Abstract
Humans exposed prenatally to ethanol can exhibit brain abnormalities and cognitive impairment similar to those seen in patients expressing mutant forms of the cell adhesion molecule L1CAM. The resemblance suggests that L1CAM may be a target for ethanol, and consistent with this idea, ethanol can inhibit L1CAM adhesion in cell lines and L1CAM-mediated outgrowth and signaling in cerebellar granule neurons. However, it is not known whether ethanol inhibits L1CAM function in other neuron types known to require L1CAM for appropriate development. Here we asked whether ethanol alters L1CAM function in neurons of the cerebral cortex. We find that ethanol does not alter axonal polarization, L1CAM-dependent axon outgrowth or branching, or L1CAM recycling in axonal growth cones. Thus, ethanol inhibition of L1CAM is highly dependent on neuronal context.
Keywords: L1CAM, alcohol, cell adhesion molecules, N-cadherin
Introduction
Prenatal exposure to ethanol can produce a variety of central nervous system abnormalities resulting in a broad spectrum of cognitive and behavioral impairments known as fetal alcohol syndrome (FAS) and fetal alcohol spectrum disorder (FASD) (Jones and Smith, 1973, Bertrand et al., 2004). The underlying anatomical substrates have been the subject of intensive investigation. Magnetic resonance imaging (MRI) and postmortem studies in humans exposed prenatally to alcohol (Jones, 1975, Riley et al., 1995., Sowell et al., 2001) and studies in experimental animal models have identified several common anatomical abnormalities that serve to highlight brain regions, areas, and epochs that are vulnerable to the effects of ethanol during development (e.g. Miller, 1986, West et al., 1986, Maier et al., 1997, Miller, 1997, Gabriel et al., 2002, Qiang et al., 2002). Far less is known about the molecular targets and cellular processes that lead to ethanol's deleterious actions. A growing body of work supports that ethanol can mediate broad effects in cells via its intercalation within the plasmalemma (Mrak, 1992, Laev et al., 1996), but can also act as a more typical pharmacological agent by engaging particular receptor populations (Hanchar et al., 2006), such as the cell adhesion molecule L1CAM (Arevalo et al., 2008).
L1CAM is a member of the immunoglobulin (Ig) superfamily that is expressed mainly in the developing nervous system and functions in a number of processes, including axon outgrowth (Lagenaur and Lemmon, 1987), fasciculation (Stallcup and Beasley, 1985), and branching (Cheng and Lemmon, 2004). The extracellular domain of L1CAM can bind in trans to itself, other L1CAM family members, integrins, extracellular matrix proteins, and neuropilin (Castellani et al., 2002, Haspel and Grumet, 2003), while the cytoplasmic domain of L1CAM binds intracellular signaling, and cytoskeletal anchoring proteins (Davis et al., 1993, Schaefer et al., 1999, Schmid et al., 2000, Dickson et al., 2002). In this way, L1CAM is able to adhere and transduce signals bi-directionally across the plasma membrane. Mutations in the human L1CAM gene can result in neurological syndromes that share characteristics with FAS, including hydrocephalus, mental retardation, and agenesis of the corpus callosum (Wong et al., 1995, Kenwrick et al., 2000). Based on these similarities, it has been hypothesized that ethanol targets L1CAM extracellular ligand-binding and/or intracellular signal-transducing functions (Ramanathan et al., 1996, Bearer et al., 1999, Tang et al., 2006, Arevalo et al., 2008).
Several studies have tested aspects of this hypothesis. Levels of ethanol consistent with social drinking were found to inhibit clustering in neuroblastoma/glioma cells that is mediated in part by L1CAM (Charness et al., 1994). Ethanol can inhibit cell-cell adhesion in certain mouse fibroblast lines transfected with human L1CAM, as well as heterotypic adhesion between rat cerebellar granule cells and L1CAM expressing NIH/3T3 cells (Charness et al., 1994, Ramanathan et al., 1996, Wilkemeyer and Charness, 1998). Low concentrations of ethanol can also inhibit neurite extension in cerebellar granule cells grown on L1CAM, but not on laminin or N-cadherin, suggesting that ethanol specifically disrupts L1CAM-mediated outgrowth (Bearer et al., 1999, Watanabe et al., 2004).
However, ethanol does not inhibit L1CAM-mediated adhesion in Drosophila S2 cells expressing either the Drosophila homolog of L1CAM (neuroglian) or human L1CAM (Vallejo et al., 1997) or in mouse myeloma cells expressing human L1CAM (Bearer et al., 1999). Thus, while there is evidence to support that L1CAM can adopt a binding interface that interacts directly with ethanol, data from cell lines suggest this interaction will be highly sensitive to cellular context (Wilkemeyer and Charness, 1998). A variety of neuron types express L1CAM during development, but it is not known whether ethanol's effects on L1CAM are unique to cerebellar granule neurons (Bearer et al., 1999, Watanabe et al., 2004) or whether they can be generalized to other neurons expressing L1CAM.
Since the development of the cerebral cortex is known to be vulnerable to alcohol and to mutations in L1CAM, we asked whether neural differentiation and several L1CAM-mediated functions in neurons of the cerebral cortex are sensitive to ethanol. We find that the development of neural polarity, and L1CAM-dependent axon extension and branching are resistant to a wide range of ethanol concentrations. Thus, ethanol does not similarly antagonize L1CAM-mediated outcomes in all neurons, and direct interactions between L1CAM and ethanol are unlikely to account for the abnormal cortical development observed in FAS. Our findings support the idea that L1CAM sensitivity to ethanol is highly dependent on cellular context.
