Abstract
Human cytidine deaminase apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) proteins have been classified as either Z1- or Z2-type cytidine deaminases on the basis of phylogenetic analysis of their catalytic domains. Despite the identification of a number of Z1-type domain-containing cytidine deaminases, only one copy of Z2-type cytidine deaminase has been detected in each of the mammalian species evaluated thus far. Z1-type human APOBEC3 proteins are known to exhibit broad activities against diverse retroelements. However, the potential role of the only human Z2-type cytidine deaminase, APOBEC3H (A3H), in the restriction of retroelements has not yet been fully characterized. Here, we demonstrate that human A3H is a potent inhibitor of non-LTR LINE-1 transposition. Interestingly, it was also as efficient as A3G in inhibiting Alu retrotransposition, despite its poor association with Alu RNA. We have further demonstrated, for the first time, that human APOBEC3DE is also a potent inhibitor of Alu retrotransposition. Variants of A3H have divergent antiviral activities against HIV-1-Vif-deficient viruses. Unlike the anti-HIV-1 cytidine deaminases A3G and A3F, A3H is moderately regulated by interferons. These observations suggest that human Z2-type cytidine deaminase A3H variants have varying intrinsic abilities to restrict retroelements and that various APOBEC3 proteins may have evolved distinct inhibitory mechanisms against retroelements.—Tan, L., Sarkis, P. T. N., Wang, T., Tian, C., Yu, X.-F. Sole copy of Z2-type human cytidine deaminase APOBEC3H has inhibitory activity against retrotransposons and HIV-1.
Keywords: Vif, L1, virus, antiviral, evolution, G to A hypermutation
The APOBEC (apolipoproteinB mRNA-editing catalytic polypeptide) family of proteins is composed of cytidine deaminases that have the ability to modify RNA/DNA targets by converting cytidines to uracils (C to U). This family of proteins includes APOBEC1, APOBEC2, APOBEC3A-H, APOBEC4, and activation-induced cytidine deaminase (AID) (1,2,3,4,5,6,7). The recent discovery of the antiviral functions of the APOBEC3 subfamily has greatly reshaped the landscape of retroviral and retroelement research. To date, seven APOBEC3 proteins (A3A-H) have been characterized in primates and shown to exhibit varying degrees of antiviral activity, ranging from no ascribed activity to potent inhibition against retroviruses and retroelements (8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37).
When A3G is successfully packaged into newly formed virions, it induces C-to-U modifications in the minus-strand viral DNA in newly infected target cells (15, 20, 22, 23, 30, 35, 36). Recent studies (38, 39) have suggested that an intact cytidine deamination domain is required for the restriction of HIV-1ΔVif viruses under conditions approximating physiological levels. It has also been reported that A3G reduces viral DNA accumulation by inhibiting reverse transcription (40,41,42,43,44), inducing the degradation of viral DNA by cellular endonucleases as a result of uracil-DNA glycosylase activity (45, 46), and/or by inhibiting the formation of proviral DNA (42, 43).
The Vif protein of HIV-1 has been shown to neutralize the antiviral activity of multiple APOBEC3 proteins through the recruitment of the cellular E3 ubiquitin ligase complex of the host (47,48,49,50,51,52,53). The resulting polyubiquitination and proteasomal degradation of APOBEC3 cytidine deaminases prevent these proteins from being incorporated into HIV-1 virions (47, 54,55,56,57,58,59). The N terminus of Vif acts to recruit A3F and A3G (55, 60,61,62,63,64).
Through phylogenetic analysis of their cytidine deaminase catalytic domains, the APOBEC3 proteins have been classified as either Z1 or Z2 domain-containing proteins. A3H, which is found downstream of A3G on chromosome 22, is a single-domain protein and the only Z2-type APOBEC3 catalytic domain that has been detected in any mammalian species analyzed thus far (65, 66). The A3H gene encodes a functional protein that displays DNA mutator activity (66), and like other APOBEC3 family members, it has been subject to significant positive selection in primates (66, 67). The driving force behind the positive selection of this protein does not appear to be mediated by modern retroviruses such as HIV-1, since studies have revealed that human A3H (hA3H) has no antiviral activity against primate lentiviruses, including HIV-1 (66, 68).
Recent studies have identified new roles for human APOBEC3 proteins in the intracellular defense against non-LTR retrotransposons (LINE-1 and Alu). Whereas A3A, A3B, A3C, and A3F have been shown to inhibit LINE-1 retrotransposition (9, 10, 16, 24, 28, 69), A3A, A3B, and A3G are inhibitors of Alu retrotransposition (9, 11, 16). While the other APOBEC3 proteins have been well characterized in terms of their ability to inhibit retroviruses and retroelements, little is known about the activity of A3H against retroelements or the reason for its apparent inability to inhibit retroviruses. We have now demonstrated that variants of human A3H can inhibit non-LTR LINE-1 and Alu retrotransposons as well as HIV-1. Furthermore, we have found that, unlike the potent antiviral inhibitors A3F and A3G, A3H is moderately induced by interferon (IFN) treatment in macrophages but not in liver cells. Thus, human A3H may play a role in the intracellular defense against retroelements.
MATERIALS AND METHODS
Plasmids
The infectious molecular clone of NL4-3ΔVif has been described previously (70). The expression vectors pCMV-A3A-HA, pCMV-A3B-HA, and pCMV-A3C-HA were kindly provided by Michael H. Malim (King’s College London School of Medicine, London, UK). The expression vectors for A3H-V5 and A3G-V5-HA were kindly provided by Yonghui Zheng (Michigan State University, East Lansing, MI, USA) and B. Matija Peterlin (Rosalind Russell Medical Research Center, University of California, San Francisco, CA, USA). The plasmids used for LINE-1 retrotransposition assays, pL1RP-EGFP and pL1RP(JM111)-EGFP, were kindly provided by Haig H. Kazazian (University of Pennsylvania School of Medicine, Philadelphia, PA, USA). The plasmids used in the Alu retrotransposition assays, AluneoTet (Alu indicator construct) and pCEP5′UTRORF2Δneo (ORF2 Δneo, LINE-1 construct lacking the ORF1 coding sequence), were kindly provided by John V. Moran (University of Michigan Medical School, Ann Arbor, MI, USA). The expression vector pCMV4-A3G-HA was obtained from the U.S. National Institutes of Health (NIH) AIDS Reagent Program. pcDNA-A3F-HA has been described previously (54). Where indicated, either pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) or VR1012 (Vical Inc., San Diego, CA, USA) vectors were used as empty vector filler DNA to equalize the total amount of transfected DNA. VR1012-A3H-HA was made by subcloning the HA-tagged A3H gene from the A3H-V5 expression vector (a kind gift of Yonghui Zheng, Michigan State University) with the following primers: A3H forward (SalI) 5′ GTACGTCGACGCCATGGCTCTGCTGACAGCCGAAACATTCCGCCTGCAG 3′ and HA tag NotI reverse 5′-GTACGCGGCCGCTCACGCGTAATCTGGGAC-3′. The boldface sequences are the restriction sites for subcloning A3H into the VR1012 vector, which contains an intron A sequence enhancer upstream of the multiple cloning site.
