Abstract
Estrogens have well-documented effects on lung development and physiology. However, the classical estrogen receptor α (ERα) is undetectable in the lung, and this has left many unanswered questions about the mechanism of estrogen action in this organ. Here we show, both in vivo and in vitro, that ERβ is abundantly expressed and biologically active in the lung. Comparisons of lungs from wild-type mice and mice with an inactivated ERβ gene (ERβ−/−) revealed decreased numbers of alveoli in adult female ERβ−/− mice and findings suggesting deficient alveolar formation as well as evidence of surfactant accumulation. Platelet-derived growth factor A (PDGF-A) and granulocyte-macrophage colony-stimulating factor (GM-CSF), key regulators of alveolar formation and surfactant homeostasis, respectively, were decreased in lungs of adult female ERβ−/− mice, and direct transcriptional regulation of these genes by ERβ was demonstrated. This suggests that estrogens act via ERβ in the lung to modify PDGF-A and GM-CSF expression. These results provide a potential molecular mechanism for the gender differences in alveolar structure observed in the adult lung and establish ERβ as a previously unknown regulator of postnatal lung development and homeostasis.
The vital function of the lung is to provide a gas-exchange surface to meet the organism's needs for oxygen uptake and carbon dioxide elimination. Several parameters in lung biology and pathology, both during development and in the adult, are sexually dimorphic. A role for estrogen in these dimorphisms was suggested in 1980 when Mendelson et al. (21) showed an estrogen-binding component in human fetal lung tissue. Lung maturation during fetal development is more rapid in female fetuses than in male fetuses, and the onset of surfactant synthesis occurs later in the male fetus. This difference appears to be mediated mainly by inhibitory effects of androgens, but stimulatory effects of estrogens have also been demonstrated (2). Postnatal sex differences in the rodent lung have been described by Massaro et al. (20). Adult females have a larger number of alveoli, smaller in size, than males, probably to allow for elevated oxygen consumption during pregnancy and lactation. This difference develops as animals reach sexual maturity and seems to be mediated mainly by estrogens (19). In the human population, women are more prone than men to developing chronic obstructive pulmonary disease (29) and incur a higher risk of developing lung cancer (13, 41), indicating that women are more susceptible to the deleterious effects of tobacco smoking. The reasons for these sex differences are unknown, but estrogens are likely to play a major role, since in animal models, there are estrogen-dependent gender differences in susceptibility towards tobacco-associated lung carcinogens (23), and furthermore, epidemiological studies suggest that hormone replacement therapy with estrogen is associated with a higher risk of lung cancer in postmenopausal women (1, 39).
Although previous data suggest that estrogens might be important in lung development, physiology, and carcinogenesis, there is very little information about estrogen receptor-dependent functions in the lung. This is most likely related to the absence of estrogen receptor alpha (ERα) in this tissue, which, for many years, was considered to be the only estrogen receptor. To better understand the role of estrogen in the lung, we have investigated the expression and physiological role of ERβ (16) in the lung. In this paper, we show that ERβ is abundantly expressed and biologically active in the lung. Comparisons of lungs from wild-type (WT) and ERβ−/− female mice indicate that this receptor modulates alveolar structure and surfactant homeostasis. Analysis of gene expression in WT and ERβ−/− female mice and studies of transcriptional regulation show that platelet-derived growth factor A (PDGF-A), which plays a pivotal role in alveolar formation (4, 18), and granulocyte-macrophage colony-stimulating factor (GM-CSF), a key regulator of surfactant homeostasis (9, 38), are both controlled at the transcriptional level by estrogens via ERβ in the lung. This provides a mechanism for the modulation of alveolar structure and surfactant homeostasis by estrogen.
MATERIALS AND METHODS
Animals.
The generation of ERβ knockout and estrogen response element (ERE) reporter mice has been described elsewhere (7, 14). All other mice were C57BL/6.
Fixation and tissue preparation.
Animals were killed through cervical dislocation, and anterior chest walls were removed. A cannula was inserted into the trachea and tied firmly in place. The trachea and lungs were infused with 4% paraformaldehyde (pH 7.4) at 20 cm H2O pressure and maintained at this pressure for 5 min or removed without intratracheal infusion. The lungs were subsequently kept in fixative overnight at 4°C. After fixation, the lungs were dehydrated through a graded series of ethanol. Finally, the right and left lungs were separated and placed into individual cassettes and embedded in paraffin. The central portions of the blocks were sectioned at 5-μm intervals, and the sections were mounted on glass slides, deparaffinized, and hydrated for staining.
Immunohistochemistry and immunofluorescence.
The cellular presence of ERα and β was detected by standard immunohistochemistry procedures as described by Patrone et al. (26) with some modifications. Briefly, for detection of ERα, the rabbit polyclonal antibody MC20 from Santa Cruz Biotechnology (Santa Cruz, Calif.) was used, and for ERβ, the chicken polyclonal ERβ 503 immunoglobulin Y (33, 40), made by immunization with ERβ 503, was used. ERβ 503 is human ERβ1, modified in its ligand-binding domain (LBD) by insertion of the rat 18-amino-acid sequence described in reference 24. The production and characterization of this ERβ-specific antibody have previously been described (33, 40). After deparaffinization and rehydration, the lung section was boiled in 10 mM citrate buffer for antigen retrieval. The cooled sections were incubated in 0.5% H2O2 to quench endogenous peroxidase. To block unspecific binding of secondary antibodies, sections were incubated in blocking solution (5% normal goat serum). Primary antibody 503 was added (1:500 dilution in blocking solution, incubated overnight at 4°C). The ERα antibody MC20 (Santa Cruz Biotechnology) was diluted 1:500, and antibodies against surfactant apoprotein A (SP-A) and the intracellular proform of surfactant apoprotein C (proSP-C) (N-19 and M-20, respectively, from Santa Cruz Biotechnology) were diluted 1:200 in blocking solution. After several washes, the Vectastain ABC kit (Vector Laboratories, Burlingame, Calif.) was used for visualization. All slices were slightly counterstained with Mayer's hematoxylin and mounted. Control experiments including incubation without primary antibodies, as well as studies with preadsorbed antibody, were all negative. Sections for immunofluorescence were deparaffinized and rehydrated. To block unspecific binding of secondary antibodies, sections were incubated in blocking solution (0.1 M lysine). Primary antibody against smooth muscle α-actin (clone 1A4; Sigma, St. Louis, Mo.) was used at a 1:500 dilution. For visualization, a secondary fluorescein isothiocyanate-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was used at a 1:100 dilution. After counterstaining with 4′,6′-diamidinio-2-phenylindole (DAPI), slides were mounted and examined with a Zeiss Axioplan 2 microscope with filters for fluorescein isothiocyanate and DAPI.
Protein extraction and Western blot analysis.
