Abstract
Although studies have shown that the Notch2 family member is critical for embryonic development, little is known concerning its role in hematopoiesis. In this study, we show that the effects of an activated form of Notch2 (N2IC) on the T-cell lineage are dosage related. High-level expression of N2IC results in the development of T-cell leukemias. In contrast, lower-level expression of N2IC does not lead to transformation but skews thymocyte development to the CD8 lineage. Underlying this skew is a dramatic enhancement in positive selection and CD8SP maturation. N2IC permits early B-cell development but blocks the maturation of conventional B2 cells at the pre-B stage, which is the limit of endogenous Notch2 protein expression in developing B cells. Most strikingly, while B2 B cell development is blocked at the pre-B-cell stage, N2IC promotes the selective development of LPS-responsive B1 B cells. This study implicates a role for Notch2 in the maturation of the CD8 lineage and suggests a novel function for Notch2 in the development of the B1 B-cell subset.
Mammalian Notch proteins (Notch1 to 4) are a highly conserved family of type 1 transmembrane receptors that regulate cell fate decisions in numerous developmental contexts (20). Since the discovery of Notch homologue expression in hematopoietic cells (25), numerous studies have revealed an important role for Notch in hematopoiesis. In particular, studies showing that the conditional inactivation of Notch1 in murine bone marrow resulted in the complete loss of T-cell development demonstrated an essential role for Notch1 in the establishment of the T-cell lineage (31). In addition, the enforced expression of an activated form of Notch1 (Notch1IC) resulted in the emergence of T-cell populations in the bone marrow of Notch1IC mice with a concomitant inhibition of B-cell development (30). More recently, inhibition of Notch signaling by transgenic expression of the Notch modifier, Lunatic Fringe, in cortical thymocytes blocked T-cell commitment and promoted B-cell development in the thymus (21). These studies have led to the model that Notch1 determines a T-versus-B-cell fate, presumably through its actions on a common lymphoid progenitor.
While it is now clear that Notch1 plays an integral role in T-cell lineage specification, controversy persists surrounding its role in later stages of T-cell development. Early studies in which expression of Notch1IC, driven by the proximal Lck promoter, resulted in skewed thymocyte development to the CD8 lineage led authors to conclude that activated Notch1 influences CD4-versus-CD8-lineage fate (32). However, subsequent studies showed transgenic expression from the proximal Lck promoter of another form of Notch1IC, which included more of its C-terminal transactivation domain, promoted maturation of both CD4 and CD8 SP cells, although the mice expressing this form of Notch1IC also developed an excess of CD8SP (7). In contrast to the findings of both of these studies, retroviral expression of Notch1IC, also with the extended transactivation domain, resulted in an apparent block in T-cell development at the CD4+ CD8+ double-positive (DP) stage (18). Though formal proof is lacking, it has been proposed that differences among the various forms of Notch1IC used in these independent studies resulted in differential expression levels of Notch1IC (18) as well as in differing propensities for tumor formation (11) and may account for the discrepant phenotypes.
In light of these studies, it was surprising that the conditional inactivation of Notch1 in double-negative (CD4− CD8−) thymocytes showed no effects on the development of either CD4SP or CD8SP, suggesting that other Notch family members might compensate in the regulation of SP thymocyte maturation in the absence of Notch1 (40). One such protein is the Notch family member Notch2, which is also expressed in hematopoietic cells, including developing thymocytes (37). Although these latter studies suggest that some functional redundancy may exist between Notch1 and Notch2 in later stages of T-cell development, numerous observations argue against complete redundancy among these proteins throughout hematopoiesis. For instance, the inability of Notch2 to compensate for the loss of Notch1 in T-lineage specification indicates that these proteins do not serve redundant roles in the early stages of T-cell development. In addition, studies have shown that while both Notch1 and Notch2 inhibit the cytokine-induced differentiation of 32D myeloid cells, they do so in a cytokine-specific manner and that structural differences in their notch cytokine response domains are responsible for this functional specificity (2). More recently, it was shown that the phosphorylation of a critical serine residue unique to the Notch2 notch cytokine response domain regulates its cytokine-specific inhibition of 32D myeloid cell differentiation (17). Despite these and other observations highlighting important differences between Notch1 and Notch2, most of what is known of Notch function in hematopoiesis continues to come from studies of the family member Notch1. Consequently, the role of Notch2 and the extent to which it serves unique functionality in hematopoiesis remain unclear.
To investigate the role of Notch2 in hematopoiesis, we analyzed Notch2 protein expression in the hematopoietic system. To obtain insight into its function, we expressed various levels of an activated form of Notch2 (N2IC) in murine hematopoietic progenitors by using a retroviral vector coexpressing green fluorescent protein (GFP) as a reporter. Transduced cells were transplanted into irradiated recipient mice and analyzed at various time points posttransplantation for the effects of N2IC on hematopoiesis. Our studies show that various expression levels of N2IC result in contrasting phenotypes for the T-cell lineage. High levels of N2IC lead to the development of highly proliferative T-cell populations, which invariably give rise to leukemias, while lower levels enhance the maturation of the CD8SP cells, skewing thymocyte development to the CD8 lineage. Furthermore, in contrast to what has been reported for Notch1, we show that N2IC permits early B-cell development but results in a block in conventional B2 B-cell development at the pre-B-cell stage. In addition to blocking maturation of B2 cells, N2IC promoted the selective development of B1 B cells. These results suggest that Notch2 plays a role in CD8SP thymocyte maturation and has a unique function in the development of the B1 B-cell subset.
MATERIALS AND METHODS
Histochemical analysis of tissue sections.
Mice generated to express an in-frame fusion of Notch2 and the Escherichia coli β-galactosidase (N2/β-Gal) have been previously described (13). Cryosections of thymus and spleen from N2/β-Gal+/− and wild-type littermates were prepared at 5-μm thicknesses and fixed in 4% glutaraldehyde in phosphate-buffered saline for 20 min at room temperature, washed in phosphate-buffered saline, and incubated overnight at 37° in solution containing 84 mM sodium phosphate (pH 7.5), 8.4 mM potassium chloride, 1 mM magnesium chloride, 3 mM potassium ferrocyanide, 3 mM potassium ferricyanide, and 1 mM X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Promega). The sections were washed and coverslipped with 90% glycerol as the mounting medium and visualized by bright-field microscopy and photographed by using a Nikon E800 fluorescent microscope with a SPOT imaging system (Diagnostic Instruments, Sterling Heights, Mich.).
Assessment of β-Gal activity by flow cytometry for Notch2 protein expression and antibodies.
Single-cell suspensions were stained with antibodies to immunophenotypic markers, washed, and loaded with fluorescein di-β-d-galactopyranoside by using a FluoReporter lacZ flow cytometry kit (Molecular Probes) as described in the manufacturer's protocol. N2 protein expression was determined by assessing β-Gal activity within specific subpopulations as indicated by the production of fluorescein. For three- and four-color analysis, cells were resuspended in propidium iodide and viable cells were electronically gated and analyzed on either a FACSCalibur (Becton Dickinson) or MoFlo (Cytomation) system. Antibodies included phycoerythrin-conjugated anti-Gr-1, anti-CD43, anti-Syn-1, anti-CD8, anti-CD4, anti-CD3, anti-HSA, anti-CD19, anti-BP-1, anti-CD5, and anti-T-cell-receptor (anti-[TCR]) β chain; APC-conjugated anti-Mac-1 and anti-CD8; PECy5-conjugated anti-B220, anti-CD44, and anti-CD4; and biotinylated anti-CD23, anti-CD25, anti-c-kit, anti-AA4.1, and anti-CD69. All antibodies were purchased from BD Pharmingen. Streptavidin-conjugated Spectral Red and Texas Red were purchased from Southern BioTech (Birmingham, Ala.). Goat anti-mouse immunoglobulin M (IgM) coupled to Cy5 was purchased from Jackson Immunoresearch Inc. (West Grove, Pa.).
Construction of retroviral vectors.