Cell culture, transfection, multinuclear-activation galactosidase indicator (MAGI) infectivity, and virion incorporation assays
Cultures of 293T cells and MAGI-CCR5 cells were maintained in Dulbecco modified Eagle medium (Invitrogen) with 10% fetal bovine serum (FBS) and gentamicin (5 μg/ml) and passaged on confluence. HeLa-HA cells were maintained in minimal essential medium (Invitrogen) with 10% FBS, l-glutamine (2 mM), nonessential amino acids (0.1 mM) and gentamicin (5 μg/ml) and passaged on confluence. Freshly isolated primary hepatocytes from anonymous donors (purchased from BD Biosciences, San Jose, CA, USA) were received within 24–48 h of isolation in 6-well plates in Hepatostim medium (BD Biosciences). Macrophages were obtained by plating freshly isolated peripheral blood mononuclear cells (PBMCs) in 6-well plates at 2 × 107 cells/ml in RPMI 1640 with 10% FBS. The following day, nonadherent cells were removed, and the medium was replaced every 2 days. Macrophages, differentiated by adherence to plastic, were used 12 days after isolation.
DNA transfections were carried out using either Lipofectamine 2000 (Invitrogen) or Fugene 6 (Roche, Indianapolis, IN, USA) for 293T and HeLa-HA cells, respectively, as described by the manufacturer. Viruses were produced from 293T cells transfected with NL4-3ΔVif in the presence of APOBEC3 expression or control empty vector for 48 h. MAGI assays for virus infectivity were performed as described previously (50). For virion incorporation, cell culture supernatants containing viruses were collected at 72 h post-transfection by removal of cellular debris through centrifugation at 3000 rpm for 10 min in a Sorvall RT 6000B centrifuge (Thermo Fisher Scientific, Waltham, MA, USA). The virus-containing supernatants were then filtered through a 0.2 μm-pore-size membrane, and the virus particles were concentrated using a 30% sucrose cushion at 100,000 g for 2 h at 4°C in a Sorvall Ultra80 ultracentrifuge (Thermo Fisher).
Cytokines
IFN-α and IFN-γ (EMD Biosciences, San Diego, CA, USA) were dissolved in PBS with 0.5% bovine serum albumin (control medium) and stored in single-use aliquots at −70°C. IFN-α was used at 1000 IU/ml and IFN-γ at 10 IU/ml. In IFN induction experiments, cells were treated with equal volumes of either IFN or control medium.
Immunoprecipitation and immunoblot analysis
At 48 h after transfection, 293T cells were harvested, washed with PBS, and lysed in lysis buffer (50 mM Tris, pH 7.5, with 150 mM NaCl, 1% Triton X-100, 400 U/ml RNase inhibitor, and complete protease inhibitor cocktail tablets) at 4°C for 1 h, followed by centrifugation at 10,000 g for 30 min. HA tag immunoprecipitation was carried out by mixing cell lysates from a T75 flask with a 50 μl bead volume of anti-HA antibody-conjugated agarose beads (Roche) and incubating the mixture at 4°C for 3 h. Samples were washed 6 times with washing buffer (20 mM Tris, pH 7.5, with 100 mM NaCl, 0.1 mM EDTA, 0.05% Tween-20, and 40 U/ml RNase inihibitor). The beads were then either eluted with 2× loading buffer or subjected to RNA extraction. The eluted materials were then analyzed by SDS-PAGE and immunoblotting with the appropriate antibodies. The anti-HA antibody-agarose conjugate, anti-HA, anti-p24, and anti-human ribosomal P antigen antibodies used have been described previously.
Sample preparation for detection of APOBEC3 binding to Alu RNA
The 293T cells were transfected with HA-tagged APOBEC3 expression vectors or empty VR1012 vector. After 48 h, the cells were collected for immunoprecipation with anti-HA antibody-conjugated agarose beads (Roche). The HA-immunoprecipitated samples were subjected to RNA extraction using Trizol reagent (Invitrogen) as described by the manufacturer. cDNA was then made from the extracted RNA using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) as described by the manufacturer. The relative binding of Alu RNA to APOBEC3 proteins was measured by quantitative real-time polymerase chain reaction (qRT-PCR) as described below.
qRT-PCR
qRT-PCR was performed according to standard protocols (71) and as described previously (66, 68). In brief, total RNA from IFN- or control-treated primary hepatocytes or primary macrophages in 6-well plate cultures was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), including an on-column DNase digestion step. One-fifth of the RNA was reverse-transcribed using the High Capacity cDNA Archive Kit, and the cDNA was diluted 1:40 (final) in the real-time PCR reaction. For detection of APOBEC3 genes in a panel of normal human tissue cDNA (Promgen, Bothell, WA, USA), 2.5 ng of each tissue-sample cDNA was used per reaction. The cDNA was amplified using TaqMan Universal PCR Master Mix (Applied Biosystems) and an ABI Prism 7000 sequence detection system (Applied Biosystems). Except for the quantification of Alu RNA and A3H (see below), the primers and probe sets were from prevalidated TaqMan gene expression assays specific for A3A, A3B, A3F, A3G, double-stranded RNA-activated protein kinase (PKR), and interferon regulatory factor-1 (IRF-1) (assay identification numbers: Hs00377444_m1, Hs00358981_m1, Hs00736570_m1, Hs00222415_m1, and Hs00169345_m1, Hs00233698_m1, respectively). Amplification of target genes was normalized to β-actin as an endogenous control [human ACTB (β-actin) endogenous control FAM/MGB probe; Applied Biosystems]. The efficiency of the PCR was tested by amplification of the target from serially diluted cDNA generated from reverse transcription of a stock set of human RNAs or from serially diluted plasmids containing the target sequence. Calculations were performed using the 2−ΔΔCT method, as described previously (71). For IFN induction experiments, gene expression was expressed as fold induction of a gene measured in the IFN-treated sample relative to the sample treated with control medium (PBS 0.5% BSA). A >2-fold induction was considered positive. Detection of Alu was performed using SYBR Green PCR Master Mix and the Alu-specific primers 5′-GGCCGGGCGCGGTGGCTCAC-3′ (forward) and 5′-TTTTTTTTTTGAGACGGAGTCTCGCTC-3′ (reverse).