All tissue handling was done at 4°C. Tissue samples were homogenized for a few seconds with a Polytron PT3100 in a buffer containing 600 mM Tris · HCl, 1 mM EDTA (pH 7.4) and protease inhibitor mixture tablets (Roche Molecular Biochemicals, Mannheim, Germany), and the homogenates were centrifuged for 1 h at 50,000 × g. To load equal amounts of protein for Western blot analysis, the protein content of the supernatant fractions was measured by a Bio-Rad (Hercules, Calif.) protein assay with bovine serum albumin as the standard. For ERβ detection, samples were precipitated with trichloroacetic acid (TCA), and the precipitate was washed with methanol. Samples were placed on dry ice for 30 min, and the proteins were recovered by centrifugation. Pellets were then dissolved in sodium dodecyl sulfate (SDS) sample buffer and loaded on the gel. For SP-A and Clara cell secretory protein (CCSP), equal amounts of protein (20 μg) were loaded. Proteins were resolved on SDS-10, 12, and 15% polyacrylamide gels for ERβ, SP-A, and CCSP, respectively. Transfer to polyvinylidene difluoride membranes was done by electroblotting in Tris-glycine buffer. The membranes were checked for equal transfer by Ponceau staining. The membranes were blocked in 10% skimmed milk in phosphate-buffered saline (PBS) buffer-0.1% NP-40. Incubation with antibodies was done at dilutions of 1:3,000 for ERβ LBD (33, 40), 1:100 for SP-A (Santa Cruz Biotechnology), and 1:3,000 for CCSP (22) in the same buffer as used for the blocking reaction. The ERβ LBD antibody is a rabbit polyclonal antibody prepared by using the LBD of human ERβ1 (amino acids 320 to 527). The production and characterization of this ERβ-specific antibody are described in references 33 and 40. All incubations were performed overnight at 4°C. After washing with PBS buffer-0.1% NP-40, horseradish peroxidase-coupled secondary antibodies (1:10,000; Santa Cruz Biotechnology) were added for 2 h at room temperature. After washing with PBS buffer-0.1% NP-40, the signals were visualized by using the enhanced chemiluminescence method (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
N-terminal sequencing of proteins.
To obtain sufficient ERβ for N-terminal amino acid sequencing, cytosol from 10 g of lung was prepared in 50 ml of the Tris-EDTA buffer described above. This was diluted 10-fold with 20 mM sodium phosphate buffer, pH 7.4, to reduce the ionic concentration. Heparin-Sepharose (1 ml) was added, and the mixture was gently rotated for 1 h at 5°C. Heparin-Sepharose was recovered by centrifugation and washed 5 times with 20 mM sodium phosphate buffer. Proteins were eluted with 1 M NaCl, precipitated with 10% TCA, washed with methanol, and resolved on SDS gels in 6 lanes. Proteins were transferred to polyvinylidene difluoride membranes, a strip was cut from one lane for detection of ERβ by Western blotting, and the rest of the membrane was stained with Coomassie brilliant blue. Protein bands corresponding to those reacting with the LBD antibody were cut from the membrane, and N-terminal sequencing was performed with an Applied Biosystems 473A protein sequencer.
Sucrose gradient sedimentation.
Tissues, frozen in liquid nitrogen, were pulverized in a dismembrator (Braun, Kronberg, Germany) in 10 mM Tris-HCl, pH 7.5, 1.5 mM EDTA, and 5 mM sodium molybdate. Cytosol was obtained by centrifugation of the homogenate at 204,000 × g. Cytosols were incubated for 3 h at 0°C with 10 nM tritiated estradiol in the presence or absence of excess radio-inert estradiol (50 nM), and the bound and unbound steroids were separated with Dextran-coated charcoal. Sucrose density gradients (10 to 30% [wt/vol] sucrose) were prepared in buffer containing 10 mM Tris-HCl, 1.5 mM EDTA, 1 mM α-monothioglycerol (Sigma), and 10 mM KCl. Samples of 200 μl were layered on 3.5-ml gradients and centrifuged at 4°C for 16 h at 300,000 × g. Successive 100-μl fractions were collected from the bottom by paraffin oil displacement, by using a collector of our own design, and assayed for radioactivity by liquid scintillation counting. For ERβ detection, samples were precipitated with TCA, and the precipitate was washed with methanol. Samples were placed on dry ice for 30 min, and the proteins were recovered by centrifugation. Pellets were then dissolved in SDS sample buffer and resolved by SDS-polyacrylamide gel electrophoresis by using 4 to 20% gradient gels.
Electrophoretic mobility shift assay.
Estrogen receptor DNA binding was measured in nuclear extracts from primary mouse Clara cells. Primary Clara cells were isolated as described by Oreffo et al. (25). From these cells, nuclear proteins were prepared and electrophoretic mobility shift assays were performed essentially as described by Cassel et al. (6). Briefly, nuclear proteins were incubated with a labeled duplexed ERE (12) (sequence, 5′-GGG TAG AGG TCA CTG TGA CCT CTC GA-3′) in binding buffer (100 mM KCl, 10 mM Tris-HCl [pH 7.5], 2 mM dithiothreitol, 5% glycerol, and 0.9 μM estradiol) with or without anti-estrogen receptor antibodies (503 and LBD, see above) (33, 40). In some experiments, unlabeled duplexed oligonucleotides were included as competitors: either the ERE-containing oligonucleotide as described above or an oligonucleotide carrying a single nucleotide substitution in one half site that has been described to disrupt DNA binding of estrogen receptors (34) (sequence [the mutated nucleotide is indicated by a lowercase letter], 5′-GGG TAG AaG TCA CTG TGA CCT CTC GA-3′).
Transgenic mice.
The transgenic mice carrying the luciferase reporter gene under the transcriptional control of an estrogen response element in front of a herpes simplex virus thymidine kinase promoter have been described elsewhere (7). Heterozygous male mice (2 months old) were injected subcutaneously with 50 μg of E2/kg of body weight or 250 μg of hydroxytamoxifen or vehicle (vegetable oil)/kg as control. At the indicated time points, the animals were sacrificed and luciferase activity was assayed as described.
Morphometry.
Sections from lungs fixed by intratracheal inflation at constant pressure as described above were chosen at random and stained with hematoxylin and eosin. From these sections, randomly selected microscopic fields were photographed at ×10 magnification with a Zeiss Axioplan 2 microscope. Five fields were analyzed per animal. The pictures were enlarged uniformly and used for morphometric analysis. All alveoli in each field were counted. The number of cells was determined by counting the number of stained nuclei. All pictures were counted by two independent observers who were unaware of the genotype of the animals. Means and standard deviations were calculated, and statistical comparisons were performed by unpaired Student's t test.
Quantitative reverse transcription-PCR.
cDNA was synthesized by using the SuperScript first-strand synthesis system for reverse transcription-PCR (Life Technologies, Paisley, United Kingdom) according to the instructions of the manufacturer. For real-time quantitative reverse transcription-PCR analysis, predeveloped TaqMan assay reagents for mouse GM-CSF mRNA and 18S rRNA (Applied Biosystems) were used according to the instructions of the manufacturer. For PDGF-A mRNA, the sequences of the primers used were 5′-GGT CCA CCA CCG CAG TGT-3′ (upper) and 5′-GGA CCT CTT TCA ATT TTG GCT TC-3′ (lower), and they were used together with the SYBR Green PCR master mix kit (Applied Biosystems) according to the instructions of the manufacturer. The specificity of the PCR product was ensured by agarose gel electrophoresis in conjunction with melting curve analysis by using the Dissociation Curves software (Applied Biosystems) according to the instructions of the manufacturer. All analyses were carried out on an ABI PRISM 7700 sequence detector (Applied Biosystems).
Transient transfection studies.
