Abstract
This study demonstrates that in malignant melanoma, elevated levels of nuclear ß-catenin in both primary tumors and metastases correlate with reduced expression of a marker of proliferation and with improved survival, in contrast to colorectal cancer. The reduction in proliferation observed in vivo is recapitulated in B16 murine melanoma cells and in human melanoma cell lines cultured in vitro with either WNT3A or small-molecule activators of ß-catenin signaling. Consistent with these results, B16 melanoma cells expressing WNT3A also exhibit decreased tumor size and decreased metastasis when implanted into mice. Genome-wide transcriptional profiling reveals that WNT3A up-regulates genes implicated in melanocyte differentiation, several of which are down-regulated with melanoma progression. These findings suggest that WNT3A can mediate transcriptional changes in melanoma cells in a manner reminiscent of the known role of Wnt/ß-catenin signaling in normal melanocyte development, thereby altering melanoma cell fate to one that may be less proliferative and potentially less aggressive. Our results may explain the observed loss of nuclear ß-catenin with melanoma progression in human tumors, which could reflect a dysregulation of cellular differentiation through a loss of homeostatic Wnt/ß-catenin signaling.
Keywords: differentiation, prognosis, metastasis, WNT5A, B16 model, microarray
Malignant melanoma accounts for <5% of all skin cancers, yet is responsible for 80% of skin cancer deaths (1). The outlook for patients with metastatic melanoma remains quite bleak, with a 5-year survival rate of only 5%–15% that has not changed significantly over decades despite intensive efforts to develop an effective therapy. Although the molecular mechanisms underlying the formation and progression of melanoma remain unresolved, recent studies have implicated Wnt signal transduction pathways in melanoma biology (2), raising the question of whether this insight can be used to develop a therapy.
Wnt genes encode a family of 19 secreted glycoproteins that act as ligands to activate receptor-mediated signaling pathways that control cell fate and differentiation, cell proliferation, and cell motility (3). The extensively characterized Wnt/ß-catenin pathway inhibits the degradation of ß-catenin, leading to its accumulation in the nucleus and to regulation of target gene expression (3). Vertebrates also have at least one other Wnt signaling pathway, often referred to as “noncanonical” Wnt signaling, that uses ß-catenin-independent signaling mechanisms and, in some contexts, actively antagonizes ß-catenin signaling (3–5). Both Wnt pathways have been implicated in cancer. Specifically, mutations leading to constitutive activation of Wnt/ß-catenin signaling are observed in colorectal cancer and in some kidney tumors (6–9), where activation of the pathway has been directly implicated in disease pathogenesis. This finding, coupled with the initial identification of vertebrate Wnt1 as an oncogene in a breast cancer screen (10) and with studies demonstrating the activation of this pathway in other cancers, has promoted the idea that elevated Wnt/ß-catenin signaling is oncogenic in most contexts (3). In colorectal carcinoma, the majority of tumors exhibit constitutive activation of Wnt/ß-catenin signaling through activating mutations of the adenomatous polyposis coli (APC) gene, and the presence of increased nuclear ß-catenin has been shown to predict cancer progression to metastasis, as well as decreased patient survival (11–14). Interestingly, although activating mutations in the ß-catenin pathway are rare in melanoma (15–20), the noncanonical pathway, often activated by WNT5A, has been implicated in melanoma metastasis (21–24).
Melanocytes arise from neural crest cells, a multipotent pool of precursors that also give rise to neuronal, glial, and cartilage lineages (25). Wnt/ß-catenin signaling is necessary and sufficient to drive neural crest cells toward a melanocyte cell fate, in large part through direct regulation of transcriptional targets, such as the homeobox gene microphthalmia transcription factor (MITF) (26–28). The observed presence of nuclear ß-catenin in the majority of benign nevi, along with the loss of nuclear ß-catenin seen with melanoma progression (29–31), support the hypothesis that activation of Wnt/ß-catenin signaling is important for cellular homeostasis in this context. Consequently, the dysregulation of specific transcriptional programs in melanocytes and nevus cells through a loss of Wnt/ß-catenin signaling may contribute to the lower survival seen in patients with tumors that lack nuclear ß-catenin (29–31).
In the present study, we found that elevated levels of nuclear ß-catenin correlate with improved survival from melanoma. This finding is paralleled in an established murine melanoma model in which activation of Wnt/ß-catenin by either WNT3A or small molecules leads to decreased proliferation in vitro and decreased tumor size in vivo. We also noted that activation of Wnt/ß-catenin signaling promotes the expression of markers of melanocyte differentiation, which correlates with our observed decrease in the expression of proliferation markers in patients with higher levels of nuclear ß-catenin. Interestingly, the same genes activated by Wnt/ß-catenin signaling are antagonized by WNT5A, which may be relevant to disease progression given the observed increased expression of WNT5A in later-stage, more aggressive melanomas (21–24). Together, these findings support the hypothesis that melanoma progression is associated with a loss of Wnt/ß-catenin signaling, leading to dysregulated cell fate and increased proliferation.