Retrotransposition assays
The LINE-1 and Alu retrotransposition assays have been previously described. The LINE-1 retrotransposition assay was carried using either pL1RP-EGFP, an active L1 retrotransposon, or the negative control pL1RP(JM111)-EGFP, an L1 retrotransposon with two misense mutations in ORF1 that have been shown to abolish retrotransposition (72). 293T cells were transfected with 1.5 μg of pL1RP-EGFP or pL1RP(JM111)-EGFP in the presence of 1.5 μg of APOBEC3-expressing or empty control vector using Lipofectamine (Invitrogen). At 5 days post-tranfection, cells were trypsinized and analyzed for positive retrotransposition events by detecting eGFP-positive cells. eGFP-expressing cells were analyzed and quantified in a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) by gating 20,000 live cells for each transfection sample.
The Alu retrotransposition assay was carried by cotransfecting 5 × 105 HeLa-HA cells plated overnight in a T-75 flask with an Alu construct tagged with a neomycin-based retrotransposition indicator cassette, an L1 construct lacking the ORF1 coding sequence (ORF2Δneo) in the presence of an APOBEC3 expression vector, or a control empty vector. Positive Alu retrotransposition events were detected by selecting for neomycin-resistant cells at 3 days post-transfection. Selection was carried out for 11–12 days before detecting neomycin-resistant colonies after fixation and staining with crystal violet.
Velocity sucrose gradient analysis
The 293T cells were transfected for 48 h with APOBEC3 expression vectors. The transfected cells were harvested, washed once with PBS, and lysed in lysis buffer (50 mM Tris, pH 7.5, with 150 mM NaCl, 1% Triton X-100, 400 U/ml RNase inhibitor, and complete protease inhibitor cocktail tablets) at 4°C for 1 h, followed by centrifugation at 1500 g for 30 min. The supernatants were then subjected to 10–30% sucrose (in PBS) density gradient ultracentrifugation using an SW40 rotor at 30,000 rpm for 4 h. Fractions were collected for immunoblotting analysis using an anti-HA monoclonal antibody to detect APOBEC3 proteins and anti-p19 to detect the larger (60S) ribosomal subunit.
RESULTS
A3H variants have different inhibitory effects on non-LTR LINE-1 retrotranposons
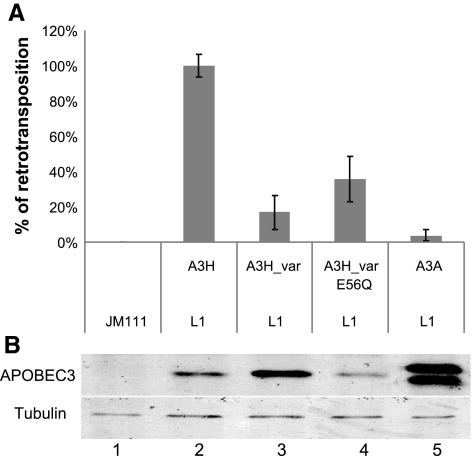
Phylogenetic analysis of the cytidine deaminase domains of the APOBEC3 proteins identifies A3H as the only Z2-type human APOBEC3 protein (65, 66; Fig. 1A). Members of the human APOBEC3 family of proteins have been shown to have varying degrees of inhibitory activity against non-LTR LINE-1 retrotransposons. To date, only the Z1-type cytidine deaminase domain-containing APOBEC3 proteins have been shown to inhibit LINE-1 retrotransposons, with A3A being the most potent inhibitor and A3G having only weak inhibitory function (9, 10, 14, 24, 28, 69, 73).
Figure 1.
A3H, the only Z2-type human APOBEC3 cytidine deaminase, is a potent inhibitor of LINE-1 retrotransposition. A) 293T cells were cotransfected with either pL1RP-EGFP or the negative control pL1RP(JM111)-EGFP in the presence of APOBEC3 expression vector. FACS was carried out to analyze for positive retrotransposition events by detecting eGFP expression in 293T cells at 5 days post-transfection. B) Cell lysates were harvested from the LINE-1 retrotransposition assay 3 days post-transfection and analyzed by SDS-PAGE, followed by immunoblotting with anti-V5 and antitubulin antibodies.
Previous reports (24, 66, 68, 74) have shown that human A3H has little activity against LINE-1 retrotransposition. However, polymorphisms of human A3H exist in the Single Nucleotide Polymorphism database at the National Center for Biotechnology Information (NCBI; Bethesda, MD, USA; http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?chooseRs=coding&go=Go&locusId=164668). In particular, G105R, K121E, and E178D have been detected and cluster with each other. The frequency of this A3H variant allele, A3H-Var, is the highest among sub-Saharan Africans (HapMap-YRI; ∼0.9) and is significantly lower in Asian populations (HapMap-HCB and HapMap-JPT) and European populations (HapMap-CEU; ∼0.3–0.4).
