Abstract
Intermittent hypoxia (IH) occurs in many pathological conditions including recurrent apneas. Hypoxia-inducible factors (HIFs) 1 and 2 mediate transcriptional responses to low O2. A previous study showed that HIF-1 mediates some of the IH-evoked physiological responses. Because HIF-2α is an orthologue of HIF-1α, we examined the effects of IH on HIF-2α, the O2-regulated subunit expression, in pheochromocytoma 12 cell cultures. In contrast to the up-regulation of HIF-1α, HIF-2α was down-regulated by IH. Similar down-regulation of HIF-2α was also seen in carotid bodies and adrenal medullae from IH-exposed rats. Inhibitors of calpain proteases (ALLM, ALLN) prevented IH-evoked degradation of HIF-2α whereas inhibitors of prolyl hydroxylases or proteosome were ineffective. IH activated calpain proteases and down-regulated the endogenous calpain inhibitor calpastatin. IH-evoked HIF-2α degradation led to inhibition of SOD2 transcription, resulting in oxidative stress. Over-expression of transcriptionally active HIF-2α prevented IH-evoked oxidative stress and restored SOD2 activity. Systemic treatment of IH-exposed rats with ALLM rescued HIF-2α degradation and restored SOD2 activity, thereby preventing oxidative stress and hypertension. These observations demonstrate that, unlike continuous hypoxia, IH leads to down-regulation of HIF-2α via a calpain-dependent signaling pathway and results in oxidative stress as well as autonomic morbidities.
Keywords: calcium signaling, hypoxia inducible factors
Sleep-disordered breathing with recurrent apneas is a leading cause of morbidity and mortality affecting an estimated 18 million people in the United States alone (1–4). Recurrent apneas are characterized by transient, repetitive cessations of breathing (≈10 sec in adults) resulting in periodic decreases in arterial blood O2 or intermittent hypoxia (IH). Patients with recurrent apneas are at risk for developing several co-morbidities including hypertension, sympathetic activation, breathing abnormalities, atherosclerosis, and stroke (4–8). Exposure of rodents to IH alone induces several co-morbidities reported in patients with recurrent apnea (9–11). However, little information is available on the molecular mechanisms underlying the morbidities associated with IH.
Hypoxia-inducible factors (HIFs) mediate transcriptional responses to low O2 (12). HIF-1 is the prototypical member of the HIF family and comprises an O2-regulated α subunit and a constitutive β subunit (13). HIF-1 transcriptional activity is induced under continuous hypoxia (CH) as a result of HIF-1α protein accumulation resulting from decreased O2-dependent proline hydroxylation, ubiquitination, and proteasomal degradation (14). Recent studies showed that IH leads to HIF-1α accumulation and utilizes signaling pathways distinct from those identified with CH (15). The importance of HIF-1 to IH-associated physiological and pathophysiological responses was studied in mice with heterozygous deficiency of HIF-1α. IH-evoked cardio-respiratory and metabolic morbidities were absent in HIF-1α heterozygous mice (16).
The O2 regulated α subunit HIF-2α, also known as endothelial PAS domain protein-1, is another member of the HIF family (17). HIF-2α shares 80% sequence homology to HIF-1α and also interacts with HIF-1β (18). Similar to HIF-1, CH leads to HIF-2α accumulation and the resulting transcriptional activation regulates several target genes that are also common to HIF-1 (19). We hypothesized that HIF-2 signaling is also affected in IH-evoked autonomic morbidities. To test this hypothesis, we studied the effects of IH on HIF-2α accumulation, a prerequisite for HIF-2 activation, in cell cultures and rodents. In contrast to the up-regulation of HIF-1α, we found that IH down-regulates HIF2α protein levels via signaling involving calpain proteases. Remarkably, systemic administration of calpain inhibitors restored HIF-2α levels in IH-exposed rats and prevented IH-induced oxidative stress and cardiovascular abnormalities.
Results
Differential Responses of HIF-1α and HIF-2α to IH.