Results
Nuclear ß-Catenin Levels Correlate with Improved Patient Survival.
In a tissue microarray composed of 343 melanoma tumor cores (118 primary tumors plus 225 recurrences/metastases), we used immunohistochemical staining followed by automated quantitative analysis (AQUA®) to measure nuclear ß-catenin levels. Fig. 1A shows representative immunofluorescent staining from 2 different tumor cores measured by AQUA (32). The tumor mask was defined by S100 staining, and nuclei were defined by labeling with DAPI. This method provides a clear distinction between nuclear and cytoplasmic/membranous ß-catenin. Nuclear ß-catenin is higher in primary tumors than in metastases (bordering on statistical significance by nonparametric t-test), suggesting that melanoma progression is associated with a loss of Wnt/ß-catenin signaling (supporting information (SI) Fig. S1C).
Fig. 1.
Nuclear ß-catenin levels predict improved survival in melanoma patients. (A) Representative tumors cores from the tissue microarray are shown to illustrate the localization of nuclear ß-catenin by AQUA. Tumor 1 (Upper panels) is representative of tumors with lower nuclear ß-catenin expression, whereas tumor 2 (Lower panels) is representative of tumors with higher nuclear ß-catenin expression. In the left panels, the orientation of the histospot used for analysis is oriented on the tumor core. The middle panels illustrate how S100 and DAPI are used to identify the cytoplasmic/membranous and nuclear compartments of the tumor, respectively. Staining with ß-catenin, shown in the right panels, is co-localized with either S100 or DAPI to generate measured values of ß-catenin staining in each subcellular compartment. (B) Primary tumors (n = 118) were stratified a priori into tertiles based on nuclear ß-catenin level (see also Fig. S1A). Patients with the highest nuclear ß-catenin levels (upper tertile) have a significantly higher survival probability by Kaplan-Meier analysis compared with those in the middle and lower tertiles (log-rank test). (C) Metastatic and recurrent tumors were separated into those with the highest nuclear ß-catenin levels (upper 20%; n = 46) and those with lower nuclear ß-catenin levels (remaining 80%; n = 179). The nuclear ß-catenin levels in the upper 20% of metastatic/recurrent tumors correspond with the levels seen in the upper tertile of primary tumors (Fig. S1B). Kaplan-Meier analysis demonstrated a significantly increased survival probability in patients with the highest nuclear ß-catenin levels (Gehan-Breslow-Wilcoxon test).
Survival probabilities were derived using Kaplan-Meier analysis after primary tumors were stratified into tertiles based on nuclear ß-catenin expression (Fig. S1A), with the upper tertile corresponding to tumors in the highest third of nuclear ß-catenin expression and the lower tertile corresponding to tumors in the lowest third of nuclear ß-catenin expression. By using these AQUA-scored tumor samples to correlate different levels of nuclear ß-catenin with survival, we found that higher expression of nuclear ß-catenin in primary tumors predicts increased survival (Fig. 1B). In addition, metastases and recurrences with the highest nuclear ß-catenin levels (Fig. S1B) also are associated with a higher survival probability (Fig. 1C). These findings provide an initial survival analysis in metastatic melanoma based on nuclear ß-catenin levels and, more importantly, suggest that even in advanced disease, activation of Wnt/ß-catenin signaling still provides a survival benefit. A detailed comparison of these results in the context of previously published analyses of nuclear ß-catenin levels in melanoma is presented in SI Discussion.
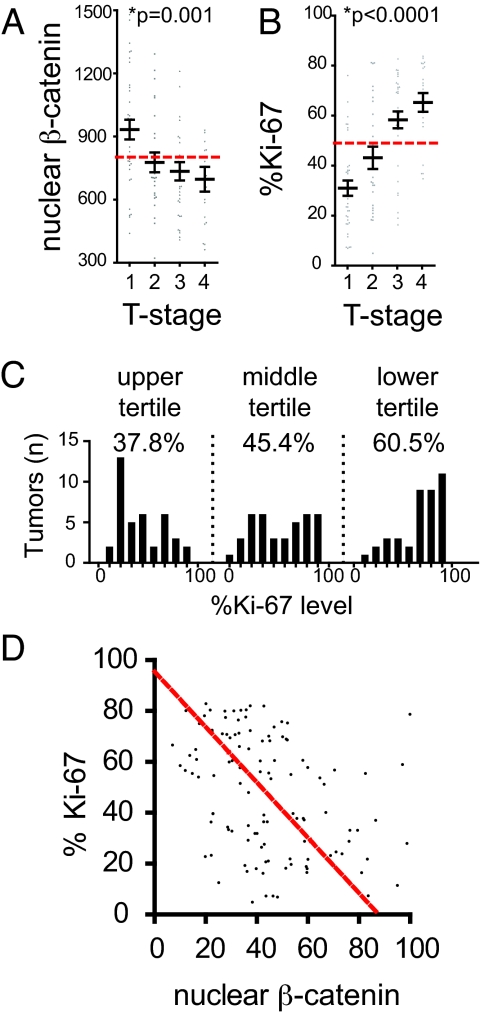
Because we had tumor depth measurements (Breslow thickness) for 113 primary tumors in our array cohort, we analyzed this subgroup of patients based on the Breslow thickness stratification used in the 2002 American Joint Committee on Cancer (AJCC) melanoma staging criteria (33). Survival analysis correlates well with AJCC tumor staging by Breslow thickness (Fig. S1D). We found a significant decrease in nuclear ß-catenin levels with increasing tumor thickness (Fig. 2A). In contrast, the tumor proliferation index, as measured by the Ki-67 marker, increased with more advanced tumor staging (Fig. 2B).
