplant. Depicted are hyperacute rejection, acute rejection (including A-grade typical perivascular cellular rejection and lymphocytic bronchiolitis), and chronic allograft rejection or bronchiolitis obliterans syndrome (BOS).
MECHANISMS OF ACUTE REJECTION
Organisms from sponges to mammals have evolved sophisticated mechanisms that permit recognition of self from non-self, enabling them to protect their integrity and respond to pathogens while tolerating their own cells. In vertebrate hosts, the advanced interplay of innate and adaptive immune systems leads to a robust response to an organ allograft in the absence of immunosuppression. This alloimmune response is predominantly driven by T cell recognition of foreign major histocompatibility complexes (MHC) (Figure 2). The MHC in humans is also referred to as Human Leukocyte Antigen (HLA) and represents a protein complex encoded by a set of very closely linked genes. The MHC regulates the immune response by presenting antigenic peptides to T cells. In transplantation, allogeneic MHC is first presented directly to recipient T cells by donor dendritic cells in the graft (the direct pathway). As donor antigen-presenting cells (APCs) die out or are destroyed, recipient dendritic cells process and present alloantigens to recipient T cells (the indirect pathway) (3).
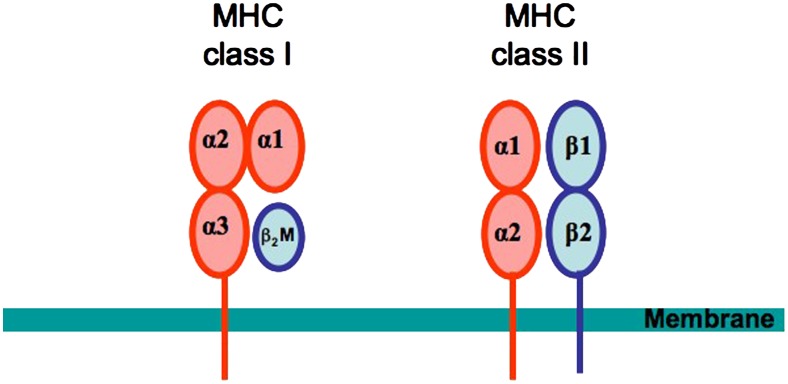
Figure 2.
Structure of major histocompatibility complex (MHC) molecules. The MHC class I molecules are composed of a heavy α chain and a light β2-microglobulin chain. The α chain is composed of three extracellular domains (α1, α2, and α3), a transmembrane-spanning domain, and a small cytoplasmic domain. The α1 and α2 domains together form a peptide-binding groove presenting peptide to CD8+ T cells. MHC Class II molecules are heterodimers with an α and a β chain. Both chains have two extracellular domains, a transmembrane domain, and a cytoplasmic domain. The α1 and β1 domains together form the peptide-binding groove presenting peptide to CD4+ T cells.
HLA genes are located on the short arm of human chromosome 6 and are traditionally divided into two classes based on historic differentiation. The classical HLA class I genes include A, B, and Cw loci, which are expressed on most nucleated cells. The classical HLA class II genes include DR, DQ, and DP genes, which are expressed constitutively on B cells, monocytes, dendritic cells, and other APCs, but can be up-regulated on a variety of other cells under inflammatory conditions. HLA class I molecules present primarily endogenous peptides to CD8+ T cells, while HLA class II molecules present primarily exogenous peptides to CD4+ T cells. The extraordinary diversity of HLA polymorphisms creates a considerable barrier to transplantation as the donor organ is quickly recognized as non-self on the basis of HLA differences with the recipient (3).
In lung transplantation, the process of allorecognition is likely augmented by local innate immune activation through endogenous tissue injury and exogenous infection as well as by an autoimmune response to cryptic self-epitopes exposed during lung damage at the time of transplantation. The precise immune mechanisms and their complex interactions that ultimately lead to the stimulation of adaptive cellular immunity and lung rejection remain to be fully elucidated. The common pathway of acute cellular rejection involves the recruitment and activation of recipient lymphocytes (predominantly effector T cells) to the lung allograft, which can result in allograft injury and loss of function (3). Consequently, successful outcomes after lung transplantation did not become a possibility until the widespread introduction into clinical practice of the calcineurin inhibitor cyclosporine, which permits a highly effective blockade of T cell activation and proliferation (4, 5).
In addition, some lung transplant recipients appear to mount a humoral response to the allograft after transplantation. Most evidence suggests that this humoral response occurs to donor MHC antigens, although other endothelial or epithelial antigens expressed in the lung may become antibody targets as well. T cells activated through indirect presentation provide help for B cell memory, antibody class switching, and affinity maturation in the presence of appropriate cytokines and co-stimulatory factors. Acute and chronic humoral rejection has recently been well described in renal transplantation (6).
Finally, using modern solid phase antibody detection techniques, it has become clear that some patients present for transplant with preformed anti-HLA antibodies, which are usually acquired through prior pregnancy, transfusions, or transplantation. Immune stimulation by prior infections or autoimmunity might contribute to the development of antibodies to alloMHC in those patients with no identifiable risk factors. These preexisting antibodies can react with donor antigens, leading to immediate graft loss (hyperacute rejection) or accelerated humoral rejection and BOS (6).
ACUTE CELLULAR REJECTION
Clinical Presentation
The diagnosis of acute cellular rejection relies on the identification of lymphocytic perivascular or peribronchiolar infiltrates in lung tissue. Many episodes of acute rejection are diagnosed in asymptomatic patients undergoing surveillance biopsies. When symptomatic, acute rejection can present with dyspnea, cough, or sputum production. More subtle signs include fever, hypoxia, and adventitious sounds on lung auscultation. Higher-grade rejection appears to cause more severe symptoms and can lead to acute respiratory distress (7).
Because of the nonspecific nature of symptoms, emphasis should be placed on objective data, mainly pulmonary function testing, in identifying patients at risk for rejection. Spirometry has been found to have a sensitivity of greater than 60% for detecting infection or rejection grade A2 and higher, but it cannot differentiate between the two (8). The usefulness of spirometry is diminished in single lung transplant recipients, as the contralateral native lung dysfunction confounds the pulmonary function test results (9). Pulmonary function testing should therefore be used as an adjunct to clinical evaluation and not as a stand-alone definitive diagnostic modality for acute lung rejection.
Radiographic imaging of lung transplant patients is useful in identifying specific causes of symptoms or decreased pulmonary function, such as focal infections or neoplasms. Findings of ground-glass opacities, septal thickening, volume loss, and pleural effusions on high-resolution chest computed tomography (CT) scans suggest acute rejection. Although early small studies attempted to demonstrate the usefulness of chest X-rays and chest CT scans in the diagnosis of rejection, more recent data shows very low sensitivity for acute rejection (as low as 35%) and no discriminatory value between rejection and other processes (10).
Despite the common clinical impression that lymphocytic pleural effusions are a hallmark of acute rejection, published data are inconclusive (11). In fact lymphocytic effusions have been documented in the absence of rejection, making it difficult to ascertain whether they mostly represent a sequela of rejection or simply a physiologic response to lung transplantation (12).
In summary, in light of the nonspecific clinical symptoms and the poor specificity of pulmonary function tests and radiographic studies, we discourage diagnosis and empiric treatment of rejection based solely on clinical signs or symptoms, consistent with the ISHLT definitions, which require histopathologic analysis of lung tissue to diagnose and grade acute lung rejection.
Diagnosis of Acute Lung Allograft Rejection
Bronchoscopy.
Bronchoscopy with transbronchial biopsies is the most important modality in the diagnosis of acute allograft rejection, and should be considered in any lung transplant recipient with allograft dysfunction. Bronchoscopy allows acute rejection to be distinguished from other potential etiologies of allograft dysfunction such as airway stenosis or infection, and routinely includes bronchoalveolar lavage (BAL) with cultures and transbronchial biopsies for histopathologic analysis. Most transbronchial biopsies are performed in the lower lobes, a practice that seems reasonable in light of data showing that different lung lobes have similar rejection grades and that, if rejection is present, the grade is usually worse in the lower lobes as compared with the upper lobes (13). The Lung Rejection Study Group (LRSG) now recommends five pieces of well-expanded alveolated lung parenchyma to provide adequate sensitivity to diagnose rejection (14). Adverse events reported with bronchoscopy in lung transplant recipients are low and include transient hypoxemia (10.5%), bleeding more than 100 ml (4%), pneumothorax (0.6–2.5%), arrhythmia (0.57%), and ventilation support (0.32%). Bronchoscopy in this setting has no reported mortality (15, 16). Because of the low procedural risk of bronchoscopy and its critical role in the diagnosis of rejection in this patient population, bronchoscopic diagnosis is preferred to empiric treatment of rejection in most circumstances.
In addition, bronchoscopy is also performed for surveillance purposes to diagnose rejection in asymptomatic lung transplant recipients. The rationale for surveillance biopsies includes the occurrence of clinically silent acute rejection, inadequate surrogate markers for acute rejection, and the relatively low risks of the bronchoscopy procedure. Grade A2 and higher acute rejection has been found in a relatively high percentage of asymptomatic patients, ranging from 22% to 39% (17, 18). The yield for acute rejection is reported from 6.1% to 31% in studies of surveillance transbronchial biopsies (15, 19) and 25% or greater in clinically indicated bronchoscopies and follow-up bronchoscopies (16). Furthermore, acute rejection can be seen even in patients that had low rates of early rejection and at more than 1 year after transplant (19).
