Abstract
The p53 tumor suppressor gene can induce either apoptosis or a permanent growth arrest (also termed senescence) phenotype in response to cellular stresses. We show that the increase in intracellular reactive oxygen species (ROS) associated with the magnitude of p53 protein expression correlated with the induction of either senescence or apoptosis in both normal and cancer cells. ROS inhibitors ameliorated both p53-dependent cell fates, implicating ROS accumulation as an effector in each case. The absence of Bax or PUMA strongly inhibited both p53-induced apoptosis and ROS increase, indicating an important role these p53 targets affecting mitochondrial function genes in p53-mediated ROS accumulation. Moreover, physiological p53 levels in combination with an exogenous ROS source were able to convert a p53 senescence response into apoptosis. All of these findings establish a critical role of ROS accumulation and mitochondrial function in p53-dependent cell fates and show that other ROS inducers can collaborate with p53 to influence these fate decisions. Thus, our studies imply that therapeutic agents that generate ROS are more likely to be toxic for normal cells than p53-negative tumor cells and provide a rationale for identifying therapeutic agents that do not complement p53 in ROS generation to ameliorate the cytotoxic side effects in normal cells.
The p53 tumor suppressor protein can trigger the onset either of reversible or permanent growth arrest (51, 52) or of apoptosis (27, 34). However, the mechanisms involved in the decision between these cellular responses are not well understood. Cell type, the presence of growth factors or oncogenes, the intensity of the stress signal, and the cellular level of p53 have been cited as important factors in determining a specific p53-induced response (7, 12, 53). Posttranslational modifications of the p53 gene have also been reported to influence the response observed. For example, p53 phosphorylation by different kinases in response to stress can select for arrest or apoptosis, suggesting the involvement of modifiers upstream of the p53 gene (29). Moreover, p53 mutants that can induce growth arrest but not apoptosis, or vice versa, have been identified (12, 49, 60), which is consistent with the concept that certain p53 gene mutations may cause selective loss of the ability to transactivate certain p53-responsive promoters (35).
Several p53-target genes have been reported to be specifically involved in apoptosis. These genes include those encoding KILLER/DR5 (56), Bax (39), IGF-BP3 (6), and, more recently, PIG3 (45), PAG608 (24), PERP (1), Noxa (43), PIDD (33), p53AIP1 (44), APAF-1 (46), ferredoxin reductase (FDXR) (23), and PUMA (41, 57). Some of the genes, like the PIG3 and FDXR genes, are involved in reactive oxygen species (ROS)-related pathways (45). Moreover, apoptosis triggered by p53 has been reported to be dependent on an increase of ROS and the release of apoptotic factors resulting from mitochondrial damage (45).
An increase in ROS has independently been implicated in cellular senescence (10). Senescence was first observed in normal human fibroblasts in culture, which have a finite replicative life span and then become permanently arrested (21). Senescent cells have higher levels of ROS than normal cells (20), and both oncogenic Ras (30) and the cyclin-dependent kinase inhibitor p21Waf1/cip1/sdi1 (36) induce senescence in association with increased intracellular ROS. It has also been reported that oxidative stress caused by sublethal doses of H2O2 (11) or hyperoxia (55) can force human fibroblasts to arrest in a senescence-like fashion (9). Loss of wild-type p53 is sufficient for these cells to escape senescence (5, 48), and it has therefore been suggested that senescence acts as a tumor suppressor mechanism to avoid the emergence of immortal cells (51, 52). In view of the implication of ROS in both apoptosis and senescence and the capacity of p53 to elevate ROS levels, we studied the involvement of ROS in p53-induced senescence and how conditions of p53 expression may modulate ROS levels to achieve different cell fate outcomes.
MATERIALS AND METHODS
Cell culture.
EJ or PC3 cells with a tetracycline (TET)-regulated expression system (17) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin-streptomycin (50 units/ml), hygromycin (100 μg/ml) and geneticin (750 μg/ml), plus 1 μg of TET per ml to repress expression of p53. Fresh medium with TET was added every 3 days. To induce p53 expression, cells were washed three times with phosphate-buffered saline (PBS) and seeded in medium in the absence of TET. HCT116 cells, normal human fibroblasts (501T), 293T cells, and mouse embryo fibroblasts were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin-streptomycin (50 units/ml). Cells were treated by adding to the medium 10 mM NAC (Sigma), 1 mM reduced glutathione (GSH; Sigma), 10 mM N-acetylalanine (NAA; Sigma), 0.2 μg of doxorubicin per ml (Sigma), or different concentrations of tert-butyl-hydroperoxide (tBH; Sigma) for the specified time. If chemical was to be removed, cells were washed twice with PBS before fresh medium was added.
Adenoviral infection.
An adenovirus containing p53 (Adp53), a generous gift of B. Vogelstein (Johns Hopkins University, Baltimore, Md.), or LacZ (AdLacZ) were amplified as previously described (22). Cells were exposed to 10 μl of the appropriately diluted virus stock.
FACS analysis.
Fluorescent stained cells were transferred to polystyrene tubes with cell strainer caps (Falcon) and subjected to a fluorescence-activated cell sorter (FACS) (FACScan; Beckton Dickinson) with Cell Quest 3.2 software (Beckton Dickinson) for acquisition and analysis. FLIH and F12A are laser channels representing green and red fluorescence, respectively.
Cell cycle analysis.
Cells were stained with propidium iodide (PI) with the CycleTEST Plus DNA reagent kit (Beckton Dickinson), according to the instructions provided by the manufacturer. FACS analysis was then performed.
Annexin and PI fluorescent staining.
Cells were washed with PBS, trypsinized, and then incubated with annexin and PI with the Annexin-V-Fluos staining kit (Boehringer Manheim), as previously reported (2, 3), followed by FACS analysis. Cells positively stained with annexin and not PI were considered apoptotic, and cells negative for the two dyes were considered live cells.
Measurement of intracellular oxidation.
Cells were incubated with 5 μg of dichlorofluorescin diacetate (DCF; Molecular Probes) per ml for 30 min at 37°C, then washed with PBS, trypsinized, and collected in 1 ml of PBS, followed by FACS analysis. Values of mean fluorescence intensity were used to plot graphs. Alternatively, a colorimetric assay to determine intracellular GSH (GT10; Oxford Biomedical Research) concentrations was performed according to the manufacturer's directions. Briefly, cells were collected, washed, and incubated with provided reagents, and then samples were measured for optical density with a spectrophotometer set at 400 nm. To specifically measure mitochondrial levels of ROS, cells were incubated with 10 μg of dihydrorhodamine 123 (DHR123; Molecular Probes) per ml for 30 min at 37°C and then washed with PBS, trypsinized, and collected in 1 ml of PBS, followed by FACS analysis. Values of mean red fluorescence intensity were used to plot graphs.
Sequencing of p53.