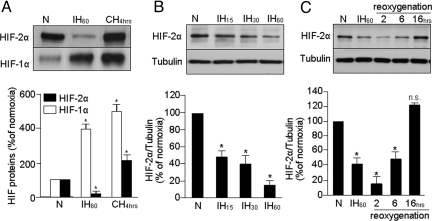
HIF-1α and HIF-2α protein levels were analyzed by immunoblot in rat pheochromocytoma 12 (PC12) cell lysates exposed to 60 cycles of IH (IH60; each cycle consisting of 30 sec of 1.5% O2 followed by 5 min of 20% O2) or CH (1.5% O2 for 4 h) or normoxia (20% O2). Basal HIF-2α expression was higher than HIF-1α (Fig. 1A). Consistent with a previous report (15), HIF-1α expression increased ≈4-fold in cells exposed to IH60. In striking contrast, HIF-2α levels markedly decreased in cells exposed to IH60 (Fig. 1A). Similar down-regulation of HIF-2α was also seen in human lung microvascular endothelial cells exposed to IH60 (data not shown). In comparison, exposing PC12 cells to 4 h of CH led to significant accumulation of both HIF-1α and HIF-2α (Fig. 1A).
Fig. 1.
IH results in HIF-2α degradation in PC12 cells. (A) Representative immunoblot of HIF-2α and HIF-1α proteins in PC12 cells exposed to normoxia (N) or to IH60 or CH for 4 h. (B) Effect of increasing cycles of intermittent hypoxia (IH15,30,60) on HIF-2α expression in PC12 cells. (C) Effect of re-oxygenation on HIF-2α following exposure to IH60. Tubulin expression was monitored as control for protein loading in B and C. (Bottom), Average data of densitometric analysis of the immunoblots presented as mean ± SEM from 4 independent experiments. *P < 0.05; n.s., not significant, P > 0.05.
HIF-2α protein progressively decreased as the duration of IH was increased from 15 to 30 to 60 cycles (Fig. 1B). O2 profiles remained constant during the course of a given experiment and medium PO2 decreased by ≈30 mmHg during each episode of hypoxia [supporting information (SI) Fig. S1A]. After terminating IH60, HIF-2α levels remained significantly down-regulated for 6 h and returned to normoxic levels after 16 h of re-oxygenation (Fig. 1C). Cell viability was not affected by IH60 as determined by lactate dehydrogenase activity (control 0.61 ± 0.15 μmol NADH oxidized min/mg/protein; IH, 0.63 ± 0.12 μmol; P > 0.05; n = 4). These observations demonstrate that, unlike the up-regulation of HIF-1α, IH leads to down-regulation of HIF-2α in a stimulus-dependent and reversible manner.
IH60 led to modest but significant up-regulation of HIF-2α mRNA in PC12 cells as assessed by real-time RT-PCR (P < 0.05; Fig. S1B). Prolyl hydroxylase (PHDs) isoforms 1–3 contribute to regulation of HIF-2α under normoxia (20). Dimethyloxalylglycine (1 mM), an inhibitor of PHDs, up-regulated HIF-2α protein in normoxic cells, yet the protein was down-regulated by 50%–60% in response to IH60 (Fig. S1 C and D). Similar results were also obtained with MG-132 (5 μM), a proteasome inhibitor (Fig. S1 C and D). These observations indicate that neither changes in HIF-2α mRNA nor protein degradation by PHDs and/or proteasome would account for IH-induced HIF-2α degradation.
Calpain Proteases Mediate IH-Induced HIF-2α Degradation.
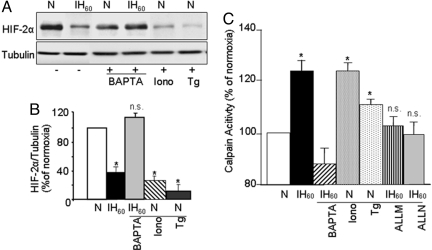
IH elevates [Ca2+]I in PC12 cells, and Ca2+ signaling is critical for HIF-1 activation by IH (15). To assess whether Ca2+ signaling contributes to HIF-2α degradation by IH, we determined the effect of altered Ca2+ levels on HIF-2α levels. As shown in Fig. 2 A and B, IH-induced HIF-2α degradation was prevented by BAPTA-AM (10 μM), a chelator of Ca2+, whereas ionomycin (1.4 μM), a Ca2+ ionophore, and thapsigargin (1.5 μM), which elevates [Ca2+]I by inhibiting (Ca2+-Mg2+)-ATPases, down-regulated HIF-2α protein in cells exposed to normoxia. These results indicate the involvement of Ca2+ signaling in IH-induced HIF-2α degradation.
Fig. 2.