Questioning the importance of surveillance bronchoscopies, a single-center retrospective study suggested that 3-year outcomes in patients who underwent only clinically indicated bronchoscopies were comparable to ISHLT data (20). However, no randomized clinical trials have ever compared different post-transplant monitoring strategies. A survey of lung transplant centers published in 1997 demonstrated that 68% of centers perform scheduled bronchoscopies, in addition to the clinically indicated and postrejection follow-up bronchoscopies (21). A common schedule consists of bronchoscopy at 1 month, 3 months, 6 months, and then on an annual basis. Regardless of whether bronchoscopy is performed for clinical indications or surveillance purposes, the incidence of acute rejection is highest within the first year after transplant, and a high clinical suspicion for acute rejection should be maintained during this time period.
Alternate diagnostic methods for acute lung rejection.
In an attempt to reduce the risk of surveillance bronchoscopies, many studies have focused on surrogates of acute lung rejection. Particular focus has been directed at identifying acute rejection biomarkers in the bronchoalveolar lavage (BAL). Many of the positive studies have been small and have not been replicated. Nevertheless, several themes stand out in this research arena. Multiple studies have considered the BAL cellular composition and its relation to rejection or infection. In the early months after transplantation, there is increased leukocytosis of the BAL as compared with nontransplant patients with high numbers of neutrophils and lymphocytes (22). Acute rejection has been associated with elevated CD8+ T cells, activated CD4+ T cells, a trend toward increased NK T cells, increased B cells, and decreased NK cells in the BAL (23). Nevertheless, no study has proven the BAL cellular composition to be adequately sensitive or specific in the discrimination of rejection from infection (24).
Small studies have found a correlation between acute rejection and elevation of interleukin-17 (25), interleukin-15 (26), and interferon-γ in the BAL (27). Recent advances in genomics offer the potential for more specific means of diagnosing rejection in lung transplantation. A pilot study of gene expression in the BAL of lung transplant recipients found that gene expression signatures related to T-lymphocyte function, cytotoxic CD8 activity, and neutrophil degranulation correlate with acute rejection (28). Additional studies are needed to validate these findings and establish whether BAL microarray determinations of “acute rejection signature” are cost effective and provide information that supplements or replaces biopsy results.
Of greater interest than analysis of the BAL would be a noninvasive means of diagnosing acute rejection without bronchoscopy. For example, the recently published studies in heart transplantation describe the use of peripheral blood gene expression profiling to identify future risk of cardiac allograft rejection (29): this methodology appears to be most useful in predicting persistent negative biopsies in patients with prior negative biopsies. A similar study is now underway in lung transplantation, known as the lung allograft rejection gene expression observational (LARGO) study. Preliminary data from almost 900 patients, similarly to the CARGO results, show differential gene expression in the lymphocyte priming and neutrophil homeostasis pathways for A0 versus ≥ A2 acute lung rejection (30). Additional data and its further validation may bring about a peripheral blood test for an acute rejection gene expression signature, which could reduce the frequency of invasive diagnostic procedures in the future.
Although no effective serum biomarkers are currently in use in clinical lung transplantation, their potential utility is illustrated in a recent study in which marked serum elevations in hepatocyte growth factor (HGF) in lung transplant recipients were associated with acute rejection. Smaller elevations in HGF also occurred with lung infection, and additional studies are needed to validate the specificity and sensitivity of HGF for acute rejection (31). In addition, in recent years, the Cylex Immune Cell Function Assay (ImmuKnow; Cylex, Inc., Columbia, MD) has been examined as a potential peripheral blood surrogate of rejection or infection. It has been approved by the U.S. Food and Drug Administration to measure global immune function in solid organ transplant recipients. This assay measures the in vitro production of adenosine triphosphate (ATP) by the patient's CD4+ T cells in response to stimulation by phytohemagglutinin-L (PHA). Several studies in kidney, liver, heart, and small bowel allograft recipients have demonstrated that low ATP levels (≤ 225 ng/ml) correlate with infection, while high levels (≥ 525 ng/ml) are associated with rejection (32). The data in lung transplantation are scarce and not very promising to date. A recent study shows that lung transplant recipients with active infection are more likely to have low ATP levels compared with stable lung transplant recipients (mean of 111 versus 283 ng/ml). While the sensitivity for infection of an ATP value of less than 225 was 93% in this study, the specificity was only 38%. In addition, the utility of ATP measurements was not assessed, as only two recipients in the patient sample had rejection (33). Another study, published in abstract form, demonstrated a very poor correlation between histologically proven rejection and the ImmuKnow assay, with 87% of the allograft rejection episodes occurring in the setting of a low to moderate ATP level (34). Based on these preliminary results, the ImmuKnow assay does not seem to have the potential to differentiate between infection and rejection in lung transplant recipients and, until more data becomes available, should not be used clinically in this patient population.
Exhaled nitric oxide (NO) is also an attractive marker of lung injury: it has been correlated with lymphocytic bronchiolitis (35) and acute rejection (36). Furthermore, in a study of inert gas single breath washout, the slope of alveolar plateau for helium (SHe) had a sensitivity of 68% for acute rejection (8).
In summary, no surrogate markers have been sufficiently validated as means to reproducibly identify patients with acute rejection and none supplant direct histopathologic examination of lung tissue. Although this remains an area of intense research, many challenges exist in developing noninvasive biomarkers to reliably identify acute rejection, including the probable heterogeneity of rejection phenotypes and lack of interobserver agreement on the histologic “gold standard” upon which the validity of any biomarker would be established, as described in the next section.
Histology and Cellular Infiltration of Acute Lung Rejection
Acute lung rejection is defined as perivascular mononuclear cellular infiltrates on histologic analysis of lung allograft tissue. Most commonly, the diagnosis is established based on transbronchial biopsies obtained by bronchoscopy. The Lung Rejection Study Group (LRSG), a workshop organized by the ISHLT, has created and revised a Working Formulation for grading acute allograft rejection. The most recent revision was published in 2007. This detailed document describes the histologic appearance of acute lung allograft rejection and outlines the grading rules for acute cellular rejection (A-grade), airway inflammation (B-grade), chronic airway rejection or bronchiolitis obliterans (C-grade), and chronic vascular rejection or accelerated graft vascular sclerosis (D-grade). This grading scheme and its key features are summarized in Table 1 (14), and illustrative images are shown in Figure 3. The A and B acute rejection grades are discussed extensively in this article, while histopathology of chronic rejection is discussed elsewhere in this issue (see pages 108–121).
TABLE 1.
PATHOLOGIC GRADING OF LUNG REJECTION
| Category | Grade | Meaning | Appearance |
|---|---|---|---|
| A: acute rejection | 0 | None | Normal lung parenchyma |
| 1 | Minimal | Inconspicuous small mononuclear perivascular infiltrates | |
| 2 | Mild | More frequent, more obvious, perivascular infiltrates, eosinophils may be present | |
| 3 | Moderate | Dense perivascular infiltrates, extension into interstitial space, can involve endothelialitis, eosinophils, and neutrophils | |
| 4 | Severe | Diffuse perivascular, interstitial, and air-space infiltrates with lung injury. Neutrophils may be present. | |
| B: airway inflammation | 0 | None | No evidence of bronchiolar inflammation |
| 1R | Low grade | Infrequent, scattered or single layer mononuclear cells in bronchiolar submucosa | |
| 2R | High grade | Larger infiltrates of larger and activated lymphocytes in bronchiolar submucosa. Can involve eosinophils and plasmacytoid cells. | |
| X | Ungradable | No bronchiolar tissue available | |
| C: Chronic airway rejection – obliterative bronchiolitis | 0 | Absent | If present describes intraluminal airway obliteration with fibrous connective tissue |
| 1 | Present | ||
| D: Chronic vascular rejection – accelerated graft vascular sclerosis | Not graded | Fibrointimal thickening of arteries and poorly cellular hyaline sclerosis of veins. Usually requires open lung biopsy for diagnosis. |
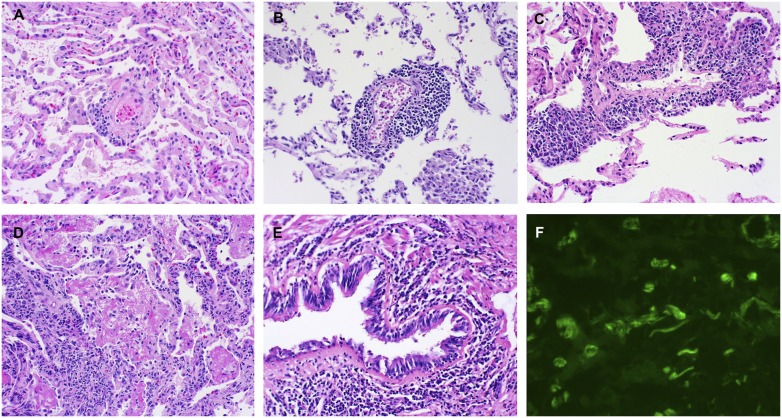
Figure 3.
Pathologic examples of acute lung allograft rejection. (A) Grade A1 acute rejection with rare perivascular lymphocytes (hematoxylin and eosin [H&E] staining; ×40). (B) Grade A2 acute rejection with a prominent perivascular mononuclear infiltrate (H&E staining; ×40). (C) Grade A3 acute rejection with extensive perivascular infiltrate extending into perivascular spaces (H&E staining; ×40). (D) Grade A4 acute rejection with a diffuse mononuclear infiltrate with lung injury (H&E staining; ×40). (E) Grade B2R (high grade) lymphocytic bronchiolitis with dense peribronchiolar mononuclear infiltrate (H&E staining; ×40). (F) Immunofluorescence on frozen lung tissue, demonstrating positive C4d staining in subendothelial space and within alveolar septae (immunofluorescent staining; ×400).
A-grade acute cellular rejection of the lung allograft.