Approximately 107 EJ cells were infected with Adp53 virus for 24 h. DNA was extracted with phenol-chloroform, precipitated, washed twice with ice-cold 70% ethanol, and then resuspended in 100 μl of Tris-EDTA buffer. PCR assays were set up in a 50-μl reaction volume with 50 ng of DNA, 1× PCR buffer, 2 mM MgCl2, 0.11 mM deoxynucleoside triphosphate, 0.30 μM of each primer (forward primer, 5′-GCAGTCAGATCCTAGCGTCGAG-3′; reverse primer, 5′-GCACCACCACACTATGTCGAAA-3′), and 1 U of platinum Taq DNA polymerase (Invitrogen). PCR was carried out for 35 cycles, with 5 cycles at 60°C and 30 cycles at 59°C. Post-PCR products were purified with a QIAquick PCR purification kit (Qiagen) according to the manufacturer's instructions. Two microliters of purified PCR product (Adp53) and the p53 plasmid used in the TET system (54) were sequenced with 2 μM forward primer, the Big Dye 1.0 sequencing kit (Applied Biosystems), and an ABI 3700 DNA Analyzer.
Immunoblot analysis.
Cells were washed twice with ice-cold PBS and lysed in EBC buffer (50 mM Tris [pH 8], 120 mM NaCl, 0.5% NP-40, 100 mM sodium fluoride, 2 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride, and 10 μg of aprotinin/ml). Lysates were cleared by centrifugation at 20,000 × g for 20 min at 4°C. Protein concentrations were then determined with a bicinchoninic acid protein assay (Pierce). Forty micrograms of total cell protein per sample was subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and transferred to an Immobilon (Milipore) polyvinylidene difluoride filter. The presence of p21 was detected with the Ab-1 monoclonal antibody (Oncogene Science), and p53 was detected with the 1801 monoclonal antibody. An ECL detection system (Amersham) was used.
Senescence-associated β-galactosidase (SA-β-gal) staining.
Cells were washed in PBS and fixed with 2% formaldehyde-0.2% glutaraldehyde in PBS for 5 min at room temperature. Plates were stained as previously described (14).
Immunocytochemistry.
Cells were seeded onto glass coverslips and infected with either AdLacZ or Adp53 for 12 h. Coverslips were rinsed with PBS, fixed with 1% paraformaldehyde in PBS for 10 min at room temperature, washed two times with PBS, incubated in precooled ethanol-acetic acid (2:1) for 5 min at −20°C, washed two times with PBS, incubated for 15 min in 3% hydrogen peroxide in PBS, and then blocked in normal horse serum for 30 min at room temperature. Cells were stained for p21 with monoclonal p53 antibody (2.5 μg/ml) overnight at 4°C. Following staining, cells were washed two times with PBS and then incubated for 30 min at room temperature in biotinylated horse anti-mouse secondary antibody (Vector Lab). Following two washes with PBS, cells were incubated in streptavidin-horseradish peroxidase (Zymed), washed three more times with PBS and once with 0.5% Triton X-100, and then incubated for 5 min in the dark with diaminobenzidine (Sigma).
RESULTS
Cell fate decisions concerning senescence or apoptosis in the same cell correlate with p53 protein levels.
To study the relationship between p53-induced senescence and apoptosis, we initially tested the p53-null EJ human bladder carcinoma cell line with a TET-regulatable p53 expression system (EJp53) (19). EJp53 cells undergo senescence-like changes in morphology when p53 expression is activated by the removal of TET from the culture medium (54). Growth arrest becomes irreversible after 4 or more days of TET removal, even after repression of p53, and is accompanied by the expression of the senescence-specific marker SA-β-gal (14).
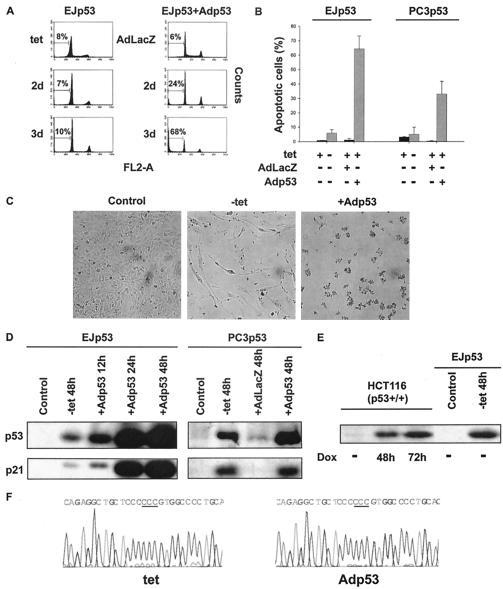
Previous studies with other cancer cell lines lacking p53 indicated that infection with an adenovirus containing p53 (Adp53) can cause apoptosis (45). To investigate the basis for these different responses, we infected EJp53 cells cultured in the presence of TET with Adp53. As shown in Fig. 1A, while TET removal induced a pronounced arrest in both G1 and G2 phases of the cell cycle, as previously reported (54), Adp53 infection resulted in an initial arrest followed by a marked increase in the apoptotic fraction (sub-G1). After 3 days, around 70% of the cells infected with Adp53 were apoptotic, whereas 90% of the same EJp53 cells induced by TET removal survived in a proliferation-arrested state. Infection of EJp53 cells with AdLacZ, a control adenovirus, did not induce apoptosis, indicating that adenovirus infection itself was not responsible for this effect. Annexin V-PI staining, a method used to detect early induction of apoptosis, confirmed these results (Fig. 1B), which also correlated with the morphological changes observed under the microscope (Fig. 1C). Whereas TET-regulatable p53 expression resulted in elongated growth-arrested cells with the morphological features of senescence (54), cells infected with Adp53 for 2 days rounded up, contracted, and lost adhesion to the plate, a result characteristic of apoptosis (13). We also analyzed p53-null PC3 human prostate cancer cells with a TET-regulatable p53 expression system (PC3p53) (31). Similar to EJp53, PC3p53 cells underwent senescence-like changes similar to those observed with EJp53 after TET removal (data not shown). In striking contrast, Adp53 infection of uninduced PC3p53 cells caused apoptosis in around 40% of the cells (Fig. 1B).
FIG. 1.