The entire intracellular domain of Notch2 (N2IC), beginning at base pair 5,248 (GenBank accession no. D32210), was generated from full-length Notch2 cDNA cloned into pTracer-CMV (a gift from Yoshio Hamada, National Institute for Basic Biology, Okasaki, Japan) by PCR by using PWO polymerase (Roche) as described in the manufacturer's protocol. N2IC/MSCV forward primer (CGCCGCCATGGTCATCATGGCCAAGCGGAAGCAAGC) and N2IC/MSCV reverse primer (TCATGCATACACCTGCATGTTGCT) were purchased from Operon (Alameda, Calif.). N2IC was blunt cloned into the EcoR1 site upstream of the IRES element of the parental murine stem cell virus (MSCV) IRES/GFP vector (16), which also served as the control. The N2IC insert was verified by DNA sequencing.
Western blotting.
NIH 3T3 fibroblasts plated to 80% confluency were transfected with full-length Notch2 (used for PCR generation of the N2IC fragment referred to above), N2IC/MSCV, or control vector by calcium phosphate coprecipitation. At 24 h, cells were lysed in Laemmli buffer and run on an 8% polyacrylamide gel. N2IC protein was detected with a goat anti-mouse antibody raised against a peptide mapping to the carboxy terminus of Notch2 (Santa Cruz Biotechnology, Inc.) and was visualized by anti-goat IgG horseradish peroxidase and enhanced chemiluminescence (Amersham Pharmacia).
Retroviral transduction of whole bone marrow and transplantations.
BOSC23 retroviral producer cells were plated at 70% confluency and transfected 24 h later with 10 μg of N2IC/MSCV or control vector DNA by calcium phosphate coprecipitation. On the day of transfection, whole bone marrow (WBM) was harvested from 6- to 8-week-old C57BL/6 mice pretreated 4 days previously with 5-flourouracil by intraperitoneal injection at a dosage of 150 mg/kg of body weight. Harvested WBM was treated for 3 min on ice with sterile ACK (0.15 M NH4Cl and 0.01 M KHCO3) to lyse red blood cells and then plated for 24 h prestimulation as previously described (6). After 24 h, WBM was plated onto transfected and irradiated (30-Gy) BOSC23 retroviral producers with or without the addition of 4 μg of Polybrene/ml for a 48-h coculture prior to transplantation. On the day of the transplant, 6- to 8-week-old congenic C57BL/6-Ly5.1 mice were lethally irradiated with 10 Gy in a split dose separated by 3 h. The transduction efficiency of WBM cocultures was assessed by fluorescence-activated cell sorter (FACS), and 2 × 105 WBM cells were injected by tail vein into irradiated recipient mice. The recipients were maintained for 3 weeks on acidified water containing neomycin sulfate (1.1 g/liter) and polymyxin B sulfate (106 U/liter).
Adoptive transfers.
DP and CD8SP populations from N2IChi WBM were isolated by fluorescence-activated cell sorting with a MoFlo (Cytomation) sorter. Cells (8 × 104) from each population were injected into the lateral tail veins of nonirradiated 6- to 8-week-old congenic C57BL/6-Ly5.1 mice. The mice were sacrificed at 6 weeks posttransfer for analysis of peripheral blood, bone marrow, spleen, and thymus.
In vivo LPS treatment.
N2IClo and empty control vector mice (12 weeks posttransplant [PT]) and wild-type C57BL/6 mice were treated with 20 μg of lipopolysaccharide (LPS) (Sigma) by intravenous injection. Untreated wild-type C57BL/6, untreated empty control vector, and untreated N2IClo mice served as negative controls. Wild-type C57BL/6 and empty control vector mice treated with the same LPS dosage served as positive controls for comparison of LPS responsiveness within the splenic IgM+ populations. At 48 h after the LPS injection, the mice were sacrificed and single-cell suspensions were prepared from harvested spleens. The cell suspensions were stained with anti-IgM and anti-Syn-1 and resuspended in propidium iodide for analysis of viable cells with FACSCalibur.
RESULTS
Notch2 protein expression is spatially restricted in spleen and thymus.
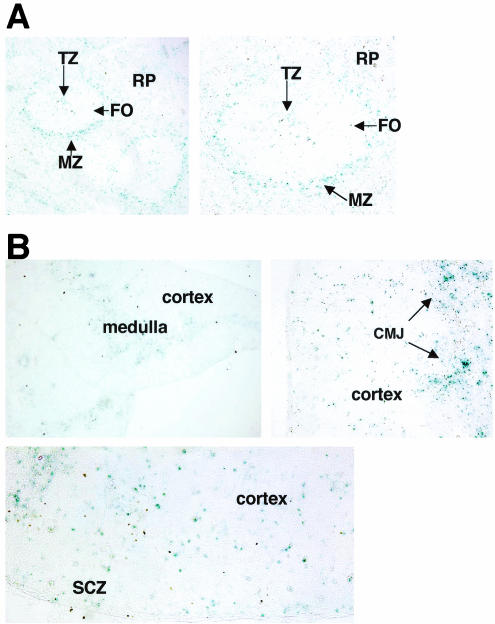
Mice were previously generated to express an in-frame fusion of Notch2 and the E. coli β-Gal protein (N2/β-Gal) under the control of the endogenous Notch2 promoter (13). Because these mice express N2/β-Gal in all cells where the endogenous Notch2 promoter is active, they provided a highly reproducible means to analyze Notch2 expression at the protein level within hematopoietic cells. To visualize the location of Notch2-expressing cells within hematopoietic organs, cryosections of spleen and thymus from Notch2+/− mice were prepared and stained by X-Gal histochemistry. Cryosections from wild-type littermates served as negative controls and exhibited no background staining. Splenic sections showed that most Notch2 expression was concentrated in cells of the marginal zone (MZ), with little protein detected in the follicular B-cell zone (FO) (Fig. 1A). Notch2-expressing cells were also dispersed throughout the red pulp. Within the splenic T-cell zone, staining was localized to small clusters of cells, while the majority of cells in this region showed no Notch2 expression. In the thymus, most Notch2+ cells aggregated in the medulla and cortico-medullary junction. Notch2+ cells were also seen to be more dispersed throughout the cortex and in small clusters localized to the cortical-subcapsulary zone (Fig. 1B).
FIG. 1.
Localization of Notch2 Protein in spleen and thymus. (A) Splenic cryosections at 100× (left panel) and 200× (right panel) magnification. (B) Thymic cryosections at 20× (left panel), 200× (right panel), and 400× (bottom) magnification. TZ, T-cell zone; RP, red pulp; CMJ, cortico-medullary junction; SCZ, cortical-subcapsulary zone. The results are representative of two independent experiments.
Notch2 is expressed throughout the myeloid lineage but is limited to distinct B-cell subsets.
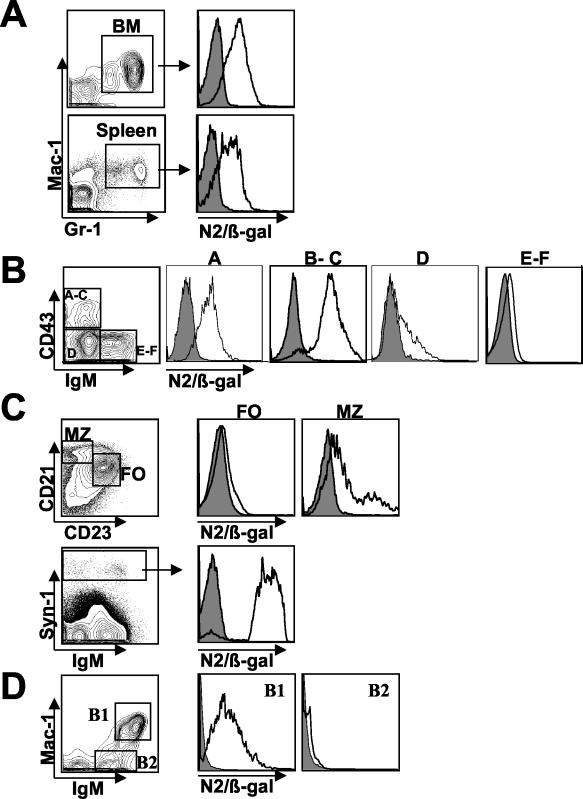
Upon confirming the presence and localization of Notch2 protein in spleen and thymus, we stained single-cell suspensions of spleen, thymus, and bone marrow with antibodies to immunophenotypic markers and assayed for β-Gal activity within subpopulations by using the fluorescent substrate fluorescein di-β-d-galactopyranoside. Analysis of the myeloid lineage showed that nearly all Mac-1+ Gr-1+ cells in both bone marrow and spleen expressed high levels of Notch2 (Fig. 2A). In developing B cells of the bone marrow, Notch2 expression was present in fraction A (B220+ CD19−) (14), which also includes non-B-cell progenitors. Protein levels increased in fractions B through C (CD19+ CD43+ gM−). Further fractionation of this population into B and C fractions by costaining with anti-BP-1 showed that the two subsets expressed Notch2 at similar levels (data not shown). Notch2 protein levels decreased in fraction D (B220+ CD43− IgM−), while little protein was detected above background in fractions E through F (B220+ CD43− IgM+) (Fig. 2B).