Ca2+ signaling involving calpain activation mediates IH-induced HIF-2α degradation. (A). Effects of altered Ca2+ levels upon HIF-2α levels in PC12 cells exposed to either normoxia (N) or IH60. Representative immunoblot of HIF-2α in cells exposed to normoxia (N) or IH60 with and without pretreatment with BAPTA-AM (10 μM), a Ca2+ chelator. Effect of ionomycin (Iono, 1.4 μM) a Ca2+ ionophore; or thapsigargin (Tg, 1.5 μM), a Ca2+-Mg2+ATPase blocker, on HIF-2α expression in PC12 cells exposed to normoxia. (B) Quantitative data from densitometric analysis from 3 experiments. (C) Activation of calpains by IH in PC12 cells. Calpain activity was determined in PC12 cells exposed to normoxia (N) or to IH60. Calpain activity increased in cells exposed to IH60, which was inhibited by BAPTA-AM and the calpain inhibitors ALLM and ALLN (10 μM each). Ionomycin (Iono, 1.4 μM) and thapsigragin (Tg, 1.5 μM), which elevate [Ca2+]I activated calpains in cells exposed to normoxia. Data presented are mean ± SEM. from 4 experiments. *P < 0.05; n.s., not significant, P > 0.05.
Elevation in [Ca2+]I activates several downstream effector molecules including the Ca2+-activated proteases calpains 1 and 2 (21). A recent study reported that calpains mediate HIF-1α degradation in pVHL-deficient renal carcinoma cells exposed to a combination of hypoxia and nitric oxide donor (22). To test the role of calpains, calpain enzyme activity was determined in PC12 cells exposed to IH60. Calpain activity was significantly elevated in IH60-exposed cells, and 10 μM BAPTA-AM prevented this effect; whereas ionomycin and thapsigargin stimulated calpain activity in cells exposed to normoxia (Fig. 2C). These observations demonstrate that the measured calpain enzyme activity requires Ca2+.
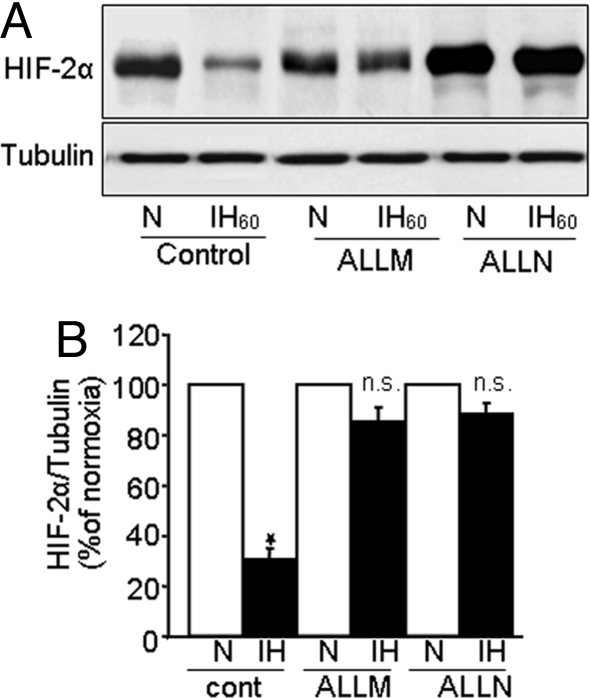
We next determined the effects of calpain inhibitors ALLN and ALLM (10 μM each) on IH-evoked calpain activation and HIF-2α degradation. Both calpain inhibitors prevented IH-induced calpain activation (Fig. 2C) as well as HIF-2α degradation (Fig. 3 A and B). Basal HIF-2α expression increased under normoxia in presence of ALLN but not ALLM (Fig. 3A), conceivably as a result of the effect of ALLN on proteosome activity (23), Therefore, ALLM was chosen in subsequent studies.
Fig. 3.
Calpain inhibitors prevent IH-induced HIF-2 degradation. (A) Representative immunoblot showing that calpain inhibitors ALLM and ALLN (10 μM each) prevent IH60-evoked HIF-2α degradation. (B) Densitometric analysis of the immunoblots presented as mean ± SEM from 4 independent experiments. *P < 0.05; n.s., not significant.