Correlation of growth arrest or apoptosis with levels of p53 expression in the same tumor cell. (A) PI staining of EJp53 cells after p53 induction for 2 or 3 days by TET removal (left column) or after infection with a 1:10 dilution of the Adp53 viral stock (right column). Control cells in the left column were cultured in TET for up to 3 days; control cells in the right column were infected with AdLacZ virus for up to 3 days. The percentages of apoptotic cells (sub-G1 fraction) are indicated. (B) Annexin-PI staining of p53-induced EJp53 and PC3p53. Cells were cultured in the presence or absence of TET for 3 days or were infected for 3 days with AdLacZ, a 1:10 dilution of Adp53 (EJp53), or undiluted Adp53 (PC3p53) in the presence of TET. Results represent the mean values of at least three different experiments, and error bars show standard deviations. (C) Morphological changes in p53-induced EJp53 cells. Cells were cultured in the presence or the absence of TET for 4 days or infected with Adp53 for 4 days. Cells were photographed with a Nikon Eclipse TE200 microscope (magnification, ×100). (D) Immunoblot analysis of p53 and p21 expression levels in lysates of control EJp53 or PC3p53 cells cultured in the presence of TET for 48 h and after p53 induction by TET removal (48 h) or Adp53 infection (12, 24, or 48 h). (E) Immunoblot analysis of p53 expression levels in lysates of HCT116 cells after 48 or 72 h of treatment with 0.2 μg of doxorubicin (Dox) per ml, compared to protein levels in control EJp53 cells cultured in the presence of TET or after 48 h following TET removal. (F) Sequences of TET and Adp53, both showing the Pro variant in position 72.
To investigate the basis for the striking differences in biologic responses to wild-type p53 expression under these different conditions, we measured the kinetics of p53 protein increase as well as levels of expression after TET removal or following Adp53 infection. As shown in Fig. 1D, Adp53 infection resulted in much higher levels of the p53 protein and of its transcriptional target, p21, than observed in either TET-induced EJp53 or PC3p53 cells. Of note, p53 levels observed after 2 days of TET removal were similar to those observed in p53 wild-type cells in response to exposure to doxorubicin, a DNA-damaging agent (Fig. 1E). Thus, the 5- to 20-fold increase in p53 levels observed with Adp53 infection appeared to be considerably higher than might be expected in response to physiological cell stress.
It has recently been shown that a polymorphism in position 72 affects the ability of p53 to induce apoptosis (16). The Arg72 variant has been reported to induce apoptosis more efficiently than the Pro72 variant. To confirm that the different responses observed with TET and Adp53 were due to p53 protein levels and not to different polymorphic variants of p53, we sequenced the constructs used in the TET system and Adp53. As shown in Fig. 1F, both constructs had the Pro variant in position 72, thus excluding this polymorphism as being responsible for the different cell fate outcomes observed.
ROS increases in p53-induced senescent and apoptotic cells.
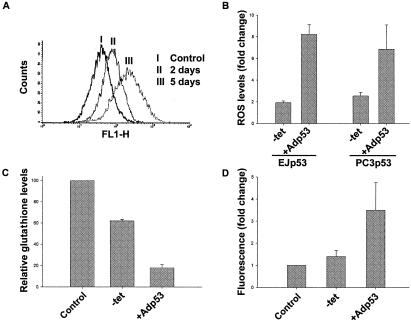
Previous studies have implicated increased ROS levels as responsible for apoptosis induced by Adp53 in DLD-1 colon cancer cells (45). We have also shown that p21 causes p53-independent ROS accumulation, which is responsible for the permanent growth arrest phenotype induced by p21 (36). To investigate the role of ROS in the senescent or apoptotic cell fates triggered by different p53 levels in EJ and PC3 cells, we measured ROS levels with the green fluorescent probe DCF, a marker of a change in general cellular oxidant accumulation (50). As shown in Fig. 2A, FACS analysis of DCF-stained EJp53 cells revealed a progressive increase in ROS levels following TET removal. After 3 days of induction, when senescent morphological changes were first observed, ROS levels in the cells had increased around twofold, with further increases by day 5, when growth arrest became irreversible. By comparison, Adp53-infected cells showed as much as an eightfold increase in ROS levels within 3 days (Fig. 2B).
FIG. 2.
ROS levels in EJp53 and PC3p53 cells after p53 induction. (A) ROS levels in EJp53 were measured by FACS analysis after they were stained with the fluorescent probe DCF. The curves correspond to control cells cultured for 5 days in the presence of TET (I) or for 2 (II) or 5 days (III) after TET removal. (B) ROS levels in EJp53 and PC3p53 cells after 3-day induction of p53 by TET removal or 3 days following infection with Adp53 at a 1:10 dilution or undiluted, respectively. Results represent mean values of three different experiments, and error bars show standard deviations. (C) Intracellular GSH levels were measured as described in Materials and Methods after 2 days of either TET removal or Adp53 infection, compared to control cells infected with AdLacZ for 2 days. Results represent mean values of two experiments, and error bars show standard deviations. (D) Measure of mitochondrial ROS levels with DHR123 in EJp53 cells after 48-h induction of p53 by TET removal or after 2-day infection with Adp53, compared to control cells infected with AdLacZ for 2 days. Results represent mean values of two experiments, and error bars show standard deviations.
We next studied whether ROS levels correlated with the decision between senescence and apoptosis in PCp53 cells. Figure 2B shows that Adp53-infected PC3p53 cells exhibited much higher ROS levels than PC3p53 cells after TET removal. As observed with EJp53 cells, the higher ROS levels correlated with induction of the apoptotic response. We also tested the effects of Adp53 in the p53-negative cancer cell line DLD-1. In this case, after 4 days of infection more than 60% of the cells were apoptotic, with ROS increases of at least sixfold (data not shown).
To extend these results, we measured the levels of intracellular GSH, one of the principal ROS buffers and a marker of oxidative stress (15). Consistent with the increase in ROS, GSH levels were decreased in EJp53 cells cultured in the absence of TET and more markedly decreased in Adp53-infected cells (Fig. 2C). Moreover, we stained these cells with DHR123, a red fluorescent dye that has been used to measure mitochondrial levels of H2O2 (8). As shown in Fig. 2D, EJp53 cells infected with Adp53 exhibited greater increases in fluorescence than cells with p53 induced by TET removal. Even though DHR123 can also reflect mitochondrial accumulation of peroxide generated elsewhere in the cell and, therefore, is not an unequivocal marker for mitochondrial ROS production (42), this result confirms that Adp53 produces higher oxidative intracellular increases and suggests a possible role of the mitochondria in ROS generation after p53 induction.
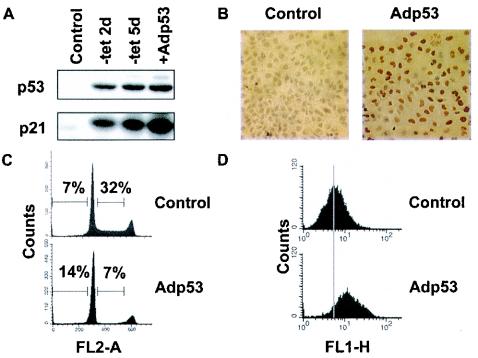
To further establish that the different cell fate outcomes were due to p53 protein levels and not to other possible variables between the TET and adenoviral models, we titrated the amount of Adp53 used to infect EJp53 cells. As shown in Fig. 3A, infection of EJp53 with an amount of virus 100-fold lower than that which induced apoptosis in these cells resulted in protein levels similar to those observed with TET removal. This concentration was sufficient to lead to p53 expression in most of the cells (Fig. 3B) but did not cause a significant increase in apoptosis. Instead, such cells showed a cell cycle arrest (Fig. 3C) similar to that seen with TET removal (see Fig. 1A). Of note, the levels of induced ROS increased around twofold (Fig. 3D), which is similar to the increase observed with TET removal, compared to the much higher ROS levels observed with cells infected with higher concentrations of Adp53 (see Fig. 2B).