FIG. 2.
Notch2 protein expression in the myeloid and B-cell lineages. Cells gated on specific lineage markers are represented as histogram overlays of negative control (filled) and N2/β-Gal (unfilled) cells. (A) Notch2 expression in Mac-1+ Gr-1+ myeloid cells derived from total bone marrow (BM) and total spleen. (B) Total bone marrow was stained with anti-B220, anti-CD19, anti-CD43, and anti-IgM antibodies. B220+ gated cells (left) were fractionated according to the method of Hardy et al. for analysis of Notch2 expression in each subset (right panels) as follows: B220+ CD19− cells (fraction A); CD19+ CD43+ IgM− cells (fractions B through C); CD19+ CD43− IgM− cells (fraction D); and CD19+ CD43− IgM+ cells (fractions E through F). (C) IgM+ gated splenocytes were further gated based on CD21 and CD23 expression for analysis of Notch2 expression in FO and MZ B cells (top panel). Notch2 expression in IgM+/− Syn-1hi plasma cells (right) derived from total spleen is shown (bottom panel). (D) Mac-1+ IgM+ B1 B cells and Mac-1− IgM+ B2 cells of the peritoneal cavity are gated for Notch2 expression. Each value is representative of the results of at least three independent experiments with two mice per analysis.
Within the distinct mature B-cell subsets of the spleen, we found high levels of Notch2 expression in marginal-zone B cells (MZ) (IgM+ CD21hi CD23lo), while detecting little protein in follicular-zone B cells (FO) (IgM+ CD21lo CD23hi) (Fig. 2C). Syn-1hi IgM+/− plasma cells also expressed high levels of Notch2 (Fig. 2C). These findings were consistent with those obtained from splenic cryosections showing Notch2+ cells localized to the marginal zone and red pulp with only slight detection in the follicular B-cell zone (Fig. 1A). Within the peritoneal cavity, we found the vast majority of B1 B cells expressed Notch2 protein, while little protein was detected in B2 B cells (Fig. 2D).
Notch2 is expressed in early T-cell development and in immature CD8+ thymocytes.
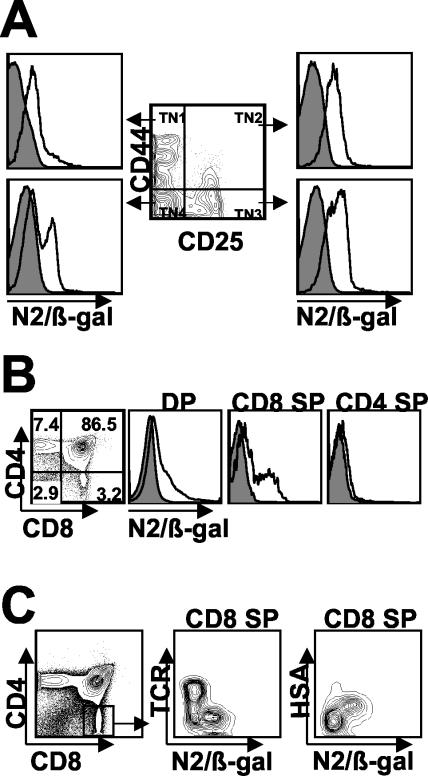
Within the various subsets of developing triple-negative (TN) thymocytes (CD3− CD4− CD8−; TN1, TN2, TN3, and TN4) (Fig. 3A), Notch2 protein was detected in the earliest population of CD44+ CD25− TNs (TN1) but was largely restricted to CD44hi precursors with no expression in CD44lo cells of this subset (data not shown). Notch2 expression increased as thymocytes progressed to the CD44+ CD25+ (TN2) and CD44− CD25+ stages (TN3) and then declined in a fraction of the CD44− CD25− cells (TN4). Within the CD4+ CD8+ DP population, expression levels were found to be age dependent, with 20 to 30% of DP thymocytes expressing Notch2 in mice 2- to-4-weeks old but only 9 to 12% expressing Notch2 in mice older than 14 weeks (Fig. 3B and data not shown). Analysis of SP subsets revealed no Notch2 expression in CD4SP but significant levels of Notch2 expression within a subset of the CD8SP population (Fig. 3B). Because this population is heterogeneous, consisting of both immature SP thymocytes (ISP) and mature CD8SP, we wished to determine whether Notch2 expression was correlated with the maturational status of the CD8SP. Indeed, we found that within this population, expression was limited to HSA+ TCRlo/− ISP thymocytes and was not detected in mature HSAint/− TCRhi CD8SP (Fig. 3C).
FIG. 3.
Notch2 protein expression in developing thymocytes. (A) CD3− CD4− CD8− gated cells (center) were further fractionated according to defined stages of early thymic development (TN1-4) for analysis of Notch2 expression within subsets (left and right). (B) Total thymus was stained with anti-CD4 and anti-CD8 for analysis of DP and SP populations. (C) Total thymus was stained with anti-CD4, anti-CD8, and either anti-TCR or anti-HSA to delineate immature from mature CD8SP. Each value is representative of the results of at least three independent experiments with two mice per analysis.
High-level expression of N2IC results in proliferative T-cell populations.
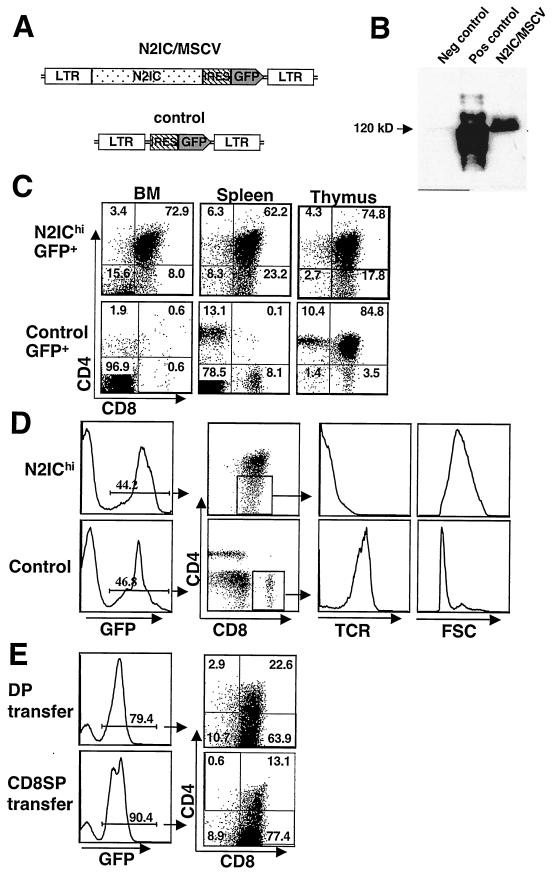
To investigate the function of Notch2 in hematopoiesis, we expressed a constitutively active form of Notch2 (N2IC) in hematopoietic cells by using an MSCV-based retroviral vector that coexpressed GFP (N2IC/MSCV). A second construct expressing only GFP served as a control vector (Fig. 4A). Western blot analysis of transfected 3T3 fibroblasts confirmed N2IC protein expression from the vector (Fig. 4B). Bone marrow harvested from 5-fluorouracil-treated C57BL/6 mice was transduced with or without the addition of Polybrene (commonly used to enhance transduction efficiency) and then transplanted into lethally irradiated congenic mice. Mice transplanted with bone marrow transduced in the presence of Polybrene expressed high levels of N2IC (N2IChi), as indicated by the mean fluorescence of GFP, while mice reconstituted with bone marrow cells transduced without Polybrene expressed low levels of N2IC (N2IClo). As previous studies have shown GFP to be a valid surrogate marker for protein expression levels (18), these mice permitted the analysis of both low- and high-level effects of N2IC on the hematopoietic system.