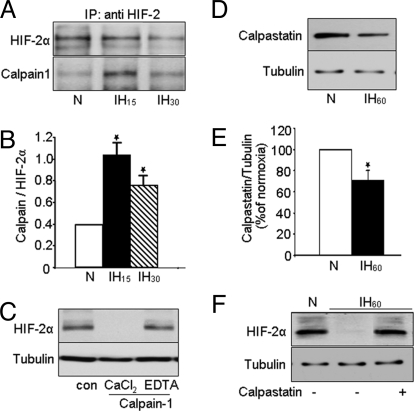
Four potential calpain binding sites were identified in HIF-1α, two at the amino terminus and the other two at the carboxy terminus (22). Comparison of the amino acid sequence of HIF-1α and HIF-2α revealed conserved calpain binding sites in the amino terminus of HIF-2α. To test whether calpains interact with HIF-2α, co-immunoprecipitation experiments were performed. For these studies, the effects of IH15 and IH30 were examined because ≈80% of HIF-2α protein was degraded in response to IH60, precluding detection of calpain/HIF-2α complex. In cells exposed to IH15 or IH30, significant amount of HIF-2α/calpain 1 complex was detected (Fig. 4 A and B) compared with normoxic controls. Parallel experiments with calpain 2 revealed inconsistent results (n = 3; data not shown).
Fig. 4.
Evidence for interactions of HIF-2α with calpain 1. (A) PC12 cells were exposed to normoxia or IH15 and IH30. Immunocomplexes precipitated with anti-HIF-2α were analyzed on 6% SDS/PAGE gels and probed with anti-HIF-2α (Top) and anti-calpain-1 (Bottom) antibodies (n = 3). (B) Densitometric analysis of the immunoblots from 3 experiments expressed as ratio of calpain to HIF-2α. (C) HIF-2α protein was analyzed by immunoblot in PC12 cell lysates incubated for 15 min with purified calpain-1 (3 mg/mL) in the presence of 1 mM CaCl2 or 1 mM CaCl2 plus 2 mM EDTA. (n = 3). (D and E) Immunoblot and densitometric data showing decreased calpastatin protein expression in PC12 cells exposed to IH60 (n = 5) compared with cells exposed to normoxia (N). (F) Representative example of an immunoblot showing that 2 μM calpastatin rescues IH60-evoked HIF-2α degradation (n = 3). Tubulin expression was determined as control for protein loading in C, D, and F.
To further establish that HIF-2α is a substrate for calpain 1, cell lysates from normoxic PC12 cells were incubated with purified calpain 1 (3 mg/mL) in the presence of CaCl2 (1 mM) with and without the Ca2+ chelator EDTA (2 mM). HIF-2α was completely degraded when cell lysate was incubated as briefly as 15 min with calpain 1 and CaCl2; addition of EDTA prevented this effect (Fig. 4C). These results, taken together with co-immunoprecipitation studies, demonstrate that HIF-2α is a substrate for calpain 1 and suggest that calpain activation contributes to HIF-2α degradation by IH.
IH Down-Regulates Calpastatin, Endogenous Inhibitor of Calpains.
In vivo calpain activity is under the regulation of calpastatin, an endogenous inhibitor of calpains (24). To test whether IH affects this endogenous inhibitor, calpastatin protein levels were analyzed by immunoblot in cells exposed to IH60 or normoxia. As shown in Fig. 4 D and E, IH decreased calpastatin levels by 30% ± 4% compared with normoxic controls (P < 0.01). More importantly, pretreating cells with calpastatin (2 μM) completely prevented IH60-evoked HIF-2α degradation (Fig. 4F) and calpain activation (data not shown). These results indicate that calpastatin down-regulation contributes in part to IH-evoked calpain activation and the ensuing HIF-2α degradation.
Down-Regulation of HIF-2α Contributes to IH-Induced Oxidative Stress.