FIG. 3.
Induction of growth arrest in EJp53 cells at low multiplicity of infection with Adp53. (A) Immunoblot analysis of p53 and p21 expression levels in lysates of EJp53 cells cultured in the presence of TET for 2 days (Control), after p53 induction by TET removal at 2 or 5 days, or at 3 days following infection with a 1:1,000 dilution of the Adp53 viral stock. (B) Immunostaining with p53 antibody of EJp53 infected with AdLacZ (Control) or Adp53. Cells were photographed with a Nikon Microphot-FXA microscope (magnification, ×200). (C) PI staining of EJp53 cells after Adp53 infection for 3 days with a low concentration of virus. Percentages indicate apoptotic cells (sub-G1 fraction) and cells in S phase. (D) ROS levels in EJp53 3 days after infection with AdLacZ (Control) or a low concentration of Adp53, as measured by DCF staining. Grey line indicates mean fluorescent values in control cells.
The p53 levels and associated ROS increases correlate with the induction of senescence or apoptosis in normal human fibroblasts.
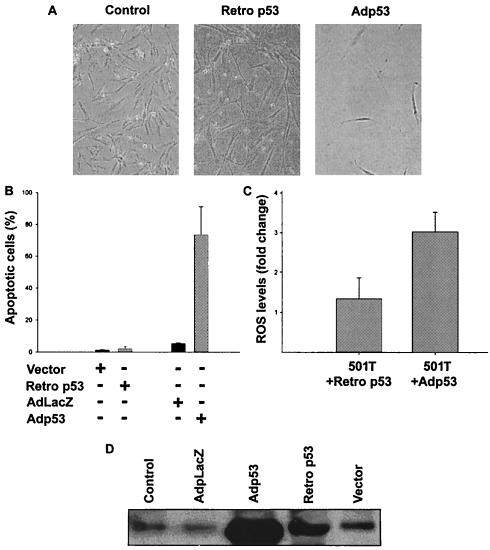
To investigate whether the responses of normal human fibroblasts to different p53 levels were comparable to those seen in cancer cells, we utilized a retrovirus containing p53 (Retrop53) or Adp53. As shown in Fig. 4A and B, fibroblasts selected following Retrop53 infection showed growth arrest, increased cell size, and intact nuclei, which are characteristic of senescence, in the absence of detectable apoptosis. In contrast, Adp53 caused apoptosis in these same fibroblasts after 3 days. ROS levels induced by Retrop53 increased around 1.5-fold over vector controls, whereas Adp53 caused a greater than threefold increase in ROS levels (Fig. 4C). These different cell responses again correlated with the increased p53 protein observed (Fig. 4D). Thus, quantitative differences in p53 expression levels correlated with ROS induction and either senescence or apoptosis in the same normal cell.
FIG. 4.
Comparison of the effects of different p53 protein levels on arrest and apoptosis in 501T normal human fibroblasts. (A) Morphological changes in 501T cells infected with a retrovirus containing a vector (Control) or p53 (Retrop53), 1 week after puromycin selection, compared to 48 h after infection with undiluted Adp53. Cells were photographed with a Nikon Eclipse TE200 microscope (magnification, ×100). (B) Percentage of apoptotic cells, as measured by annexin-PI staining, in 501T cells after infection with Retrop53 (1 week after selection) or Adp53 (3 days) (grey bars). Control cells (black bars) correspond to infection with a retrovirus containing a vector or AdLacZ, respectively. Results reflect mean values of at least two different experiments, and error bars show standard deviations. (C) Increase in intracellular ROS levels in 501T cells after infection with Retrop53 or Adp53. Control cells were infected with a retrovirus containing a vector or AdLacZ, respectively. Results reflect mean values of at least two different experiments, and error bars show standard deviations. (D) Immunoblot analysis of p53 expression levels in lysates of 501T cells after infection with AdLacZ, Adp53 (2 days after selection), Retrop53, or a vector containing retrovirus (1 week after selection).
The influence of Bax and PUMA on p53-induced ROS and apoptosis.
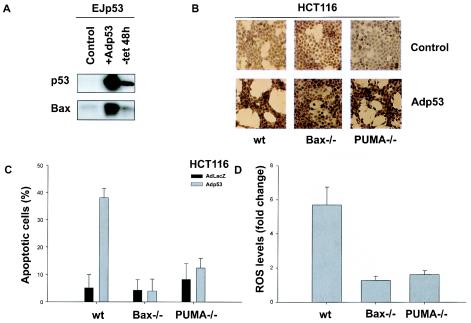
The proapoptotic Bax gene is a Bcl-2 family member that has been reported to increase mitochondrial membrane permeability (26, 37). It has been shown to be upregulated by DNA damage and p53 (39), and the requirement for Bax in p53-induced apoptosis has been proposed to be cell context dependent (4, 7, 40). Moreover, EJp53 cells infected with Adp53 exhibited higher Bax protein levels than observed in EJp53 cells after TET removal (Fig. 5A). PUMA is a BH3-only protein that binds to Bcl-2 and Bcl-XL and is directly regulated by p53 (41, 57). When overexpressed, PUMA causes cytochrome c release from the mitochondria and induces apoptosis. To test the role of both Bax and PUMA in Adp53-induced ROS and apoptosis, we used HCT116 cells with either of these genes inactivated by somatic gene targeting (59). As shown in Fig. 5B, immunostaining revealed a similarly high percentage of cells (>50%) expressing p53 in each cell line in response to Adp53. Both Bax−/− and PUMA−/− cells were significantly more resistant to apoptosis after infection with Adp53 than wild-type parental cells, a result that is consistent with previous reports (Fig. 5C) (59). Of note, the accumulation of ROS in response to p53 was also markedly reduced in the absence of either Bax or PUMA (Fig. 5D). These findings further correlate with the magnitude of ROS accumulation in response to p53 in determining apoptosis and implicate Bax and PUMA as important effectors of p53-induced ROS, suggesting a mitochondrial role in generating ROS after p53 upregulation.
FIG. 5.