Experimental Procedures
Cell Culture
All protocols were carried out in accordance with guidelines set by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee and in accordance with the National Institutes of Health guidelines for care and use of laboratory animals. Cultures of cortical neurons were prepared from embryonic day 18 Sprague Dawley rats as described previously (Mintz et al, 2008). Neurons were taken from somatosensory cortex, dissociated, and plated on glass coverslips or glass bottomed dishes (MatTek, Ashland, MA) coated with poly-L-lysine and L1CAM-Fc or Ncad-Fc (see below). Neurons were maintained in Neurobasal (Invitrogen, Carlsbad, CA) in the absence of glia. For cultures of cerebellar granule neurons, we used a protocol described by Hatten and colleagues (1998) (Hatten et al., 1998). Cerebella were dissected from P6 rat brains in calcium and magnesium free phosphate buffered saline (pH 7.4), dissociated by trituration following an incubation in trypsin (10mg/ml) and DNAse (1mg/ml), and plated in glass bottom dishes in Basal Media Eagle (Invitrogen) supplemented with glucose, penicillin-streptomycin, glutamine, and 10% horse serum. At P6, the vast majority of cerebellar neurons are granule cells. For both granule cell and cortical cultures, four hours following plating, ethanol was added at the concentrations indicated: 0, 12.5, 25, 50 and 100mM, with the highest concentration being near the maximum of physiologically observed levels (Majchrowicz and Mendelson, 1970, Pauly et al., 1999). Two different paradigms were employed. In the first method, glass bottomed dishes were made into sealed chambers by the addition of a glass cloning ring topped with a second coverslip using vacuum grease. In the second method, ethanol was added to 6 mLs medium in conventional 60 mm petris dishes and maintained in an incubator having the same concentration of ethanol in the water pan (Bearer et al., 1999). Ethanol concentrations were measured using UV absorbance and an enzymatic ethanol level determination kit (Roche, Basel) as per the manufacturer's instructions. After 12, 24, and 48h ethanol concentrations were ≈ 55.3mM, ≈ 44.3mM, and ≈ 42.8mM respectively.
Substrates
To generate substrates, plasmids encoding L1CAM-Fc (gift of Martin Grumet) or N-cadherin-Fc (gift of Takeshi Sakurai) fusion proteins were transiently transfected into HEK293 cells, and secreted proteins were harvested after 3-5 days of incubation in low IgG media. Fc proteins were purified using a Protein A Sepharose column (Pharmacia/Amersham, Pittsburgh, PA) (Fransen et al., 1998, Sakurai et al., 1998). Identity was confirmed by Western blot, and purity was assessed by Coomassie staining. Protein concentration was determined using a Bradford Assay (BioRad, Hercules, CA). PLL-coated coverslips were incubated with goat anti-rabbit Fc-specific antibodies, then with 1000 ng of purified L1CAM-Fc or Ncad-Fc, a concentration that is saturating for cortical neurite outgrowth (determined by dilution series) and within the range used for other neuron types (e.g. (Kamiguchi and Yoshihara, 2001). Following recommendations of Kamiguchi and Yoshihara, 2001, culture supernatants of transfected HEK293 cells were used for most experiments instead of affinity purified fusion proteins, because the supernatant proved to contain 1-3μg/ml of Fc fusion protein—a saturating concentration for neural growth—and was a more stable environment for the fusion protein.
Immunocytochemistry and Microscopy
Unless otherwise noted, neurons were fixed with 4% paraformaldehyde/4% sucrose solution, permeabilized with 0.25% Triton-X, and incubated overnight at 4°C with mouse anti- β-tubulin TuJ1 antibody (1/2000, Millipore, Billerica, MA). Labeling was visualized with anti-mouse fluorescence tagged secondary antibodies (Jackson ImmunoResearch, West Grove, PA). F-actin was visualized using fluorescence tagged phalloidin (Invitrogen). For L1CAM immunolabeling, mouse anti-rat L1CAM (ASCS4, Developmental Studies Hybridoma Bank, Iowa City, IA) was added to unpermeabilized neurons.
In order to determine axon lengths and branching patterns, an observer blinded to the conditions mapped axonal arbors using a Zeiss Axiophot microscope equipped with a cooled CCD camera (Dage Corp, Stanford, CT) and a Ludl automated stage driven by Neurolucida software (MicroBrightfield Inc., Williston, VT). Axons were defined as the process exceeding the length of all others by at least 10μm (Goslin and Banker, 1989). At 48h, minor processes are commonly quite short (30μm or less) and axon lengths commonly exceed those of minor processes by several tens of microns (Goslin and Banker, 1989, Goslin et al., 1990, Esch et al., 1999). Axon length was determined by NeuroExplorer software (MicroBrightfield) as the sum of all segments. The branch number represents the total number of discrete segments comprising an axonal tree, with segments less than 5 μm excluded. Nodes are the points at which branches form (a node gives rise to two branches at least 5 μm in length). Raw data were analyzed in Excel (Microsoft, Redmond, WA) using one- or two-way ANOVA or t-test, and graphs for figures were generated in Prism (GraphPad, San Diego, CA). In all experiments, at least ten neurons were analyzed per group, and experiments were repeated in separate cultures at least two times. Images for figures were imported into Photoshop (Adobe Inc., San Jose, CA) for figure preparation.
L1CAM Endocytosis
Neurons were incubated live in anti-L1CAM (ASCS4) diluted in home media for 15 minutes, 37°C, and then fixed as described above. Sur face epitopes were blocked with an unlabeled anti-mouse secondary antibody and the cells were then fixed and permebilized as described above. Internalized ASCS4 was labeled with anti-mouse Rhodamine Red (Vector) and F-actin was labeled using Oregon Green 488 phalloidin (Invitrogen). Neurons were imaged using a Nikon Diaphot 300 with a 60× 1.4 N.A. objective and analyzed using MetaMorph (Molecular Devices, Downington, PA). Growth cones were visualized and traced using actin staining. L1CAM signal in growth cones was then thresholded and data were exported into Excel. Statistical analysis was also performed using Excel 2003 (Microsoft).
Results
Ethanol and cortical neuron differentiation
L1CAM and N-cadherin both promote neurite extension from cerebellar granule cells, but ethanol abrogates the enhanced growth on L1CAM at very low concentrations and modulates N-cadherin-mediated growth only at concentrations exceeding physiological levels (Bearer et al., 1999). Since L1CAM and N-cadherin are expressed at high levels on the surface of most axons in the developing cortex (Mintz et al, 2003, Huntley and Benson, 1999), we asked whether ethanol also selectively inhibits L1CAM-mediated cortical axon extension. Cortical neurons were dissociated and plated in glass chambers on a substrate of either L1CAM or N-cadherin, both of which promote cortical axon growth (Castellani et al, 2000(Castellani et al., 2000), Poskanzer et al, 2003). After neurons had attached (4h after plating), a range of ethanol concentrations was added (final concentration 12.5 – 100 mM), and the chambers were sealed. After 48h, neurons were fixed and double-labeled to visualize actin filaments and neuronal tubulin (Fig. 1), and an observer blinded to the exposure conditions traced the entire axonal arbor using Neurolucida.