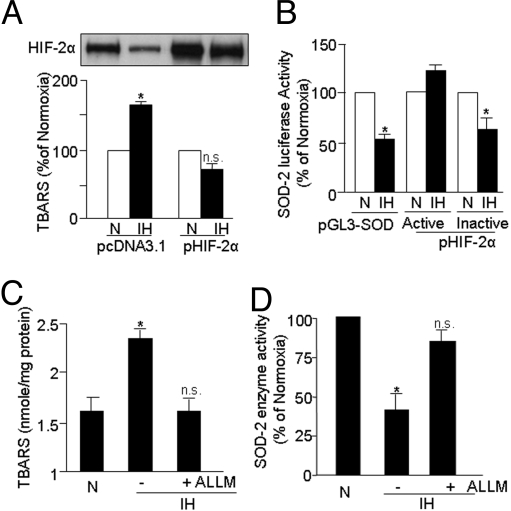
IH induces oxidative stress in cell cultures and in rodents (25). Because HIF-2α deficiency also leads to oxidative stress (26), we hypothesized that HIF-2α down-regulation contributes to IH-evoked oxidative stress. To test this possibility, PC12 cells were transfected with either pHIF-2α (i.e., over-expression) or empty vector (i.e., control) expression plasmid, and then were exposed to either IH60 or normoxia. The levels of thio-barbituric acid reactive substances (TBARS) were monitored as an index of oxidative stress along with HIF-2α protein levels. As shown in Fig. 5A, in cells transfected with an empty vector, IH resulted in elevated TBARS levels and down-regulation of HIF-2α. In contrast, in cells over-expressing HIF-2α, TBARS levels were unchanged by IH60 and HIF-2α degradation was less pronounced compared with cells transfected with empty vector.
Fig. 5.
Down-regulation of HIF-2α contributes to IH-induced oxidative stress as a result of decreased SOD2 promoter trans-activation and enzyme activity. (A) Over-expression of HIF-2α prevents IH-evoked oxidative stress and HIF-2α down-regulation in PC12 cells. TBARS levels were monitored as an index of oxidative stress in cells transiently transfected with an empty vector (pCDNA3.1; control) or pHIF-2α plasmid, and then exposed to normoxia (N) or to IH60. (B) Transcriptionally active HIF-2α prevents IH-induced inhibition of SOD2 promoter activity. SOD2 promoter activity was determined in PC12 cells transiently transfected with the reporter plasmid pGL3-SOD-Luciferase and co-transfected with pIRES-WT HIF2 (transcriptionally active) and pIRES-IA HIF2 (transcriptionally inactive) expression plasmids. Cells were exposed to normoxia (N) or IH60 and SOD2 promoter activity was determined by luciferase activity in cell lysates. (C and D) Treatment of cells with the calpain inhibitor ALLM (10 μM) prevents the IH60-evoked increase in TBARS (index of oxidative stress) as well as the decrease in SOD2 enzyme activity. Control cells were exposed to normoxia (N). Data presented are mean ± SEM. from 3 to 5 experiments. *P < 0.05; n.s., not significant, P > 0.05.
Oxidative stress is a consequence of increased generation of reactive oxygen species (ROS) by pro-oxidants and/or of decreased degradation of ROS by anti-oxidants including anti-oxidant enzymes (AOEs). A previous study reported that HIF-2 activates the major AOE genes including copper/zinc and manganese superoxide dismutases (Cu/ZnSOD and MnSOD encoded by SOD1 and SOD2, respectively), catalase (CAS1), and glutathione peroxidase 1 (GPX1) (26). Analysis of the AOE activities in IH60-exposed PC12 cells revealed significant decrease in SOD2 activity (60% ± 5%; P < 0.01; n = 5), whereas other AOE activities showed either no change (CAS1 and GPX; P > 0.05; n = 5) or inconsistent effects (SOD1; P > 0.05; n = 5).
To test whether the decreased SOD2 activity of IH-exposed PC12 cells is caused by transcriptional down-regulation of SOD2, PC12 cells were transfected with a SOD2 promoter linked to a luciferase reporter plasmid or pGL3-basic plasmid (control), and then were exposed either to IH60 or to normoxia. SOD2 promoter activity was down-regulated by 50% ± 1% in cells exposed to IH60; this effect was prevented by co-transfection with a transcriptionally active, but not with an inactive, HIF-2α plasmid (Fig. 5B). These observations suggest that HIF-2α degradation contributes to IH-induced oxidative stress by leading to insufficient induction of SOD2 transcription. A calpain inhibitor, ALLM (10 μM), that prevented HIF-2α degradation also abolished IH-evoked increase in TBARS levels (Fig. 5C) and prevented the inhibition of SOD2 activity in cells exposed to IH60- (Fig. 5D).
HIF-2α Degradation Contributes to IH-Evoked Autonomic Morbidity.