Comparison of ROS levels and apoptosis in Adp53-infected Bax- and PUMA-null cells. (A) Immunoblot analysis of p53 and Bax expression levels in lysates of EJp53 cells after infection with a 1:10 dilution of Adp53 (2 days) or TET removal for 2 days. (B) Immunostaining with p53 antibody of HCT116 cells (wild type, Bax−/−, or PUMA−/−) infected with AdLacZ (Control) or undiluted Adp53. Cells were photographed with a Nikon Microphot-FXA microscope (magnification, ×200). (C) Apoptosis levels in wild-type, Bax−/−, and PUMA−/− HCT116 cells infected with AdLacZ or Adp53 for 3 days, as measured by Annexin-PI staining. (D) Relative ROS levels in wild-type, Bax−/−, and PUMA−/− HCT116 cells infected with Adp53, compared to ROS levels in the same cells infected with AdLacZ. Results represent mean values of at three different experiments, and error bars show standard deviations. wt, wild type.
ROS inhibition partially blocks senescence and apoptosis in EJp53 cells.
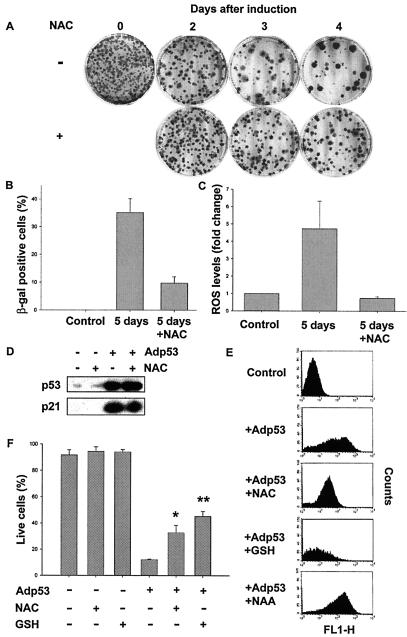
To investigate the contribution of ROS to p53-induced senescence and apoptosis, we tested whether the antioxidant N-acetylcysteine (NAC), a reduced GSH provider and a direct scavenger of ROS (10, 47), was able to protect cells from the permanent growth arrest phenotype. Around 100 EJp53 cells were plated, and p53 was induced by TET removal for up to 4 days. TET was then added back to the medium, and cells were cultured for another 2 weeks for analysis of colony formation. It has been shown that the longer such cells are exposed to p53, the fewer colonies that can be recovered following p53 down-regulation (54). Figure 6A shows that the addition of 10 mM NAC to the culture medium increased the number of cells able to escape p53-induced senescence, suggesting that inhibition of ROS protected them from this outcome. The fraction of cells that scored positive for the senescence marker SA-β-gal was also reduced in EJp53-induced cells cultured in the presence of NAC (Fig. 6B). DCF staining of these cells confirmed that NAC treatment significantly reduced ROS accumulation (Fig. 6C). NAC did not induce cell death at the concentration utilized (see Fig. 6F). As a further control, 10 mM NAA, a structural analogue of NAC without antioxidant activity, had no effect on colony recovery (data not shown). All of these results implied that ROS accumulation was an important mediator of p53-induced senescence.
FIG. 6.
Effect of antioxidants on p53-induced senescence and apoptosis. (A) Representative plates from a colony formation assay, in which around 100 EJp53 cells were plated. Cells were maintained in the absence of TET for 0 (Control), 2, 3, or 4 days, and then TET was added again to the medium. Cells were cultured for 14 more days followed by 10% formalin fixation and Giemsa staining. Where indicated (plus sign), 10 mM NAC was added at the beginning of the experiment and at each subsequent medium change, until TET was added back to the medium. (B) Percentage of SA-β-gal-positive cells in EJp53 control cells cultured in the presence of TET and after 5 days of p53 induction in the presence or absence of 10 mM NAC. (C) ROS levels in the same cells were measured by DCF staining. Results represent mean values of at least two different experiments, and error bars show standard deviations. (D) Immunoblot analysis of p53 and p21 expression levels in lysates of EJp53 cells infected with Adp53 in the presence or absence of 10 mM NAC. (E) ROS levels in EJp53 cells cultured in the presence of TET (Control) and infected with Adp53 in the presence or absence of 10 mM NAC, 1 mM GSH or 10 mM NAA. (F) Anenxin-PI staining showing the percentage of live cells in control EJp53 cultured in presence of TET for 2 days, with 10 mM NAC or 1 M GSH added to the media for the same period, and following infection with Adp53 for 3 days in the presence or absence of the same concentrations of NAC or GSH. Results represent mean values of three different experiments, and error bars show standard deviations. *, statistical analysis of Adp53 versus Adp53 + NAC (P < 0.00001); **, statistical analysis of Adp53 versus Adp53 + GSH (P < 0.000001).
We next tested the effects of antioxidants on p53-induced apoptosis. As shown in Fig. 6D, treatment with NAC did not affect p53 or Bax protein levels induced by Adp53 infection. Treatment of EJp53 with either 10 mM NAC or 1 mM reduced GSH prior to Adp53 infection significantly inhibited the magnitude of the increase in ROS levels compared to the increase observed in the absence of antioxidants, whereas the inactive NAC analogue NAA had no effect on ROS accumulation (Fig. 6E). NAC and GSH treatment also resulted in a statistically significant increase in surviving cells after 24 h (Fig. 6F). Because at higher concentrations antioxidants had adverse effects on cell survival, it was not possible to determine whether further neutralization of ROS could be achieved, and if so, whether the magnitude of the p53 apoptotic response would be further reduced.
Exogenous ROS cooperates with physiological levels of induced p53 to convert a senescent response into apoptosis.
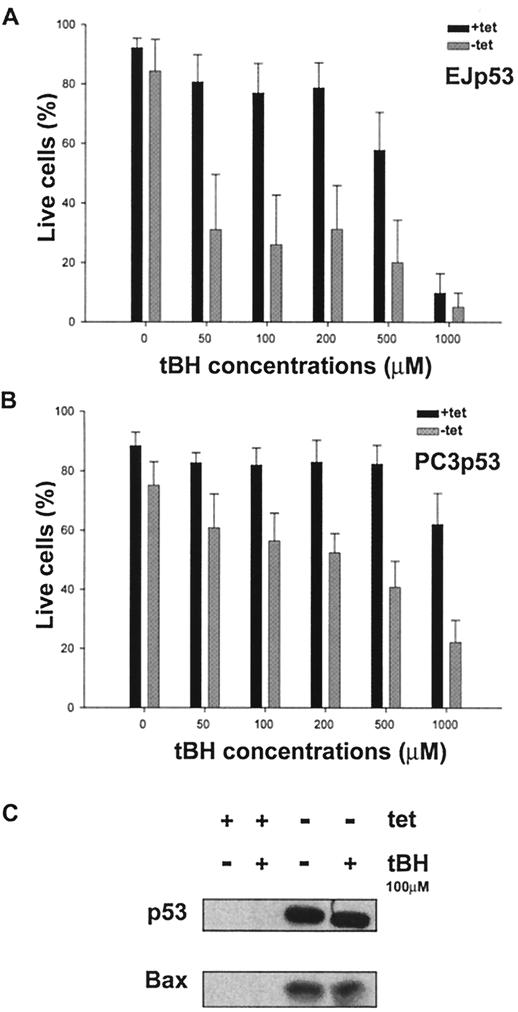
The above results demonstrated that high, nonphysiological levels of p53 were needed to induce apoptosis in the same cells in which physiological levels of this tumor suppressor caused permanent growth arrest. Moreover, these outcomes could be directly correlated with the magnitude of p53-induced ROS. Thus, we reasoned that exposure of TET-regulated EJp53 cells to an exogenous source of ROS might be sufficient to alter their fate so as to convert a senescent response to apoptosis. To test this possibility, we used tBH, an organic hydroperoxide that causes oxidative stress (58). In combination with physiological levels of p53 induced by 24 h of TET removal, tBH was able to induce apoptosis in EJp53 cells that would otherwise have undergone the senescence process (Fig. 7A). Moreover, such cells were significantly more sensitive to tBH-induced cell death than uninduced cells. Whereas treatment with 50 μM tBH resulted in survival of less than 20% of the p53-induced EJp53 cells, greater than 500 μM was required to cause comparable cell death in uninduced EJp53 cells. As shown in Fig. 7B, PC3p53 cells were less sensitive than EJp53 cells to tBH-induced apoptosis. However, induction of p53 to physiological levels in these TET-regulatable cells also increased significantly their sensitivity to the apoptotic outcome. These results establish that exogenous ROS can cooperate with physiological levels of p53 to convert a senescence response to apoptosis.