A549 cells were routinely cultured in Dulbecco's minimal essential medium (GibcoBRL, Paisley, United Kingdom) supplemented with 10% fetal bovine serum, 1% l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Twenty-four hours before transfection, cells were seeded in 12-well plates. Transient transfections were carried out as previously described (27). Briefly, the Lipofectamine Plus reagent (GibcoBRL) was used in serum, phenol red, and antibiotic-free media according to the instructions of the manufacturer. The 1.8-kb PDGF-A promoter-luciferase reporter gene construct and the 1.6-kb GM-CSF promoter-luciferase reporter gene construct were kind gifts from David M. Kaetzel (Department of Molecular and Biomedical Pharmacology, College of Medicine, University of Kentucky, Lexington, Ky.) and Peter Cockerill (Division of Human Immunology, Hanson Centre For Cancer Research, Institute for Medical and Veterinary Science, Adelaide, Australia), respectively. Each well received 200 ng of the reporter plasmid and 5 ng of pSG5-ERβ (expressing the mouse ERβ) (28), or the parental vector, as indicated. Twenty nanograms of cytomegalovirus-β-galactosidase plasmid (constitutively expressing β-galactosidase) was included as a control for transfection efficiency. Serum-containing medium with the addition of hormones (10 nM 17β-estradiol, 250 nM ICI 182,780) as indicated was added 3 h posttransfection, and the cells were incubated for 24 h before harvest. Data are presented as inductions (n-fold) of luciferase activity corrected for the internal standard and represent the means ± standard deviations of the results from three independent experiments performed in duplicate. The activity of the luciferase reporter transfected without estrogen receptor-expressing plasmid and without hormone treatment was arbitrarily set to 1.
RESULTS
ERβ is the predominant estrogen receptor in the lung.
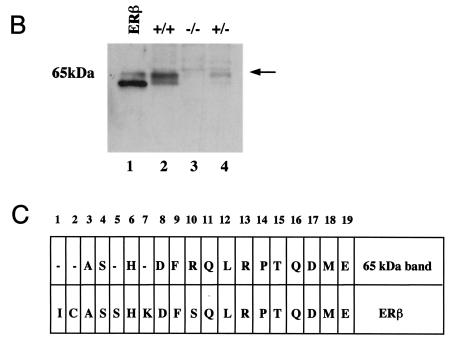
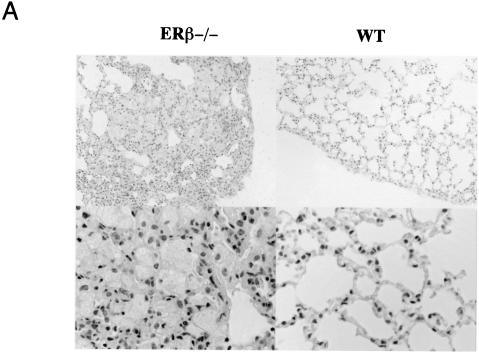
As outlined in the introduction, previous data indicate that estrogens affect lung development, physiology, and carcinogenesis. However, there is very little information about the mechanisms of estrogen action and estrogen receptor expression in the normal lung. The lung has been estimated to consist of 40 or more different cell types (5). For this reason, we started our investigations of a potential estrogen action in the lung by analyzing estrogen receptor expression by immunohistochemistry on adult mouse lung sections. As seen in Fig. 1A, upper panel, extensive nuclear ERβ staining was observed both in the bronchiolar epithelium and in the alveolar region. In the bronchiolar epithelium, the majority of cells express ERβ, indicating the presence of this nuclear receptor in both ciliated cells and nonciliated secretory Clara cells, the predominant cell populations in this area of the lung. The pattern of expression in the alveolar region is compatible with the presence of ERβ in both alveolar type II cells and in type I cells. No ERα staining could be detected (Fig. 1A, lower panel; mouse mammary gland inset as positive control). No differences with regard to ER staining were observed when sections from male and female lungs were compared (data not shown). Western blotting with lung extracts confirmed the expression of ERβ (Fig. 1B, lane 2). In contrast, lung extracts from knockout mice lacking ERβ (14) (Fig. 1B, lane 3) exhibited no immunoreactivity, corroborating the specificity of the antibody. N-terminal sequence analysis of the bands from SDS gels further confirmed ERβ expression and showed that the doublet around 65 kDa corresponds to the two 530- and 549-amino-acid ERβ isoforms (10). These data are in agreement with previous analyses of estrogen receptor mRNA in the rat lung by reverse transcription-PCR (15). They show that ERβ is highly expressed in the adult mouse lung and also indicate that ERβ is the predominant pulmonary estrogen receptor.
FIG. 1.
ERβ is highly expressed in the lung. (A) Immunohistochemistry for ERβ in mouse lung. Lungs obtained from WT and ERβ−/− mice were fixed by intratracheal inflation, sectioned, and stained with antibodies for ERβ (upper panel) and ERα (lower panel). Counterstaining was done with hematoxylin. Alveolar type I (arrowhead) and type II (open arrow) cells as well as bronchiolar epithelial cells (filled arrow) are indicated in the upper panel. The inset in the lower panel is a section of mouse mammary gland used as a positive control for ERα immunostaining. (B) Western blot analysis for ERβ in mouse lung extracts. Lane 1, recombinant ERβ short form, included as a positive control; lanes 2 to 4, lung extracts from ERβ+/+, ERβ−/−, and ERβ+/− mice. (C) N-terminal sequencing of ERβ immunoreactive bands. The upper line represents the result of N-terminal sequencing of the area corresponding to the 65-kDa doublet shown in panel B. The lower line is the amino acid sequence of mouse ERβ.
ERβ present in the lung binds estradiol and DNA in vitro.
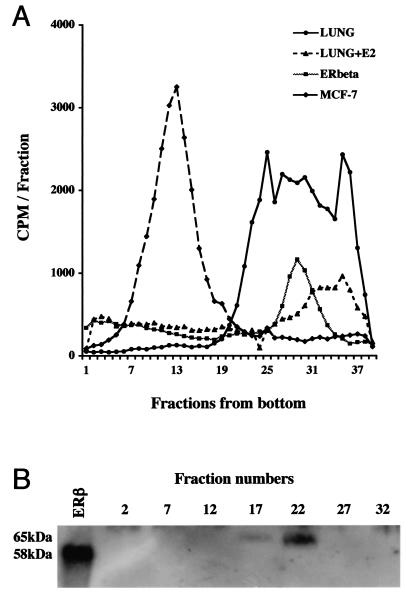
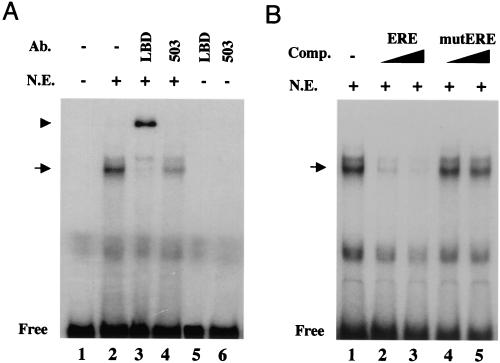
To investigate whether lung ERβ is biologically active, we investigated its potential to bind its natural ligand 17β-estradiol, as well its capability to interact with an ERE in vitro. Sucrose density gradient fractionation of lung extracts showed specific estradiol binding (at 10 nM) in the 4S region (Fig. 2A). Western blot analysis revealed ERβ immunoreactivity in corresponding fractions (Fig. 2B). Extracts from Sf9 cells overexpressing ERβ (40) exhibited estradiol binding in the 4S region as well (Fig. 2A). As expected, in extracts from MCF-7 cells, ERα immunoreactivity and estrogen binding were detected in an 8S peak. In the lung, no ERα immunoreactivity or 8S estradiol-binding peak was detected (Fig. 2A and data not shown). DNA binding was analyzed in electrophoretic mobility shift assays with an oligonucleotide containing a consensus ERE (12). A shift was observed when incubations were performed with nuclear extracts from isolated murine Clara cells (Fig. 3A, lane 2). When antibodies directed against the LBD of ERβ or the entire protein (503) were included (Fig. 3A, lanes 3 and 4, respectively), the shift was clearly diminished, and in the case of the LBD antibody, a supershift appeared, together indicating that the shift contains ERβ. The specificity of the shifted complex was demonstrated as these bands were efficiently abolished by competition with unlabeled homologous oligonucleotide (Fig. 3B, lanes 2 and 3) while no competition was observed upon inclusion of unlabeled oligonucleotide carrying a single nucleotide substitution described to disrupt DNA binding by estrogen receptors (34) (Fig. 3B, lanes 4 to 5). These results corroborate the finding that ERβ is the major estrogen receptor expressed in the lung and demonstrate that it is functional with regard to ligand binding and DNA binding in in vitro assays.