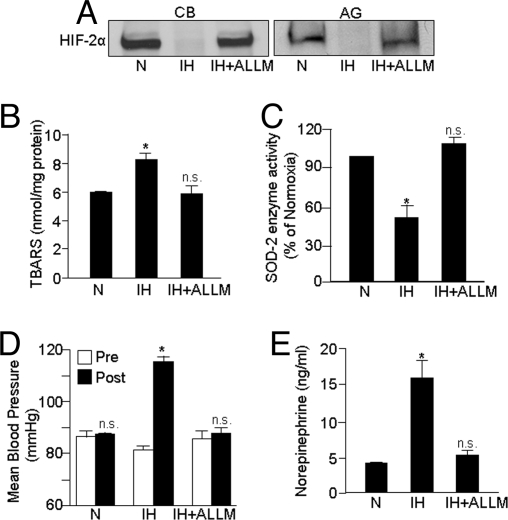
Our cell culture results demonstrate that IH degrades HIF-2α via calpain activation and HIF-2α degradation results in insufficient activation of SOD2, thereby leading to increased oxidative stress. Rodents exposed to IH exhibit hypertension and elevated plasma catecholamines, and anti-oxidant treatment prevents these responses (10, 16). We therefore evaluated whether IH down-regulates HIF-2α in rats exposed to IH and if rescuing HIF-2α degradation by calpain inhibitors prevents IH-evoked oxidative stress and hypertension. Adult rats were systemically treated daily with either ALLM (1 mg/kg/day; i.p) or saline solution (i.e., vehicle) every day for 10 days before they were exposed to IH. HIF-2α expression was determined in the carotid body, the primary chemoreceptor for arterial PO2, which mediates reflex increases in sympathetic nervous system activity, as well as in adrenal medullae, the major source of catecholamines, which contributes to hypertension during IH. As shown in Fig. 6A, HIF-2α was down-regulated in carotid bodies and adrenal medullae of IH-exposed rats, and this effect was prevented by treating IH-exposed rats with ALLM. Similarly, ALLM treatment prevented IH-induced increases in TBARS levels and decreases in SOD2 activity in adrenal medullae (Fig. 6 B and C). More importantly, arterial blood pressures and plasma norepinephrine levels, which are significantly elevated in IH-exposed rats, were restored to control values following ALLM treatment (Fig. 6 D and E).
Fig. 6.
Systemic administration of the calpain inhibitor ALLM prevents HIF-2α degradation, oxidative stress, inhibition of SOD2 activity, and autonomic dysfunction in rats exposed to IH. Adult rats were exposed to 10 days of IH and were treated daily with either saline solution (IH) or ALLM (1 mg/kg/d i.p; IH+ALLM). Control experiments were performed on rats exposed to normoxia (N). ALLM prevented the following IH-evoked changes: (A) HIF-2α degradation in carotid bodies (CB) and adrenal glands (AG), (B and C) Elevations in TBARS (index of oxidative stress) levels and SOD-2 enzyme activity in adrenal medullae, and (D and E) Increases in arterial blood pressure and plasma norepinephrine levels. Data presented in B–E are mean ± SEM from 8 rats in each group. *P < 0.05; n.s., not significant, P > 0.05.
Discussion
Our results demonstrate diametrically opposed effects of IH on HIF-2α and HIF-1α. Upon IH exposure, HIF-1α was up-regulated and HIF-2α was down-regulated. The observed effects are remarkable considering that HIF-2α is an orthologue of HIF-1α. The effects of IH can be elicited not only in cell cultures, but also in tissues from IH-exposed rats. The changes in HIF-2α protein levels were stimulus-dependent and reversed slowly after terminating IH. The effects are unique to IH because CH up-regulated both HIF-1α and HIF-2α in cell cultures. Thus, IH has a differential effect upon these two structurally related HIF transcriptional activators.
Although both IH and CH lead to HIF-1α accumulation, the signaling mechanisms associated with IH requires complex Ca2+signaling involving phospholipase Cγ, PKC, and mammalian target of rapamycin activation (15). Activation of NADPH oxidase and the ensuing elevation of [Ca2+]i are upstream signaling events for triggering IH-evoked changes in HIF-1α (15). The present results also demonstrate a critical role for Ca2+ signaling in IH-evoked HIF-2α degradation. However, unlike HIF-1α accumulation that involves PKC activation, IH-induced HIF-2α degradation is mediated by activation of Ca2+activated proteases, calpain(s), via down-regulation of an endogenous inhibitor, calpastatin. Whether IH-evoked calpain activation also requires NADPH oxidase remains to be determined.