FIG. 7.
Comparison of the effects of exogenous ROS on p53-induced or uninduced cells. (A) Percentages of live cells in EJp53 24 h after treatment with the oxidant tBH. Cells cultured in the presence (black bars) or absence (grey bars) of TET for 24 h were then treated with different concentrations of tBH for 2 h. (B) Percentages of live cells in PCp53 24 h after treatment with tBH. Cells cultured in the presence (black bars) or absence (grey bars) of TET for 24 h were then treated with different concentrations of tBH for 2 h. Results represent mean values of four different experiments, and error bars show standard deviations. (C) Immunoblot analysis of p53 and Bax expression levels in lysates of EJp53 cells cultured for 24 h in the presence or absence of TET, followed by treatment for 2 h with 100 μM tBH.
Oxidative stress is known to induce p53 expression (11), and we and others have shown that p53 induces ROS (45). To test the possibility of the existence of a positive feedback loop between ROS and p53, we measured p53 protein levels in induced and uninduced EJp53 cells after tBH treatment. At sublethal concentrations, tBH did not alter p53 or Bax protein levels (Fig. 7C). These results indicate that oxidants do not change p53 levels or activity with respect to Bax induction in EJp53 cells.
DISCUSSION
The tumor suppressor p53 is an important sensor of cellular stress conditions, including DNA damage, hypoxia, survival factor deprivation, mitogenic oncogenes, and telomere shortening. Various outcomes can be observed following p53 activation in response to such stresses, including reversible growth arrest, permanent growth arrest or apoptosis (27, 32, 34). In fact, it is thought that the need for a cell to escape these cell fates in order to become a cancer cell accounts for the high frequency at which p53 function is lost in cancers. Thus, elucidation of the p53 signaling pathways involved in each of these different cellular responses has potentially important implications for understanding cellular aging and cancer as well as for therapeutic approaches aimed at counteracting these pathological processes. In the present studies, we manipulated p53 expression levels in different cell types in the absence of other stimuli in an effort to identify those p53 functions critically involved in determining cell fate decisions with respect to senescence and apoptosis.
At physiological levels of expression similar to those induced by DNA damage, p53 expression alone induced a growth arrest phenotype in EJ cells, which under conditions of TET-regulated expression became permanent by 4 to 5 days despite subsequent p53 down-regulation. In contrast, a p53 adenovirus vector, which resulted in p53 protein expression levels that were at least 10- to 20-fold greater than could be observed in DNA-damaged wild-type p53-containing cells, induced rapid and efficient apoptosis. Our results show that when p53 was induced in the same cell context and in the absence of other stimuli, the magnitude of p53 expression alone determined the decision with respect to these cell fates; this result is consistent with previous reports that demonstrate that the intracellular level of p53 can influence the decision between arrest and apoptosis (12). These findings help to explain the differences in cell fates observed in various studies where Adp53 was compared to other p53 expression systems.
It has been previously shown that p53 induces ROS accumulation (45), and a number of genes induced by p53 are associated with the metabolism of ROS (45). We observed that in cells undergoing senescence in response to p53 at physiological expression levels, there was a reproducible two- to fivefold increase in ROS accumulation. Moreover, NAC, a free radical scavenger, was able both to ameliorate ROS accumulation and to partially rescue the same cells from the permanent growth arrest phenotype. These findings implicate ROS accumulation in determining p53-induced senescence. At the high p53 levels capable of inducing an apoptotic response, we observed a more rapid appearance and a greater magnitude of ROS accumulation in the same cells that underwent p53-induced senescence at physiological p53 expression levels.
Previous studies have indicated that Adp53 induces ROS associated with decreased levels of cardiolipin, a component of the mitochondrial membrane that is sensitive to oxidative damage (45). Moreover, overexpression of ferredoxin reductase, a p53-induced gene, resulted in its localization to the mitochondrial membrane associated with mitochondrial accumulation of ROS (23). In our present studies, an Adp53 apoptotic response could be ameliorated by NAC and GSH but not by inactive analogues, and ROS inhibition by antioxidants correlated with a decreased magnitude of apoptosis. All these results strongly imply that the level of p53 protein overexpression and the p53-induced elevation of intracellular ROS importantly influence the decision between senescence and apoptosis in a given cell.
It has been reported that p53 can induce the expression of Bax, a proapoptotic gene product of the Bcl-2 family. Bax has been proposed to control mitochondrial membrane permeability either by forming a channel in the outer mitochondrial membrane or by regulating the opening and closing of the permeability transition pore (37). After the disruption of the membrane, mitochondrial proteins including cytochrome c and Smac/DIABLO are released into the cytosol, activating caspase-9 and inhibiting antiapoptotic proteins, which leads to activation of the downstream effector caspases of the apoptotic cascade (40). The requirement of Bax by p53 to induce apoptosis has been proposed to be context dependent. Previous studies have shown that the absence of Bax did not suppress γ-irradiation-induced p53-dependent apoptosis in mouse lymphocytes (28). On the other hand, chemotherapy-induced p53-dependent apoptosis in Bax−/− primary mouse embryo fibroblasts was shown to be attenuated, although not completely suppressed (38). In accordance with these findings, we showed that the absence of Bax impaired an Adp53-mediated apoptotic response. Our results further show that the absence of Bax significantly inhibited the accumulation of intracellular ROS. Similar results were observed in the absence of PUMA. PUMA has been reported to be an exclusively mitochondrial protein that binds to members of the Bcl family (57). These data, together with our results showing increased mitochondrial oxidation, indicate important roles of Bax and PUMA in ROS accumulation in response to p53 upregulation. Since adaptive changes may occur in cells in which there has been a knockout expression of any given gene, further studies will be needed to fully elucidate the roles of Bax and PUMA in the p53-dependent elevation of intracellular ROS.