Fig. 1. Ethanol exposure and neuron morphology.
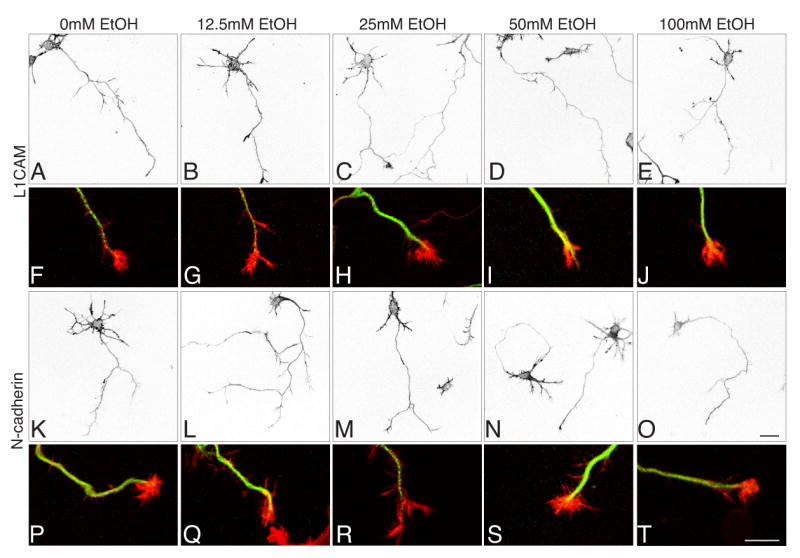
Confocal images of cortical neurons plated on L1CAM (A-J) or N-cadherin (K-T) in sealed chambers in the presence 0-100mM EtOH. Rhodamine-phalloidin labels the F-actin cytoskeleton in A-E and K-O; red in F-J and P-T. Neuronal tubulin is shown in green in F-J. In all concentrations of ethanol and on either substrate, neurons are well-differentiated and display the normal range of morphologies. Magnification bars (0, T) = 20μm.
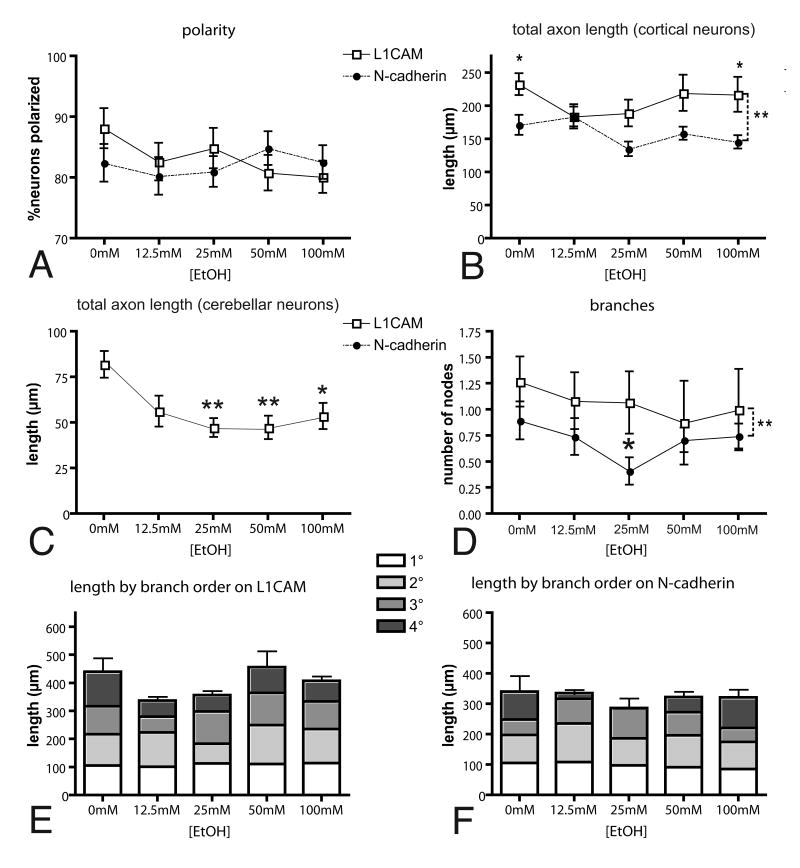
When neurons first begin to differentiate, they have multiple, short neurites any of which can become the axon. Between 12 and 48h in culture, most neurons polarize: one process extends more rapidly and reaches a length 5 or more μm greater than its neighbors. This fast-growing process rapidly acquires the characteristics of an axon. The remaining neurites subsequently differentiate into dendrites. Given the close relationship between rapid growth and the acquisition of neural polarity, we first asked whether neurons grown on L1CAM or N-cadherin and exposed to ethanol were polarized by 48h. At all ethanol concentrations and on either L1CAM or N-cadherin substrates, more than 80% of the neurons were polarized (see Methods; Figure 2A). Neither substrate nor ethanol altered the percentage of neurons that developed an axon.
Fig. 2. Quantitative effects of ethanol on axon outgrowth and branching.
Neurons were plated and grown on either L1CAM or N-cadherin as indicated and exposed to ethanol at the indicated concentrations for 48h (A, B, D-F) or 12h (C). (A) Similar proportions of neurons developed an axon on L1CAM (open squares) and N-cadherin (closed circles) at all concentrations of ethanol. (B) Axons grew significantly longer on L1CAM than on N-cadherin (2-way ANOVA, **p < 0.0001; *p < 0.05, Bonferroni post-test), but there was no effect of ethanol on the total length of axons on either substrate (p = 0.1963). (C) In contrast to cortical neurons, axon outgrowth in cerebellar granule cells grown for 12h on L1CAM is inhibited by ethanol (ANOVA, p = 0.0022; **p < 0.01; *p < 0.05, Bonferoni post test relative to control) (D) Axon branching, measured by the numbers of nodes is not altered by exposure to ethanol in neurons growing on L1CAM, but is reduced at 25mM in neurons growing on N-cadherin (ANOVA and Dunnet's post hoc test, *p < 0.05). There are also significantly fewer branches on N-cadherin than on L1CAM (2-way ANOVA, **p=0.01; Bonferroni post-test, p < 0.05). (E, F) Ethanol exposure did not significantly alter the length of axon branches of a particular order in neurons grown on L1CAM or N-cadherin, but on the latter, there is a strong trend toward shorter distal branches at 12.5-50mM.