Perivascular mononuclear infiltrates with or without interstitial mononuclear cells are thought to represent the typical acute lung allograft rejection. Increasing thickness of the mononuclear cell cuff around vessels with increasing mononuclear invasion into the interstitial and alveolar spaces is what determines the A-grade (Table 1 and Figures 3A–3D). The majority of these mononuclear cells are T cells, although a few studies have described increased populations of B cells or eosinophils (14, 37, 38).
Studies from 2005 evaluated the inter- and intrareader reliability of this grading scheme. Two studies found relatively good interreader agreements for the A grades (kappa of 0.65 and 0.73) (39, 40), but this could not be replicated in another study in which the kappa was 0.47 in spite of dichotomization of the A-grades to A0/A1 versus A2/A3/A4 (41). Intrareader agreement for acute rejection has been found to be good: 0.65 and 0.795 (39, 41). Infection can complicate the diagnosis: viral infection in particular can cause mononuclear inflammation. In addition, alveolar damage with macrophage and fibrin accumulation was found to be present in 80% of transbronchial biopsies in the first 6 months after transplant, further increasing interreader pathologist discordance (40). In general, the LRSG recommends grading rejection only after the exclusion of infection.
B-grade airway inflammation.
Mononuclear airway inflammation (Figure 3E) has been recognized since the early years of lung transplantation. However, establishing a reliable and relevant grading scheme for it has been problematic for several reasons: many transbronchial biopsies contain no or minimal airway tissue, airway biopsies are susceptible to tangential cutting and other artifacts, and airway inflammation often accompanies infection. Prior revisions of the pathology working formulation generated by the LRSG initially recommended calling airway inflammation as present or absent and subsequently expanded the grading to 5 grades from 0 to 4 (as is currently used for A-grading). However, the interreader reliability for grading airway inflammation (B-grades) was found to be very low with kappas of approximately 0.3 (39, 41). For this reason, the LRSG has now simplified B-grading to four possible grades: B0 for no airway inflammation, B1R for low-grade small airway inflammation (R standing for “revised” so as not to confuse it with the prior grading scheme), B2R for high-grade small airway inflammation, and BX for ungradable airway inflammation (Table 1). This nomenclature is to be used for grading noncartillaginous small airways only after rigorous exclusion of infection (14).
Clinical Significance of Acute Rejection
Multiple studies demonstrate that acute rejection is the major risk factor for the development of chronic airflow obstruction: A single episode of acute rejection, as well as increased frequency and severity of acute rejection, increase the risk for BOS (2). For example, a single ≥ A2 rejection has been found to increase the hazard ratio (HR) for development of BOS up to 2.1 (P = 0.014), recurrent rejection increased this HR up to 3.4 (P < 0.001), and late rejection up to 4.8 (P = 0.003). However, lack of prospective validation of this data prevents direct clinical application of such numbers (2). An area of controversy has been the significance of minimal acute rejection (A1) or of a single solitary perivascular infiltrate. In the early years of lung transplantation, A1 rejection was usually discounted and not treated. Studies have found that minimal acute rejection (grade A1) increases the risk of higher-grade subsequent rejections (grade ≥ A2) (42, 43) and of subsequent BOS with HR of 2.5 (95% confidence interval [CI], 1.32–4.76; P = 0.005) for BOS stage 1 and 3.49 (95% CI, 1.26–9.67; P = 0.02) for BOS stage 2 (44). The finding of a solitary perivascular monocytic infiltrate was followed by worsening acute rejection in all four untreated patients in one study, while the treatment of such a solitary infiltrate in nine patients resulted in improvement of rejection score (45). Furthermore, based on multiple studies, grade B lymphocytic bronchiolitis is now also known to be an important risk factor for BOS (relative risk 1.62 for BOS stage 1; 95% CI, 1.31–2.00; P < 0.001) (46) and death, independent of acute vascular rejection (2, 46).
Large airway inflammation, as determined by endobronchial biopsies, is by definition excluded from the B-grading of acute airway rejection. Although large airway inflammation can represent infectious tracheo-bronchitis (47), most studies of endobronchial biopsies have demonstrated the presence of noninfectious CD8+ T cell rich inflammation, distinct from the inflammation seen on transbronchial biopsies and in the BAL (48, 49). In addition, gene expression has been found to be discordant between endobronchial biopsy cells and transbronchial biopsy tissue, suggesting that A-grade rejection is a process different from large airway inflammation (50). Thus, although lymphocytic inflammation is frequently seen on endobronchial biopsies, its clinical or prognostic significance remains unclear and there is no demonstrated link between lymphocytic bronchitis seen on endobronchial biopsies and lymphocytic bronchiolitis or bronchitis seen on transbronchial biopsies.
The role of atypical cellular composition in acute rejection is not well understood. Eosinophils can accompany acute lung rejection and are recognized in the revised LRSG acute rejection definition (Table 1) (14). The presence of eosinophils has been found to portend a poor prognosis and to be a risk factor for BOS in small single-center studies (51). High proportions of B cells have been shown to accompany steroid-resistant rejection in lung transplant patients (37). The mechanisms by which B cells contribute to refractory rejection are not entirely clear, although they might reflect ongoing humoral rejection, perhaps explaining the diminished responsiveness to standard immunosuppression. Mast cells have been identified in acute rejection biopsies of increasing A-grade but, again, their role has not been elucidated (52). Additional immunohistochemical analysis of the mononuclear cells that participate in acute rejection is another important area of future research.
Risk Factors for Acute Rejection
Despite a large number of studies addressing risk factors for BOS, very few studies have focused specifically on factors that predict the development of acute rejection.
HLA mismatching.
It is generally thought that the intensity of host alloimmune response is related to recipient recognition of differences with the donor HLA antigens, and that this process drives acute lung allograft rejection. Consistent with this idea, several studies have shown that an increasing degree of HLA mismatch increases the risk of acute rejection (53–55). However, this effect is not consistent across all HLA loci or studies. Mismatches at the HLA-DR, HLA-B (53), and HLA-A (54) loci, as well as a combination of all three loci (55), appear important. However, these single-center studies simply do not have enough patients or power to fully assess the clinical significance of HLA matching, since in any single-center study there are relatively few patients with a high degree of matching. A similar lack of replicability has been seen in the evaluation of HLA mismatching as a risk factor for BOS. Although several single-center studies have shown that increased HLA mismatching predicts BOS (56), a registry analysis of 3,549 lung transplant recipients contradicts this data (54). In addition, the ISHLT registry has not found a correlation between HLA matching and survival (1). Thus, while HLA mismatching between donor and recipient likely contributes to the immunologic basis for acute rejection, it is difficult to discern from existing studies if a mismatch at a particular locus or if different degrees of mismatch significantly alter the overall risk for acute rejection.
Immunosuppression.
Despite the introduction of new immunosuppressants in the maintenance regimens of lung transplant recipients, no agents have been associated with a clear reduction in acute rejection rates. Current regimens commonly employ a calcineurin inhibitor, corticosteroids, and mycophenolate mofetil (MMF) or azathioprine as third agent (4). Several studies suggest that there may be lower incidence of acute rejection with tacrolimus as opposed to cyclosporine (57, 58). Self-reported ISHLT registry data also supports the idea of decreased acute rejection episodes with tacrolimus compared with cyclosporine, but no difference is seen between MMF and azathioprine (1).
Surprisingly, very few studies have directly examined the link between serum levels of immunosuppression and acute rejection. High levels of immunosuppression, as measured indirectly by a positive blood Epstein-Barr virus (EBV) PCR, have been found to correlate with lower incidence of acute rejection (59). Furthermore, lung transplant recipients who develop one episode of early high-grade acute rejection appear to be more likely to develop additional acute rejection episodes within the first year after lung transplant, suggesting that patients with prior rejection should be treated with more aggressive immunosuppressive regimens or dosing (43).
Infections.
In solid organ transplantation, viral infections have long been thought to modulate the immune system and heighten alloreactivity. Indeed, a high incidence of acute rejection has been found in lung transplant recipients after community-acquired respiratory tract infections with human influenza virus, respiratory syncytial virus (RSV), rhinovirus, coronavirus, and parainfluenza virus (60–62). Although cytomegalovirus (CMV) is considered a potential risk factor for bronchiolitis obliterans syndrome, studies directly linking CMV infection or CMV prophylaxis strategies with acute rejection have been inconsistent (2). In one study, Chlamydia pneumoniae infection was linked to the development of acute rejection and BOS (63).
Recipient factors.
Several host genetic characteristics have been identified as risk factors for acute lung rejection. A genotype leading to increased interleukin-10 production may protect against acute rejection (64) and a multidrug-resistant genotype (MDR1 C3435T) appears to predispose to persistent acute rejection resistant to immunosuppressive treatment (65).
We have also developed and pursued the hypothesis that genetic variation in innate pattern recognition receptors modulates the development of acute rejection after lung transplantation and found reduced acute rejection with a variant in Toll-like receptor 4 (TLR4) that blunts the innate immune response and increased rejection with a CD14 variant that augments the innate response (66, 67). Collectively, these studies provide considerable support for the overall hypothesis that the constant interplay between the environment and pulmonary innate immunity modulates adaptive alloimmunity after lung transplantation.
The effect of age on acute rejection appears to be bimodal, with the lowest incidence of acute rejection in infancy (below age 2) (68) and increased risk during childhood as compared with adulthood (69). The incidence of acute rejection in older lung transplant recipients (age 65 or higher) does not seem to change (70); in fact, an increased rate of infections in older lung transplant recipients is thought to contribute to an increased mortality detected at one center, arguing for reduced immunosuppression in these patients (71).
Multi-organ and living-lobar transplants.