A major question in cancer therapeutics is the impact in a high fraction of tumors of nonfunctional p53 on the ability of specific agents to selectively target the tumor as opposed to normal cells. Our observations that p53 induces ROS accumulation, which plays an important role in both senescence and apoptosis cell fate decisions, led us to ask whether p53 at physiological levels induced by cellular stresses could cooperate with an exogenous ROS source to favor an apoptotic outcome. By the use of TET-regulatable p53-containing tumor cells, we observed that this was indeed the case. At physiological p53 protein levels capable of triggering senescence, an apoptotic response was observed when the p53-induced increase in intracellular ROS levels was complemented by an exogenous ROS source, which itself was not able to induce apoptosis in the absence of p53.
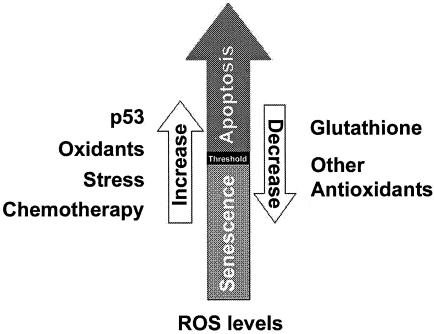
These findings suggest the existence of a threshold of cellular oxidation above which the apoptotic program is initiated. This threshold may vary between cell types or as a function of other physiological factors. However, the balance between all the ROS inducers and the antioxidants present in the cell at a given moment is likely crucial in determining cell fate decisions (Fig. 8). Certain agents used in cancer therapy, such as γ-irradiation and doxorubicin, have the ability to induce ROS (18). Based on our present findings, those therapeutic agents that cooperate with p53 in ROS generation would likely be more toxic for wild-type p53-containing normal than p53-negative tumor cells. These results provide a rationale for identifying therapeutic agents that do not complement p53 in ROS generation, which would ameliorate the cytotoxic side effects of such chemicals in normal cells.
FIG. 8.
The balance between all the oxidants (like p53, certain kinds of stress, or chemotherapeutics) and antioxidants present in the cell in a given moment can determine, by controlling intracellular ROS levels, whether a cell fate response would be senescence or apoptosis. Apoptosis is induced only if intracellular oxidation reaches a certain threshold.
Acknowledgments
We thank B. Vogelstein (Johns Hopkins University, Baltimore, Md.) for generously providing HCT116 Bax−/− cells and Adp53, I. George from the Mount Sinai Flow Cytometry Core Facility, and L. Goldin and J. Leung for technical assistance.
S. Macip received support from the Ministerio de Educacion y Cultura of Spain and is a recipient of a postdoctoral fellowship from the Forchheimer foundation. P. Berggren received support from the Swedish Cancer Society (Cancerfonden 477-BO2-01SAA). This work was supported in part by National Institutes of Health grants CA80058 and CA85214 (to S.A.A.) and CA78356 and CA82211 (to S.W.L.).
REFERENCES
- 1.Attardi, L. D., E. E. Reczek, C. Cosmas, E. G. Demicco, M. E. McCurrach, S. W. Lowe, and T. Jacks. 2000. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14:704-718. [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry, J. P., A. Blaecke, S. Lecoanet-Henchoz, P. Jeannin, N. Herbault, G. Caron, V. Moine, and J. Y. Bonnefoy. 1999. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry 37:197-204. [DOI] [PubMed] [Google Scholar]
- 3.Bartkowiak, D., S. Hogner, H. Baust, W. Nothdurft, and E. M. Rottinger. 1999. Comparative analysis of apoptosis in HL60 detected by annexin-V and fluorescein-diacetate. Cytometry 37:191-196. [DOI] [PubMed] [Google Scholar]
- 4.Bates, S., and K. H. Vousden. 1999. Mechanisms of p53-mediated apoptosis. Cell Mol. Life Sci. 55:28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond, J. A., F. S. Wyllie, and D. Wynford-Thomas. 1994. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 9:1885-1889. [PubMed] [Google Scholar]
- 6.Buckbinder, L., R. Talbott, S. Velasco-Miguel, I. Takenaka, B. Faha, B. R. Seizinger, and N. Kley. 1995. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377:646-649. [DOI] [PubMed] [Google Scholar]
- 7.Burns, T. F., and W. S. El-Deiry. 1999. The p53 pathway and apoptosis. J. Cell Physiol. 181:231-239. [DOI] [PubMed] [Google Scholar]
- 8.Cai, J., and D. P. Jones. 1998. Superoxide in apoptosis: mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 273:11401-11404. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Q., and B. N. Ames. 1994. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl. Acad. Sci. USA 91:4130-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Q., A. Fischer, J. D. Reagan, L. J. Yan, and B. N. Ames. 1995. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA 92:4337-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Q. M., J. C. Bartholomew, J. Campisi, M. Acosta, J. D. Reagan, and B. N. Ames. 1998. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 332:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, X., L. J. Ko, L. Jayaraman, and C. Prives. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10:2438-2451. [DOI] [PubMed] [Google Scholar]
- 13.Coleman, M. L., and M. F. Olson. 2002. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 9:493-504. [DOI] [PubMed] [Google Scholar]
- 14.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolphin, D., R. Poulson, and O. Avramovic (ed.). 1989. Glutathione: chemical, biochemical, and medical aspects. John Wiley & Sons, New York, N.Y.