FIG.4.
A high level of N2IC results in proliferative T-cell populations. (A) Schematic of retroviral vector expressing N2IC and GFP (N2IC/MSCV) and control vector expressing GFP alone (bottom). (B) Western blot showing N2IC expression from the retroviral vector. NIH 3T3 fibroblasts were transfected with N2IC/MSCV, control vector, or full-length Notch2 as the positive control for the anti-Notch2 antibody. (C) GFP+ gated populations from bone marrow, spleen, and thymus of N2IChi and control mice 6 weeks PT stained for CD4 and CD8 expression. (D) Immature phenotype of N2IChi CD8+ cells 6 weeks PT. Total spleen was stained with anti-CD4, anti-CD8, and anti-TCR. TCR expression levels and forward scatter (FSC) properties of GFP+ CD8+ gated splenocytes from N2IChi and control mice are shown (right). The values shown in panels C and D are representative of the results for N2IChi mice (n = 12) analyzed in three independent experiments. (E) DP cells (8 × 104) (top) and CD8SP cells (bottom) FACS-purified from N2IChi bone marrow 26-days PT were adoptively transferred into nonirradiated recipients. Recipient bone marrow 6-weeks postadoptive transfer shows extensive in vivo expansion. The values shown are representative of the results for N2IChi mice (n = 5 for each transferred population) analyzed in two independent experiments.
Initial analyses of Mac-1+ Gr-1+ myeloid lineage cells did not reveal any gross abnormalities in bone marrow or spleen (data not shown). In contrast, N2IC had a marked impact on both B- and T-lymphoid development. In N2IChi mice, CD4+ CD8+ T-cell populations were present in bone marrow and were followed by the emergence of CD8SP of an immature phenotype (Fig. 4C). These populations invariably gave rise to leukemias between 6- to 10-weeks PT, a finding similar to those of previous studies that used an activated form of Notch1 driven by the long terminal repeat of a retroviral vector (30). T-cell populations observed in N2IChi bone marrow were observed simultaneously in peripheral blood and spleen as early as 12-days PT and were present in the absence of thymic reconstitution. The reconstitution of N2IChi thymus with GFP+ thymocytes did not occur until later time points (>4 weeks PT) and was in association with substantive expansion in the periphery. In these cases, the GFP+ thymocyte population did not include early double-negative thymocyte subsets and was indistinguishable from the T-cell populations observed in bone marrow and spleen (Fig. 4C and data not shown). Very few mature CD4SP were observed within the GFP+ population of N2IChi mice. The CD8SP that were observed in thymus and the periphery were TCR− and large by forward-scatter analysis, indicating an immature phenotype (Fig. 4D). In contrast to these results, the control mice transplanted with WBM transduced in the presence of Polybrene displayed long-term reconstitution in both the lymphoid and myeloid lineages and exhibited no signs of pathology, as indicated by analyses greater than 14-weeks PT.
Expanding T-cell populations in the bone marrow may have resulted from an ongoing skew in hematopoiesis toward the T-cell lineage or by the seeding of proliferating T cells following transplantation. In order to assess their proliferative potential, 8 × 104 GFP+ CD4+ CD8+ or GFP+ CD8SP cells FACS-purified from N2IChi bone marrow 26 days PT were adoptively transferred into recipient mice and then monitored by peripheral blood analysis for their in vivo expansion. Aggressive T-cell leukemias comprising the majority of bone marrow cells developed in all recipient mice (n = 5) within 6 weeks PT, demonstrating that the T-cell populations in N2IChi bone marrow were highly proliferative (Fig. 4E). The fact that rapid expansion was achieved from purified T cells indicates that these populations in N2IChi bone marrow were self generating and did not require input of stem cell progenitors for either their maintenance or their expansion.
Low-level expression of N2IC skews thymocyte development to the CD8 lineage.
To assess the effects of N2IC on the T-cell lineage in the absence of leukemic cells, more-detailed analyses were done on mice expressing lower levels of N2IC (N2IClo). These mice developed only small (or no) DP T-cell populations in the periphery and remained free from leukemia as long as 10 months PT, thus permitting the analyses of both T- and B-lymphoid compartments.
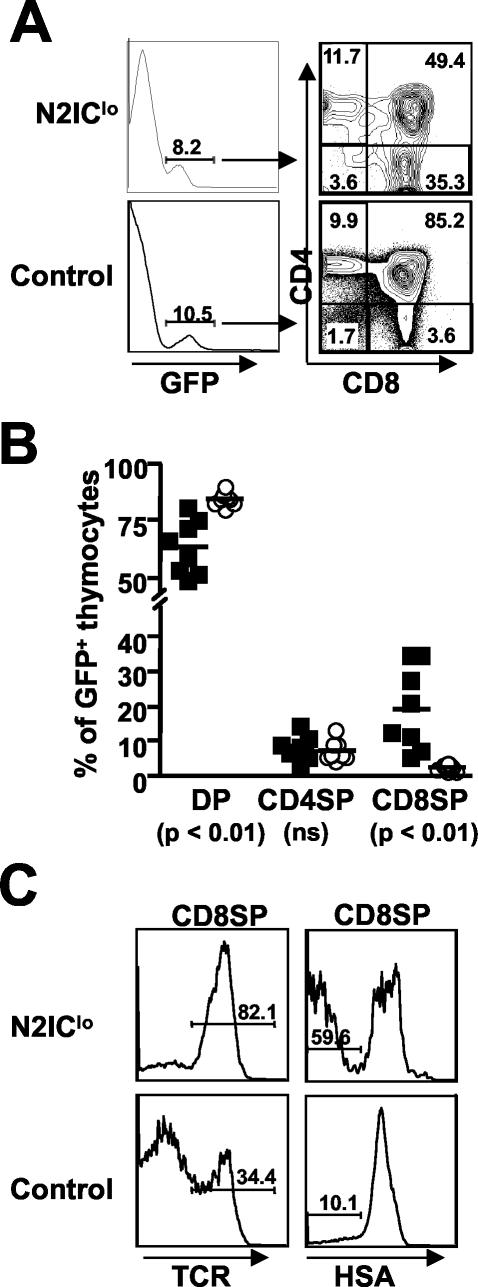
Within the GFP+ thymocyte population of N2IClo mice, there was a decrease in the relative frequency of DP thymocytes, with a concomitant increase in CD8SP, compared to that of the control mice (Fig. 5A). This skew in thymic development varied in degree but was present in all N2IClo mice analyzed (n = 8) (Fig. 5B). Because the CD8SP population in thymus is heterogeneous and consists of both the ISP (TCRlo/− HSA+) and mature CD8SP (TCRhi HSAint/−), it was not clear whether N2IClo was blocking or otherwise delaying the progression of the ISP to the DP stage or whether N2IClo was enhancing the progression of DP thymocytes to the SP stage. We therefore stained thymocytes with antibodies to markers that identify the maturational status of CD8+ thymocytes. Analysis of this population showed a significantly higher frequency of TCRhi HSAint/− thymocytes than in the controls, indicating a relative increase in mature thymocytes (Fig. 5C). These results, taken with the decrease in DP thymocytes and increase in CD8SP, indicate that N2IClo enhances the progression of DP thymocytes to the SP stage of development and that this effect is preferential to the CD8 lineage.
FIG. 5.
Low level of N2IC skews thymocyte development to the CD8 lineage. (A) N2IClo thymus analyzed at 4- to 6-weeks PT. N2IClo and control thymus were stained with anti-CD4 and anti-CD8.(B) Data compiled from two independent experiments (n = 8) showing the frequencies of DP and SP populations in N2IClo (▪) and control mice (○). (C) Expression of TCR and HSA on GFP+ CD8+ gated thymocytes from N2IClo and control thymus stained with anti-CD4, anti-CD8, and either anti-TCR or anti-HSA.
N2IClo enhances positive selection.