FIG. 2.
ERβ in the lung binds ligand. (A) Sucrose density gradient assay for 17β-estradiol binding. Extracts from mouse lung (LUNG), mouse lung incubated with an excess of unlabeled estradiol (LUNG+E2), Sf9 cells overexpressing ERβ (ERbeta), and MCF-7 cells (MCF-7) were fractionated on sucrose density gradients and assayed for binding of tritiated estradiol. (B) Western blot analysis for ERβ in fractions from sucrose gradient sedimentation of mouse lung extracts. Fractions analyzed by Western blotting are indicated. Recombinant ERβ short form (58 kDa) was included as a positive control in the first lane (ERβ).
FIG. 3.
ERβ in the lung binds DNA. (A) Electrophoretic mobility shift assay with ERβ antibodies. Extracts from isolated lung epithelial cells were analyzed by using a consensus ERE (12) as a probe. The arrow indicates the position of the retarded complex, and “Free” indicates the position of free unbound probe. Antibody (Ab.) directed against the LBD was included in lane 3 and caused the appearance of a supershift (arrowhead) and the disappearance of the retarded complex. Inclusion of the 503 antibody also clearly diminished the retarded complex (lane 4). Addition of the respective antibodies to the probe alone did not affect the migration of the probe (lanes 5 and 6). (B) Competition (Comp.) with unlabeled homologous and mutated oligonucleotides. To establish specific binding, increasing concentrations of unlabeled homologous oligonucleotide (ERE) were added in 50- to 100-fold excesses (lanes 2 and 3) or unlabeled mutated oligonucleotide carrying a single nucleotide substitution in one half-site that has been described to disrupt DNA binding by estrogen receptors (mutERE) (34) was added in a 50- to 100-fold excess (lanes 4 and 5). +, present; −, absent; N.E., nuclear extract.
Estrogen receptors in the lung confer estrogen responsiveness in vivo.
To extend our studies of the biological activity of ERβ in the lung, we investigated the estrogen responsiveness of the lung in vivo. For this purpose, we used transgenic mice in which a luciferase reporter gene is under the control of an ERE. The development of these mice and their use in assessing tissue-specific estrogen-mediated transcriptional activity have been reported previously (7). Male mice were used to avoid background activation from endogenous estrogen. As shown in Fig. 4A, a robust stimulation of reporter gene expression in the lung occurred 6 h after estradiol treatment. Treatment with the antiestrogen tamoxifen blocked estradiol activation, confirming estrogen receptor-dependence of the reporter gene activation (Fig. 4B). These results clearly show the presence of a transcriptionally active estrogen receptor in the lung. Based on the above data demonstrating the presence of ERβ, but not ERα, in the lung we conclude that the activation of the ERE-luciferase reporter gene in the lungs of these mice is most likely mediated by ERβ. Together, these in vitro and in vivo experiments show that the lung contains functional ERβ and is estrogen responsive, suggesting ERβ as a mediator of estrogen effects in the lung.
FIG. 4.
Estrogen receptors in the lung confer estrogen responsiveness in vivo. The effects of estrogen on luciferase reporter gene activity in lungs from ERE-luc transgenic mice are shown. Male transgenic mice carrying the luciferase reporter gene under the control of an estrogen response element were injected with vehicle (Cont) or 17β-estradiol (E2), and lung luciferase activity was assayed at different time points (A) or with estradiol or estradiol plus hydroxytamoxifen (Tam) at 6 h (B). Bars represent averages ± standard deviations of 4 to 10 individual animals assayed in duplicates. *, P < 0.01 by Student's t test; RLU, luminescence units per milligram of protein.
Adult female mice lacking ERβ show altered alveolar structure.
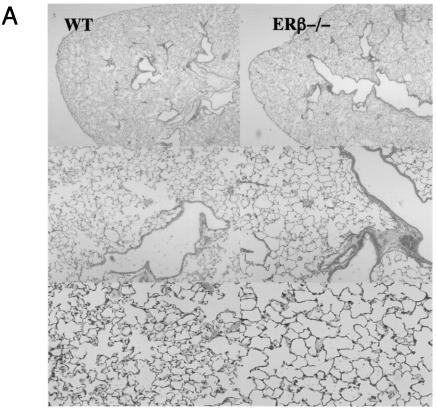
To gain insight into the role of estrogens and ERβ in the lung we utilized knockout mice lacking ERβ (ERβ−/−) (14) and compared the lungs of these mice with lungs from WT mice. Since gender differences in alveolar structure have been reported (20), we initially compared the histology of lungs of ERβ−/− and WT mice. These comparisons revealed clear differences in alveolar structure. Lungs from adult female ERβ−/− mice have larger and fewer alveoli than their WT littermates (Fig. 5A), and morphometric analyses corroborated significant changes in alveolar number (Table 1). No differences between ERβ−/− and WT lungs were observed in male mice. To investigate when the difference between ERβ−/− and WT female lungs appears during development, the histology of WT and ERβ−/− lungs was compared at embryonic day 19, postnatal day 14, and 4 weeks and 3 months of age. No differences in lung morphology were observed at the embryonic stage or in mice of 14 days or 4 weeks of age. However, in the 3-month-old mice, altered alveolar structure was evident (Table 1). This indicates that the phenotype in female lungs develops after sexual maturation. The morphometric analysis also demonstrated that the ratio between cell number and alveolar number is not changed in ERβ−/− lungs, suggesting that the differences in alveolar structure are more related to structural alterations than to changes in cellular proliferation. No morphological changes were observed in the conducting airways. From the morphometric analysis, it was also evident that the adult female lung has a larger number of alveoli than that of the male (Table 1). This gender difference in alveolar structure is in accordance with the previous observations by Massaro et al. (20). In addition, our morphometric analysis revealed that, with regard to alveolar number, the female ERβ−/− lung is strikingly similar to the male lung (Table 1). Thus, we conclude that the gender differences in alveolar number and size, evident in sexually mature animals, do not occur in the ERβ−/− mice.