Fig. 2.
Activated Wnt/ß-catenin signaling is associated with decreased proliferation. (A) Nuclear ß-catenin level was measured in primary tumors staged by Breslow depth according to AJCC criteria. Tumor depth increases from T1 to T4. Bars representing the mean and SEM for tumors in each stage indicate decreasing nuclear ß-catenin level with increasing tumor depth. (B) In comparison, the proliferative index of tumors, as measured by %Ki-67, increases with tumor depth. Gray dots represent individual tumors, and the dotted red line indicates the average for the entire cohort. The changes in nuclear ß-catenin and Ki-67 were highly significant by ANOVA followed by a posttest for linear trend. (C) Histograms binned by 10% increments reveal the distribution of %Ki-67 within tumors stratified into tertiles by nuclear ß-catenin level. Note the increased number of tumors with higher %Ki-67 in the presence of lower nuclear ß-catenin levels (lower tertile), compared with the larger number of tumors with lower %Ki-67 seen in the presence of higher nuclear ß-catenin levels (upper tertile). The mean %Ki-67 for each tertile (shown above the histograms) increased significantly with lower expression of nuclear ß-catenin (*P < 0.0001 by ANOVA with posttest for linear trend). (D) Levels of nuclear ß-catenin and %Ki-67 in individual tumors were analyzed by Deming regression, revealing a slope of -1.089 ± 0.2374, suggesting that higher levels of nuclear ß-catenin are associated with decreased %Ki-67. In contrast, Deming regression comparing α-catenin and %Ki-67 revealed a slope not significantly different from zero (Fig. S2B).
Strikingly, distribution histograms of %Ki-67 staining in primary tumors stratified by nuclear ß-catenin expression showed a statistically significant shift toward increased proliferation (elevated %Ki-67 staining) in the groups with lower nuclear ß-catenin levels (Fig. 2C). No significant difference in the distribution of α-catenin was seen within these tertiles (Fig. S2A). Ki-67 exhibited similar results within tumors to another proliferative marker, PCNA (Fig. S2C). A Deming regression of nuclear ß-catenin and %Ki-67 within primary tumors is shown in Fig. 2D (slope = -1.089 ± 0.2374; P < .0001). In contrast, no relationship was found between α-catenin expression and %Ki-67 staining (Fig. S2B). Together, our data from patient tumors support a model in which activation of Wnt/ß-catenin signaling is associated with decreased proliferation.
Activation of Wnt/ß-Catenin Signaling Is Correlated with Decreased Proliferation of Melanoma Cells.
We next investigated whether Wnts elicit changes in melanoma cells cultured in vitro that might be consistent with our clinical observations. Because melanoma tumors express WNT3A (Fig. S3A), which plays a pivotal role in the regulation of melanocyte biology (28, 34), as well as WNT5A, which is elevated in melanoma metastases (21–24), we transduced B16-F1 mouse melanoma cells with lentivirus constructs encoding WNT3A, WNT5A, or a GFP control. We designate the resulting cell lines B16:GFP, B16:WNT3A, and B16:WNT5A, respectively.
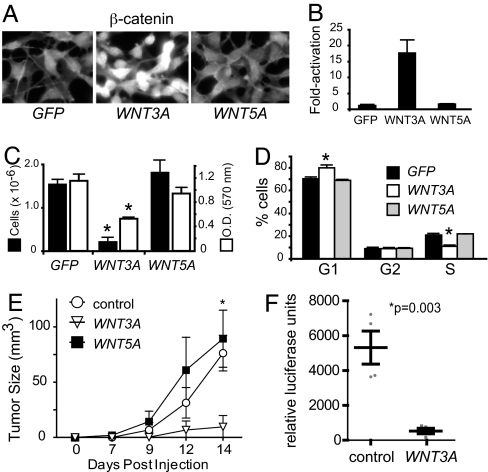
Scoring cells for nuclear accumulation of ß-catenin revealed that only B16:WNT3A cells, not B16:WNT5A or B16:GFP cells, exhibit elevated ß-catenin (Fig. 3A). Expression of WNT5A was confirmed by immunoblot analysis (data not shown). As a positive control, we noted that conditioned media (CM) from B16:WNT3A cells, but not from B16:WNT5A cells or B16:GFP cells, activate a ß-catenin–responsive reporter in UACC1273 melanoma cells (Fig. 3B), confirming that B16:WNT3A cells secrete active WNT3A. We then found that B16:WNT3A cells exhibit marked up-regulation of the ß-catenin target gene Axin2 (35) compared with B16:GFP and B16:WNT5A cells (Fig. 4).