The presence of multiple organs from the same donor is generally believed to provide an immunologic advantage and lead to lower rates of rejection. Decreased rejection has been shown for grafted kidney, liver, and heart in combined heart-kidney, liver-kidney, and heart-lung transplant recipients, although this benefit does not seem to translate into prolonged graft or recipient survival (72, 73). The data regarding lung rejection in the presence of a second organ remain inconclusive. One study showed decreased acute lung rejection in heart-lung transplant recipients (73), while other studies have disputed this finding (74, 75). In addition, the ISHLT registry reports similar rejection-related mortality of lung and heart-lung recipients (1). The protective effects of the liver in lung-liver recipients is still debated as well, due partly to the rarity of this transplant procedure. Two of three published small case series of lung-liver recipients show very low lung rejection rates: 30% versus the expected greater than 50% seen in lung-only recipients (76). This immune protection has not been apparent in studies of living-lobar lung recipients, where incidence of acute rejection does not appear to be significantly different from baseline, although lower rates of BOS have been described (77).
Summary.
While the precise mechanisms that lead to acute rejection after lung transplantation are uncertain, it appears that the recipient response to the allograft is modulated by the degree of HLA mismatch between donor and recipient and environmental factors, including both the nature and intensity of immunosuppression as well as local allograft exposures and infection. Recently, we have demonstrated that genetic variation in the recipient's innate response act in concert with these other factors to further regulate the development of acute rejection. Clearly, additional genetic and environmental factors not yet identified likely influence the complex host response to lung allotransplantation.
Treatment of Acute Lung Rejection
Treatment of acute lung allograft rejection consists of increased immunosuppression. There has been consensus that grades A2 and higher require treatment. Early in the clinical practice of lung transplantation, there was debate whether to treat grade A1 and isolated B-grade airway inflammation. In light of recent evidence that grade A1 rejection and lymphocytic bronchiolitis are major risk factors for BOS, treatment seems prudent.
Although a more in-depth discussion of immunosuppression can be found elsewhere in this issue, we present a brief overview of acute rejection treatment. The mainstay of treatment for acute lung rejection is pulse-steroids. Several studies from the 1990s showed successful resolution or improvement of acute rejection after high-dose steroid treatment (37, 78). There is no data to clearly guide dosing of the pulse steroids; a standard dose is 500 mg of methylprednisolone intravenously (4), although centers use doses that range from 125 mg per day up to 1,000 mg per day. Duration of treatment also varies but typically includes at least three doses followed by an oral prednisone taper.
A major challenge in lung transplantation has been the treatment of resistant, persistent, or recurrent rejection that gets diagnosed on follow-up transbronchial biopsies. A repeat course of corticosteroids is one option. Several studies support switching from cyclosporine to tacrolimus for treatment of persistent acute rejection (79, 80). Many centers use pulse treatments with a polyclonal antithymocyte globulin (ATG), anti–interleukin-2 receptor (IL2R) antagonists, or muromonab-CD3 (OKT3) (81). A recent report demonstrated the utility of alemtuzumab, an anti-CD52 monoclonal antibody, in the treatment of refractory acute rejection in a small cohort of patients who previously failed treatment with ATG (38).
Inhaled immunosuppressants are appealing for treatment of acute rejection. Inhaled corticosteroids have not been found to have a role in the treatment of acute lung rejection. Inhaled cyclosporine data were initially encouraging, with small studies showing resolution of acute rejection and decrease in BAL inflammatory markers (82, 83). Additional research is needed to justify the use of inhaled cyclosporine in the treatment of acute rejection, especially given the high incidence of side effects from the inhalation carrier polyethylene glycol. Other therapies that have been considered in the management of severe or persistent acute rejection include extracorporeal photopheresis (84) and total lymphoid irradiation (85).
The relationship between acute rejection, its current treatments, and the eventual occurrence of BOS is an area of considerable interest. In many studies, acute rejection is identified as a risk factor for BOS despite patients undergoing treatment with corticosteroids for acute rejection. On the one hand, it is possible that this treatment delays the onset of BOS. On the other hand, in light of the recent explosion of knowledge regarding the importance of regulatory T cells in transplantation tolerance, it is conceivable that certain rejection treatments have adverse effects on tolerance and regulatory mechanisms, changing alloreactivity in unexpected ways. A more mechanistic understanding of acute rejection and its relationship to BOS is needed to develop new and innovative means to improve outcomes in lung transplantation.
HUMORAL REJECTION
Antibody-mediated allograft rejection is an increasingly recognized entity in lung transplantation. Early observations were based on the phenomenon of hyperacute rejection, where pre-existent donor-specific antibodies lead to complement activation and rapid graft loss. With the advent of improved crossmatching before transplant, the incidence of hyperacute rejection in all organs has decreased. However, acute or chronic antibody-mediated lung rejection is an emerging and controversial subject. With the development of improved antibody detection techniques, allograft-specific antibodies have been implicated in both acute and chronic kidney as well as heart rejection, and recent data have expanded the concept to lung transplantation. This section will discuss emerging issues in humoral lung rejection, including humoral sensitization both before and after lung transplantation as well as pathologic features of humoral rejection, which can occur with or without the presence of detectable antibodies.
Detection of HLA Antibodies
Technologies used for HLA antibody screening and identification include complement-dependent cytotoxicity (CDC) and solid phase technologies such as ELISA, flow cytometry, and the Luminex bead array assays.
Complement dependent cytotoxicity (CDC) methodology.
The CDC assay can be used for HLA serologic typing, antibody screening and identification, and direct crossmatching. The assay principle is that the specific reactivity between serum antibody and cell surface antigen will activate complement, causing cell death. Dead cells can subsequently be identified under the microscope using vital dyes for cell staining.
For HLA serologic typing using CDC technology, a panel of alloantisera and/or monoclonal antibodies with known specificities is used to determine the type of HLA antigens on the donor's cell surface. This serologic HLA typing is the most error prone due to lack of specific antibodies and cross-reactivity of sera. For solid organ transplantation, most HLA laboratories currently perform HLA typing using sequence-specific primers and reversed sequence-specific oligonucleotide probes for more precise DNA-based detection.
The real-time “prospective crossmatch” using the CDC assay consists in incubating the actual recipient serum with actual donor leukocytes to identify antibody binding and cell death. In lung transplantation, due to the short ischemia time allowed between organ harvest and transplantation, it is impossible, in most cases, to perform a prospective crossmatch to determine the compatibility between the donor and the potential recipient. Therefore, a stored panel of lymphocytes with known HLA types can be used. In this setting, the Panel Reactive Antibody (PRA) is the percentage of lymphocytes from the given panel that are recognized by the patient's anti-HLA antibodies. The PRA percentage and specificity can be determined for a recipient at the time of evaluation and then compared with the HLA typing of a potential donor at the time of organ harvest. However, due to expression of multiple HLA antigens on each cell, it is difficult to definitely determine the fine antibody specificities by this method, leading to an overall low sensitivity and specificity for the CDC detection system.
Solid phase technologies for antibody screening and identification.
These technologies are significantly more sensitive and specific than the CDC-based assays. Their common feature is the use of a solid matrix coated with purified HLA antigens obtained from either cell lines or recombinant technology. Routinely used solid phase technologies include ELISA, flow cytometry, and Luminex, which detect both complement-fixing antibodies and noncomplement-fixing antibodies.
ELISA is the least sensitive method of the solid phase methodologies. It uses a microtiterplate on which the surface of each well is pre-coated with purified HLA antigens. After incubation with patient serum and a secondary antibody, a chromogenic reaction visualizes the specific antibody–antigen bindings.
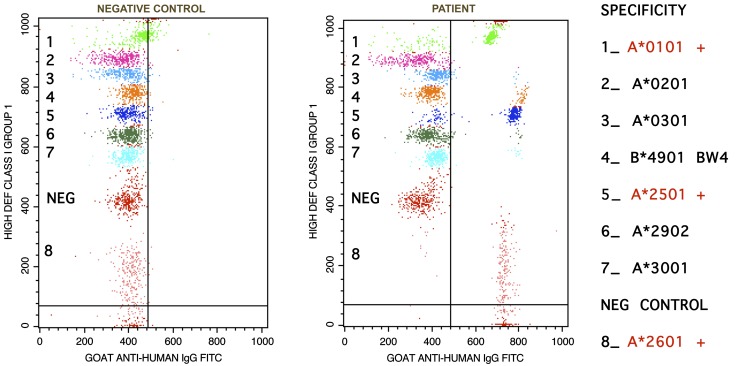
By measuring indirect immunofluorescence, both flow cytometry and Luminex detect HLA antibody binding to microparticles coated with purified HLA antigens or recombinant single antigens. Antibody screening by flow cytometry, also referred to as Flow PRA (Figure 4), uses a panel of 30 populations of beads coated with HLA antigens extracted from 30 individual donors. Once a patient's PRA is determined to be positive or high, the actual HLA specificity of a recipient's anti-HLA antibodies needs to be determined. The flow cytometric single antigen bead assay allows very accurate identification of specific HLA antibodies using beads coated with recombinant HLA single antigens (Figure 5) (86). The most recently developed solid phase methodology for single antigen detection is the Luminex single antigen bead array assay, which can simultaneously detect a maximum of 100 different colored beads in suspension with a different HLA antigen bound to each colored bead, providing an even better coverage of the diversity of HLA antigens.