FIG. 5.
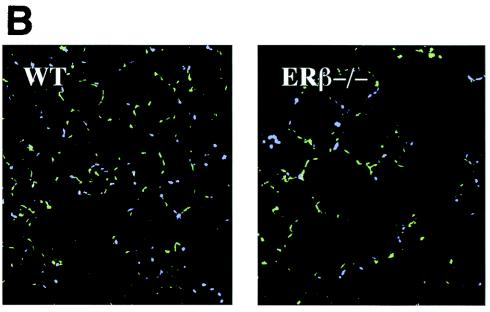
Structural changes in lungs from mice lacking ERβ. (A) Histology of lungs from WT and ERβ−/− female mice. Lungs obtained from WT and ERβ−/− mice were fixed by intratracheal inflation at constant pressure, sectioned, and stained with hematoxylin-eosin. Microscopic fields were selected at random and photographed at different magnifications. (B) Immunostaining for smooth muscle α-actin in WT and ERβ−/− mouse lungs. Lungs obtained from WT and ERβ−/− female mice were fixed and stained with antibody for smooth muscle α-actin. After nuclear counterstaining with DAPI, sections were examined with a fluorescence microscope.
TABLE 1.
Morphometric analysis of WT and ERβ−/− mouse lungsa
| Mouse description | No. of alveoli/field | No. of nuclei/no. of alveoli |
|---|---|---|
| Female, 4 wk | ||
| WT | 338 ± 19 | 2.66 ± 0.04 |
| ERβ−/− | 317 ± 56 (NS) | 2.69 ± 0.10 (NS) |
| Female, 3 mo | ||
| WT | 433 ± 86 | 2.70 ± 0.26 |
| ERβ−/− | 309 ± 44* | 2.75 ± 0.36 (NS) |
| Male, 3 mo | ||
| WT | 306 ± 27 | 2.71 ± 0.36 |
| ERβ−/− | 301 ± 16 (NS) | 2.67 ± 0.18 (NS) |
Data are means ± standard deviations (n = 4). *, P < 0.05; NS, not significant (unpaired Student's t test).
Alveolar formation is dependent on specialized mesenchymal cells in the walls of the alveolar sacs (18). In the mouse lung parenchyma, smooth muscle α-actin is a specific marker for these cells. To investigate whether these specialized mesenchymal cells were affected in ERβ−/− mice, we stained WT and ERβ−/− lungs for smooth muscle α-actin. The staining pattern for these cells was changed in accordance with the altered alveolar structure. In the WT lung, stained cells were more evenly distributed compared to the staining pattern in the ERβ−/− lung (Fig. 5B), indicating that the positioning of these cells is affected in lungs from ERβ−/− mice. This suggests that a deficiency in alveolar formation underlies the differences in alveolar number. Together, these results indicate that the lack of ERβ renders the female lung unresponsive to estrogen during sexual maturation and thus the normal increase in alveolar number does not occur in the lungs of female ERβ−/− mice.
Surfactant accumulation in mice lacking ERb.
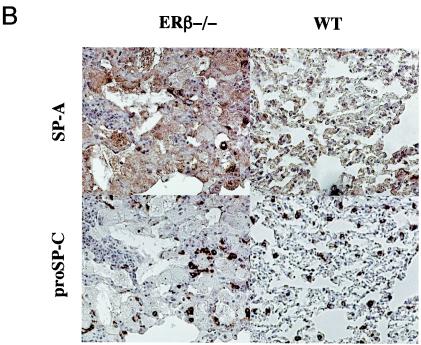
Histological examination of lungs from 1-year-old mice revealed amorphous, acellular, lightly eosinophilic material present inside the alveolar spaces of the female ERβ−/− mice (Fig. 6A). This material stained positive for SP-A, one of the major surfactant proteins (17) (Fig. 6B), indicating accumulation of surfactant components. As a marker for the surfactant-producing alveolar type II cells, an antibody specific for proSP-C (17) was used to stain serial sections. However, no differences were noted in the number, or staining intensity, of the type II cells (Fig. 6B). Also, the proSP-C specific antibody failed to stain the accumulated material. The reactivity of the accumulated material with the antibody against SP-A, together with the absence of reactivity for the intracellular proSP-C, suggests that the material represents extracellular accumulation of surfactant inside the alveolar spaces. Accumulation was observed in four of five 1-year-old female ERβ−/− mice investigated. In contrast, no evidence of surfactant accumulation was observed in 1-year-old WT female or male mice or in ERβ−/− male mice (five animals examined per group). As demonstrated above, ERβ is expressed at high levels in both bronchiolar and alveolar epithelial cells in the lung. Thus, we also analyzed a marker for bronchiolar epithelial cells, the CCSP (37), to investigate whether loss of ERβ affected bronchiolar epithelial cell function as well. However, no differences in the levels or patterns of expression were observed (data not shown). These results suggest that alveolar homeostasis is affected in female lungs lacking ERβ, resulting in accumulation of surfactant components.
FIG. 6.
Surfactant accumulation in mice lacking ERβ. (A) Histology of lungs from 1-year-old WT and ERβ−/− female mice. Lungs obtained from WT and ERβ−/− mice were fixed by immersion, sectioned, and stained with hematoxylin-eosin. (B) Immunostaining for SP-A and proSP-C in 1-year-old WT and ERβ−/− female mouse lungs. Lungs obtained from WT and ERβ−/− female mice were fixed, and serial sections were stained with antibodies for SP-A and proSP-C. Counterstaining was done with hematoxylin.
PDGF-A and GM-CSF are reduced in the lungs of mice lacking ERβ.
Lungs of female ERβ−/− mice thus exhibit alterations in alveolar number and surfactant homeostasis. PDGF-A and GM-CSF are two signaling molecules central for these aspects of lung homeostasis. PDGF-A is a major regulator of alveolar formation. Mice with a targeted disruption of the PDGF-A gene show a marked decrease in the number of alveoli because of defects in alveolar formation as a consequence of deficiencies in the specialized interstitial cells forming the alveolar walls (cells that express smooth muscle α-actin) (4, 18). GM-CSF, on the other hand, is critical in the regulation of lung surfactant. Disruption of the gene for GM-CSF in mice results in severe abnormalities in alveolar homeostasis with gross accumulation of surfactant (9, 38). We therefore compared the expression of these two signaling molecules in the lungs of WT and ERβ−/− female mice. For this purpose, PDGF-A and GM-CSF expression were analyzed by quantitative real-time reverse transcription-PCR. The results in Table 2 show that both PDGF-A and GM-CSF mRNAs were significantly lower in lungs from female ERβ−/− mice than in WT littermates. In male mice, no differences were detected between WT and ERβ−/− mice. PDGF-A mRNA levels in male mice were similar to the levels in ERβ−/− female mice, in accordance with the similarity in alveolar number between the male and female ERβ−/− mouse lungs. With regard to GM-CSF expression, male lungs instead exhibited mRNA levels similar to those of female WT lungs, in agreement with the absence of surfactant accumulation in the male WT and ERβ−/− lungs. In light of the central role of these factors in regulation of alveolar structure and surfactant homeostasis, these results suggest that the phenotypic alterations observed in female ERβ−/− mice may be related to diminished PDGF-A and GM-CSF signaling in the lungs.