We found that A3H-Var is a highly effective inhibitor of non-LTR LINE-1 retrotransposition when compared with A3H (Fig. 1A) using a well-established cell culture-based LINE-1 retrotransposition assay (72, 75, 76). This inhibitory effect of A3H against LINE-1 retrotransposition was as potent as that of A3A (Fig. 1A). Thus, our data suggest that the strong inhibitory activity of the Z1-type cytidine deaminases against non-LTR LINE-1 retrotransposon is also preserved in the Z2-type cytidine deaminase A3H-Var. A single amino acid substitution of the active site of A3H-Var reduced its anti-LINE-1 activity (Fig. 1A). It has been previously reported (10, 69) that mutations in the active site of A3A also reduce its anti LINE-1 retrotransposon activity. However, APOBEC3 proteins have not been shown to induce cytidine deamination in newly synthesized LINE-1 DNA. It is possible that the active sites of APOBEC3 may be important for LINE-1 inhibition and not for inducing deamination but for maintaining its interaction with cofactors.
A3H and A3DE are also inhibitors of Alu retrotransposition
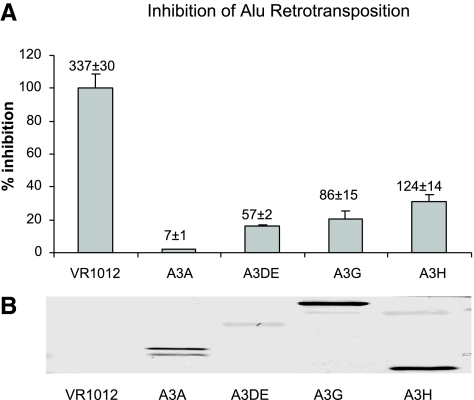
Alu elements belong to the family of short interspersed elements that make up ∼11% of the human genome (77, 78). Since recent studies (9, 11, 16) have shown that the Z1-type cytidine deaminases A3A, A3B, and A3G can inhibit Alu retrotransposition in an ORF1-independent manner, we asked whether A3H, with a Z2-type cytidine deaminase domain, had similar inhibitory activity. The Alu retrotransposition assay has been described previously (16). HeLa-HA cells (16) were cotransfected with an Alu construct tagged with a neomycin-based retrotransposition indicator cassette plus an LINE-1 construct lacking the ORF1 coding sequence (ORF2 Δneo) in the presence of either an APOBEC3 expression vector or a control empty vector, VR1012. Positive Alu retrotransposition events were detected by staining for neomycin-resistant colonies at 11–12 days postselection.
We found that A3H was able to inhibit Alu retrotransposition in an ORF1-independent manner, producing an ∼65% decrease in Alu retrotransposition events in cells cotransfected with the Alu indicator cassette plus ORF2Δneo and A3H when compared with cells cotransfected with the empty control vector, VR1012 (Fig. 2A). A3A and A3G, which were previously demonstrated to be inhibitors of Alu retrotransposition (9, 11, 16), showed inhibition levels of ∼98 and ∼73%, respectively (Fig. 2A). In a parallel transfection assay, APOBEC3 proteins were found to be expressed at similar levels in the Alu retrotransposition assay (Fig. 2B). The inhibition phenomenon seen against both of the non-LTR retrotransposons, LINE-1, and Alu (Figs. 1 and 2), strongly arguing that the Z2-type cytidine deaminase A3H maintains some of the functional characteristics previously observed for the Z1-type cytidine deaminases.
Figure 2.
A3H is an inhibitor for Alu retrotransposition. A) HeLa-HA cells were cotransfected with an Alu construct tagged with the retrotransposition indicator cassette, a LINE-1 construct that lacks ORF1 coding sequence (ORF2Δneo), and an APOBEC3 expression or control empty vector. At 3 days post-transfection, the cells were subjected to neomycin selection for 11–12 days to select for neomycin-resistant colonies that were indicative of positive retrotransposition events. B) Cell lysates were harvested and analyzed by SDS-PAGE, followed by immunoblotting with anti-HA antibodies.
Human APOBEC3DE (A3DE) has moderate anti-HIV-1 activity, and we have previously reported that human A3DE has potent inhibitory activity against LINE-1 retrotransposition (69). To determine whether A3DE can also inhibit Alu retrotransposition, we preformed the Alu retrotransposition assay described above in the presence of A3DE. Human A3DE proved to be a potent inhibitor of Alu retrotransposition, with a >80% decrease in Alu retrotransposition events seen for cells cotransfected with the Alu indicator cassette plus ORF2Δneo and A3DE when compared with cells cotransfected with the empty control vector (Fig. 2A). Considering that A3DE was expressed at a level much lower than that of the other human APOBEC3 proteins we tested (Fig. 2B), these data suggest that A3DE is one of the most potent inhibitors of Alu retrotransposition among the human APOBEC3 cytidine deaminases.
A3H associates with intracellular high molecular mass (HMM) complexes but not with Alu RNA
A3G has been reported to be associated either with enzymatically inactive HMM ribonucleoprotein complexes in 293T cells (70) and activated CD4+ T lymphocytes (79) or with enzymatically active low molecular mass (LMM) complexes in resting CD4+ T lymphocytes (79). The LMM form of A3G in resting CD4+ T lymphocytes is active against incoming HIV-1 (79). Since HMM A3G ribonucleoprotein complexes restrict Alu retrotransposition and are also found to associate with Staufen RNA-transporting granules and Ro/La ribonucleoprotein complexes containing Alu and hY RNAs, this inhibitory activity of A3G against LINE-1-dependent Alu retrotransposition has been suggested to be mediated through the sequestration of Alu RNA in the cytoplasm HMM complexes away from the LINE-1 machinery in the nucleus (11).
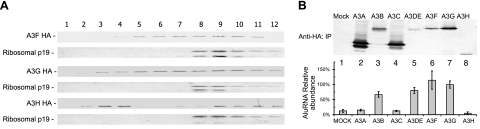
To determine whether the association with HMM complexes is involved in restricting Alu retrotransposition by A3H, various APOBEC3 protein samples were purified through velocity sucrose gradients and analyzed by anti-HA immunoblotting to detect tagged APOBEC3 proteins. A3F, A3G, and A3H were all found to form HMM complexes in 293T cells (Fig. 3A, fractions 8–12). This ability of A3H to associate with HMM complexes in 293T cells appears to suggest that A3H may resemble A3G in being able to associate with HMM complexes containing Alu RNAs.
Figure 3.