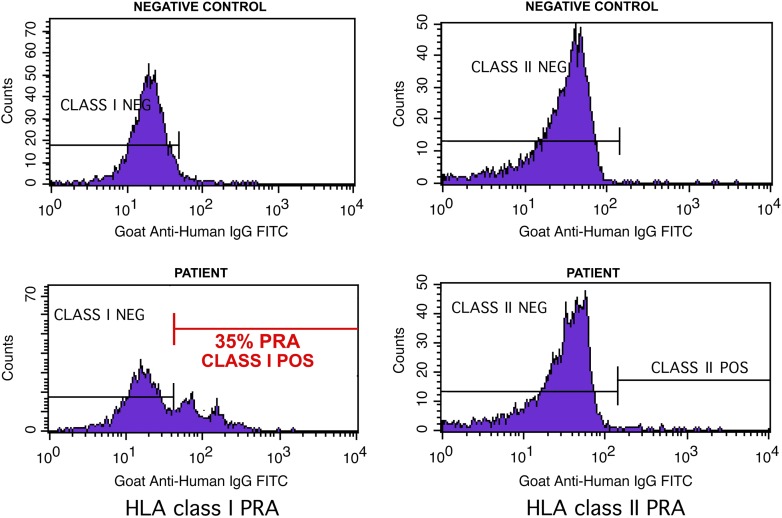
Figure 4.
Flow cytometric antibody screening for measurement of panel reactive antibody (PRA). FlowPRA beads are coated with purified human leukocyte antigens (HLA). After incubation with patient serum, and subsequent staining with fluorescein isothiocyanate (FITC)-labeled anti-human IgG, flowPRA beads were analyzed on a flow cytometer. Beads with antibody binding have greater fluorescence intensity as represented by the rightward channel shift compared with the negative control. A percentage value of PRA is calculated based on the area of peak shifted. This patient demonstrated a PRA of 35% for HLA class I and 0% for HLA class II. The double peak in the positive flow histogram is due to different bead populations emitting fluorescence of different intensity.
Figure 5.
Flow cytometric single antigen (SA) bead assay for detection of a group of anti-HLA class I specific antibodies. Each SA bead is coated with multiple copies of a single recombinant HLA antigen. After incubation with patient serum and subsequent staining with FITC-labeled anti-human IgG, SA beads were analyzed on a flow cytometer. A rightward shift of the beads in the patient sample (right-hand plot), as compared with the negative control (left-hand plot), indicates antibody specificity to the HLA antigens analyzed. This patient has anti-A1, A25, and A26 specific antibodies. For this patient, A1, A25, and A26 represent unacceptable antigens.
Antibodies may still be present at a level of detection below the sensitivity of the methodology and/or against antigens not represented by the screening reagents. However, it is believed that antibodies that remain undetected by current methods are mostly “weak antibodies” and may not be clinically relevant. Nevertheless, the most definitive compatibility test remains the real-time crossmatch of the recipient serum with the potential donor cells. Flow crossmatch, whereby actual donor cells are incubated with recipient serum and bound antibodies are then tagged with secondary fluorescent anti-IgG antibodies, has been proven to be up to 10 to 250 times more sensitive than a CDC crossmatch (87).
Pre-Transplant Considerations for Sensitized Patients
One of the major goals in donor selection is to avoid HLA antigens against which the potential recipient has preformed antibodies. About 10 to 15% of lung transplant recipients are presensitized to HLA antigens (88). The recent development of very sensitive and specific solid phase flow cytometry and Luminex-based methodologies has allowed for accurate detection of antibody specificities in sensitized recipients. These technologies identify “unacceptable donor antigens” that should be avoided at the time of transplant. When a donor becomes available, information about the donor HLA antigens and the recipient antibodies is compared, constituting a “virtual crossmatch” and allowing for the real-time prospective crossmatch to be waived. We and others have demonstrated this virtual crossmatch approach has significantly shortened the waiting time for presensitized recipients, and correlates highly with crossmatch results performed at the time of transplant (89, 90). In addition, patients undergoing retransplantation evaluation should be carefully screened for presensitization.
Post-Transplant Considerations in Sensitized Recipients
Even though “unacceptable antigens” are avoided during the virtual crossmatch, patients with positive pre-transplant PRA are at higher risk for post-transplant complications. Their post-transplant PRA can stay stable or increase via generation of either donor-specific or non–donor-specific anti-HLA antibodies. Similarly, patients that had negative PRA screening tests before transplant can develop de novo non–donor-specific or donor-specific anti-HLA antibodies after transplant.
Using modern sensitive antibody detection techniques, recent studies have consistently demonstrated increased incidence of acute rejection (a threefold increase in one study) (91), persistent rejection, increased BOS (HR, 3.19; 95% CI, 1.41–7.12; P = 0.005) (92) or worse overall survival (median of 1.5 yr versus 5.2) (93) in patients with anti-HLA antibodies. This effect is apparent both with pre-transplant HLA sensitization and with the development of de novo anti-HLA donor-specific antibodies after transplantation (92).
The importance of donor specificity and target antigens in humoral rejection is not well understood. The risk of poor outcome may be heightened in the setting of donor-specific antibodies and positive retrospective crossmatches (93). However, patients with positive PRA, with negative crossmatches, and without specificity to mismatched donor HLA antigens have also been found to be at increased risk for poor outcome. On the one hand, non–donor-specific antibodies that are present might cross react with the donor HLA or antibodies specific to donor HLA might be rapidly absorbed in the lung allograft before their detection in the sera. Alternatively, other non-HLA antibodies could contribute to graft injury. For example, de novo autoimmunity after lung transplantation against type V collagen (94) and K-α1 tubulin expressed on airway epithelial cells has been shown to predispose to BOS (95). Another study demonstrated the presence of antiendothelial antibody directed against donor antigens in the absence of anti-HLA antibodies (96).
It remains unclear exactly how often post-transplant PRAs should be measured and to what extent humoral rejection occurs among lung transplant recipients. Additional research is needed to more precisely define the significance of antibody to donor HLA, to third party HLA, or to self-antigens after lung transplantation.
Mechanisms of Post-Transplant Humoral Injury
The mechanisms by which antibody promotes lung allograft injury remain poorly understood. Antibody binding to alloMHC or other endothelial or epithelial targets in the lung could lead to activation of the complement cascade with complement deposits leading to endothelial cell injury, production of proinflammatory molecules, and recruitment of inflammatory cells. Complement-independent antibody-mediated mechanisms can also induce endothelial cell activation without cell injury, leading to increased gene expression and subsequent proliferation (6). As demonstrated by in vitro studies, anti-HLA antibodies can cause proliferation of airway epithelial cells as well, producing fibroblast-stimulating growth factors (97), potentially contributing to the generation of obliterative airway lesions.
Pathologic Patterns of Humoral Lung Rejection
Hyperacute rejection after lung transplantation has been rarely reported because of the use of crossmatching and careful avoidance of unacceptable donor antigens. The characteristic pathologic appearance of hyperacute lung rejection includes small vessel vasculitis and necrosis, intra-alveolar hemorrhage, and diffuse alveolar damage, with the common identification of platelet and fibrin thrombi, capillary congestion with neutrophils and macrophages, as well as antibody deposition on endothelial surfaces, vascular walls, alveolar spaces, and septae (98).
Early reports of pulmonary capillaritis suggested that there is a separate form of atypical rejection, presumably due to humoral rejection that is poorly responsive to steroids, with some responsiveness to plasmapheresis (99). More recent studies have attempted to evaluate immunoglobulin and complement deposits in the subendothelial space. Septal capillary deposits of immunoglobulins and complement products such as C1q, C3d, C4d (Figure 3F), and C5b-9 have been described in association with anti-HLA antibodies (100, 101) as well as allograft dysfunction and BOS (102, 103).
The concept of a specific histopathologic syndrome associated with humoral rejection remains controversial. Recent studies question the relation between complement or immunoglobulin staining and allograft rejection (104, 105). Others demonstrate that C3d and C4d staining can occur in lung transplant patients with nonalloimmune lung injury such as infection and PGD with no evidence of anti-HLA antibodies (103). Differences in staining techniques between different laboratories may further explain some of the inconsistencies in the published data.
The LRSG report on the working formulation for the diagnosis of lung rejection remains very cautious in discussing the pathologic appearance of humoral rejection. The consensus is that capillary injury can be detected on lung allograft biopsies, although it can be a nonspecific finding. Findings of small vessel intimitis or endothelialitis, along with immunohistochemical staining for complements, should raise the suspicion for humoral rejection (14). Although such pathologic findings have been reported without evidence of anti-HLA antibodies and visa versa, the presence in one patient of both anti-HLA antibodies and pathologic findings suspicious for humoral rejection should be seen as strong evidence for humoral rejection.
Prevention and Therapy for Antibody-mediated Rejection
Plasmapheresis is the mainstay for antibody removal from the circulation and has been shown to lead to clinical improvement in other solid organ transplants with humoral rejection as well as in lung transplant recipients with pulmonary capillaritis unresponsive to steroids (106). However, it is usually reserved for severe cases of suspected humoral rejection, given the side effects and difficulties of administration. Intravenous immunoglobulin (IVIG) is one of the most common therapies used to decrease antibody-mediated immunity. IVIG causes B cell apoptosis, reduces B cell numbers, and down-regulates several B cell surface antigens. It also blocks binding of donor-reactive antibodies and may inhibit complement activation. It has a relatively low side effect profile. The peritransplant use of IVIG and plasmapheresis at our institution in 12 presensitized patients led to elimination of antibodies in 85.7% for class I anti-HLA antibodies and 33.3% of class II anti-HLA antibodies, stronger improvements in pulmonary function, more than a 50% decrease in acute rejection episodes, and greater freedom from BOS (90% treated versus 50% untreated at 5 yr by Kaplan-Meir estimates) compared with the 23 presensitized patients who did not get desensitization therapy (88). Rituximab, an anti-CD20 monoclonal antibody that causes B cell depletion, has been proven effective in the treatment of presensitized renal transplant recipients in conjunction with IVIG (6, 107).
Despite new highly sensitive measures to screen for anti-HLA antibodies and evidence that such antibodies are detrimental to the allograft, optimal monitoring and treatment parameters for humoral rejection after transplant remain uncertain. Further studies are needed to determine whether IVIG, plasmapheresis, or rituximab alter the risk for chronic allograft dysfunction in sensitized patients.