Fig. 3.
Wnt/ß-catenin activation is associated with decreased proliferation in melanoma. (A) Immunofluorescent staining demonstrates increased nuclear ß-catenin in B16 cells expressing WNT3A, but not in cells expressing either GFP or WNT5A. (B) CM from B16:WNT3A cells activated a Wnt/ß-catenin reporter in UACC1273 human melanoma cells, indicating that these cells secrete active WNT3A. No activation was seen with CM isolated from B16:GFP or B16:WNT5A cells. (C) Proliferation of B16:GFP, B16:WNT3A, or B16:WNT5A cells was measured by hematocytometry after 6 days of culture (black bars, left y-axis) or by MTT assay after 3 days of culture (white bars, right y-axis). Bars represent the average and standard deviation of 3–6 biological replicates. The inhibition of proliferation seen with WNT3A cells was highly significant by ANOVA with both proliferation assays (*P < 0.001). (D) In cell cycle analysis, B16:WNT3A cells were decreased in S phase and increased in G1 compared with B16:GFP or B16:WNT5A cells. Bars indicate the average and standard deviation of 3 biological replicates, and the data shown are representative of 5 individual experiments, each with at least 3 biological replicates per condition. The changes observed in B16:WNT3A cells are extremely significant by ANOVA (*P < 0.001). (E) Tumor grafts demonstrate that B16 cells expressing WNT3A form smaller tumors than cells expressing GFP or WNT5A. Data are expressed as mean and standard deviation from 4 mice for each cell line tested. The experiment shown is representative of 4 independent experiments with the same result, with each experiment involving at least 4 mice for each cell line tested. The decrease in tumor size with WNT3A was found to be highly significant by ANOVA at 14 days postimplantation (*P = 0.004). (F) Metastases to the popliteal sentinel lymph node bed were evaluated by firefly luciferase assay, demonstrating significantly decreased metastases in tumors expressing WNT3A. Bars represent mean and standard deviation, and the data shown are representative of 4 independent experiments.
Fig. 4.
Activation of Wnt/ß-catenin signaling alters cell fate in melanoma cells. (A) B16:GFP, B16:WNT3A, or B16:WNT5A cells were isolated at equivalent confluency, spun down, and photographed in a 96-well plate, demonstrating the marked difference in pigmentation seen in melanoma cells expressing WNT3A. Increased pigmentation also was seen in B16 cells treated for 4 days with WNT3A-conditioned L-cell media (L-WNT3A) compared with control L-cell media (L-control). (B) Whole genome expression profiles of B16:WNT3A or B16:WNT5A cells were initially compared with gene expression in B16:GFP cells, with 3 biological replicates analyzed for each cell line. The heatmap illustrates the differences between the most significant regulated genes in B16:WNT3A cells compared with B16:WNT5A cells by subsequent unpaired t-test. Genes that were among the most significantly regulated in B16:WNT3A cells are listed with their normalized fold-change [log (2)] compared with B16:GFP cells shown in parentheses; these include known Wnt/ß-catenin targets, genes involved in melanocyte and neural crest differentiation, and genes implicated in melanoma prognosis or therapy. (C) Various genes were selected for validation using quantitative RT-PCR (qRT-PCR), including genes implicated in melanocyte differentiation (Met, Kit, Sox9, Mitf, Si/Gp100) and melanoma biology (Trpm1, Kit, Mme, Mlze), as well as known Wnt target genes (Axin2, Met, Sox9). All genes that were up-regulated in B16:WNT3A cells by transcriptional profiling were up-regulated by qRT-PCR, whereas genes that were down-regulated in B16:WNT3A cells on the array (Mlze, Mme) also were down-regulated by qRT-PCR. Genes up-regulated in B16:WNT3A cells were universally down-regulated in the B16:WNT5A cells, providing evidence that WNT5A can antagonize transcription of Wnt/ß-catenin gene targets in melanoma cells, even in the absence of WNT3A. Data are expressed as log (2)–transformed fold-change (with standard error) compared with B16:GFP cells and are representative of 3 or more experiments with similar results. (D) Gene changes induced in B16:WNT3A cells were antagonized on treatment with ß-catenin siRNA (20 nM) compared with control siRNA (20 nM). Data are expressed as log (2)–transformed fold-change (with standard error) in cells treated with ß-catenin siRNA compared with control siRNA.