A3H, like A3F and A3G, forms HMM complexes but, in contrast, to A3G, does not bind endogenous Alu RNA efficiently. A) Cell lysates from 293T cells transfected for 72 h with APOBEC3 expression or control empty vector were subjected to 10–30% continuous velocity sucrose gradient fractionation. Fractions were collected, and samples were subjected to SDS-PAGE, followed by immunoblotting with anti-HA and anti-ribosomal p19 antibodies. B) 293T cells were transfected with A3G-HA, A3H-HA expression vector, or empty control vector. At 3 days post-transfection, cell lysates were immunoprecipitated with anti-HA affinity matrix. cDNA was then made from RNA extracted by Trizol, followed by qRT-PCR to detect endogenous Alu RNA binding to the APOBEC3-HA proteins. Samples from immunoprecipitation with anti-HA affinity matrix to detect endogenous Alu RNA binding were analyzed by SDS-PAGE, followed by immunoblotting with anti-HA.
Since the mechanism by which A3H inhibits Alu has yet to be identified, we wondered whether examining the interaction of the APOBEC3 proteins with Alu RNA would reveal some useful insights into the process. Therefore, we transfected 293T cells with HA-tagged A3G and A3H expression vectors or a control empty vector for 48 h and then analyzed the resulting complexes by anti-HA affinity matrix immunoprecipitation and RNA extraction. To analyze the interaction of endogenous Alu RNA with different APOBEC3 proteins, we carried out qRT-PCR amplification using Alu-specific primers and cDNA made from the extracted RNAs. Consistent with a previous study (11), we detected an interaction between A3G and Alu RNAs (Fig. 3B, lane 7). We saw only a minimal amount of non-specific binding of Alu RNAs to the assay system in the absence of APOBEC3 proteins (Fig. 3B, lane 1). Although A3G and A3H inhibited Alu retrotransposition at similar levels (73 and 65%, respectively; Fig. 2A), A3H interacted poorly with the Alu RNAs (Fig. 3B, lane 8). In fact, the level of Alu RNAs associated with A3H (Fig. 3B, lane 8) was not significantly higher than that for the negative control sample (lane 1). Immunoblotting analysis showed relatively comparable levels of A3G-HA and A3H-HA in the immunoprecipitation samples (Fig. 3B, top panel). Thus, although A3H has been observed to associate with HMM complexes, its less efficient interaction with endogenous Alu RNAs suggests that it does not inhibit Alu retrotransposition by sequestering Alu RNA. We have also observed that the most potent inhibitor of Alu retrotransposition, A3A, also did not interact with Alu RNAs compared with A3G (Fig. 3B, bottom panel).
A3H variants have different inhibitory effects on HIV-1
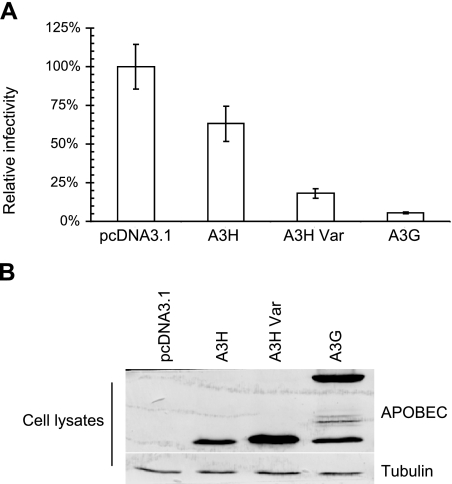
Although APOBEC3 proteins such as A3F and A3G are potent inhibitors of retroviruses, including HIV-1, human A3H is ineffective against primate lentiviruses (66, 68). We also found that A3H had only weak anti-HIV-1 activity (Fig. 4A). However, A3H-Var had more potent anti-HIV-1 activity than A3H (Fig. 4A). The lack of antiviral activity of A3H has previously been attributed to its low expression levels when compared with other potent antiviral APOBEC3 proteins (66). A3H-Var expressed at a higher level than A3H (Fig. 4B), which could potentially explain its more potent anti-HIV-1 activity than A3H.
Figure 4.
Anti-HIV-1 activity of APOBEC3 proteins. A) NL4-3ΔVif viruses were produced in 293T cells cotransfected with NL4-3ΔVif and APOBEC3 expression or control empty vector. After 48 h, virus was collected and assayed for virus infectivity using MAGI indicator cells. Relative infectivity was expressed as a percentage relative to the infectivity obtained in the absence of APOBEC3 (i.e., with empty vector control, pcDNA3.1). B) Virus-producing 293T cells were harvested and analyzed by SDS-PAGE, followed by immunoblotting to detect intracellular levels of APOBEC3-HA proteins, with tubulin as a loading control.
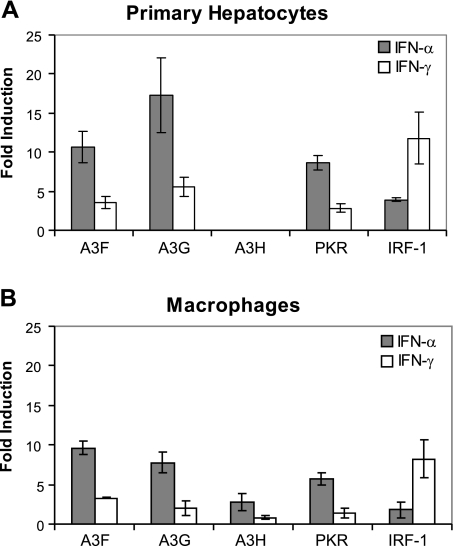
A3G and A3F may have evolved to function as antiviral factors, since their anti-HIV-1 and anti-HBV activities have been found to be enhanced by interferons in hepatocytes and macrophages (80,81,82,83,84). In accordance with the previously published literature, we found that primary hepatocytes and macrophages treated with IFN-α showed a robust induction of A3F and A3G as well as a previously identified IFN-α inducible gene, PKR (Fig. 5). Primary hepatocytes and macrophages treated with IFN-γ showed modest increases in A3F and A3G induction when compared with the induction of the IFN-γ inducible IRF-1. We observed, however, that unlike A3G and A3F, A3H was not expressed or induced by either IFN-α or IFN-γ in hepatocytes (Fig. 5A). A3H was moderately up-regulated (∼3-fold) in macrophages by IFN-α (Fig. 5B). However, A3H was not induced by IFN-γ in macrophages (Fig. 5B).
Figure 5.