We next asked whether there were detectable differences in overall neuronal morphology defined by the microtubule network and actin cytoskeleton. In both control and ethanol exposed neurons, F-actin filaments revealed well-defined axons and minor processes studded with filopodia (A-E; K-O). Tubulin staining varied in intensity from neuron to neuron, but showed no systematic variation corresponding to ethanol concentration or substrate (Fig. 1F-J; P-T). Actin and tubulin together revealed a variety of growth cone morphologies, but on both N-cadherin and L1CAM and under all exposure conditions, growth cones having well defined lamellipodia and filopodia were common (Fig. 1F-J; P-T).
Effects of ethanol on cortical axon outgrowth and branching
We determined whether ethanol alters axon extension by first assessing the average lengths of cortical axons and all of their branches. Axons were significantly longer on L1CAM than on N-cadherin (similar to what is observed in hippocampal neurons, Esch et al, 2000), but there were no differences in either group that could be attributed to their exposure to ethanol (Fig. 2B). As a positive control, we measured axon outgrowth from cerebellar granule neurons grown on L1CAM and exposed to various concentrations of ethanol for 12h. Consistent with previous work, ethanol significantly inhibits granule cell axon extension (Fig. 2C) (Bearer et al, 1999; Watanabe et al, 2004).
L1CAM can also promote axonal branching (Cheng and Lemmon, 2004), and consistent with this, the numbers of branch nodes for cortical axons grown on L1CAM were significantly greater than for those on N-cadherin (Fig. 2D), but at no concentration did ethanol alter axon branching on L1CAM (Fig. 2D, E). At 25mM, however, ethanol significantly decreased numbers of branch nodes in axons grown on N-cadherin. This decrease appears to rest principally with a loss of the most distal branches (Fig.2D, F).
Given the overall lack of an effect of ethanol on L1CAM-mediated cortical axon outgrowth and branching, we asked whether a ramped exposure to ethanol, in which ethanol concentrations diminish over time as they would be expected to in vivo, might yield different outcomes. Four hours after plating we added 100mM ethanol to neurons in a standard tissue culture dish containing a total volume of 6mls as well as to the water supply in the tissue culture incubator (as has been described previously, Bearer et al., 1999). Under these conditions, ethanol concentration decreases to about 40% the start value over 24h (see Experimental Procedures). Similar to what we observe following a sustained exposure, total axon length per neuron is not affected by ethanol (Fig. 3A). However, this diminishing ramped exposure to ethanol induces a significant increase in branch numbers (Fig. 3B). This increase appears to be spread throughout the axonal arbor, as numerical increases are seen at every level in the propensity for a given branch order to generate a branch (Fig. 3C). However, the increase is quite modest as it is not accompanied by increased outgrowth or correspondingly diminished branch segment lengths (Fig. 3D).
Fig. 3. Effects of ramped exposure on L1CAM-mediated axon outgrowth and branching.
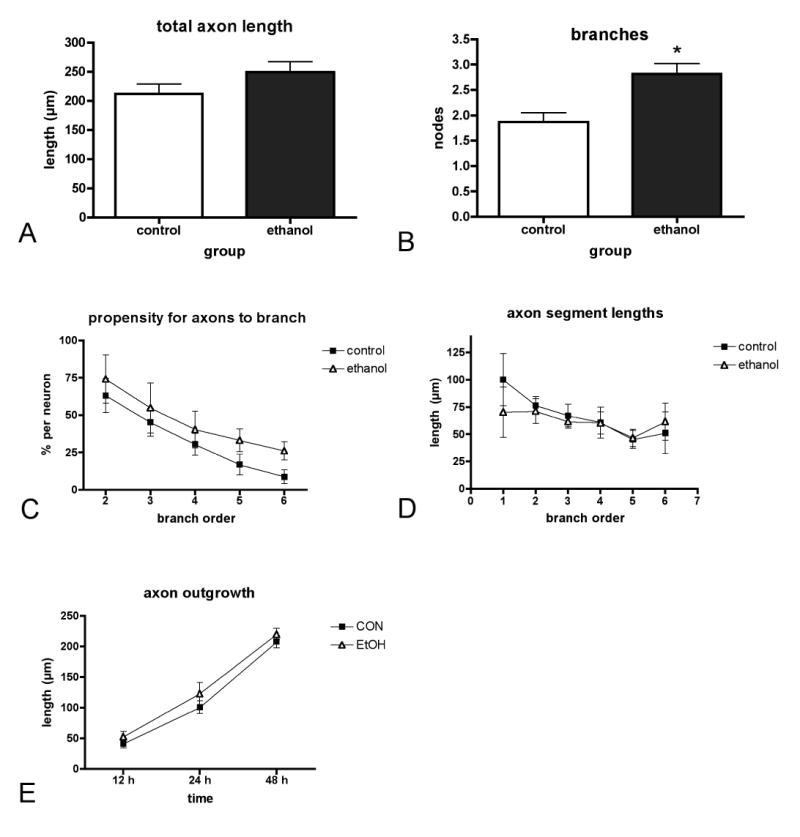
Neurons were plated on L1CAM and grown for 48h under conditions in which the exposure started at 100mM ethanol and ended at 40mM. Under these conditions, total length was unchanged (A), but numbers of nodes increased significantly in neurons exposed to ethanol (t-test, *p < 0.01). The increase in branching is evenly distributed throughout the axonal arbor as can be seen by an increase in the propensity to form an axonal branch at each order, but this does not reach significance (2-way ANOVA, p = .0951 for etoh vs. control) (C). Axon length does not decrease at every level as one might predict supporting the very modest nature of the branching increase (D). Total axon length was not significantly different in neurons exposed to 100mM ethanol and examined at 12h, 24h, or 48h compared to unexposed neurons (2-way ANOVA, p = .3870 for etoh vs. control) (E).