TABLE 2.
Levels of PDGF-A and GM-CSF mRNA in WT and ERβ−/− mouse lungsa
| Mouse description | PDGF-A | GM-CSF |
|---|---|---|
| Female | ||
| WT | 2.89 ± 0.75 | 1.36 ± 0.20 |
| ERβ−/− | 1.23 ± 0.95* | 0.90 ± 0.07** |
| Male | ||
| WT | 1.17 ± 0.46 | 1.56 ± 0.23 |
| ERβ−/− | 1.25 ± 0.62 (NS) | 1.30 ± 0.50 (NS) |
Data are means ± standard deviations (n = 3 to 4). *, P < 0.05; , P < 0.01; NS, not significant (unpaired Student's t test).
Transcriptional regulation of PDGF-A and GM-CSF by estrogen in lung epithelial cells.
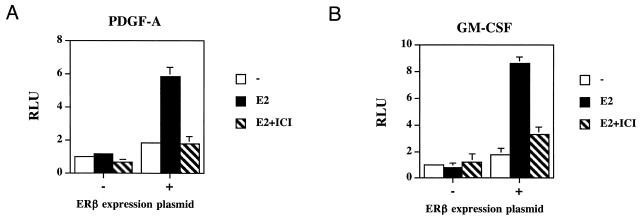
The decreased levels of PDGF-A and GM-CSF in ERβ−/− lungs suggest estrogen and ERβ as important regulators of these factors in the lung. Estrogen-dependent stimulation of both factors has been demonstrated in reproductive organs (11, 32); the nature of this regulation has, however, not been investigated. In the lung, estrogen may directly regulate the expression of these factors, since both are expressed in the bronchiolar and alveolar epithelial cells (3, 31), the same cells that express ERβ, enabling direct transcriptional activation by ERβ. The effects of estrogen on the PDGF-A and GM-CSF promoters were, therefore, examined in transient transfection experiments. For this purpose, the ERβ-negative lung epithelial cell line A549 was transfected with a reporter gene driven by a 1.8-kb PDGF-A promoter fragment or a 1.6-kb GM-CSF promoter fragment. When an ERβ expression plasmid was cotransfected in these cells, expression of the reporter driven by the PDGF-A promoter fragment increased up to sixfold (Fig. 7A) and the GM-CSF promoter was stimulated up to ninefold by estradiol (Fig. 7B). Neither promoter was stimulated by estradiol when ERβ expression plasmid was omitted. The ERβ-dependent response to estradiol was, in both cases, blocked by addition of the pure antiestrogen ICI 182,780. These data demonstrate an ERβ-dependent estrogen responsiveness of the PDGF-A and GM-CSF promoters in lung epithelial cells and indicate that response elements reside within 1.8 and 1.6 kb, respectively, from the start sites of transcription. A computer search for consensus EREs (12) revealed 9 of 13 and 10 of 13 matches in these parts of the PDGF-A and GM-CSF promoters, respectively. This indicates that estrogens may directly influence the transcription of these genes in the lung. PDGF-A and GM-CSF are key regulators of alveolar formation and surfactant homeostasis, respectively. Thus, these results, taken together, suggest that the phenotypic alterations observed in female ERβ−/− mice may be related to a lack of proper estrogen regulation of these genes by ERβ. This provides a possible mechanism behind the observed lung phenotype in ERβ−/− mice.
FIG. 7.
Transcriptional regulation of PDGF-A and GM-CSF by ERβ in transient transfections of lung epithelial cells. The lung epithelial cell line A549 was transfected with a luciferase reporter gene under the control of a 1.8-kb fragment of the PDGF-A promoter (A) or with the same reporter under the control of a 1.6-kb fragment of the GM-CSF promoter (B). Both promoters were tested in the absence (−) or presence (+) of ERβ expression plasmid. Cells were treated with 17β-estradiol (E2) or E2 and the pure antiestrogen ICI 182,780 (ICI). RLU, luciferase luminescence units per β-galactosidase units.
DISCUSSION
This study shows, in vivo and in vitro, that ERβ is the functional estrogen receptor in the lung; it is abundantly expressed in the lung epithelium and biologically active. Lungs of adult female mice with a targeted disruption of the ERβ gene exhibit decreased numbers of alveoli as a result of deficient alveolar formation after sexual maturation. In addition, female ERβ−/− mice exhibit evidence of surfactant accumulation. PDGF-A and GM-CSF, key regulators of alveolar formation and surfactant homeostasis, respectively, are decreased in lungs of adult female ERβ−/− mice and were found to be transcriptionally regulated by ERβ. Together, this provides a possible mechanism behind the observed lung phenotype in ERβ−/− mice. The data presented here suggest that ERβ has a role in the alveolar formation occurring in the sexually mature female and indicate ERβ as an important factor in postnatal lung development and homeostasis.
Alveolar formation (or alveologenesis) occurs postnatally by the formation of alveolar septa. Formation of these septa is dependent on a specialized subset of mesenchymal cells that also deposit elastin, the molecule providing elasticity to the lung (18, 30). Adult virgin female rats and mice have a larger number of alveoli, smaller in size, than males. These differences probably exist to meet the metabolic demands of reproduction (20). They are first seen after the animals have reached sexual maturity and seem to be mediated mainly by estrogens (19). Our morphometric analysis shows that this increase in alveolar number does not occur in the female ERβ−/− mice. It seems likely that the absence of ERβ renders the lung unresponsive to the elevated circulating estrogen levels in the sexually mature female. In males, no differences in alveolar structure were detected between WT and ERβ−/− mice. An explanation for this sexual dimorphism is that at sexual maturity females have higher levels of circulating estrogens than males. Furthermore, aromatase, the enzyme converting androgen to estrogen, is lacking in the male mouse lung (36), making it unlikely that there would be any substantial local production of estrogens in the male lung. In addition, recently presented results from the mice carrying the ERE-luciferase reporter gene reveal that whereas transcriptional activation can occur independent of hormone in some organs, this does not occur in the lung (8). Together, this supports the notion that the presence of circulating estrogens acting via ERβ in the sexually mature female is the major determinant of the observed lung phenotype.
The extracellular signaling molecule PDGF-A is a key regulator of alveologenesis, as demonstrated in mice carrying a targeted disruption of the PDGF-A gene. PDGF-A−/− mice exhibit complete failure of alveolar septation and loss of elastin expression and die postnatally due to pulmonary problems (4). Further analysis of these mice suggests that PDGF-A from the lung epithelium is crucial for the proliferation, migration, and elastin deposition of the mesenchymal cells central for alveologenesis. In the absence of PDGF-A, these mesenchymal cells do not take their correct positions and fail to deposit elastin, resulting in failure of alveolar formation (18). In light of these observations, our findings of reduced PDGF-A expression and changed positioning of the mesenchymal cells in the ERβ−/− female lung, together with transcriptional regulation of the PDGF-A promoter via ERβ in lung epithelial cells, provide a mechanistic explanation for the decreased number of alveoli in ERβ−/− female mice. That the lung phenotype of female ERβ−/− mice is less severe than that of PDGF-A−/− is to be expected, as estrogens have their main role in alveolar development after sexual maturation and serve to induce the increase in alveolar number observed at this time. This is in line with the study by Massaro et al. (20), in which estrogen is proposed to cause the increase in alveolar number in female rodents after sexual maturity. Our results provide a potential mechanism for this effect, and we speculate that estrogens act via ERβ in lung epithelial cells to directly modify PDGF-A expression and thereby influence alveologenesis (Fig. 8). In conclusion, our data suggest that, in the absence of ERβ, estrogen-dependent up-regulation of PDGF-A will not occur and, therefore, mesenchymal cells will not be stimulated to form additional alveoli, resulting in the loss of the estrogen-dependent increase in alveolar number occurring in the sexually mature female.