From the co-immunoprecipitation experiments, calpain 1, which is activated by nano- to micromolar Ca2+, appears more important for IH-evoked HIF-2α degradation than calpain 2, which requires millimolar Ca2+. Why do calpain(s) selectively target HIF-2α rather than HIF-1α under IH conditions? Basal expression of HIF-2α is high in PC12 cells (Fig. 1). We speculate that, during IH exposure, Ca2+-evoked calpain activation depletes preexisting HIF-2α protein before de novo HIF-1α protein accumulation via complex Ca2+ signaling and mammalian target of rapamycin activation (15).
We propose that calpain-induced down-regulation of HIF-2α contributes to IH-evoked oxidative stress, at least in part via transcriptional down-regulation of the HIF-2 target gene SOD2. Our observation that calpain inhibitors restore HIF-2α protein levels and prevent oxidative stress in cell culture supports this model. Studies on rodent models revealed that oxidative stress and the ensuing ROS-mediated signaling accompanying IH is critical for much of the IH-evoked autonomic morbidities including hypertension (25). Prevention of the autonomic changes in IH-exposed rats by calpain inhibitors confirm the findings from cell culture studies and further demonstrate that IH-induced HIF-2α degradation has important functional consequences in intact animals.
A substantial population of adult humans and premature infants experience IH as a consequence of sleep-disordered breathing (1–4). The present observations on the effects of IH on HIF-2α, taken together with earlier observations on HIF-1α-deficient mice (16), provide new insights into the molecular mechanism(s) underlying IH-evoked oxidative stress and the resulting cardiovascular changes associated with recurrent apneas. IH is also encountered under several other clinical scenarios including ischemia/re-perfusion injury, in which ROS signaling and oxidative stress also play an important pathophysiological role (27). Whether HIF-2α inactivation contributes to increased generation of ROS and oxidative stress under these conditions remains to be determined.
Methods
Exposures of Cell Cultures and Rats to IH.
PC12 cells were cultured in Dulbecco modified Eagle medium supplemented with 10% horse serum, 5% FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) under 10% CO2 and 90% air (20% O2) at 37 °C. Before all experiments, the cells were serum starved for 16 h in antibiotic-free medium. In the experiments involving treatment with drugs, cells were preincubated for 30 min with either drug or vehicle. Cell cultures were exposed to IH (1.5% O2 for 30 sec followed by 20% O2 for 5 min at 37 °C) as described previously (15). O2 levels were monitored by an electrode (Lazar) placed in the tissue culture medium and ambient O2 levels were monitored by an O2 analyzer (LB2; Beckman). Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed on adult male rats (Sprague-Dawley, 150–200 g). Rats were exposed to 10 d of IH for 8 h per day as described previously (16). In the experiments involving calpain inhibitor, rats were treated with ALLM (Calbiochem, 1 mg/kg dissolved in saline solution; i.p.) or with saline solution (i.e., controls) every day before IH exposure.
Immunoblot Assay.
Cell or tissue extracts (20 μg) were fractionated by 6% polyacrylamide-SDS gel electrophoresis and immunoblots of HIF-1 and 2α were performed as described (15). For co-immunoprecipitation experiments, cells were lysed in RIPA buffer and incubated with anti-HIF-2α antibody overnight at 4 °C. The immune complexes were precipitated using protein A/G agarose beads (Santa Cruz Biotechnology) and dissolved in 2× SDS lysis buffer. Samples were analyzed on 6% SDS/PAGE gels and immunoblotted with anti-HIF-2α or calpain-1 or 2 antibodies as described (15).
Real-Time RT-PCR Assay.
PC12 cell samples exposed to normoxia or IH were used to generate total RNA followed by first-strand cDNA. Aliquots of cDNA were used in quantitative real-time RT-PCR with rat-specific primers detecting either HIF-2 α or β-actin using SYBR as a fluorogenic binding dye as described previously (26).
Measurements of Calpain Activity.
Calpain activity was measured using an Innozyme calpain 1/2 Kit (CBA054; Calbiochem) per manufacturer's instructions. Briefly, following IH exposure, cells were lysed in 50 mM Tris-Cl (pH 7.4), 0.5 mM EDTA, 0.05% β-mercaptoethanol, 1 mm PMSF, and protease inhibitor mixture (Sigma), and calpain activity was measured using purified calpain as standard. Values were normalized to protein content and expressed as percent of normoxic controls.