When hippocampal neurons are grown on poly-L-lysine and exposed to ethanol, their rates of axon extension differ over time such that axon initiation is delayed relative to unexposed neurons, but later axon growth is more rapid and can compensate (Lindsley et al., 2003). To determine whether there was an early effect on L1CAM-mediated axon extension in cortical neurons that was no longer evident at 48h, we compared total axon length in control and ethanol exposed groups after 12, 24 and 48h, but we observed no significant differences (Fig. 3E).
Effects of ethanol on L1CAM localization and endocytosis
L1CAM is polarized to axons, and changes in L1CAM polarization would be predicted to influence its function. In neurons plated on L1CAM, we asked whether exposure to ethanol altered this pattern. We find L1CAM is distributed evenly among all processes at 12h and becomes polarized to nearly all axons within 48h. There were no differences between neurons exposed to 0 or 100mM ethanol (Fig. 4).
Fig. 4. Ethanol does not alter axonal expression of L1CAM.
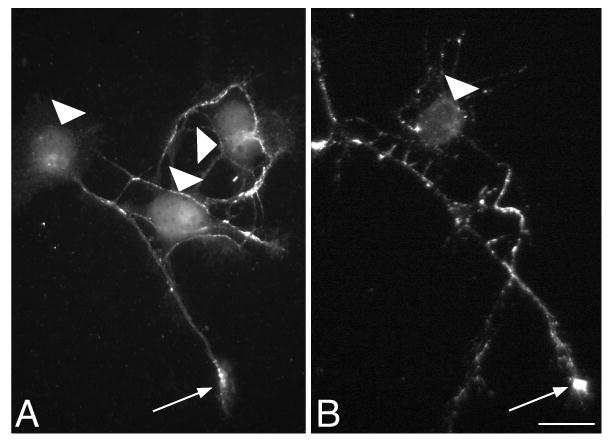
Neurons plated on L1CAM were exposed to 0 (A) or 100mM (B) ethanol for 48h. In unpermeabilized neurons, both show patches of L1CAM labeling that are enriched in growth cones (arrow) and greatly reduced over cell bodies (arrowheads). The pattern and degree of polarity is similar in both conditions. Magnification bar = 20μm.
In addition to its functions as an adhesion protein, L1CAM is also a signaling protein. Its contributions to axon growth are due in part to its internalization and initiation of MAP kinase signaling cascades (Schaefer et al., 1999, Schmid et al., 2000). In neurons grown on L1CAM substrates, L1CAM is internalized in the central domain of growth cones where it can initiate signaling and then is recycled and re-inserted in the growth cone periphery (Kamiguchi and Lemmon, 2000). While there is no effect of ethanol on L1CAM-mediated axon extension, we asked whether ethanol might alter L1CAM recycling and the initiation of signaling in growth cones. To test this, we stimulated L1CAM internalization by incubating neurons with anti-L1CAM for 15 minutes at 37°C (Kamiguchi and Lemmon, 2000, Schmid et al., 2000) and asked whether previous exposure to 100mM ethanol for 24h altered the ability of growth cones to internalize L1CAM. After fixation, surface epitopes were adsorbed with an excess of unlabeled secondary antibody, and the internalized pool of L1CAM was labeled with a tagged secondary following mild permeabilization (Kamiguchi and Lemmon, 2000). Similar to dorsal root ganglion growth cones, cortical neurons internalize L1CAM in the central domain of growth cones (Kamiguchi and Lemmon, 2000, Mintz et al., 2008) (Fig. 5A-C). To ensure the specificity of the assay L1CAM internalization on L1CAM was compared to that on poly-L-lysine (PLL). Consistent with previous work (Kamiguchi and Lemmon, 2000), the area of internalized L1CAM in growth cones on L1CAM substrates greatly exceeds that on PLL (1 ± 0.25 vs. 2.6 ± 0.4, t-test, p = 0.03). We find that 24h exposure to ethanol alters neither the area nor intensity of internalized L1CAM in growth cones (Fig. 5D-H). Extending the ethanol exposure to 72h yielded similar outcomes. These data indicate that like L1CAM-mediated outgrowth and branching, neither L1CAM localization nor L1CAM internalization is notably disrupted by sustained exposure to ethanol.
Fig. 5. Ethanol does not impact L1CAM internalization.
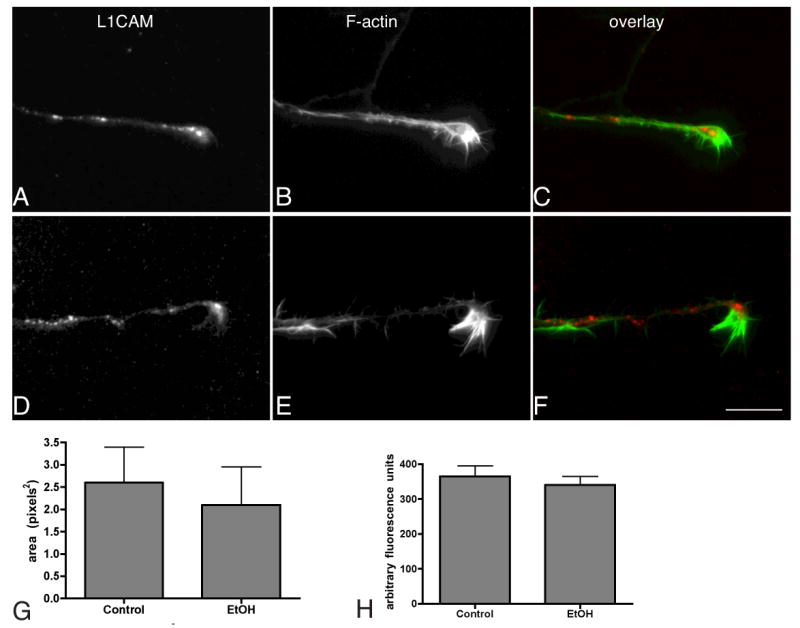
Neurons were plated on L1CAM substrates, exposed to 0 (A-C) or 100mM ethanol (D-F) for 24h, and then L1CAM internalization was stimulated using anti-L1CAM (see text). The internalized L1CAM is shown in A and D, (red in C and F) in relation to phalloidin labeled axonal growth cones (B, E and green in C, F). The area and intensity of the internalized puncta were measured and the averages are shown in the bar graphs in G and H ± sem (t-tests, p > 0.05; n = 3 experiments, 20 neurons each experiment). Magnification bar = 20μm.