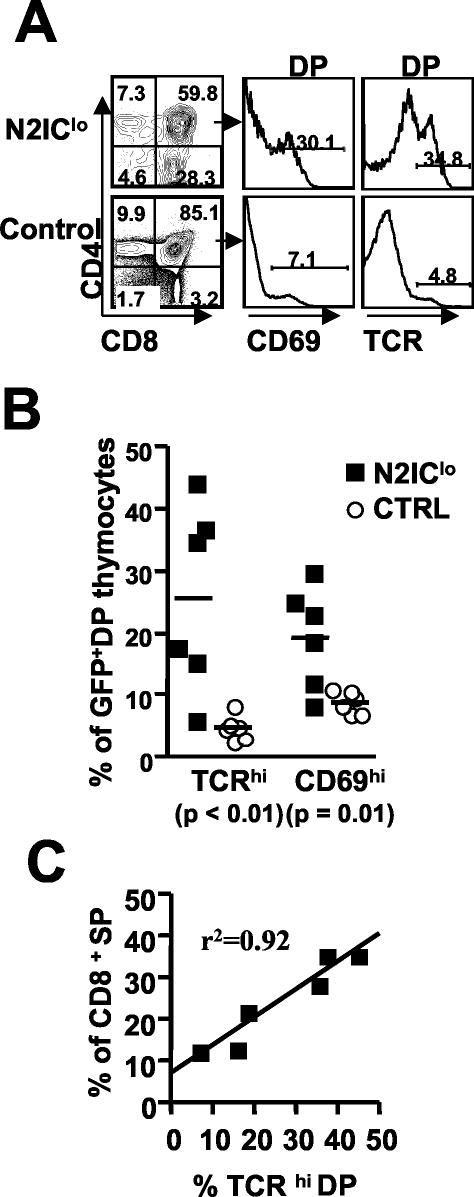
Within the DP stage of normal thymocyte development, increased levels of TCR and upregulation of CD69 are indicative of thymocytes undergoing TCR/MHC interaction (33), and recent studies have indicated that the progression of DP thymocytes through a TCRhi stage is a differentiation pathway preferentially taken by CD8 lineage-committed DP thymocytes during the course of their development (5). Therefore, we questioned whether N2IClo had any effect on selective events at the DP stage that might contribute to the higher frequency of mature CD8SP. Analyses of DP thymocytes showed a significantly higher frequency of TCRhi thymocytes in N2IClo mice than in the controls. (Fig. 6A). Consistent with these results, the N2IClo DP population also showed a significantly higher frequency of CD69hi cells, which is indicative of postselected thymocytes (Fig. 6A). The frequencies of postselected thymocytes varied but were increased in all N2IClo mice analyzed (Fig. 6B). Furthermore, the frequency of these cells was linearly correlated with the degree to which thymocyte development was skewed to the CD8 lineage, with higher frequencies of TCRhi DP thymoctes associated with the most severe developmental skewing (Fig. 6C). These results indicate that N2IClo enhances the positive selection of DP thymocytes and, taken with the dramatic skew toward the CD8 lineage, suggest that Notch2 affects the maturation, and possibly the selection, of the CD8SP.
FIG. 6.
N2IClo results in increased frequencies of TCRhi CD69hi DP thymocytes. (A) Total thymus was stained with anti-CD4, anti-CD8, and either anti-TCR or anti-CD69. (B) Data compiled from two independent experiments (n = 6) showing the frequencies of CD69hi and TCRhi cells within GFP+ DP gated thymocytes. (C) Linear correlation between the frequency of TCRhi DP and CD8SP. The data was compiled from two independent experiments (n = 6). Ctrl, control.
N2IC leads to B2 B-cell developmental block.
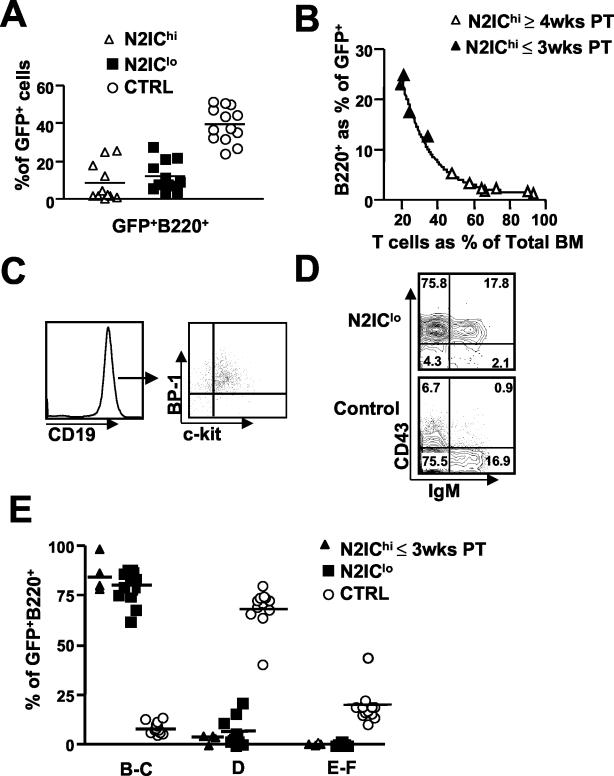
Although B220+ cells were always present within the GFP+ population of all N2IC mice regardless of N2IC expression levels, their relative frequencies were severely reduced compared to those of the controls (Fig. 7A). Analyses of N2IChi mice at early time points (≤3 weeks PT), prior to substantive T cell expansion, showed similar frequencies in B220+ cells for N2IChi and N2IClo mice. However, relative frequencies of B220+ cells in N2IChi mice declined over time as T-cell populations expanded (Fig. 7B). Furthermore, at late stages of leukemic progression (>6 weeks PT), all lineages of both donor and recipient-derived origin were suppressed, as is often the case under leukemic conditions (data not shown). Analyses of the B lineage at early time points PT revealed similar phenotypes for B-cell development for both N2IChi and N2IClo mice (described below).
FIG. 7.
N2IC permits B-cell commitment but inhibits B2 B-cell development at the pre-B-cell stage. (A) Frequency of B220+ cells expressed as a percentage of the GFP+ population in N2IClo, N2IChi, and control bone marrow (BM) analyzed 3- to 6-weeks PT. (B) Decline in frequency of B220+ cells within the GFP+ population of N2IChi bone marrow as T cells expand. (C) CD19 expression on GFP+ B220+ gated cells (left) and BP-1 and c-kit expression on CD19+ gated cells (right) from N2IClo bone marrow. (D) Representative expression profile for CD43 and IgM on B220+ GFP+ gated cells from N2IClo and control bone marrow. (E) Compiled data showing the frequencies of Hardy fraction subsets expressed as percentages of GFP+ B220+ derived from N2IClo and control bone marrow analyzed 3- to 6-weeks PT and of N2IChi bone marrow analyzed ≤ 3-weeks PT. The data were compiled from analyses of N2IClo mice (n = 12) from three independent experiments and N2IChi mice (n = 4) from two independent experiments. Ctrl, control.
Because of the general suppression of hematopoiesis associated with leukemic progression, more detailed analyses of B-cell development were done on N2IClo mice, with results confirmed in N2IChi mice at early time points PT. In contrast to a previous study where an activated form of Notch1 completely inhibited B lymphopoiesis (30), expression of N2IC allowed development of the earliest B-lineage subsets. FACS analysis of N2IClo bone marrow indicated that the vast majority of GFP+ B220+ cells were also CD19+ BP-1+ c-kit+, confirming commitment to the B-cell lineage and maturation to the fraction C (pre-B) stage (Fig. 7C). Further analyses showed that N2IC-developing B-cells were largely CD25− (data not shown), indicating that development was restricted to an early stage of fraction C. There was no apparent reduction in the frequencies or absolute numbers of fraction B and C cells in N2IC mice compared to those of the control mice (data not shown). Similar results were obtained from analyses performed as long as 14 weeks PT, indicating that B-cell subsets were derived from long-term stem cell activity rather than from the transduction of early progenitors that were already committed to the B-cell lineage. These results indicate that, in contrast to the Notch1 family member, N2IC permits early B-cell development regardless of N2IC expression levels.
Although early B-cell subsets appeared unimpaired, N2IC had a profound impact on later stages of B-cell development. Within the GFP+ B220+ population of N2IClo bone marrow, there was a severe reduction in Hardy fractions D through F (14) in the bone marrow of all N2IClo mice analyzed from three independent experiments (n = 12) (Fig. 7D and E). The absence of these fractions, which represent the majority of B220+ B-lineage cells in bone marrow, accounts for the severe reduction in B220+ cells in all N2IC mice. Collectively, these results show that the low frequencies of B220+ cells within the GFP+ population of N2IC mice were not due to a block in B-cell commitment but rather were due to a block in developmental progression from the fraction C to the fraction D stage.