Measurements of TBARS.
TBARS levels were determined essentially as described previously (16). Malondialdehyde was used as standard. The values were normalized to protein and expressed as nmol/mg protein.
Measurements of AOE Activities.
Mitochondrial and cytosol fractions were isolated from cells or tissue extracts by differential centrifugation as described (28). Superoxide dismutase activity was measured in the cytosol (SOD1) and mitochondrial (SOD2) fractions using an SOD assay kit (WST, S311–10; Dojondo Molecular Technologies). The SOD activity was calculated per milligram of protein and expressed as inhibition rate percent per the manufacturer's instructions. Glutathione peroxidase (703102) and catalase (707002) activities were measured using enzyme assay kits (Cayman Chemical). Protein concentration was estimated using a Bio-Rad protein assay kit. The enzyme activities were expressed as nanomoles per minute per milligram of protein.
SOD-2 Luciferase Reporter Gene Assay.
Plasmids pGL3-basic reporter alone (luc), SOD promoter in pGL3, WT HIF-2α, and inactive HIF-2α expression constructs in pIRES-hrGFP (Stratagene) were described previously (26). Cells were transfected with plasmid DNA using Lipofectamine (Invitrogen) reagent. Transiently transfected cells were starved in serum-free growth medium for 18 h and exposed to either 20% O2 or IH60. Cells were harvested in 100 μL of lysis buffer (100 mM potassium phosphate [pH 7.4], 0.2% Triton X-100, and 1 mM DTT). Relative luminescent light units were recorded in a microplate luminometer (Turner Biosystems) using a Bright-Glo luciferase assay system (Promega). The relative units are calculated, normalized per milligram protein, and expressed as percent of control values.
Measurements of Blood Pressure and Plasma Norepinephrine.
Arterial blood pressure was monitored non-invasively by the tail-cuff method in un-sedated rats as described previously (16). Basal blood pressure was recorded during normoxia. Arterial blood samples were collected from urethane anesthetized rats (1.2 g/kg; i.p.) in heparinized vials (heparin, 30 IU/mL; n = 6). Plasma was separated and norepinephrine was extracted with acid-activated alumina. DHBA (3,4-dihydroxybenzylamine) was used as an internal standard and norepinephrine levels were determined by HPLC/electrochemical detection as described previously (16).
Statistical Analysis.
Data are expressed as mean ± SEM from 3 to 5 independent experiments each performed in triplicate. Statistical analysis was performed by ANOVA, and P values <0.05 were considered significant.
Supplementary Material
Acknowledgment.
This research was supported by National Institutes of Health Heart, Lung and Blood Institute Grants HL-90554 and HL-76537.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811018106/DCSupplemental.
References
- 1.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Marshall NS, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto FJ, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, et al. Sleep-disordered breathing and cardiovascular disease. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Drager LF, et al. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 8.Yaggi HK, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- 11.Savransky V, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. Essays Biochem. 2007;43:1–15. doi: 10.1042/BSE0430001. [DOI] [PubMed] [Google Scholar]
- 15.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng YJ, et al. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 18.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmquist-Mengelbier L, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Appelhoff RJ, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich P. The intriguing Ca2+ requirement of calpain activation. Biochem Biophys Res Commun. 2004;323:1131–1133. doi: 10.1016/j.bbrc.2004.08.194. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Köhl R, Herr B, Frank R, Brüne B. Calpain mediates a von Hippel-Lindau protein-independent destruction of hypoxia-inducible factor-1alpha. Mol Biol Cell. 2006;17:1549–1558. doi: 10.1091/mbc.E05-08-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikebe T, Takeuchi H, Jimi E, Beppu M, Shinohara M, Shirasuna K. Involvement of proteasomes in migration and matrix metalloproteinase-9 production of oral squamous cell carcinoma. Int J Cancer. 1998;77:578–585. doi: 10.1002/(sici)1097-0215(19980812)77:4<578::aid-ijc18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Wendt A, Thompson VF, Goll DE. Interaction of calpastatin with calpain: a review. Biol Chem. 2004;385:465–472. doi: 10.1515/BC.2004.054. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 26.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 27.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JC, Clark JB. Preparation of synaptic and nonsynaptic mitochondria from mammalian brain. Methods Enzymol. 1979;55:51–60. doi: 10.1016/0076-6879(79)55008-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.