Regulation of APOBEC3 genes by IFNs. A) Primary hepatocytes were treated with IFN-α (1000 IU/ml), IFN-γ (10 IU/ml), or control medium. After 16 h, RNA was isolated from the cells and converted to cDNA. Gene expression of A3F, A3G, A3H, PKR, and IRF-1 was measured by qRT-PCR of the cDNA and normalized to β-actin expression. Graph depicts the fold induction of normalized gene expression in IFN-treated over control-treated cells. Error bars represent triplicate samples. B) Primary macrophages were treated with IFN-α (1000 IU/ml), IFN-γ (10 IU/ml), or control medium. After 8 h, RNA was isolated from the cells and converted to cDNA. Gene expression of A3F, A3G, A3H, PKR, and IRF-1 was measured by qRT-PCR of the cDNA and normalized to β-actin expression. Graph depicts the fold induction of normalized gene expression in IFN-treated over control-treated cells. Error bars represent triplicate samples.
DISCUSSION
In this study, we report the anti-LINE-1 and anti-Alu activities of the only human Z2-type cytidine deaminase, A3H. Recent studies (24, 74) have shown that cotransfection of A3H and LINE-1 retrotranposon indicator constructs produces no significant inhibition of LINE-1 retrotransposition by A3H. Our data also indicate that A3H has weak anti-LINE-1 activity. In contrast, we found A3H-Var inhibited LINE-1 retrotransposition by ∼85% (Fig. 1). Thus, different naturally occurring A3H variants have divergent potencies against LINE-1 retrotransposition. Interestingly, differences in LINE-1 retrotransposition controlled by other APOBEC3 proteins have been observed. For example, in the case of A3F, two groups have reported that A3F does not affect LINE-1 retrotransposition (9, 16), whereas others have reported that A3F inhibits this process (10, 24, 28, 69).
In the current study, we demonstrated for the first time that A3H and A3DE have the ability to restrict Alu retrotransposition. A3G, A3A, and A3B are also potent restrictors of Alu retrotransposition (9, 11, 16), but A3C and A3F are reported to be ineffective (9, 16). We observed that A3H was almost as effective as A3G in restricting Alu retrotransposition when these cytidine deaminases were expressed at similar levels. A3DE was expressed at a lower level than A3G but was more effective than A3G in terms of Alu restriction. Thus, A3DE is apparently a particularly potent inhibitor of non-LTR retrotransposons.
To date, several members of the APOBEC3 family have been shown to be inhibitors of the non-LTR retrotransposons, LINE-1 and Alu. However, the exact mechanism of action of the APOBEC3 family members against these retroelements has yet to be resolved. Recent work (11) has revealed that HMM A3G complexes contain significant amounts of endogenous retroelements, Alu and small hY RNAs, and that HMM A3G complexes restrict Alu retrotransposition. It was hypothesized that cytoplasmic HMM A3G complexes act to inhibit LINE-1-dependent Alu retrotransposition by sequestering Alu RNAs away from the LINE-1 machinery in the nucleus. However, A3H and A3A have been reported to be localized to both the cytoplasm and the nucleus and to act as potent inhibitors of Alu retrotransposition. We found that A3H inhibited Alu retrotransposition as efficiently as A3G did (Fig. 2). However, unlike A3G, A3H interacted poorly with Alu RNAs (Fig. 3). Furthermore, A3A was the most potent inhibitor of Alu retrotransposition among the human APOBEC3 proteins tested (Fig. 2). A3A does not interact efficiently with Alu RNAs (Fig. 3) nor does it form intracellular HMM complexes (69). Thus, A3A and A3H restrict Alu retrotransposition through a mechanism that apparently does not involve the sequestration of Alu RNA within HMM complexes. Several groups have reported that A3G is localized to P-bodies and stress granules, which are sites of mRNA storage and metabolism, raising the question of whether P-bodies and/or stress granules play a role in A3G-mediated Alu retrotransposition. A3A was much more potent than A3G in terms of its suppression of Alu retrotransposition, but it interacts poorly with P-bodies and mRNA-containing HMM complexes (69), suggesting that A3A-mediated suppression of Alu retrotransposition is not linked to P-bodies. These results together suggest that different APOBEC3 proteins may have evolved distinct inhibitory mechanisms against Alu retroelements. It is reasonable to hypothesize that APOBEC3 cytidine deaminases such as A3H and A3A have evolved to inhibit Alu movement by interfering with components of the LINE-1 machinery and/or host factors that are required for Alu retrotransposition.
In agreement with two previous studies (66, 68), we found that human A3H had only weak anti-HIV-1 activity in our analysis (Fig. 4A). It has been reported that the weak antiviral activity of A3H can be enhanced by increasing its intracellular expression using an intron-containing expression vector or by increasing its protein length via the removal of a premature stop codon in the coding sequence (85). This A3H allele is prevalent among Caucasian and Asian populations. Interestingly, an A3H variant, A3H-Var, containing amino acid substitutions at positions 105, 121, and 178 also exists in human populations and is prevalent among sub-Saharan Africans. A3H-Var has more potent anti-HIV-1 activity than does A3H. Whether A3H variants with divergent anti-HIV-1 activities can influence the rate of HIV-1 infection and/or disease progression remains to be determined.
Acknowledgments
We thank A. Zhen, R. Markham, E. Ehrlich, A. Niewiadomska and J. Garcia-Perez for advice and technical assistance; Michael H. Malim (King’s College London School of Medicine, London, UK), Yonghui Zheng (Michigan State University, East Lansing, MI, USA), John V. Moran (University of Michigan Medical School, Ann Arbor, MI, USA), and Haig H. Kazazian (University of Pennsylvania School of Medicine, Philadelphia, PA, USA) for critical reagents; Dr. M. Emerman for sharing the A3H preprint before publication; and D. McClellan for editorial assistance. MAGI-CCR5 cells, pCMV-Apobec3G-HA, and the NL4-3ΔVif constructs were obtained through the AIDS Research Reagents Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH. This work was supported by grants from NIH (AI062644 and AI071769) and funding from the National Science Foundation of China (NSFC-30425012) and Cheung Kong Scholars Program Foundation of the Chinese Ministry of Education to X.-F.Y.