N2IC promotes the selective development of B1 B cells.
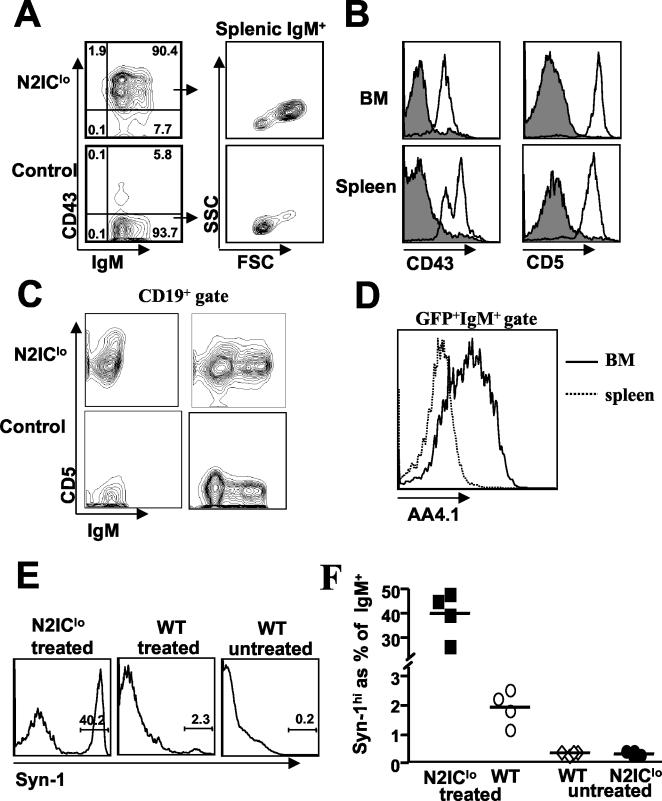
While mature B2 cells were absent in all N2IC mice regardless of N2IC levels, B220+ cells coexpressing IgM and CD43 were present at a significant frequency (between 10 to 20%) of the GFP+ B220+ bone marrow population (Fig. 7D). IgM+ CD43+ cells were found in peripheral blood and represented the vast majority of the GFP+ B-cell population within N2IClo spleen (Fig. 8A). Further analyses showed that these cells exhibited large forward- and side-scatter properties and expressed intermediate to high levels of CD5 in addition to CD43 (Fig. 8B). This phenotype defines the B1 B-cell subset that resides at low frequency in adult spleen and at a greater frequency in the adult peritoneal cavity (22). The generation of B1 B-cells led to a three- to sevenfold increase in the absolute number of B1 B cells in the spleens of N2IC mice and were not a result of clonal expansion, as both kappa and lambda light chains were expressed at appropriate ratios within the GFP+ B220+ population (data not shown). Analysis at 13 days PT showed that developing sIgM− B progenitors expressed CD5 protein (Fig. 8C), consistent with recent unpublished observations that developing fetal B1 B cells express CD5 protein prior to surface IgM expression (P. Kincade et al., personal communication). CD5+ IgM+ cells present in bone marrow were also AA4.1+, indicating that these cells were newly formed, as opposed to recirculating mature B cells (Fig. 8D). The apparent de novo production of B1 B cells persisted over time, as similar results were obtained from analyses of B-cell development as long as 14 weeks PT. Finally, similar results were obtained from studies of N2IChi mice analyzed at early time points PT (data not shown), indicating that the generation of the B1 B-cell phenotype was not a function of N2IC expression levels.
FIG. 8.
N2IC promotes the selective development of B1 B cells. (A) Total spleen was stained with anti-B220, anti-CD43, and anti-IgM. GFP+ B220+ gated cells from N2IClo and control spleen are shown as contour plots (left) with frequencies shown in each quadrant. The forward scatter (FSC)/side scatter (SSC) properties of GFP+ IgM+ gated splenocytes are shown (right). (B) Total spleen was stained with anti-IgM and either anti-CD43 or anti-CD5. Expression of CD43 and CD5 on GFP+ IgM+ gated splenocytes is displayed as histogram overlays of N2IClo (unfilled) and control (filled) cells. (C) Analysis at 13 (left)- and 21 (right)-days PT showing CD5 expression on CD19+ gated cells from N2IC and control bone marrow. (D) AA4.1 expression on GFP+ IgM+ bone marrow cells from N2IClo mice displayed as an overlay of GFP+ IgM+ cells from N2IClo spleen. (E) Representative histogram showing the upregulation of Syn-1 48 h after an LPS injection. GFP+ IgM+ gated splenocytes from treated N2IClo mice and IgM+ gated splenocytes from treated C57BL/6 mice (WT) are shown. For comparison, IgM+ gated splenocytes from untreated wild-type C57BL/6 mice are also shown. (F) Compiled data from two independent experiments (n = 4). The results for untreated N2IClo mice showed frequencies of Syn-1hi cells similar to those for untreated wild-type C57BL/6 mice but are not shown in panel E.
Upon bacterial LPS stimulation, B1 B cells differentiate into plasmablasts (Syn-1hi) within 48 to 72 h (24, 28). To determine whether B1 B cells generated in N2IC mice were responsive to LPS challenge in vivo, we treated N2IClo with 20 μg of LPS by intravenous injection and analyzed splenocytes by flow cytometry at 48 h postinjection for the upregulation of Syn-1. To determine the relative response of N2IC IgM+ cells to LPS, empty control vector (control) and wild-type C57BL/6 mice were treated with the same LPS dosage and analyzed in parallel. Wild-type C57BL/6, control vector, and N2IClo mice untreated with LPS served as negative controls. As shown in Fig. 8E and summarized in Fig. 8F, nearly half of splenic GFP+ IgM+ cells from N2IClo mice showed high levels of Syn-1, compared to 1 to 2% of IgM+ cells from wild-type C57BL/6 mice treated with the same LPS dosage, demonstrating the dramatically increased frequency of LPS-responsive B1 B cells in the N2IClo GFP+ population (n = 4). Response levels of GFP+ IgM+ cells in treated empty control vector mice were similar to those for treated wild-type C57BL/6 mice and are not shown. These results, taken with the above, suggest that N2IC inhibits the development of conventional B2 cells but promotes the selective development of B1 B cells.
DISCUSSION
It has been proposed that the discrepant phenotypes obtained in previous Notch1IC studies may have been due to differing levels of Notch1IC expressed by the various constructs, though formal proof is lacking. Our studies show that various levels of N2IC result in similar contrasting phenotypes for the T-cell lineage and may offer some resolution to the present controversy arising from these discrepant Notch1 studies. Also, our findings may shed light on why the conditional inactivation of Notch1 in double-negative thymocytes did nothing to impair CD8SP development (40). If Notch1 and Notch2 function in redundant roles in late stages of T-cell development, the loss of either factor would not lead to any discernible phenotype. Studies in which the function of all Notch members were inhibited through the use of gamma-secretase inhibitors demonstrated the necessity for Notch function in the development of the CD8 lineage (8, 12).
The fact that different expression levels of N2IC resulted in such contrasting phenotypes suggests that levels of Notch2 signaling may have important consequences for thymocyte development. In mice expressing high levels of N2IC, CD8+ T-cell populations with variable levels of CD4 developed in the bone marrow and spleen and gave rise to leukemias between 6 to 10 weeks posttransplantation, similar to results obtained in a previous study of mice expressing an activated form of Notch1 (30). However, in N2IChi mice, peripheral T-cell populations were shown to be highly proliferative, as demonstrated by their aggressive expansion in vivo upon adoptive transfer. Because GFP+ thymocytes lacked early double-negative thymocytes and were phenotypically indistinguishable from peripheral T-cell populations, it is likely that N2IChi thymocytes were the result of thymic seeding by these same proliferative cells observed in the periphery. Therefore, what appeared to be a block in thymocyte development at the DP stage may reflect disregulation imparted by transformation, thus making it difficult to define a physiologic role for Notch2 in normal thymocyte development from these mice.