Discussion
Based on similarities between humans having mutations in the gene encoding L1CAM and those exposed prenatally to ethanol, it has been hypothesized that ethanol disrupts L1CAM function during early brain development (Charness et al., 1994, Ramanathan et al., 1996). The most compelling findings in support of this hypothesis are that ethanol at physiologically relevant concentrations reduces L1CAM-mediated outgrowth in developing cerebellar granule neurons (Bearer et al., 1999, Watanabe et al., 2004) and that the extracellular domain of L1CAM can bind to ethanol (Arevalo et al., 2008). These data suggest that L1CAM functions may be broadly targeted by ethanol in the developing brain. However, we find that in cortical neurons several L1CAM functions including L1CAM-dependent axon outgrowth, branching, and its recycling in growth cones are resistant to physiological concentrations of ethanol. Our findings suggest that the neuronal context in which L1CAM is expressed greatly influences its vulnerability to ethanol.
Early exposure to ethanol affects neocortical differentiation and function. Cortical axons crossing the midline appear to be particularly vulnerable as MRI and postmortem studies in humans that have been exposed prenatally to ethanol often show agenesis, displacement or decreased callosal area (Coles, 2006, Fryer et al., 2006). Callosal defects are also common to many animal models of FAS with reduced area observed in some models (Chernoff, 1977, Wainwright and Gagnon, 1985, Zimmerberg and Mickus, 1990, Moreland et al., 2002) and increased axon numbers in others (Miller, 1997, Miller et al., 1999). Callosal defects, and in particular, agenesis are common to many humans having mutations in L1CAM as well as to certain strains of mice lacking L1CAM (Dahme et al., 1997). Thus, it would seem to be a strong possibility that ethanol might inhibit L1CAM function in cortical neurons.
However ethanol, at concentrations ranging from 12.5 - 100mM does not alter axon morphology or the ability of cortical axons to extend on either L1CAM or N-cadherin. Axons on L1CAM are longer than those on N-cadherin, similar to what has been observed in hippocampal neurons (Esch et al., 2000), but this substrate-dependent difference exceeds any effects that can be attributed to ethanol exposure. These findings are notably distinct from observations in cerebellar granule cells where as little as 3mM ethanol inhibits L1CAM-dependent neurite extension (Bearer et al., 1999, Watanabe et al., 2004), and suggest that L1CAM sensitivity to ethanol depends on the neuronal context.
L1CAM may play an even more important role in axon branching than outgrowth. Cerebellar neurons expressing any one of several L1CAM mutations that are known to be associated with abnormal brain development show decreased axon branching, while very few of the mutations show decreased length (Cheng and Lemmon, 2004). Experiments in which L1CAM-cytoskeletal interactions have been altered also show changes in axonal branching (Dickson et al., 2002, Cheng et al., 2005). Consistent with a role in branching, our results show that L1CAM promotes axon branching in cortical neurons, as axons grown on L1CAM are more highly branched than those grown on N-cadherin. However, sustained exposure to ethanol did not inhibit branching on L1CAM at any concentration. When ethanol concentrations were allowed to diminish over time, there was a slight increase in branch number throughout the axonal arbor suggesting a rebound effect, but the lack of a corresponding decrease in individual axon segment lengths or increase in total length indicates this effect is exceedingly modest. There was a small, but significant decrease in branching in neurons grown on N-cadherin in the presence of 25mM ethanol, but this outcome was not observed at any other concentration.
While ethanol does not detectably alter L1CAM-dependent axon growth or branching, it remains possible that it impacts cortical axon development in ways we would not have detected in our experiments. For example, hippocampal neurons grown on poly-L-lysine and exposed to ethanol also show no changes in net axon growth, but exhibit altered growth dynamics (Lindsley et al., 2003); and septal explants can elaborate axons in the presence of ethanol, but fail to target hippocampal tissue (Heaton et al., 1994). This latter example may be particularly relevant as it outlines an alternative means by which ethanol could target L1CAM: Septal fibers are directed toward the hippocampus in part by their responsiveness to Sema3A (Pascual et al., 2005), a guidance cue that binds a receptor complex, which can include L1CAM(Castellani et al., 2000).
L1CAM can also mediate a variety of functions outside of outgrowth and branching that are initiated by L1CAM internalization in growth cones (Schaefer et al., 1999, Kamiguchi and Yoshihara, 2001, Mintz et al., 2008). Ligand-induced L1CAM internalization activates the MAP kinase cascade in endosomes (Schaefer et al., 1999, Schmid et al., 2000) and significantly, in cerebellar granule cells levels of L1CAM-stimulated ERK1/2 phosphorylation levels are diminished by ethanol (Tang et al., 2006). However, we find that 24 - 72h pre-exposure to ethanol does not diminish the ability of neurons to undergo ligand-induced L1CAM internalization. It is possible that neurons recover their ability to internalize L1CAM since ethanol was removed at the start of the assay, but we have found that L1CAM internalization in the continuous presence of ethanol yields similar results (Mintz and Benson, unpublished data).