References
- Bieniasz P D. Intrinsic immunity: a front-line defense against viral attack. Nat Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- Goff S P. Retrovirus restriction factors. Mol Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Harris R S, Liddament M T. Retroviral restriction by APOBEC proteins. Nat Rev Immunol. 2004;4:868–877. doi: 10.1038/nri1489. [DOI] [PubMed] [Google Scholar]
- Navarro F, Landau N R. Recent insights into HIV-1 Vif. Curr Opin Immunol. 2004;16:477–482. doi: 10.1016/j.coi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Rogozin I B, Basu M K, Jordan I K, Pavlov Y I, Koonin E V. APOBEC4, a new member of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases predicted by computational analysis. Cell Cycle. 2005;4:1281–1285. doi: 10.4161/cc.4.9.1994. [DOI] [PubMed] [Google Scholar]
- Rose K M, Marin M, Kozak S L, Kabat D. Transcriptional regulation of APOBEC3G, a cytidine deaminase that hypermutates human immunodeficiency virus. J Biol Chem. 2004;279:41744–41749. doi: 10.1074/jbc.M406760200. [DOI] [PubMed] [Google Scholar]
- Turelli P, Trono D. Editing at the crossroad of innate and adaptive immunity. Science. 2005;307:1061–1065. doi: 10.1126/science.1105964. [DOI] [PubMed] [Google Scholar]
- Bishop K N, Holmes R K, Sheehy A M, Davidson N O, Cho S J, Malim M H. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Bogerd H P, Wiegand H L, Hulme A E, Garcia-Perez J L, O'Shea K S, Moran J V, Cullen B R. Cellular inhibitors of long interspersed element 1 and Alu retrotransposition. Proc Natl Acad Sci U S A. 2006;103:8780–8785. doi: 10.1073/pnas.0603313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lilley C E, Yu Q, Lee D V, Chou J, Narvaiza I, Landau N R, Weitzman M D. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Chiu Y L, Witkowska H E, Hall S C, Santiago M, Soros V B, Esnault C, Heidmann T, Greene W C. High-molecular-mass APOBEC3G complexes restrict Alu retrotransposition. Proc Natl Acad Sci U S A. 2006;103:15588–15593. doi: 10.1073/pnas.0604524103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle B P, Schafer A, Cullen B R. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology. 2005;339:281–288. doi: 10.1016/j.virol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Esnault C, Millet J, Schwartz O, Heidmann T. Dual inhibitory effects of APOBEC family proteins on retrotransposition of mammalian endogenous retroviruses. Nucleic Acids Res. 2006;34:1522–1531. doi: 10.1093/nar/gkl054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault C, Heidmann O, Delebecque F, Dewannieux M, Ribet D, Hance A J, Heidmann T, Schwartz O. APOBEC3G cytidine deaminase inhibits retrotransposition of endogenous retroviruses. Nature. 2005;433:430–433. doi: 10.1038/nature03238. [DOI] [PubMed] [Google Scholar]
- Harris R S, Bishop K N, Sheehy A M, Craig H M, Petersen-Mahrt S K, Watt I N, Neuberger M S, Malim M H. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Hulme A E, Bogerd H P, Cullen B R, Moran J V. Selective inhibition of Alu retrotransposition by APOBEC3G. Gene. 2007;390:199–205. doi: 10.1016/j.gene.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Takaori-Kondo A, Shindo K, Abudu A, Fukunaga K, Uchiyama T. APOBEC3G targets specific virus species. J Virol. 2004;78:8238–8244. doi: 10.1128/JVI.78.15.8238-8244.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer M, Bittner A, Schnierle B S. Human APOBEC3G incorporation into murine leukemia virus particles. Virology. 2005;337:175–182. doi: 10.1016/j.virol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Langlois M A, Beale R C, Conticello S G, Neuberger M S. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic Acids Res. 2005;33:1913–1923. doi: 10.1093/nar/gki343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecossier D, Bouchonnet F, Clavel F, Hance A J. Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science. 2003;300:1112. doi: 10.1126/science.1083338. [DOI] [PubMed] [Google Scholar]
- Liddament M T, Brown W L, Schumacher A J, Harris R S. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schrofelbauer B, Navarro F, Konig R, Bollman B, Munk C, Nymark-McMahon H, Landau N R. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Muckenfuss H, Hamdorf M, Held U, Perkovic M, Lower J, Cichutek K, Flory E, Schumann G G, Munk C. APOBEC3 proteins inhibit human LINE-1 retrotransposition. J Biol Chem. 2006;281:22161–22172. doi: 10.1074/jbc.M601716200. [DOI] [PubMed] [Google Scholar]
- Noguchi C, Ishino H, Tsuge M, Fujimoto Y, Imamura M, Takahashi S, Chayama K. G to A hypermutation of hepatitis B virus. Hepatology. 2005;41:626–633. doi: 10.1002/hep.20580. [DOI] [PubMed] [Google Scholar]
- Rosler C, Kock J, Malim M H, Blum H E, von Weizsacker F. Comment on “Inhibition of hepatitis B virus replication by APOBEC3G.”. Science. 2004;305:1403. doi: 10.1126/science.1101974. [DOI] [PubMed] [Google Scholar]
- Schumacher A J, Nissley D V, Harris R S. APOBEC3G hypermutates genomic DNA and inhibits Ty1 retrotransposition in yeast. Proc Natl Acad Sci U S A. 2005;102:9854–9859. doi: 10.1073/pnas.0501694102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenglein M D, Harris R S. APOBEC3B and APOBEC3F inhibit L1 retrotransposition by a DNA deamination-independent mechanism. J Biol Chem. 2006;281:16837–16841. doi: 10.1074/jbc.M602367200. [DOI] [PubMed] [Google Scholar]
- Suspene R, Guetard D, Henry M, Sommer P, Wain-Hobson S, Vartanian J P. Extensive editing of both hepatitis B virus DNA strands by APOBEC3 cytidine deaminases in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102:8321–8326. doi: 10.1073/pnas.0408223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suspene R, Sommer P, Henry M, Ferris S, Guetard D, Pochet S, Chester A, Navaratnam N, Wain-Hobson S, Vartanian J P. APOBEC3G is a single-stranded DNA cytidine deaminase and functions independently of HIV reverse transcriptase. Nucleic Acids Res. 2004;32:2421–2429. doi: 10.1093/nar/gkh554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Response to comment on “Inhibition of hepatitis B virus replication by APOBEC3G.”. Science. 2004;305:1403b. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- Turelli P, Mangeat B, Jost S, Vianin S, Trono D. Inhibition of hepatitis B virus replication by APOBEC3G. Science. 2004;303:1829. doi: 10.1126/science.1092066. [DOI] [PubMed] [Google Scholar]