In vitro cell proliferation studies using the MTT cell proliferation assay or manual cell counts showed that B16:WNT3A cells exhibit significantly decreased proliferation compared with B16:GFP or B16:WNT5A cells (Fig. 3C). This finding was paralleled in human cell lines (Fig. S3B and C). Cell cycle analysis revealed that B16:WNT3A cells exhibit an increased population in G1, with a decreased population in S phase, compared with either B16:GFP or B16:WNT5A cells (Fig. 3D). Furthermore, in vivo tumor grafts of B16:WNT3A cells exhibit decreased tumor growth (Fig. 3E) compared with either B16:GFP or B16:WNT5A cells, along with decreased metastasis (Fig. 3F). No differences in apoptosis were seen in tumor grafts by TUNEL staining (data not shown). Together, these data suggest that activation of Wnt/ß-catenin signaling correlates with decreased proliferation of melanoma cells.
Activation of Wnt/ß-catenin signaling by the GSK-3 inhibitors lithium chloride or 6-bromoindirubin-3′-oxime (BIO) also results in decreased proliferation of cultured B16 (Fig. S4) and human melanoma cells (Fig. S3D), supporting the hypothesis that decreased proliferation in these cells is due to activation of Wnt/ß-catenin signaling. Our observation that this decreased proliferation cannot be appreciably rescued by either the soluble antagonist Dickkopf-1 (DKK1) or ß-catenin–targeted siRNA (Fig. S5) suggests that this cellular change may reflect a commitment to an altered cell fate, which would be consistent with Wnt/ß-catenin signaling regulating cell fate in other contexts as well (36).
Activation of Wnt/ß-Catenin Signaling Up-Regulates Markers of Melanocyte Differentiation.
We found that activation of Wnt/ß-catenin signaling in B16-F1 melanoma cells by either lentiviral transduction of WNT3A or treatment of cells with CM containing WNT3A results in increased pigmentation not seen in cells transduced with lentivirus encoding GFP or WNT5A (Fig. 4A, Left) or cells treated with control CM (Fig. 4A, Right). These phenotypic changes, coupled with the observed decreased proliferation and altered cell cycle profile (Fig. 3 C and D), led us to hypothesize that activation of Wnt/ß-catenin signaling leads to changes in cell fate.
To test a prediction of this hypothesis, we performed genome-wide transcriptional profiling to determine whether expression of WNT3A in lentiviral-transduced B16-F1 cells alters the expression of genes reflecting cell fates. Using gene expression in B16:GFP cells as a reference, we established profiles of genes regulated in B16:WNT3A and B16:WNT5A cells, and then further identified genes that exhibited the highest variance between these 2 groups to focus on genes regulated by WNT3A. Among the most highly significant genes elevated by WNT3A (Fig. 4B) are Axin2 (35) and Tcf7 (37), which are direct targets of Wnt/ß-catenin signaling; Mme and Mlze, down-regulated genes previously linked to melanoma progression (38, 39); Mitf, which is linked to pigment cell fate; and Trpm1, Met, Sox9, and Kit, which are highly expressed during melanocyte and neural crest development (40). The complete list of significantly regulated genes is presented in Dataset S1. To confirm the array data, we measured levels of selected transcripts by quantitative RT-PCR (Fig. 4C). We found that changes in gene expression are antagonized by ß-catenin siRNA, confirming that the effects of WNT3A on gene expression are specific (Fig. 4D). Thus, the transcriptional profiling supports the hypothesis, evident from visual examination of cells (Fig. 4A), that activation of Wnt/ß-catenin signaling by WNT3A promotes the adoption of characteristics of melanocyte differentiation by melanoma cells.
Previous studies reporting increased expression of WNT5A observed in later-stage, more aggressive melanomas have focused on the potential role of noncanonical Wnt signaling in regulating cell motility (21–24, 41, 42). Besides regulating cell motility, WNT5A antagonizes Wnt/ß-catenin signaling in developmental models and other contexts (4). Consistent with this activity, we found that WNT5A expression antagonizes the expression of genes regulated by WNT3A (Fig. 4C). This finding suggests that the antagonism of Wnt/ß-catenin signaling by WNT5A may contribute to the loss of Wnt/ß-catenin homeostasis seen with melanoma progression.
Discussion
The exact role of Wnt/ß-catenin signaling in melanoma remains controversial, although previous reports suggesting that activation of Wnt/ß-catenin signaling can promote proliferation in different cancers, including in cultured melanoma cells (36, 43), has led to speculation that this pathway may be involved in early aspects of melanoma formation and progression through regulation of tumor growth. In support of the idea that Wnt/ß-catenin activation does not necessarily promote proliferation in melanocytic cells, a recent study found that forced transgenic expression of a stabilized constitutively active ß-catenin mutant (ß-catSTA) in mice did not increase proliferation of melanocytes or precursor melanoblasts (44). That study also found that restricting the expression of ß-catSTA to melanocytes, in the absence of activated Nras, did not lead to any melanomas over a 2-year period; however, the presence of both ß-catSTA and activated Nras led to melanomas with high penetrance and short latency, suggesting that in the context of this model, activation of Wnt/ß-catenin signaling can potentially promote melanoma formation (44). Because activating mutations such as ß-catSTA are rare in melanoma (15–20), and because the great majority of benign nevi do not progress to melanoma despite exhibiting nuclear ß-catenin (29), this model may not entirely recapitulate the role of Wnt/ß-catenin signaling in human melanoma development and progression. In addition, this model does not explain the observed increased survival (Fig. 1) seen in patients with tumors exhibiting higher nuclear ß-catenin levels (29), which is accompanied by decreased, not increased, proliferation (Fig. 2).