- 16.Dumont, P., J. I. Leu, A. C. Della Pietra III, D. L. George, and M. Murphy. 2003. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 33:357-365. [DOI] [PubMed] [Google Scholar]
- 17.Fang, L., M. Igarashi, J. Leung, M. M. Sugrue, S. W. Lee, and S. A. Aaronson. 1999. p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 18:2789-2797. [DOI] [PubMed] [Google Scholar]
- 18.Gewirtz, D. A. 1999. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 57:727-741. [DOI] [PubMed] [Google Scholar]
- 19.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen, T. M., D. L. Yowe, J. C. Bartholomew, C. M. Wehr, K. L. Do, J. Y. Park, and B. N. Ames. 1997. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. USA 94:3064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayflick, L., and P. Moorehead. 1961. The serial cultivation of human diploid strains. Exp. Cell Res. 25:585-621. [DOI] [PubMed] [Google Scholar]
- 22.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang, P. M., F. Bunz, J. Yu, C. Rago, T. A. Chan, M. P. Murphy, G. F. Kelso, R. A. Smith, K. W. Kinzler, and B. Vogelstein. 2001. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 7:1111-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israeli, D., E. Tessler, Y. Haupt, A. Elkeles, S. Wilder, R. Amson, A. Telerman, and M. Oren. 1997. A novel p53-inducible gene, PAG608, encodes a nuclear zinc finger protein whose overexpression promotes apoptosis. EMBO J. 16:4384-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, T. M., Z. X. Yu, V. J. Ferrans, R. A. Lowenstein, and T. Finkel. 1996. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 93:11848-11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnstone, R. W., A. A. Ruefli, and S. W. Lowe. 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153-164. [DOI] [PubMed] [Google Scholar]
- 27.Kastan, M. B., O. Onyekwere, D. Sidransky, B. Vogelstein, and R. W. Craig. 1991. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51:6304-6311. [PubMed] [Google Scholar]
- 28.Knudson, C. M., K. S. Tung, W. G. Tourtellotte, G. A. Brown, and S. J. Korsmeyer. 1995. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270:96-99. [DOI] [PubMed] [Google Scholar]
- 29.Kurimasa, A., H. Ouyang, L. J. Dong, S. Wang, X. Li, C. Cordon-Cardo, D. J. Chen, and G. C. Li. 1999. Catalytic subunit of DNA-dependent protein kinase: impact on lymphocyte development and tumorigenesis. Proc. Natl. Acad. Sci. USA 96:1403-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, A. C., B. E. Fenster, H. Ito, K. Takeda, N. S. Bae, T. Hirai, Z. X. Yu, V. J. Ferrans, B. H. Howard, and T. Finkel. 1999. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274:7936-7940. [DOI] [PubMed] [Google Scholar]
- 31.Lee, S. W., L. Fang, M. Igarashi, T. Ouchi, K. P. Lu, and S. A. Aaronson. 2000. Sustained activation of Ras/Raf/mitogen-activated protein kinase cascade by the tumor suppressor p53. Proc. Natl. Acad. Sci. USA 97:8302-8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 33.Lin, Y., W. Ma, and S. Benchimol. 2000. Pidd, a new death-domain-containing protein, is induced by p53 and promotes apoptosis. Nat. Genet. 26:122-127. [DOI] [PubMed] [Google Scholar]
- 34.Lowe, S. W., E. M. Schmitt, S. W. Smith, B. A. Osborne, and T. Jacks. 1993. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847-849. [DOI] [PubMed] [Google Scholar]
- 35.Ludwig, R. L., S. Bates, and K. H. Vousden. 1996. Differential activation of target cellular promoters by p53 mutants with impaired apoptotic function. Mol. Cell. Biol. 16:4952-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macip, S., M. Igarashi, L. Fang, A. Chen, Z. Q. Pan, S. W. Lee, and S. A. Aaronson. 2002. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 21:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinou, J. C., and D. R. Green. 2001. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2:63-67. [DOI] [PubMed] [Google Scholar]
- 38.McCurrach, M. E., T. M. Connor, C. M. Knudson, S. J. Korsmeyer, and S. W. Lowe. 1997. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyashita, T., and J. C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80:293-299. [DOI] [PubMed] [Google Scholar]
- 40.Moll, U. M., and A. Zaika. 2001. Nuclear and mitochondrial apoptotic pathways of p53. FEBS Lett. 493:65-69. [DOI] [PubMed] [Google Scholar]
- 41.Nakano, K., and K. H. Vousden. 2001. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7:683-694. [DOI] [PubMed] [Google Scholar]
- 42.Nemoto, S., K. Takeda, Z. X. Yu, V. J. Ferrans, and T. Finkel. 2000. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell. Biol. 20:7311-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053-1058. [DOI] [PubMed] [Google Scholar]
- 44.Oda, K., H. Arakawa, T. Tanaka, K. Matsuda, C. Tanikawa, T. Mori, H. Nishimori, K. Tamai, T. Tokino, Y. Nakamura, and Y. Taya. 2000. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102:849-862. [DOI] [PubMed] [Google Scholar]
- 45.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 46.Robles, A. I., N. A. Bemmels, A. B. Foraker, and C. C. Harris. 2001. APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res. 61:6660-6664. [PubMed] [Google Scholar]
- 47.Roederer, M., F. J. Staal, P. A. Raju, S. W. Ela, and L. A. Herzenberg. 1990. Cytokine-stimulated human immunodeficiency virus replication is inhibited by N-acetyl-l-cysteine. Proc. Natl. Acad. Sci. USA 87:4884-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogan, E. M., T. M. Bryan, B. Hukku, K. Maclean, A. C. Chang, E. L. Moy, A. Englezou, S. G. Warneford, L. Dalla-Pozza, and R. R. Reddel. 1995. Alterations in p53 and p16INK4 expression and telomere length during spontaneous immortalization of Li-Fraumeni syndrome fibroblasts. Mol. Cell. Biol. 15:4745-4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowan, S., R. L. Ludwig, Y. Haupt, S. Bates, X. Lu, M. Oren, and K. H. Vousden. 1996. Specific loss of apoptotic but not cell-cycle arrest function in a human tumor derived p53 mutant. EMBO J. 15:827-838. [PMC free article] [PubMed] [Google Scholar]
- 50.Royall, J. A., and H. Ischiropoulos. 1993. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 302:348-355. [DOI] [PubMed] [Google Scholar]
- 51.Sager, R. 1991. Senescence as a mode of tumor suppression. Environ. Health Perspect. 93:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 53.Sionov, R. V., and Y. Haupt. 1999. The cellular response to p53: the decision between life and death. Oncogene 18:6145-6157. [DOI] [PubMed] [Google Scholar]
- 54.Sugrue, M. M., D. Y. Shin, S. W. Lee, and S. A. Aaronson. 1997. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA 94:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Zglinicki, T., G. Saretzki, W. Docke, and C. Lotze. 1995. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 220:186-193. [DOI] [PubMed] [Google Scholar]
- 56.Wu, G. S., T. F. Burns, E. R. McDonald III, W. Jiang, R. Meng, I. D. Krantz, G. Kao, D. D. Gan, J. Y. Zhou, R. Muschel, S. R. Hamilton, N. B. Spinner, S. Markowitz, G. Wu, and W. S. el-Deiry. 1997. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 17:141-143. [DOI] [PubMed] [Google Scholar]
- 57.Yu, J., L. Zhang, P. M. Hwang, K. W. Kinzler, and B. Vogelstein. 2001. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7:673-682. [DOI] [PubMed] [Google Scholar]
- 58.Zamzami, N., I. Marzo, S. A. Susin, C. Brenner, N. Larochette, P. Marchetti, J. Reed, R. Kofler, and G. Kroemer. 1998. The thiol crosslinking agent diamide overcomes the apoptosis-inhibitory effect of Bcl-2 by enforcing mitochondrial permeability transition. Oncogene 16:1055-1063. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]
- 60.Zhu, J., S. Zhang, J. Jiang, and X. Chen. 2000. Definition of the p53 functional domains necessary for inducing apoptosis. J. Biol. Chem. 275:39927-39934. [DOI] [PubMed] [Google Scholar]