CONCLUSIONS
Since the first successful human lung transplants, acute rejection, defined by perivascular or peribronchiolar mononuclear inflammation, was recognized to occur in a majority of patients. Rejection was thought to occur as a result of alloreactive T lymphocytes responding directly or indirectly to donor antigen. In recent years, however, a much more complex picture of lung transplant rejection has emerged. In addition to HLA diversity, genetic variation in innate immune pattern recognition receptors also influences the risk for post-transplant rejection. Furthermore, humoral rejection occurs, characterized by the presence of antibody to the donor HLA or histopathologic evidence of local pulmonary complement activation. Finally, environmental stimuli, such as pulmonary infections, interact directly with the lung allograft and contribute to the development of rejection. Thus, while lung allograft rejection appears to share many features with other solid organ transplants, it is clear that other factors specific to the lung and its constant environmental interactions also contribute to the very high prevalence of lung allograft rejection. A greater understanding of the heterogeneity of lung rejection is critical to developing therapies that target the precise biological mechanisms of rejection and ultimately improve long-term lung transplant outcomes.
Sources of financial support: 5 KL2 RR024127–03, 1P50-HL084917–01, 1 K24 HL91140–01A2.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the international society for heart and lung transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant 2007;26:782–795. [DOI] [PubMed] [Google Scholar]
- 2.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: asystematic review of recent publications. J Heart Lung Transplant 2002;21:271–281. [DOI] [PubMed] [Google Scholar]
- 3.Snyder LD, Palmer SM. Immune mechanisms of lung allograft rejection. Semin Respir Crit Care Med 2006;27:534–543. [DOI] [PubMed] [Google Scholar]
- 4.Lau CL, Palmer SM, D'Amico TA, Tapson VF, Davis RD. Lung transplantation at Duke University Medical Center. Clin Transpl 1998;327–340. [PubMed]
- 5.Sarahrudi K, Carretta A, Wisser W, Senbaklavaci O, Ploner M, Neuhauser P, Dobrovits M, Miwai Marta G, Papp A, Klepetko W. The value of switching from cyclosporine to tacrolimus in the treatment of refractory acute rejection and obliterative bronchiolitis after lung transplantation. Transpl Int 2002;15:24–28. [DOI] [PubMed] [Google Scholar]
- 6.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol 2005;5:807–817. [DOI] [PubMed] [Google Scholar]
- 7.De Vito Dabbs A, Hoffman LA, Iacono AT, Zullo TG, McCurry KR, Dauber JH. Are symptom reports useful for differentiating between acute rejection and pulmonary infection after lung transplantation? Heart Lung 2004;33:372–380. [DOI] [PubMed] [Google Scholar]
- 8.Van Muylem A, Melot C, Antoine M, Knoop C, Estenne M. Role of pulmonary function in the detection of allograft dysfunction after heart-lung transplantation. Thorax 1997;52:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker FS, Martinez FJ, Brunsting LA, Deeb GM, Flint A, Lynch JP III. Limitations of spirometry in detecting rejection after single-lung transplantation. Am J Respir Crit Care Med 1994;150:159–166. [DOI] [PubMed] [Google Scholar]
- 10.Gotway MB, Dawn SK, Sellami D, Golden JA, Reddy GP, Keith FM, Webb WR. Acute rejection following lung transplantation: limitations in accuracy of thin-section CT for diagnosis. Radiology 2001;221:207–212. [DOI] [PubMed] [Google Scholar]
- 11.Judson MA, Handy JR, Sahn SA. Pleural effusion from acute lung rejection. Chest 1997;111:1128–1130. [DOI] [PubMed] [Google Scholar]
- 12.Shitrit D, Izbicki G, Fink G, Bendayan D, Aravot D, Saute M, Kramer MR. Late postoperative pleural effusion following lung transplantation: characteristics and clinical implications. Eur J Cardiothorac Surg 2003;23:494–496. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Iacono AT, Yousem SA. The anatomic distribution of acute cellular rejection in the allograft lung. Ann Thorac Surg 2000;69:1529–1531. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229–1242. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, Glanville AR. Prospective analysis of 1,235 transbronchial lung biopsies in lung transplant recipients. J Heart Lung Transplant 2002;21:1062–1067. [DOI] [PubMed] [Google Scholar]
- 16.Chan CC, Abi-Saleh WJ, Arroliga AC, Stillwell PC, Kirby TJ, Gordon SM, Petras RE, Mehta AC. Diagnostic yield and therapeutic impact of flexible bronchoscopy in lung transplant recipients. J Heart Lung Transplant 1996;15:196–205. [PubMed] [Google Scholar]
- 17.Guilinger RA, Paradis IL, Dauber JH, Yousem SA, Williams PA, Keenan RJ, Griffith BP. The importance of bronchoscopy with transbronchial biopsy and bronchoalveolar lavage in the management of lung transplant recipients. Am J Respir Crit Care Med 1995;152:2037–2043. [DOI] [PubMed] [Google Scholar]
- 18.Trulock EP, Ettinger NA, Brunt EM, Pasque MK, Kaiser LR, Cooper JD. The role of transbronchial lung biopsy in the treatment of lung transplant recipients: an analysis of 200 consecutive procedures. Chest 1992;102:1049–1054. [DOI] [PubMed] [Google Scholar]
- 19.Chakinala MM, Ritter J, Gage BF, Lynch JP, Aloush A, Patterson GA, Trulock EP. Yield of surveillance bronchoscopy for acute rejection and lymphocytic bronchitis/bronchiolitis after lung transplantation. J Heart Lung Transplant 2004;23:1396–1404. [DOI] [PubMed] [Google Scholar]
- 20.Valentine VG, Taylor DE, Dhillon GS, Knower MT, McFadden PM, Fuchs DM, Kantrow SP. Success of lung transplantation without surveillance bronchoscopy. J Heart Lung Transplant 2002;21:319–326. [DOI] [PubMed] [Google Scholar]
- 21.Kukafka DS, O'Brien GM, Furukawa S, Criner GJ. Surveillance bronchoscopy in lung transplant recipients. Chest 1997;111:377–381. [DOI] [PubMed] [Google Scholar]
- 22.Tiroke AH, Bewig B, Haverich A. Bronchoalveolar lavage in lung transplantation. State of the art. Clin Transplant 1999;13:131–157. [DOI] [PubMed] [Google Scholar]
- 23.Gregson AL, Hoji A, Saggar R, Ross DJ, Kubak BM, Jamieson BD, Weigt SS, Lynch JP III, Ardehali A, Belperio JA, et al. Bronchoalveolar immunologic profile of acute human lung transplant allograft rejection. Transplantation 2008;85:1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynaud-Gaubert M, Thomas P, Gregoire R, Badier M, Cau P, Sampol J, Giudicelli R, Fuentes P. Clinical utility of bronchoalveolar lavage cell phenotype analyses in the postoperative monitoring of lung transplant recipients. Eur J Cardiothorac Surg 2002;21:60–66. [DOI] [PubMed] [Google Scholar]
- 25.Vanaudenaerde BM, Dupont LJ, Wuyts WA, Verbeken EK, Meyts I, Bullens DM, Dilissen E, Luyts L, Van Raemdonck DE, Verleden GM. The role of interleukin-17 during acute rejection after lung transplantation. Eur Respir J 2006;27:779–787. [DOI] [PubMed] [Google Scholar]
- 26.Bhorade SM, Yu A, Vigneswaran WT, Alex CG, Garrity ER. Elevation of interleukin-15 protein expression in bronchoalveolar fluid in acute lung allograft rejection. Chest 2007;131:533–538. [DOI] [PubMed] [Google Scholar]
- 27.Ross DJ, Moudgil A, Bagga A, Toyoda M, Marchevsky AM, Kass RM, Jordan SC. Lung allograft dysfunction correlates with gamma-interferon gene expression in bronchoalveolar lavage. J Heart Lung Transplant 1999;18:627–636. [DOI] [PubMed] [Google Scholar]
- 28.Patil J, Lande JD, Li N, Berryman TR, King RA, Hertz MI. Bronchoalveolar lavage cell gene expression in acute lung rejection: development of a diagnostic classifier. Transplantation 2008;85:224–231. [DOI] [PubMed] [Google Scholar]
- 29.Mehra MR, Kobashigawa JA, Deng MC, Fang KC, Klingler TM, Lal PG, Rosenberg S, Uber PA, Starling RC, Murali S, et al. Clinical implications and longitudinal alteration of peripheral blood transcriptional signals indicative of future cardiac allograft rejection. J Heart Lung Transplant 2008;27:297–301. [DOI] [PubMed] [Google Scholar]
- 30.Keshavjee S, Berry G, Marboe CC, Wilt JS, Trulock EP, Corris PA, Doyle RL, McCurry KR, Arcasoy SM, Davis RD, et al. Refining the identification of discriminatory genes for rejection in lung transplantation: the largo study. J Heart Lung Transplant 2007;26:S185–S186. [Google Scholar]
- 31.Aharinejad S, Taghavi S, Klepetko W, Abraham D. Prediction of lung-transplant rejection by hepatocyte growth factor. Lancet 2004;363:1503–1508. [DOI] [PubMed] [Google Scholar]
- 32.Kowalski RJ, Post DR, Mannon RB, Sebastian A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero M, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation 2006;82:663–668. [DOI] [PubMed] [Google Scholar]