Furthermore, our transcriptional profiling reveals that activation of the Wnt/ß-catenin pathway by WNT3A led to up-regulation of Trpm1, Kit, Met, and Mlana. These genes, which are associated with normal melanocyte differentiation, were recently identified as part of a transcriptional signature that is lost in aggressive melanomas compared with normal melanocytes (45), supporting a model in which the loss of Wnt/ß-catenin–regulated genes is associated with both de-differentiation and melanoma progression. Consistent with this hypothesis, the loss of TRPM1 has been linked to decreased survival and increased risk of metastasis (46–48), and the ability of Wnt/ß-catenin signaling to rescue the transcriptional regulation of TRPM1 and other genes lost with melanoma progression provides further evidence that loss of Wnt/ß-catenin homeostasis may play a direct role in melanoma progression. Our findings also suggest that WNT5A, which is expressed at higher levels with melanoma progression (21–24), may directly contribute to this dysregulation by antagonizing Wnt/ß-catenin transcriptional targets.
Given the established role of Wnt/ß-catenin signaling as a main regulator of cell fate in the melanocytic lineage, our results invite speculation that WNT3A may be altering the differentiation of melanoma cells in this model. This hypothesis is supported by our observations that melanoma cells expressing WNT3A exhibit properties suggestive of more highly differentiated melanocytic cells, including (i) a high degree of pigmentation; (ii) alterations in the cell cycle, leading to decreased proliferation; (iii) up-regulation of melanocytic genes; and (iv) formation of smaller tumors in mice. The concept of manipulating the differentiation of tumor cells in melanoma and other cancers is not new (49), and in fact intensive research has focused on markers to identify potential melanoma cancer stem cells, because this population provides an ideal target for such a therapeutic strategy (50). The forced differentiation of these tumor-initiating cells would ideally promote cell fates that are more benign (i.e., slower growing or less metastatic) or, alternatively, more treatable.
Methods
Cell Lines.
B16-F1 murine melanoma cells expressing firefly luciferase were used as the parental line for experiments described in this report (51). Human melanoma UACC1273 and M93047 cell lines were a generous gift from Dr. Ashani Weeraratna (National Institute of Aging, Baltimore, MD) (24). The human melanoma cell lines A375, A2058, Mel 29.6, and Mel501 were a generous gift from Cassian Yee (Fred Hutchinson Cancer Research Institute, Seattle, WA). Sequences for human WNT3A and WNT5A were amplified by PCR and cloned into third-generation lentiviral vectors derived from backbone vectors that were a generous gift of Dr. Luigi Naldini (52). These lentiviral vectors contain an EF1-alpha promoter driving a bi-cistronic message encoding human Wnt isoforms plus GFP. Cells were sorted by FACS for GFP expression, with the goal of obtaining cells with approximately equivalent levels of GFP expression.
Cell Culture.
B16 murine melanoma cells and human melanoma lines were cultured in DMEM supplemented with 2% FBS and 1% antibiotic/antimycotic (Invitrogen) (51). All cell lines were cultured in the presence of 0.02% Plasmocin (InvivoGen). Synthetic siRNAs (Invitrogen) were transfected into cultured cells at a final concentration of 20 nM using Lipofectamine 2000 (Invitrogen). For validation experiments using ß-catenin siRNA in mouse melanoma cells, a total of 20 nM siRNA was used, consisting of an equimolar mix of 2 sequences, CUGUCUGUCUGCUCUAGCA(dTdT) and CUGUUGGAUUGAUUCGAAA(dTdT).
CM and Measurement of Wnt Pathway Activation Using a Reporter Assay.
CM was collected from subconfluent melanoma cell lines and tested for its ability to activate Wnt/ß-catenin signaling in UACC1273 cells stably transduced with a previously described Wnt/ß-catenin–responsive firefly luciferase reporter and a constitutive Renilla luciferase gene used for normalization (7). CM from B16 melanoma cells was spun down to clear cell debris and then incubated with reporter cells overnight. Activation of the Wnt/ß-catenin reporter was measured using a dual luciferase reporter assay kit (Promega).
Cell Proliferation Assays.