FIG. 8.
Proposed model for mechanisms of estrogen signaling in the lung epithelium. The observed phenotypic consequences from the disruption of estrogen signaling in ERβ−/− lungs are indicated by bracketed arrows.
When mice lacking GM-CSF were generated, an unanticipated role of GM-CSF signaling in surfactant homeostasis was uncovered. These mice exhibited severe abnormalities in the alveolar region of the lung with accumulation of surfactant and increased levels of surfactant proteins (9, 38). Further studies of GM-CSF signaling in surfactant homeostasis suggest that GM-CSF, locally produced by the lung epithelium and acting mainly on alveolar macrophages, is essential for normal surfactant clearance (31). In 1-year-old female ERβ−/− mice, we found evidence of surfactant accumulation in the alveolar spaces. These findings prompted us to investigate GM-CSF signaling in female ERβ−/− mice, revealing decreased GM-CSF expression. On the basis of these results, together with the transfection studies demonstrating that estrogens can regulate the GM-CSF promoter via ERβ, we speculate that estrogens act via ERβ in lung epithelial cells to directly modify GM-CSF expression and thereby influence surfactant homeostasis (Fig. 8). Again, the phenotype of ERβ−/− mice is less severe than that of GM-CSF−/− mice, probably because the levels of GM-CSF in ERβ−/− lungs are reduced and not completely abolished.
Taken together, our results provide new mechanistic insights regarding estrogen action in the lung. We have formulated a speculative model shown in Fig. 8, where we propose that estrogen acts through ERβ in the lung epithelium and influences the transcription of PDGF-A and GM-CSF. This can give further insight into the effects of estrogen on lung carcinogenesis, since PDGF-A is highly mitogenic for a large number of different cell types in vitro (3) and GM-CSF has been suggested to stimulate proliferation of alveolar type II cells in in vivo mouse models (31). Our data thus provide new information that could help in understanding the gender differences in lung cancer. In the human population, women are more susceptible than males to the deleterious effects of tobacco smoking, are more prone to develop chronic obstructive pulmonary disease, and incur a higher risk of lung cancer (29, 41). There also appears to be a sexual dimorphism regarding types of lung cancer (13, 35). The reasons for these differences are unknown, but estrogens are likely to play a major role, since in animal models, there are estrogen-dependent sex differences in susceptibility towards tobacco-associated lung carcinogens (23). Furthermore, epidemiological studies suggest that hormone replacement therapy with estrogen is associated with a higher risk of lung cancer in postmenopausal women (1, 39). In the present study, we show that estrogen directly regulates the PDGF-A and GM-CSF promoters via ERβ in lung cells. Our demonstration of direct estrogen regulation of these potent growth factors in the lungs provides new vistas for the investigation of the mechanisms underlying the observed gender differences in lung cancer.
In conclusion, the data presented in this paper give new mechanistic insights regarding estrogen action in the lung, as summarized in Fig. 8, including an understanding of the observed gender differences in postnatal lung development and structure. This provides a basis for further studies aimed at understanding the sex differences in common and severe lung disorders such as chronic obstructive pulmonary disease and lung cancer. Perhaps increased knowledge in this field may also help uncover new possibilities for treatment of these diseases.
Acknowledgments
This work was supported by grants from the Swedish Research Council (grant no. 14677 and 14678), the Swedish Cancer Society, the Åke Wiberg Research Foundation, the Swedish Society for Medical Research, and KaroBio AB.
We thank Peter Cockerill and David M. Kaetzel for reagents needed for this study. We are grateful for the skilled technical assistance provided by Lena Nordlund-Möller and Christina Thulin and for the very valuable suggestions and help from Gil-Jin Shim, Shigehira Saji, Zhang Weihua, Sari Mäkelä, and Tove Berg.
REFERENCES
- 1.Adami, H. O., I. Persson, R. Hoover, C. Schairer, and L. Bergkvist. 1989. Risk of cancer in women receiving hormone replacement therapy. Int. J. Cancer 44:833-839. [DOI] [PubMed] [Google Scholar]
- 2.Ballard, P. L. 1989. Hormonal regulation of pulmonary surfactant. Endocr. Rev. 10:165-181. [DOI] [PubMed] [Google Scholar]
- 3.Betsholtz, C., and E. W. Raines. 1997. Platelet-derived growth factor: a key regulator of connective tissue cells in embryogenesis and pathogenesis. Kidney Int. 51:1361-1369. [DOI] [PubMed] [Google Scholar]
- 4.Bostrom, H., K. Willetts, M. Pekny, P. Leveen, P. Lindahl, H. Hedstrand, M. Pekna, M. Hellstrom, S. Gebre-Medhin, M. Schalling, M. Nilsson, S. Kurland, J. Tornell, J. K. Heath, and C. Betsholtz. 1996. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85:863-873. [DOI] [PubMed] [Google Scholar]
- 5.Breeze, R. G., and E. B. Wheeldon. 1977. The cells of the pulmonary airways. Am. Rev. Respir. Dis. 116:705-777. [DOI] [PubMed] [Google Scholar]
- 6.Cassel, T. N., L. Norlund-Möller, O. Andersson, J.-Å. Gustafsson, and M. Nord. 2000. C/EBPalpha and C/EBPdelta activate the Clara cell secretory protein gene through interaction with two adjacent C/EBP-bindng sites. Am. J. Respir. Cell Mol. Biol. 22:469-480. [DOI] [PubMed] [Google Scholar]
- 7.Ciana, P., G. Di Luccio, S. Belcredito, G. Pollio, E. Vegeto, L. Tatangelo, C. Tiveron, and A. Maggi. 2001. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol. Endocrinol. 15:1104-1113. [DOI] [PubMed] [Google Scholar]
- 8.Ciana, P., M. Raviscioni, P. Mussi, E. Vegeto, I. Que, M. G. Parker, C. Lowik, and A. Maggi. 2003. In vivo imaging of transcriptionally active estrogen receptors. Nat. Med. 9:82-86. [DOI] [PubMed] [Google Scholar]
- 9.Dranoff, G., A. D. Crawford, M. Sadelain, B. Ream, A. Rashid, R. T. Bronson, G. R. Dickersin, C. J. Bachurski, E. L. Mark, J. A. Whitsett, and R. C. Mulliugan. 1994. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 264:713-716. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua, S. A., R. Schiff, I. Parra, W. E. Friedrichs, J. L. Su, D. D. McKee, K. Slentz-Kesler, L. B. Moore, T. M. Willson, and J. T. Moore. 1999. Expression of wild-type estrogen receptor beta and variant isoforms in human breast cancer. Cancer Res. 59:5425-5428. [PubMed] [Google Scholar]