In contrast to findings for N2IChi mice, DP T-cell populations were generally not observed outside of the thymus in mice expressing lower levels of N2IC. Consistent with the absence of these proliferative populations, N2IClo mice remained free from pathology as long as 10 months posttransplantation, thus permitting the assessment of N2IC effects on thymocyte development in the absence of leukemia. Within the GFP+ thymocyte population of N2IClo mice, there was a decrease in the frequency of DP thymocytes, with a concomitant increase in CD8SP thymocytes. These results are consistent with previous reports in which an activated form of Notch1IC driven by the proximal Lck promoter influenced a CD4-versus-CD8-lineage outcome (32). Further analyses of the GFP+ CD8SP population in N2IClo mice showed an increased frequency in mature versus immature thymocytes, indicating that the increase in CD8SP was due to enhanced progression from the DP stage rather than a block in ISP progression. In addition, N2IClo also resulted in a higher frequency of CD69hi TCRhi thymocytes, suggesting that activated Notch2 enhances positive selection (33). These results are particularly interesting in light of recent studies which showed that overexpression of CD69 in developing thymocytes resulted in enhanced positive selection, possibly by facilitating the trafficking of thymocytes to the medulla, where maturation of SP thymocytes takes place (10, 27). It is possible that Notch2 signaling during selective events, either directly or indirectly, upregulates expression of CD69 on DP thymocytes, which leads to an increase in postselected thymocytes. If this effect were specific to cells fated to the CD8 lineage, then enhanced migration to the medulla might be one means by which N2IC facilitates CD8SP maturation. Another intriguing possibility, not mutually exclusive, is suggested by recent studies which indicate that the progression of DP thymocytes through a TCRhi stage is a differentiation pathway preferentially taken by CD8 lineage-committed DP thymocytes during the course of their development (5). In this case, the higher frequencies of TCRhi DP thymocytes in N2IClo thymus may reflect the preferred pathway taken by developing CD8 lineage-committed thymocytes, a population that is somehow enhanced by activated Notch2. The striking correlation between the frequency of TCRhi DP thymocytes and the increase in CD8SP supports this possibility.
Previous studies have suggested that Notch1IC determines a T-versus-B-lineage outcome. In one study, mice expressing Notch1IC from a retroviral vector developed T-cell populations in the bone marrow with a concomitant inhibition in B lymphopoiesis (30). These results led the authors to conclude that activated Notch1 determined a T-versus-B-cell-fate decision, presumably through its action on a common lymphoid progenitor residing in bone marrow. The persistence and expansion of T-cell populations in N2IC mice did not appear to result from ongoing hematopoiesis, since their aggressive expansion in vivo upon adoptive transfer revealed these populations to be self generating. While these results do not exclude the possibility that a skew in hematopoiesis to the T-cell lineage was contributing to the presence of T-cell populations in N2IChi bone marrow, they suggest the alternative possibility that populations in bone marrow (and spleen) may have resulted from in situ proliferation of cells seeding these locations following transplantation.
Recent studies provide strong evidence that Delta-1-like induced Notch signaling can act on fetal-derived hematopoietic stem cells to suppress B-cell development in favor of T-cell development (36). The observation that activated Notch2 did not prevent the development of early B-lineage subsets suggests that ligand engagement of Notch members other than Notch2 are involved in a B-versus-T-cell-fate decision. This possibility is supported by previous studies which demonstrated that thymopoiesis is abolished upon conditional inactivation of Notch1, indicating that the Notch2 member cannot compensate at this stage of T-cell development (31). Our data show that endogenous Notch2 protein is highly expressed in pro-B cells, which are dependent on contact with bone marrow stroma shown to express Notch ligands (19, 37). Collectively, these findings indicate that Notch2, in contrast to the Notch1 family member, does not suppress B lymphopoiesis and may even suggest an as-yet-undefined function for Notch2 in early B-cell development.
The apparent block in B2 B-cell development in N2IC mice at the pre-B cell stage may be explained by a number of possibilities. In the retroviral system, N2IC expression continues beyond the normal limit of Notch2 protein expression in developing B cells, which in itself may reflect the necessity for Notch2 to be downregulated in order for conventional B2 B-cell development to progress. Based on their forward-scatter properties, N2IC-expressing fraction C cells were found to be large blasting cells, a finding which is consistent with the rapid proliferation associated with this stage (15). Because exit from the cell cycle is believed to be a prerequisite for the initiation of immunoglobulin light chain gene rearrangement and progression to the fraction D stage, it may be that constitutive Notch2 signaling blocks development at this critical step by sustaining cells in the cycle. In addition, NF-κB activity, which is important in the regulation of the fraction C to D transition (35), has been shown to be targeted by the Notch signaling pathway (1, 4, 29, 38).
An alternative explanation for the block in B-cell development may be that Notch signaling directly induces apoptosis in developing B cells. Though this possibility is consistent with findings of previous studies which showed that Notch1IC induces apoptosis in immature DT40 B cells (26), it is not likely that such an effect would reflect a physiologic role for Notch2 in B-cell development, given the high level of endogenous Notch2 protein expressed in pro- and pre-B cells.
A final intriguing possibility for the apparent block in B lymphopoiesis is suggested by the selective development in N2IC mice of CD5+ B1 B cells. Whether CD5+ B1 B cells constitute a distinct lineage from B2 cells or whether CD5 expression levels represent differential states of activation within a single B-cell lineage is still controversial (34). Without this clear distinction, it is difficult to definitively conclude how activated Notch2 results in the exclusive development of B1 B cells. In terms of a strength-of-signal model, it may be that activated Notch2 modulates pre-BCR signaling such that only B1 B-cell development is favored. From the view of distinct lineages, activated Notch2 might reset B lymphopoiesis in favor of an alternative B1 B-cell developmental program.
Finally, our data showing that Notch2 is expressed in both MZ B and B1 B cells but not in FO B or B2 B cells of the peritoneal cavity further support the notion that the generation of MZ B and B1 B-cell subsets may to some degree share common pathways (23, 39). For instance, there is evidence that members of the NF-κB pathway, which are targeted by Notch signaling, may be involved in the development of both MZ B and B1 B-cell subsets (3, 9). Therefore, it is possible that the selective development of B1 B cells in N2IC mice reflects a determining role for Notch2 in the development of this B-cell subset, possibly by the modulation of NF-κB activity or of other critical factors shown to be associated with altered MZ B and B1 B-cell subset development.
The results presented in this study not only demonstrate the contrasting outcomes resulting from different levels of activated Notch2 but also implicate a role for Notch2 in the maturation of the CD8SP. The profound effect of N2IC on the B-cell lineage, which results in the exclusive development of B1 B cells, also suggests a link between Notch2 signaling and the development of this important B-cell subset.
Acknowledgments
We thank Lisa Jia and Larry Gartland for their invaluable technical help. We are particularly grateful to Casey Weaver for critical reading of the manuscript. We especially wish to thank Flavius Martin and John Kearney for their technical expertise and many helpful discussions.
This work was supported by a Howard Hughes faculty development award to C.A.K. (grant no. 53000281) and a Basic Mechanisms in Lung Disease Predoctoral Training grant to C.M.W (grant no. HL07553). Work by C.S.S. was supported by an Immunology Diseases and Basic Immunology postdoctoral training grant (grant no. AIT3207051).