For cell counts by hematocytometry, cells were seeded at a uniform density (usually between 10,000 and 25,000 cells per well) in 12- or 24-well tissue culture plates in the appropriate media. After 3–7 days, the cells were trypsinized, resuspended in the appropriate media, and counted. Dead cells were identified by staining with 0.4% Trypan blue and excluded from hematocytometry measurements. Cell proliferation experiments were performed with a minimum of 6 biological replicates. Similar results were observed for all cell lines using the MTT assay (ATCC), performed according to the manufacturer's protocol. Cell cycle analysis was performed using DAPI staining and flow cytometry. For experiments using lithium chloride or BIO, cells were treated for 24–72 h before the MTT assay.
Immunohistochemistry and Immunoblot Studies.
These protocols were performed using standard techniques. Specific antibodies and dilutions used are described in detail in SI Methods.
Genome-Wide Transcriptional Profiling with Agilent Microarrays and PCR Validation.
RNA isolated by standard methods was labeled and analyzed by Agilent whole mouse genome 2-channel arrays, using B16:GFP cells as the reference sample. These protocols, including primer sequences for quantitative RT-PCR validation, are described in detail in SI Methods.
Tumor Microarrays and Statistical Analysis.
Tumor microarrays were analyzed similar to previously published protocols (32, 53). These protocols are described in detail in SI Methods.
In Vivo Tumor Inoculation and Measurement of Lymph Node Metastasis.
Footpad injections of transduced B16 melanoma cells and measurement of popliteal lymph node and lung metastasis was performed as described previously (51). All animal studies were performed using Institutional Animal Care and Use Committee protocols as approved by the applicable institutional review boards.
Supplementary Material
Acknowledgments.
E.M. was supported as a Medical Fellow, M.M. as an Associate, and R.M. as an Investigator by the Howard Hughes Medical Institute. A.L. is funded by a National Institutes of Health–German Research Foundation Research Career Transition Award. Research support was provided in part by intramural research funds from the Center for Cancer Research (to S.H. and A.L.). A.C. received funding support through a Research Scholar Award from the American Skin Association, a Career Award from the Dermatology Foundation, and a K08 Career Development Award from the National Cancer Institute. We thank Sancy Leachman, Pamela Cassidy, and Brett Milash at the University of Utah for their assistance in facilitating our microarray studies. We also thank the staff of the University of Washington Department of Biostatistics for their consultative guidance in our statistical analysis.
Footnotes
Conflict of interest statement: D.L.R. is a founder, stockholder, and consultant for HistoRx, the exclusive licensee of the Yale-owned AQUA patent.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811902106/DCSupplemental.
References
- 1.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 2.Weeraratna AT. A Wnt-er wonderland: The complexity of Wnt signaling in melanoma. Cancer Metastasis Rev. 2005;24:237–250. doi: 10.1007/s10555-005-1574-z. [DOI] [PubMed] [Google Scholar]
- 3.Chien AJ, Moon RT. WNTS and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–457. doi: 10.2741/2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidinger G, Moon RT. When Wnts antagonize Wnts. J Cell Biol. 2003;162:753–755. doi: 10.1083/jcb.200307181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veeman MT, Axelrod JD, Moon RT. A second canon: Functions and mechanisms of beta-catenin–independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 6.Rivera MN, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms' tumor. Science. 2007;315:642–645. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- 7.Major MB, et al. Wilms' tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 8.Koesters R, et al. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 1999;59:3880–3882. [PubMed] [Google Scholar]
- 9.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 10.Rijsewijk F, et al. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 11.Lugli A, et al. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: A tissue microarray–based analysis. Histopathology. 2007;50:453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401–1408. doi: 10.1158/1078-0432.ccr-0157-03. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S, et al. Nuclear beta-catenin accumulation as a prognostic factor in Dukes' D human colorectal cancers. Oncol Rep. 2004;12:245–251. [PubMed] [Google Scholar]
- 14.Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer. 2002;95:2479–2486. doi: 10.1002/cncr.10986. [DOI] [PubMed] [Google Scholar]
- 15.Omholt K, Platz A, Ringborg U, Hansson J. Cytoplasmic and nuclear accumulation of beta-catenin is rarely caused by CTNNB1 exon 3 mutations in cutaneous malignant melanoma. Int J Cancer. 2001;92:839–842. doi: 10.1002/ijc.1270. [DOI] [PubMed] [Google Scholar]
- 16.Pollock PM, Hayward N. Mutations in exon 3 of the beta-catenin gene are rare in melanoma cell lines. Melanoma Res. 2002;12:183–186. doi: 10.1097/00008390-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Reifenberger J, et al. Molecular genetic analysis of malignant melanomas for aberrations of the WNT signaling pathway genes CTNNB1, APC, ICAT and BTRC. Int J Cancer. 2002;100:549–556. doi: 10.1002/ijc.10512. [DOI] [PubMed] [Google Scholar]