- 33.Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER. Cylex immuknow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant 2008;27:990–994. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Nizami Nazih Zuhdi I. Correlation of cylex immuknow assay with lung allograft biopsy. J Heart Lung Transplant 2007;26:S175. [Google Scholar]
- 35.De Soyza A, Fisher AJ, Small T, Corris PA. Inhaled corticosteroids and the treatment of lymphocytic bronchiolitis following lung transplantation. Am J Respir Crit Care Med 2001;164:1209–1212. [DOI] [PubMed] [Google Scholar]
- 36.Silkoff PE, Caramori M, Tremblay L, McClean P, Chaparro C, Kesten S, Hutcheon M, Slutsky AS, Zamel N, Keshavjee S. Exhaled nitric oxide in human lung transplantation: a noninvasive marker of acute rejection. Am J Respir Crit Care Med 1998;157:1822–1828. [DOI] [PubMed] [Google Scholar]
- 37.Yousem SA, Martin T, Paradis IL, Keenan R, Griffith BP. Can immunohistological analysis of transbronchial biopsy specimens predict responder status in early acute rejection of lung allografts? Hum Pathol 1994;25:525–529. [DOI] [PubMed] [Google Scholar]
- 38.Reams BD, Musselwhite LW, Zaas DW, Steele MP, Garantziotis S, Eu PC, Snyder LD, Curl J, Lin SS, Davis RD, et al. Alemtuzumab in the treatment of refractory acute rejection and bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant 2007;7:2802–2808. [DOI] [PubMed] [Google Scholar]
- 39.Chakinala MM, Ritter J, Gage BF, Aloush AA, Hachem RH, Lynch JP, Patterson GA, Trulock EP. Reliability for grading acute rejection and airway inflammation after lung transplantation. J Heart Lung Transplant 2005;24:652–657. [DOI] [PubMed] [Google Scholar]
- 40.Colombat M, Groussard O, Lautrette A, Thabut G, Marrash-Chahla R, Brugiere O, Mal H, Leseche G, Fournier M, Degott C. Analysis of the different histologic lesions observed in transbronchial biopsy for the diagnosis of acute rejection: clinicopathologic correlations during the first 6 months after lung transplantation. Hum Pathol 2005;36:387–394. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson A, Flint J, English J, Vedal S, Fradet G, Chittock D, Levy RD. Interpretation of transbronchial lung biopsies from lung transplant recipients: Inter- and intraobserver agreement. Can Respir J 2005;12:75–77. [DOI] [PubMed] [Google Scholar]
- 42.Burton CM, Iversen M, Scheike T, Carlsen J, Andersen CB. Minimal acute cellular rejection remains prevalent up to 2 years after lung transplantation: a retrospective analysis of 2697 transbronchial biopsies. Transplantation 2008;85:547–553. [DOI] [PubMed] [Google Scholar]
- 43.DeVito Dabbs A, Hoffman LA, Iacono AT, Wells CL, Grgurich W, Zullo TG, McCurry KR, Dauber JH. Pattern and predictors of early rejection after lung transplantation. Am J Crit Care 2003;12:497–507. [PubMed] [Google Scholar]
- 44.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation 2005;80:1406–1413. [DOI] [PubMed] [Google Scholar]
- 45.Kim DW, Dacic S, Iacono A, Grgurich W, Yousem SA. Significance of a solitary perivascular mononuclear infiltrate in lung allograft recipients with mild acute cellular rejection. J Heart Lung Transplant 2005;24:152–155. [DOI] [PubMed] [Google Scholar]
- 46.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008;177:1033–1040. [DOI] [PubMed] [Google Scholar]
- 47.Husain AN, Siddiqui MT, Montoya A, Chandrasekhar AJ, Garrity ER. Post-lung transplant biopsies: an 8-year loyola experience. Mod Pathol 1996;9:126–132. [PubMed] [Google Scholar]
- 48.Irani S, Gaspert A, Vogt P, Russi EW, Weder W, Speich R, Boehler A. Inflammation patterns in allogeneic and autologous airway tissue of lung transplant recipients. Am J Transplant 2005;5:2456–2463. [DOI] [PubMed] [Google Scholar]
- 49.Ward C, Snell GI, Orsida B, Zheng L, Williams TJ, Walters EH. Airway versus transbronchial biopsy and bal in lung transplant recipients: different but complementary. Eur Respir J 1997;10:2876–2880. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Golden JA, Dolganov G, Jones KD, Donnelly S, Weaver T, Caughey GH. Transcript signatures of lymphocytic bronchitis in lung allograft biopsy specimens. J Heart Lung Transplant 2005;24:1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholma J, Slebos DJ, Boezen HM, van den Berg JW, van der Bij W, de Boer WJ, Koeter GH, Timens W, Kauffman HF, Postma DS. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med 2000;162:2221–2225. [DOI] [PubMed] [Google Scholar]
- 52.Yousem SA. The potential role of mast cells in lung allograft rejection. Hum Pathol 1997;28:179–182. [DOI] [PubMed] [Google Scholar]
- 53.Schulman LL, Weinberg AD, McGregor C, Galantowicz ME, Suciu-Foca NM, Itescu S. Mismatches at the HLA-dr and HLA-b loci are risk factors for acute rejection after lung transplantation. Am J Respir Crit Care Med 1998;157:1833–1837. [DOI] [PubMed] [Google Scholar]
- 54.Quantz MA, Bennett LE, Meyer DM, Novick RJ. Does human leukocyte antigen matching influence the outcome of lung transplantation? An analysis of 3,549 lung transplantations. J Heart Lung Transplant 2000;19:473–479. [DOI] [PubMed] [Google Scholar]
- 55.Wisser W, Wekerle T, Zlabinger G, Senbaclavaci O, Zuckermann A, Klepetko W, Wolner E. Influence of human leukocyte antigen matching on long-term outcome after lung transplantation. J Heart Lung Transplant 1996;15:1209–1216. [PubMed] [Google Scholar]
- 56.Sundaresan S, Mohanakumar T, Smith MA, Trulock EP, Lynch J, Phelan D, Cooper JD, Patterson GA. HLA-a locus mismatches and development of antibodies to hla after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation 1998;65:648–653. [DOI] [PubMed] [Google Scholar]
- 57.Treede H, Klepetko W, Reichenspurner H, Zuckermann A, Meiser B, Birsan T, Wisser W, Reichert B. Tacrolimus versus cyclosporine after lung transplantation: a prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. J Heart Lung Transplant 2001;20:511–517. [DOI] [PubMed] [Google Scholar]
- 58.Hachem RR, Yusen RD, Chakinala MM, Meyers BF, Lynch JP, Aloush AA, Patterson GA, Trulock EP. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant 2007;26:1012–1018. [DOI] [PubMed] [Google Scholar]
- 59.Ahya VN, Douglas LP, Andreadis C, Arnoldi S, Svoboda J, Kotloff RM, Hadjiliadis D, Sager JS, Woo YJ, Pochettino A, et al. Association between elevated whole blood Epstein-Barr virus (ebv)-encoded rna ebv polymerase chain reaction and reduced incidence of acute lung allograft rejection. J Heart Lung Transplant 2007;26:839–844. [DOI] [PubMed] [Google Scholar]
- 60.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, Johnson G, Ayers M, Siegal D, Humar A. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant 2005;5:2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vilchez RA, Dauber J, McCurry K, Iacono A, Kusne S. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant 2003;3:116–120. [DOI] [PubMed] [Google Scholar]
- 62.Garantziotis S, Howell DN, McAdams HP, Davis RD, Henshaw NG, Palmer SM. Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001;119:1277–1280. [DOI] [PubMed] [Google Scholar]
- 63.Glanville AR, Gencay M, Tamm M, Chhajed P, Plit M, Hopkins P, Aboyoun C, Roth M, Malouf M. Chlamydia pneumoniae infection after lung transplantation. J Heart Lung Transplant 2005;24:131–136. [DOI] [PubMed] [Google Scholar]
- 64.Zheng HX, Burckart GJ, McCurry K, Webber S, Ristich J, Iacono A, Dauber J, McDade K, Grgurich W, Zaldonis D, et al. Interleukin-10 production genotype protects against acute persistent rejection after lung transplantation. J Heart Lung Transplant 2004;23:541–546. [DOI] [PubMed] [Google Scholar]
- 65.Zheng HX, Zeevi A, McCurry K, Schuetz E, Webber S, Ristich J, Zhang J, Iacono A, Dauber J, McDade K, et al. The impact of pharmacogenomic factors on acute persistent rejection in adult lung transplant patients. Transpl Immunol 2005;14:37–42. [DOI] [PubMed] [Google Scholar]
- 66.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, Schwartz DA. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med 2005;171:780–785. [DOI] [PubMed] [Google Scholar]
- 67.Palmer SM, Klimecki W, Yu L, Reinsmoen NL, Snyder LD, Ganous TM, Burch L, Schwartz DA. Genetic regulation of rejection and survival following human lung transplantation by the innate immune receptor cd14. Am J Transplant 2007;7:693–699. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim JE, Sweet SC, Flippin M, Dent C, Mendelhoff E, Huddleston CB, Trinkhaus K, Canter CE. Rejection is reduced in thoracic organ recipients when transplanted in the first year of life. J Heart Lung Transplant 2002;21:311–318. [DOI] [PubMed] [Google Scholar]
- 69.Scott JP, Whitehead B, de Leval M, Helms P, Smyth RL, Higenbottam TW, Wallwork J. Paediatric incidence of acute rejection and obliterative bronchiolitis: a comparison with adults. Transpl Int 1994;7:S404–S406. [DOI] [PubMed] [Google Scholar]
- 70.Mahidhara R, Bastani S, Ross DJ, Saggar R, Lynch J III, Schnickel GT, Gjertson D, Beygui R, Ardehali A. Lung transplantation in older patients? J Thorac Cardiovasc Surg 2008;135:412–420. [DOI] [PubMed] [Google Scholar]