- 11.Gray, K., B. Eitzman, K. Raszmann, T. Steed, A. Geboff, J. McLachlan, and M. Bidwell. 1995. Coordinate regulation by diethylstilbestrol of the platelet-derived growth factor-A (PDGF-A) and -B chains and the PDGF receptor alpha- and beta-subunits in the mouse uterus and vagina: potential mediators of estrogen action. Endocrinology 136:2325-2340. [DOI] [PubMed] [Google Scholar]
- 12.Klein-Hitpass, L., G. U. Ryffel, E. Heitlinger, and A. C. Cato. 1988. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 16:647-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kmietowicz, Z. 1998. Women at double risk of small cell lung cancer. BMJ 317:1614. [Google Scholar]
- 14.Krege, J. H., J. B. Hodgin, J. F. Couse, E. Enmark, M. Warner, J. F. Mahler, M. Sar, K. S. Korach, J. A. Gustafsson, and O. Smithies. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 95:15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuiper, G. G., B. Carlsson, K. Grandien, E. Enmark, J. Haggblad, S. Nilsson, and J. A. Gustafsson. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863-870. [DOI] [PubMed] [Google Scholar]
- 16.Kuiper, G. G. J. M., E. Enmark, M. Pelto-Huikko, S. Nilsson, and J.-Å. Gustafsson. 1996. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 93:5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroki, Y., and D. R. Voelker. 1994. Pulmonary surfactant proteins. J. Biol. Chem. 269:25943-25946. [PubMed] [Google Scholar]
- 18.Lindahl, P., L. Karlsson, M. Hellstrom, S. Gebre-Medhin, K. Willetts, J. K. Heath, and C. Betsholtz. 1997. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124:3943-3953. [DOI] [PubMed] [Google Scholar]
- 19.Massaro, G. D., J. P. Mortola, and D. Massaro. 1996. Estrogen modulates the dimensions of the lung's gas-exchange surface area and alveoli in female rats. Am. J. Physiol. 270:L110-L114. [DOI] [PubMed] [Google Scholar]
- 20.Massaro, G. D., J. P. Mortola, and D. Massaro. 1995. Sexual dimorphism in the architecture of the lung's gas-exchange region. Proc. Natl. Acad. Sci. USA 92:1105-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendelson, C. R., P. C. MacDonald, and J. M. Johnston. 1980. Estrogen binding in human fetal lung tissue cytosol. Endocrinology 106:368-374. [DOI] [PubMed] [Google Scholar]
- 22.Nord, M., M. Låg, T. N. Cassel, M. Randmark, R. Becher, H. J. Barnes, P. E. Schwarze, J.-Å. Gustafsson, and J. Lund. 1998. Regulation of CCSP (PCB-BP/Uteroglobin) expression in primary cultures of lung cells - involvement of C/EBP. DNA Cell Biol. 17:481-492. [DOI] [PubMed] [Google Scholar]
- 23.Noronha, R. F., and C. M. Goodall. 1984. The effects of estrogen on single dose dimethylnitrosamine carcinogenesis in male inbred Crl/CDF rats. Carcinogenesis 5:1003-1007. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa, S., S. Inoue, T. Watanabe, A. Orimo, T. Hosoi, Y. Ouchi, and M. Muramatsu. 1998. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor of estrogen action in human. Nucleic Acids Res. 26:3505-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oreffo, V. I., A. Morgan, and R. J. Richards. 1990. Isolation of Clara cells from the mouse lung. Environ. Health Perspect. 85:51-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrone, C., S. Andersson, L. Korhonen, and D. Lindholm. 1999. Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc. Natl. Acad. Sci. USA 96:10905-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettersson, K., F. Delaunay, and J. A. Gustafsson. 2000. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene 19:4970-4978. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson, K., K. Grandien, G. G. Kuiper, and J. A. Gustafsson. 1997. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol. Endocrinol. 11:1486-1496. [DOI] [PubMed] [Google Scholar]
- 29.Prescott, E., A. M. Bjerg, P. K. Andersen, P. Lange, and J. Vestbo. 1997. Gender difference in smoking effects on lung function and risk of hospitalization for COPD: results from a Danish longitudinal population study. Eur. Respir. J. 10:822-827. [PubMed] [Google Scholar]
- 30.Prodhan, P., and T. B. Kinane. 2002. Developmental paradigms in terminal lung development. Bioessays 24:1052-1059. [DOI] [PubMed] [Google Scholar]
- 31.Reed, J. A., and J. A. Whitsett. 1998. Granulocyte-macrophage colony-stimulating factor and pulmonary surfactant homeostasis. Proc. Assoc. Am. Physicians 110:321-332. [PubMed] [Google Scholar]
- 32.Robertson, S. A., G. Mayrhofer, and R. F. Seamark. 1996. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol. Reprod. 54:183-196. [DOI] [PubMed] [Google Scholar]
- 33.Saji, S., E. V. Jensen, S. Nilsson, T. Rylander, M. Warner, and J. A. Gustafsson. 2000. Estrogen receptors alpha and beta in the rodent mammary gland. Proc. Natl. Acad. Sci. USA 97:337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwabe, J. W., L. Chapman, J. T. Finch, and D. Rhodes. 1993. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell 75:567-578. [DOI] [PubMed] [Google Scholar]
- 35.Sekine, I., Y. Nishiwaki, T. Yokose, K. Nagai, K. Suzuki, and T. Kodama. 1999. Young lung cancer patients in Japan: different characteristics between the sexes. Ann. Thorac. Surg. 67:1451-1455. [DOI] [PubMed] [Google Scholar]
- 36.Simpson, E. R., Y. Zhao, V. R. Agarwal, M. D. Michael, S. E. Bulun, M. M. Hinshelwood, S. Graham-Lorence, T. Sun, C. R. Fisher, K. Qin, and C. R. Mendelson. 1997. Aromatase expression in health and disease. Recent Prog. Horm. Res. 52:185-213. [PubMed] [Google Scholar]
- 37.Singh, G., and S. L. Katyal. 2000. Clara cell proteins. Ann. N. Y. Acad. Sci. 923:43-58. [DOI] [PubMed] [Google Scholar]
- 38.Stanley, E., G. J. Lieschke, D. Grail, D. Metcalf, G. Hodgson, J. A. Gall, D. W. Maher, J. Cebon, V. Sinickas, and A. R. Dunn. 1994. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA 91:5592-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taioli, E., and E. L. Wynder. 1994. Re: Endocrine factors and adenocarcinoma of the lung in women. J. Natl. Cancer Inst. 86:869-870. [DOI] [PubMed] [Google Scholar]
- 40.Weihua, Z., S. Makela, L. C. Andersson, S. Salmi, S. Saji, J. I. Webster, E. V. Jensen, S. Nilsson, M. Warner, and J. A. Gustafsson. 2001. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc. Natl. Acad. Sci. USA 98:6330-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zang, E. A., and E. L. Wynder. 1996. Differences in lung cancer risk between men and women: examination of the evidence. J. Natl. Cancer Inst. 88:183-192. [DOI] [PubMed] [Google Scholar]