- 18.Rubinfeld B, et al. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 19.Worm J, Christensen C, Gronbaek K, Tulchinsky E, Guldberg P. Genetic and epigenetic alterations of the APC gene in malignant melanoma. Oncogene. 2004;23:5215–5226. doi: 10.1038/sj.onc.1207647. [DOI] [PubMed] [Google Scholar]
- 20.Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER. Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol. 1999;154:325–329. doi: 10.1016/s0002-9440(10)65278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weeraratna AT, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 22.Weeraratna AT, et al. Generation and analysis of melanoma SAGE libraries: SAGE advice on the melanoma transcriptome. Oncogene. 2004;23:2264–2274. doi: 10.1038/sj.onc.1207337. [DOI] [PubMed] [Google Scholar]
- 23.Da Forno PD, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 24.Bittner M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 25.Crane JF, Trainor PA. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol. 2006;22:267–286. doi: 10.1146/annurev.cellbio.22.010305.103814. [DOI] [PubMed] [Google Scholar]
- 26.Vance KW, Goding CR. The transcription network regulating melanocyte development and melanoma. Pigment Cell Res. 2004;17:318–325. doi: 10.1111/j.1600-0749.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 27.Larue L, Kumasaka M, Goding CR. Beta-catenin in the melanocyte lineage. Pigment Cell Res. 2003;16:312–317. doi: 10.1034/j.1600-0749.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 28.Dorsky RI, Raible DW, Moon RT. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000;14:158–162. [PMC free article] [PubMed] [Google Scholar]
- 29.Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 30.Kageshita T, et al. Loss of beta-catenin expression associated with disease progression in malignant melanoma. Br J Dermatol. 2001;145:210–216. doi: 10.1046/j.1365-2133.2001.04336.x. [DOI] [PubMed] [Google Scholar]
- 31.Maelandsmo GM, Holm R, Nesland JM, Fodstad O, Florenes VA. Reduced beta-catenin expression in the cytoplasm of advanced-stage superficial spreading malignant melanoma. Clin Cancer Res. 2003;9:3383–3388. [PubMed] [Google Scholar]
- 32.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JA. The revised American Joint Committee on Cancer staging system for melanoma. Semin Oncol. 2002;29:361–369. doi: 10.1053/sonc.2002.34115. [DOI] [PubMed] [Google Scholar]
- 34.Fang D, et al. Defining the conditions for the generation of melanocytes from human embryonic stem cells. Stem Cells. 2006;24:1668–1677. doi: 10.1634/stemcells.2005-0414. [DOI] [PubMed] [Google Scholar]
- 35.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 37.Roose J, et al. Synergy between tumor-suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 38.Watabe K, et al. Structure, expression and chromosome mapping of MLZE, a novel gene which is preferentially expressed in metastatic melanoma cells. Jpn J Cancer Res. 2001;92:140–151. doi: 10.1111/j.1349-7006.2001.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bilalovic N, et al. CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression. Mod Pathol. 2004;17:1251–1258. doi: 10.1038/modpathol.3800174. [DOI] [PubMed] [Google Scholar]
- 40.Loftus SK, et al. Informatic selection of a neural crest–melanocyte cDNA set for microarray analysis. Proc Natl Acad Sci U S A. 1999;96:9277–9280. doi: 10.1073/pnas.96.16.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dissanayake SK, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial-to-mesenchymal transition. J Biol Chem. 2007;282:17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widlund HR, et al. Beta-catenin–induced melanoma growth requires the downstream target Microphthalmia-associated transcription factor. J Cell Biol. 2002;158:1079–1087. doi: 10.1083/jcb.200202049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delmas V, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu B, Kim DS, Deluca AM, Alani RM. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS ONE. 2007;2:e594. doi: 10.1371/journal.pone.0000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan LM, et al. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001;19:568–576. doi: 10.1200/JCO.2001.19.2.568. [DOI] [PubMed] [Google Scholar]
- 47.Duncan LM, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- 48.Hammock L, et al. Chromogenic in situ hybridization analysis of melastatin mRNA expression in melanomas from American Joint Committee on Cancer stage I and II patients with recurrent melanoma. J Cutan Pathol. 2006;33:599–607. doi: 10.1111/j.1600-0560.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- 49.Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Influence of the microenvironment on melanoma cell fate determination and phenotype. Cancer Res. 2006;66:7833–7836. doi: 10.1158/0008-5472.CAN-06-0731. [DOI] [PubMed] [Google Scholar]
- 50.Zabierowski SE, Herlyn M. Melanoma stem cells: The dark seed of melanoma. J Clin Oncol. 2008;26:2890–2894. doi: 10.1200/JCO.2007.15.5465. [DOI] [PubMed] [Google Scholar]
- 51.Murakami T, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–7334. [PubMed] [Google Scholar]
- 52.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreizenbeck GM, Berger AJ, Subtil A, Rimm DL, Gould Rothberg BE. Prognostic significance of cadherin-based adhesion molecules in cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2008;17:949–958. doi: 10.1158/1055-9965.EPI-07-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.