- 71.Gutierrez C, Al-Faifi S, Chaparro C, Waddell T, Hadjiliadis D, Singer L, Keshavjee S, Hutcheon M. The effect of recipient's age on lung transplant outcome. Am J Transplant 2007;7:1271–1277. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, Ghobrial RM, Yersiz H, Hiatt JR, Busuttil RW. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg 2006;141:735–741. (discussion 741–732). [DOI] [PubMed] [Google Scholar]
- 73.Pinderski LJ, Kirklin JK, McGiffin D, Brown R, Naftel DC, Young KR Jr, Smith K, Bourge RC, Tallaj JA, Rayburn BK, et al. Multi-organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant 2005;24:1828–1833. [DOI] [PubMed] [Google Scholar]
- 74.Keenan RJ, Bruzzone P, Paradis IL, Yousem SA, Dauber JH, Stuart RS, Griffith BP. Similarity of pulmonary rejection patterns among heart-lung and double-lung transplant recipients. Transplantation 1991;51:176–180. [DOI] [PubMed] [Google Scholar]
- 75.Moffatt-Bruce SD, Karamichalis J, Robbins RC, Whyte RI, Theodore J, Reitz BA. Are heart-lung transplant recipients protected from developing bronchiolitis obliterans syndrome? Ann Thorac Surg 2006;81:286–291. (discussion 291). [DOI] [PubMed] [Google Scholar]
- 76.Grannas G, Neipp M, Hoeper MM, Gottlieb J, Luck R, Becker T, Simon A, Strassburg CP, Manns MP, Welte T, et al. Indications for and outcomes after combined lung and liver transplantation: a single-center experience on 13 consecutive cases. Transplantation 2008;85:524–531. [DOI] [PubMed] [Google Scholar]
- 77.Starnes VA, Bowdish ME, Woo MS, Barbers RG, Schenkel FA, Horn MV, Pessotto R, Sievers EM, Baker CJ, Cohen RG, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg 2004;127:114–122. [DOI] [PubMed] [Google Scholar]
- 78.Clelland C, Higenbottam T, Stewart S, Otulana B, Wreghitt T, Gray J, Scott J, Wallwork J. Bronchoalveolar lavage and transbronchial lung biopsy during acute rejection and infection in heart-lung transplant patients: studies of cell counts, lymphocyte phenotypes, and expression of HLA-dr and interleukin-2 receptor. Am Rev Respir Dis 1993;147:1386–1392. [DOI] [PubMed] [Google Scholar]
- 79.Vitulo P, Oggionni T, Cascina A, Arbustini E, D'Armini AM, Rinaldi M, Meloni F, Rossi A, Vigano M. Efficacy of tacrolimus rescue therapy in refractory acute rejection after lung transplantation. J Heart Lung Transplant 2002;21:435–439. [DOI] [PubMed] [Google Scholar]
- 80.Sarahrudi K, Estenne M, Corris P, Niedermayer J, Knoop C, Glanville A, Chaparro C, Verleden G, Gerbase MW, Venuta F, et al. International experience with conversion from cyclosporine to tacrolimus for acute and chronic lung allograft rejection. J Thorac Cardiovasc Surg 2004;127:1126–1132. [DOI] [PubMed] [Google Scholar]
- 81.Shennib H, Massard G, Reynaud M, Noirclerc M. Efficacy of OKT3 therapy for acute rejection in isolated lung transplantation. J Heart Lung Transplant 1994;13:514–519. [PubMed] [Google Scholar]
- 82.Keenan RJ, Iacono A, Dauber JH, Zeevi A, Yousem SA, Ohori NP, Burckart GJ, Kawai A, Smaldone GC, Griffith BP. Treatment of refractory acute allograft rejection with aerosolized cyclosporine in lung transplant recipients. J Thorac Cardiovasc Surg 1997;113:335–340. (discussion 340–331). [DOI] [PubMed] [Google Scholar]
- 83.Iacono A, Dauber J, Keenan R, Spichty K, Cai J, Grgurich W, Burckart G, Smaldone G, Pham S, Ohori NP, et al. Interleukin 6 and interferon-gamma gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation 1997;64:263–269. [DOI] [PubMed] [Google Scholar]
- 84.Dall'Amico R, Murer L. Extracorporeal photochemotherapy: a new therapeutic approach for allograft rejection. Transfus Apheresis Sci 2002;26:197–204. [DOI] [PubMed] [Google Scholar]
- 85.Valentine VG, Robbins RC, Wehner JH, Patel HR, Berry GJ, Theodore J. Total lymphoid irradiation for refractory acute rejection in heart-lung and lung allografts. Chest 1996;109:1184–1189. [DOI] [PubMed] [Google Scholar]
- 86.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 2003;75:43–49. [DOI] [PubMed] [Google Scholar]
- 87.McKenna RM, Takemoto SK, Terasaki PI. Anti-HLA antibodies after solid organ transplantation. Transplantation 2000;69:319–326. [DOI] [PubMed] [Google Scholar]
- 88.Appel JZ III, Hartwig MG, Davis RD, Reinsmoen NL. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to hla antigens. Hum Immunol 2005;66:378–386. [DOI] [PubMed] [Google Scholar]
- 89.Appel JZ III, Hartwig MG, Cantu E III, Palmer SM, Reinsmoen NL, Davis RD. Role of flow cytometry to define unacceptable HLA antigens in lung transplant recipients with HLA-specific antibodies. Transplantation 2006;81:1049–1057. [DOI] [PubMed] [Google Scholar]
- 90.Taylor CJ, Smith SI, Morgan CH, Stephenson S, Key T, Jones P, Watson C, Jacques B, Welsh KI, Bradley JA. Selective omission of the donor cross-match before renal transplantation: efficacy, safety and effects on cold storage time. Transplantation 2000;69:719–723. [DOI] [PubMed] [Google Scholar]
- 91.Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, Spichty KJ, Yousem SA, Burckart G, Dauber JH, et al. Hla-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant 2004;23:1135–1141. [DOI] [PubMed] [Google Scholar]
- 92.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, Savik K, Reinsmoen NL. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation 2002;74:799–804. [DOI] [PubMed] [Google Scholar]
- 93.Hadjiliadis D, Chaparro C, Reinsmoen NL, Gutierrez C, Singer LG, Steele MP, Waddell TK, Davis RD, Hutcheon MA, Palmer SM, et al. Pre-transplant panel reactive antibody in lung transplant recipients is associated with significantly worse post-transplant survival in a multicenter study. J Heart Lung Transplant 2005; 24(7, Suppl)S249–S254. [DOI] [PubMed] [Google Scholar]
- 94.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 2007;117:3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of k-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol 2008;180:4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Magro CM, Klinger DM, Adams PW, Orosz CG, Pope-Harman AL, Waldman WJ, Knight D, Ross P Jr. Evidence that humoral allograft rejection in lung transplant patients is not histocompatibility antigen-related. Am J Transplant 2003;3:1264–1272. [DOI] [PubMed] [Google Scholar]
- 97.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class i antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol 2003;64:521–529. [DOI] [PubMed] [Google Scholar]
- 98.Masson E, Stern M, Chabod J, Thevenin C, Gonin F, Rebibou JM, Tiberghien P. Hyperacute rejection after lung transplantation caused by undetected low-titer anti-HLA antibodies. J Heart Lung Transplant 2007;26:642–645. [DOI] [PubMed] [Google Scholar]
- 99.Badesch DB, Zamora M, Fullerton D, Weill D, Tuder R, Grover F, Schwarz MI. Pulmonary capillaritis: a possible histologic form of acute pulmonary allograft rejection. J Heart Lung Transplant 1998;17:415–422. [PubMed] [Google Scholar]
- 100.Ionescu DN, Girnita AL, Zeevi A, Duquesnoy R, Pilewski J, Johnson B, Studer S, McCurry KR, Yousem SA. C4d deposition in lung allografts is associated with circulating anti-HLA alloantibody. Transpl Immunol 2005;15:63–68. [DOI] [PubMed] [Google Scholar]
- 101.Miller GG, Destarac L, Zeevi A, Girnita A, McCurry K, Iacono A, Murray JJ, Crowe D, Johnson JE, Ninan M, et al. Acute humoral rejection of human lung allografts and elevation of c4d in bronchoalveolar lavage fluid. Am J Transplant 2004;4:1323–1330. [DOI] [PubMed] [Google Scholar]
- 102.Magro CM, Abbas AE, Seilstad K, Pope-Harman AL, Nadasdy T, Ross P Jr. C3d and the septal microvasculature as a predictor of chronic lung allograft dysfunction. Hum Immunol 2006;67:274–283. [DOI] [PubMed] [Google Scholar]
- 103.Westall GP, Snell GI, McLean C, Kotsimbos T, Williams T, Magro C. C3d and c4d deposition early after lung transplantation. J Heart Lung Transplant 2008;27:722–728. [DOI] [PubMed] [Google Scholar]
- 104.Saint Martin GA, Reddy VB, Garrity ER, Simpson K, Robinson JA, Adent JK, Husain AN. Humoral (antibody-mediated) rejection in lung transplantation. J Heart Lung Transplant 1996;15:1217–1222. [PubMed] [Google Scholar]
- 105.Wallace WD, Reed EF, Ross D, Lassman CR, Fishbein MC. C4d staining of pulmonary allograft biopsies: an immunoperoxidase study. J Heart Lung Transplant 2005;24:1565–1570. [DOI] [PubMed] [Google Scholar]
- 106.Astor TL, Weill D, Cool C, Teitelbaum I, Schwarz MI, Zamora MR. Pulmonary capillaritis in lung transplant recipients: treatment and effect on allograft function. J Heart Lung Transplant 2005;24:2091–2097. [DOI] [PubMed] [Google Scholar]
- 107.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, Peng A, Villicana R, Jordan SC. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 2008;359:242–251. [DOI] [PubMed] [Google Scholar]