REFERENCES
- 1.Bellavia, D., A. F. Campese, E. Alesse, A. Vacca, M. P. Felli, A. Balestri, A. Stoppacciaro, C. Tiveron, L. Tatangelo, M. Giovarelli, C. Gaetano, L. Ruco, E. S. Hoffman, A. C. Hayday, U. Lendahl, L. Frati, A. Gulino, and I. Screpanti. 2000. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 19:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigas, A., D. I. Martin, and L. A. Milner. 1998. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol. Cell Biol. 18:2324-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cariappa, A., H. C. Liou, B. H. Horwitz, and S. Pillai. 2000. Nuclear factor kappa B is required for the development of marginal zone B lymphocytes. J. Exp. Med. 192:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, P., A. Zlobin, V. Volgina, S. Gottipati, B. Osborne, E. J. Simel, L. Miele, and D. I. Gabrilovich. 2001. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J. Immunol. 167:4458-4467. [DOI] [PubMed] [Google Scholar]
- 5.Correia-Neves, M., D. Mathis, and C. Benoist. 2001. A molecular chart of thymocyte positive selection. Eur. J. Immunol. 31:2583-2592. [DOI] [PubMed] [Google Scholar]
- 6.de Guzman, C. G., A. J. Warren, Z. Zhang, L. Gartland, P. Erickson, H. Drabkin, S. W. Hiebert, and C. A. Klug. 2002. Hematopoietic stem cell expansion and distinct myeloid developmental abnormalities in a murine model of the AML1-ETO translocation. Mol. Cell Biol. 22:5506-5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deftos, M. L., E. Huang, E. W. Ojala, K. A. Forbush, and M. J. Bevan. 2000. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 13:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerfler, P., M. S. Shearman, and R. M. Perlmutter. 2001. Presenilin-dependent gamma-secretase activity modulates thymocyte development. Proc. Natl. Acad. Sci. USA 98:9312-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagarasan, S., N. Watanabe, and T. Honjo. 2000. Generation, expansion, migration and activation of mouse B1 cells. Immunol. Rev. 176:205-215. [DOI] [PubMed] [Google Scholar]
- 10.Feng, C., K. J. Woodside, B. A. Vance, D. El-Khoury, M. Canelles, J. Lee, R. Gress, B. J. Fowlkes, E. W. Shores, and P. E. Love. 2002. A potential role for CD69 in thymocyte emigration. Int. Immunol. 14:535-544. [DOI] [PubMed] [Google Scholar]
- 11.Fowlkes, B. J., and E. A. Robey. 2002. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J. Immunol. 169:1817-1821. [DOI] [PubMed] [Google Scholar]
- 12.Hadland, B. K., N. R. Manley, D. Su, G. D. Longmore, C. L. Moore, M. S. Wolfe, E. H. Schroeter, and R. Kopan. 2001. Gamma-secretase inhibitors repress thymocyte development. Proc. Natl. Acad. Sci. USA 98:7487-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada, Y., Y. Kadokawa, M. Okabe, M. Ikawa, J. R. Coleman, and Y. Tsujimoto. 1999. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development 126:3415-3424. [DOI] [PubMed] [Google Scholar]
- 14.Hardy, R. R., C. E. Carmack, S. A. Shinton, J. D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy, R. R., Y. S. Li, D. Allman, M. Asano, M. Gui, and K. Hayakawa. 2000. B-cell commitment, development and selection. Immunol. Rev. 175:23-32. [PubMed] [Google Scholar]
- 16.Hawley, R. G. 1994. High-titer retroviral vectors for efficient transduction of functional genes into murine hematopoietic stem cells. Ann. N. Y. Acad. Sci. 716:327-330. [DOI] [PubMed] [Google Scholar]
- 17.Ingles-Esteve, J., L. Espinosa, L. A. Milner, C. Caelles, and A. Bigas. 2001. Phosphorylation of Ser2078 modulates the Notch2 function in 32D cell differentiation. J. Biol. Chem. 276:44873-44880. [DOI] [PubMed] [Google Scholar]
- 18.Izon, D. J., J. A. Punt, L. Xu, F. G. Karnell, D. Allman, P. S. Myung, N. J. Boerth, J. C. Pui, G. A. Koretzky, and W. S. Pear. 2001. Notch1 regulates maturation of CD4+ and CD8+ thymocytes by modulating TCR signal strength. Immunity 14:253-264. [DOI] [PubMed] [Google Scholar]
- 19.Jones, P., G. May, L. Healy, J. Brown, G. Hoyne, S. Delassus, and T. Enver. 1998. Stromal expression of Jagged 1 promotes colony formation by fetal hematopoietic progenitor cells. Blood 92:1505-1511. [PubMed] [Google Scholar]
- 20.Kimble, J., and P. Simpson. 1997. The LIN-12/Notch signaling pathway and its regulation. Annu. Rev. Cell Dev. Biol. 13:333-361. [DOI] [PubMed] [Google Scholar]
- 21.Koch, U., T. A. Lacombe, D. Holland, J. L. Bowman, B. L. Cohen, S. E. Egan, and C. J. Guidos. 2001. Subversion of the T/B lineage decision in the thymus by lunatic fringe-mediated inhibition of Notch-1. Immunity 15:225-236. [DOI] [PubMed] [Google Scholar]
- 22.Martin, F., and J. F. Kearney. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory.” Immunol. Rev. 175:70-79. [PubMed] [Google Scholar]
- 23.Martin, F., and J. F. Kearney. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12:39-49. [DOI] [PubMed] [Google Scholar]
- 24.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 25.Milner, L. A., R. Kopan, D. I. Martin, and I. D. Bernstein. 1994. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood 83:2057-2062. [PubMed] [Google Scholar]
- 26.Morimura, T., R. Goitsuka, Y. Zhang, I. Saito, M. Reth, and D. Kitamura. 2000. Cell cycle arrest and apoptosis induced by Notch1 in B cells. J. Biol. Chem. 275:36523-36531. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, T., D. J. Kasprowicz, M. Yamashita, L. A. Schubert, G. Gillard, M. Kimura, A. Didierlaurent, H. Koseki, and S. F. Ziegler. 2002. The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J. Immunol. 168:87-94. [DOI] [PubMed] [Google Scholar]
- 28.Oliver, A. M., F. Martin, and J. F. Kearney. 1999. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198-7207. [PubMed] [Google Scholar]
- 29.Oswald, F., S. Liptay, G. Adler, and R. M. Schmid. 1998. NF-κB2 is a putative target gene of activated Notch-1 via RBP-Jκ. Mol. Cell Biol. 18:2077-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pui, J. C., D. Allman, L. Xu, S. DeRocco, F. G. Karnell, S. Bakkour, J. Y. Lee, T. Kadesch, R. R. Hardy, J. C. Aster, and W. S. Pear. 1999. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 11:299-308. [DOI] [PubMed] [Google Scholar]
- 31.Radtke, F., A. Wilson, G. Stark, M. Bauer, J. van Meerwijk, H. R. MacDonald, and M. Aguet. 1999. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10:547-558. [DOI] [PubMed] [Google Scholar]
- 32.Robey, E., D. Chang, A. Itano, D. Cado, H. Alexander, D. Lans, G. Weinmaster, and P. Salmon. 1996. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell 87:483-492. [DOI] [PubMed] [Google Scholar]
- 33.Robey, E., and B. J. Fowlkes. 1994. Selective events in T cell development. Annu. Rev. Immunol. 12:675-705. [DOI] [PubMed] [Google Scholar]
- 34.Rothstein, T. L. 2002. Cutting edge commentary: two B-1 or not to be one. J. Immunol. 168:4257-4261. [DOI] [PubMed] [Google Scholar]
- 35.Scherer, D. C., J. A. Brockman, H. H. Bendall, G. M. Zhang, D. W. Ballard, and E. M. Oltz. 1996. Corepression of RelA and c-rel inhibits immunoglobulin kappa gene transcription and rearrangement in precursor B lymphocytes. Immunity 5:563-574. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt, T. M., and J. C. Zuniga-Pflucker. 2002. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17:749-756. [DOI] [PubMed] [Google Scholar]
- 37.Walker, L., A. Carlson, H. T. Tan-Pertel, G. Weinmaster, and J. Gasson. 2001. The notch receptor and its ligands are selectively expressed during hematopoietic development in the mouse. Stem Cells 19:543-552. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., L. Shelly, L. Miele, R. Boykins, M. A. Norcross, and E. Guan. 2001. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J. Immunol. 167:289-295. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J. H., N. Avitahl, A. Cariappa, C. Friedrich, T. Ikeda, A. Renold, K. Andrikopoulos, L. Liang, S. Pillai, B. A. Morgan, and K. Georgopoulos. 1998. Aiolos regulates B cell activation and maturation to effector state. Immunity 9:543-553. [DOI] [PubMed] [Google Scholar]
- 40.Wolfer, A., T. Bakker, A. Wilson, M. Nicolas, V. Ioannidis, D. R. Littman, P. P. Lee, C. B. Wilson, W. Held, H. R. MacDonald, and F. Radtke. 2001. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat. Immunol. 2:235-241. [DOI] [PubMed] [Google Scholar]