Together our findings indicate that sustained exposure to a wide range of ethanol concentrations does not significantly impact a variety of L1CAM-dependent functions in cortical neurons in dissociated culture. Thus, with respect to ethanol-mediated inhibition of L1CAM function, cerebellar neurons are highly sensitive and cortical neurons are resistant, just as L1CAM adhesion produced by the exogenous expression of L1CAM is inhibited by ethanol in certain cell lines, and not others (Ramanathan et al., 1996, Vallejo et al., 1997, Wilkemeyer and Charness, 1998, Bearer et al., 1999). Why should L1CAM respond differently? A likely possibility is that L1CAM sensitivity to ethanol is highly dependent on cellular context (Wilkemeyer and Charness, 1998). L1CAM can engage a variety of cis-binding partners, such as neuropilin1, (Castellani et al., 2000, Castellani et al., 2002), FGF receptor (Williams et al., 1994), and TAG-1/axonin-1 (Buchstaller et al., 1996). Such interactions would be expected to alter the availability of ethanol binding sites on L1CAM by direct competition or by changing the conformation adopted by L1CAM itself, which appears to be able to bind ethanol in only one of its two binding conformations (Schurmann et al., 2001, Arevalo et al., 2008). Cis-interactions could also influence the relative distribution of L1CAM into membrane microdomains (Nakai and Kamiguchi, 2002, Szabo et al., 2007) or L1CAM intracellular binding interactions, which would alter the stability of L1CAM in the membrane and its participation in various signaling networks (Gil et al., 2003, Mintz et al., 2008). Alternately, the domains of L1CAM that are critical for neuronal development may vary with cell type and context, thus changing effective targets for ethanol toxicity. While studies in non-neuronal cells suggest that ethanol may disrupt homophilic binding (Ramanathan et al, 1996), mice expressing a mutant L1CAM incapable of homophilic binding do not display the axon guidance defects that are prominent in mice lacking L1CAM (Itoh et al., 2004). It is also possible that other L1CAM family members such as CHL1 (Holm et al., 1996, Demyanenko et al., 2004) compensate for the some of the effects of ethanol on L1CAM functions that we assessed.
The development of the cerebral cortex is clearly affected by ethanol exposure and while L1CAM may not be a target in the same way that it is in granule cells, it may contribute indirectly to altered neuronal responsiveness or over a different time course. Other molecules may be more sensitive to ethanol in cortical neurons including other adhesion proteins like NCAM and integrins, as well as neurotrophins, which show altered expression or actions in the cerebral cortex in response to ethanol (Minana et al., 2000, Siegenthaler and Miller, 2004, Siegenthaler and Miller, 2006). Such cell type specificity undoubtedly contributes to some of the variability that is observed in neuronal responses to ethanol exposure.
Acknowledgments
We would like to acknowledge the technical assistance of Meghann Burke, Chun-Ying Ko, Kin Yip Chien, and Evan Gelb. This study was supported by APIRE/Janssen Resident Psychiatric Research Scholar Award (2004-6), AMA Foundation Seed Grant, NRSA MD/PhD predoctoral fellowship, and NIAAA R01AA01497.
List of Abbreviations
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorders
- HEK
human embryonic kidney
- L1CAM
L1 cell adhesion molecule
- MAP kinase
mitogen activated protein kinase
- MRI
magnetic resonance imaging
- NCAM
neural cell adhesion molecule
- PLL
poly-L-lysine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, Charness ME, Miller KW. An alcohol binding site on the neural cell adhesion molecule L1. Proc Natl Acad Sci U S A. 2008;105:371–375. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Floyd R, Weber M, O'Connor M, Riley E, Johnson K, Cohen D. Centers for Diesease Control and Prevention. Atlanta, GA: 2004. National Task Force on FAS/FAE: Guidelines for Referral and Diagnosis. [Google Scholar]
- Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, Sonderegger P. Cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Castellani V, De Angelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. Embo J. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME, Safran RM, Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269:9304–9309. [PubMed] [Google Scholar]
- Cheng L, Itoh K, Lemmon V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J Neurosci. 2005;25:395–403. doi: 10.1523/JNEUROSCI.4097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Lemmon V. Pathological missense mutations of neural cell adhesion molecule L1 affect neurite outgrowth and branching on an L1 substrate. Mol Cell Neurosci. 2004 doi: 10.1016/j.mcn.2004.08.005. In press. [DOI] [PubMed] [Google Scholar]
- Chernoff GF. The fetal alcohol syndrome in mice: an animal model. Teratology. 1977;15:223–229. doi: 10.1002/tera.1420150303. [DOI] [PubMed] [Google Scholar]
- Coles CD. Prenatal alcohol exposure and human development. In: Miller MW, editor. Brain Development: Normal Processes and the Effects of Alcohol and Nicotine. New York: Oxford University Press; 2006. pp. 123–142. [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, Maness PF. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron. 2004;44:423–437. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dickson TC, Mintz CD, Benson DL, Salton SR. Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J Cell Biol. 2002;157:1105–1112. doi: 10.1083/jcb.200111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci. 1999;19:6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29:215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- Fransen E, D'Hooge R, Van Camp G, Verhoye M, Sijbers J, Reyniers E, Soriano P, Kamiguchi H, Willemsen R, Koekkoek SKE, De Zeeuw CI, De Deyn PP, Van der Linden A, Lemmon V, Kooy RF, Willems PJ. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Human molecular genetics. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Spadoni AD, Riley EP. Influence of alcohol on structure of the developing human brain. In: Miller MW, editor. Brain Development: Normal Processes and the Effects of Alcohol and Nicotine. New York: Oxford University Press; 2006. pp. 143–152. [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JHP, Banker G. Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci. 1990;10:588–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel J, Grumet M. The L1CAM extracellular region: a multi-domain protein with modular and cooperative binding modes. Front Biosci. 2003;8:s1210–1225. doi: 10.2741/1108. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Gao WQ, Morrison ME, Mason CA. The Cerebellum: Purification and coculture of identified cell populations. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge: MIT Press; 1998. pp. 419–459. [Google Scholar]
- Heaton MB, Paiva M, Swanson DJ, Walker DW. Responsiveness of cultured septal and hippocampal neurons to ethanol and neurotrophic substances. J Neurosci Res. 1994;39:305–318. doi: 10.1002/jnr.490390308. [DOI] [PubMed] [Google Scholar]
- Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, Montag D, Schachner M. Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur J Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Cheng L, Kamei Y, Fushiki S, Kamiguchi H, Gutwein P, Stoeck A, Arnold B, Altevogt P, Lemmon V. Brain development in mice lacking L1-L1 homophilic adhesion. J Cell Biol. 2004;165:145–154. doi: 10.1083/jcb.200312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Lemmon V. Recycling of the cell adhesion molecule L1 in axonal growth cones. J Neurosci. 2000;20:3676–3686. doi: 10.1523/JNEUROSCI.20-10-03676.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshihara F. The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones. J Neurosci. 2001;21:9194–9203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Human molecular genetics. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- Laev H, Hungund BL, Karpiak SE. Cortical cell plasma membrane alterations after in vitro alcohol exposure: prevention by GM1 ganglioside. Alcohol. 1996;13:187–194. doi: 10.1016/0741-8329(95)02045-4. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci U S A. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley TA, Kerlin AM, Rising LJ. Time-lapse analysis of ethanol's effects on axon growth in vitro. Brain Res Dev Brain Res. 2003;147:191–199. doi: 10.1016/j.devbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E, Mendelson JH. Blood concentrations of acetaldehyde and ethanol in chronic alcoholics. Science. 1970;168:1100–1102. doi: 10.1126/science.168.3935.1100. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of prenatal exposure to ethanol on callosal projection neurons in rat somatosensory cortex. Brain Res. 1997;766:121–128. doi: 10.1016/s0006-8993(97)00533-7. [DOI] [PubMed] [Google Scholar]
- Miller MW, Astley SJ, Clarren SK. Number of axons in the corpus callosum of the Mature macaca nemestrina: increases caused by prenatal exposure to ethanol. J Comp Neurol. 1999;412:123–131. [PubMed] [Google Scholar]
- Minana R, Climent E, Barettino D, Segui JM, Renau-Piqueras J, Guerri C. Alcohol exposure alters the expression pattern of neural cell adhesion molecules during brain development. J Neurochem. 2000;75:954–964. doi: 10.1046/j.1471-4159.2000.0750954.x. [DOI] [PubMed] [Google Scholar]
- Mintz CD, Carcea I, McNickle DG, Dickson TC, Ge Y, Salton SRJ, B DL. ERM proteins regulate axon responsiveness to Sema3A. J Comp Neurol. 2008;510:351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland N, La Grange L, Montoya R. Impact of in utero exposure to EtOH on corpus callosum development and paw preference in rats: protective effects of silymarin. BMC complementary and alternative medicine. 2002;2:10. doi: 10.1186/1472-6882-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE. Opposite effects of dimethyl sulfoxide and ethanol on synaptic membrane fluidity. Alcohol. 1992;9:513–517. doi: 10.1016/0741-8329(92)90089-s. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Kamiguchi H. Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner. J Cell Biol. 2002;159:1097–1108. doi: 10.1083/jcb.200209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Pozas E, Soriano E. Role of class 3 semaphorins in the development and maturation of the septohippocampal pathway. Hippocampus. 2005;15:184–202. doi: 10.1002/hipo.20040. [DOI] [PubMed] [Google Scholar]
- Pauly T, Dahmen N, Szegedi A, Wetzel H, Bol GF, Ferdinand K, Hiemke C. Blood ethanol levels and adenylyl cyclase activity in lymphocytes of alcoholic patients. Biol Psychiatry. 1999;45:489–493. doi: 10.1016/s0006-3223(98)00075-4. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Wilkemeyer MF, Mittal B, Perides G, Charness ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133:381–390. doi: 10.1083/jcb.133.2.381. published erratum appears in J Cell Biol 1996 Jun;133(5):1139-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Roonprapunt C, Grumet M. Purification of Ig-fusion proteins from medium containing Ig. Biotechniques. 1998;25:382–385. doi: 10.2144/98253bm09. [DOI] [PubMed] [Google Scholar]
- Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J Biol Chem. 1999;274:37965–37973. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J Neurosci. 2000;20:4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann G, Haspel J, Grumet M, Erickson HP. Cell adhesion molecule L1 in folded (horseshoe) and extended conformations. Mol Biol Cell. 2001;12:1765–1773. doi: 10.1091/mbc.12.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Transforming growth factor beta1 modulates cell migration in rat cortex: effects of ethanol. Cereb Cortex. 2004;14:791–802. doi: 10.1093/cercor/bhh039. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Mechanisms of ethanol-induced alterations in neuronal migration. In: Miller MW, editor. Brain Development: Normal Processes and the Effects of Alcohol and Nicotine. New York: Oxford University Press; 2006. pp. 216–229. [Google Scholar]
- Stallcup WB, Beasley L. Involvement of the nerve growth factor-inducible large external glycoprotein (NILE) in neurite fasciculation in primary cultures of rat brain. Proc Natl Acad Sci U S A. 1985;82:1276–1280. doi: 10.1073/pnas.82.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Tang N, He M, O'Riordan MA, Farkas C, Buck K, Lemmon V, Bearer CF. Ethanol inhibits L1 cell adhesion molecule activation of mitogen-activated protein kinases. J Neurochem. 2006;96:1480–1490. doi: 10.1111/j.1471-4159.2006.03649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo Y, Hortsch M, Dubreuil RR. Ethanol does not inhibit the adhesive activity of Drosophila neuroglian or human L1 in Drosophila S2 tissue culture cells. J Biol Chem. 1997;272:12244–12247. doi: 10.1074/jbc.272.18.12244. [DOI] [PubMed] [Google Scholar]
- Wainwright P, Gagnon M. Moderate prenatal ethanol exposure interacts with strain in affecting brain development in BALB/c and C57BL/6 mice. Exp Neurol. 1985;88:84–94. doi: 10.1016/0014-4886(85)90115-3. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Yamazaki M, Miyazaki H, Arikawa C, Itoh K, Sasaki T, Maehama T, Frohman MA, Kanaho Y. Phospholipase D2 functions as a downstream signaling molecule of MAP kinase pathway in L1-stimulated neurite outgrowth of cerebellar granule neurons. J Neurochem. 2004;89:142–151. doi: 10.1111/j.1471-4159.2004.02308.x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Charness ME. Characterization of ethanol-sensitive and insensitive fibroblast cell lines expressing human L1. J Neurochem. 1998;71:2382–2391. doi: 10.1046/j.1471-4159.1998.71062382.x. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wong EV, Kenwrick S, Willems P, Lemmon V. Mutations in the cell adhesion molecule L1 cause mental retardation. Trends Neurosci. 1995;18:168–172. doi: 10.1016/0166-2236(95)93896-6. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Mickus LA. Sex differences in corpus callosum: influence of prenatal alcohol exposure and maternal undernutrition. Brain Res. 1990;537:115–122. doi: 10.1016/0006-8993(90)90347-e. [DOI] [PubMed] [Google Scholar]