- Wiegand H L, Doehle B P, Bogerd H P, Cullen B R. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Chen D, Konig R, Mariani R, Unutmaz D, Landau N R. APOBEC3B and APOBEC3C are potent inhibitors of simian immunodeficiency virus replication. J Biol Chem. 2004;279:53379–53386. doi: 10.1074/jbc.M408802200. [DOI] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin J M, Landau N R. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nat Struct Mol Biol. 2004;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz R J, Zhang C, Arunachalam S C, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y H, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin B M. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J Virol. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi E, Opi S, Takeuchi H, Khan M, Goila-Gaur R, Kao S, Strebel K. Enzymatically active APOBEC3G is required for efficient inhibition of human immunodeficiency virus type 1. J Virol. 2007;81:13346–13353. doi: 10.1128/JVI.01361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A J, Hache G, Macduff D A, Brown W L, Harris R S. The DNA deaminase activity of human APOBEC3G is required for Ty1, MusD, and human immunodeficiency virus type 1 restriction. J Virol. 2008;82:2652–2660. doi: 10.1128/JVI.02391-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Cen S, Niu M, Saadatmand J, Kleiman L. Inhibition of formula-primed reverse transcription by human APOBEC3G during human immunodeficiency virus type 1 replication. J Virol. 2006;80:11710–11722. doi: 10.1128/JVI.01038-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K N, Holmes R K, Malim M H. Antiviral potency of APOBEC proteins does not correlate with cytidine deamination. J Virol. 2006;80:8450–8458. doi: 10.1128/JVI.00839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbisa J L, Barr R, Thomas J A, Vandegraaff N, Dorweiler I J, Svarovskaia E S, Brown W L, Mansky L M, Gorelick R J, Harris R S, Engelman A, Pathak V K. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, Yu X F. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J Virol. 2007;81:7238–7248. doi: 10.1128/JVI.02584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S M, Emerman M. Uracil DNA glycosylase is dispensable for human immunodeficiency virus type 1 replication and does not contribute to the antiviral effects of the cytidine deaminase Apobec3G. J Virol. 2006;80:875–882. doi: 10.1128/JVI.80.2.875-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrofelbauer B, Yu Q, Zeitlin S G, Landau N R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79:10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chen K, Zhang C, Huang S, Zhang H. Virion-associated uracil DNA glycosylase-2 and apurinic/apyrimidinic endonuclease are involved in the degradation of APOBEC3G-edited nascent HIV-1 DNA. J Biol Chem. 2007;282:11667–11675. doi: 10.1074/jbc.M606864200. [DOI] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu X F. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Mehle A, Goncalves J, Santa-Marta M, McPike M, Gabuzda D. Phosphorylation of a novel SOCS-box regulates assembly of the HIV-1 Vif-Cul5 complex that promotes APOBEC3G degradation. Genes Dev. 2004;18:2861–2866. doi: 10.1101/gad.1249904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Xiao Z, Ehrlich E S, Yu X, Yu X F. Selective assembly of HIV-1 Vif-Cul5-elonginB-elonginC E3 ubiquitin ligase complex through a novel SOCS box and upstream cysteines. Genes Dev. 2004;18:2867–2872. doi: 10.1101/gad.1250204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Xiao Z, Ehrlich E, Yu Y, Liu B, Zheng S, Yu X F. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc Natl Acad Sci U S A. 2005;102:11444–11449. doi: 10.1073/pnas.0502440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle A, Thomas E R, Rajendran K S, Gabuzda D. A zinc-binding region in Vif binds Cul5 and determines cullin selection. J Biol Chem. 2006;281:17259–17265. doi: 10.1074/jbc.M602413200. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Ehrlich E, Yu Y, Luo K, Wang T, Tian C, Yu X F. Assembly of HIV-1 Vif-Cul5 E3 ubiquitin ligase through a novel zinc-binding domain-stabilized hydrophobic interface in Vif. Virology. 2006;349:290–299. doi: 10.1016/j.virol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Ehrlich E, Luo K, Xiong Y, Yu X F. Zinc chelation inhibits HIV Vif activity and liberates antiviral function of the cytidine deaminase APOBEC3G. FASEB J. 2007;21:217–222. doi: 10.1096/fj.06-6773com. [DOI] [PubMed] [Google Scholar]
- Liu B, Sarkis P T, Luo K, Yu Y, Yu X F. Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase. J Virol. 2005;79:9579–9587. doi: 10.1128/JVI.79.15.9579-9587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M, Rose K M, Kozak S L, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- Sheehy A M, Gaddis N C, Malim M H. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2003;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Takaori-Kondo A, Kobayashi M, Tomonaga M, Izumi T, Fukunaga K, Sasada A, Abudu A, Miyauchi Y, Akari H, Iwai K, Uchiyama T. Ubiquitination of APOBEC3 proteins by the Vif-Cullin5-ElonginB-ElonginC complex. Virology. 2006;344:263–266. doi: 10.1016/j.virol.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Conticello S G, Harris R S, Neuberger M S. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Simon V, Zennou V, Murray D, Huang Y, Ho D D, Bieniasz P D. Natural variation in Vif: differential impact on APOBEC3G/3F and a potential role in HIV-1 diversification. PLoS Pathog. 2005;1:e6. doi: 10.1371/journal.ppat.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Yu X, Zhang W, Wang T, Xu R, Yu X F. Differential requirement for conserved tryptophans in human immunodeficiency virus type 1 Vif for the selective suppression of APOBEC3G and APOBEC3F. J Virol. 2006;80:3112–3115. doi: 10.1128/JVI.80.6.3112-3115.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrofelbauer B, Senger T, Manning G, Landau N R. Mutational alteration of human immunodeficiency virus type 1 Vif allows for functional interaction with nonhuman primate APOBEC3G. J Virol. 2006;80:5984–5991. doi: 10.1128/JVI.00388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R A, Pathak V K. Identification of two distinct human immunodeficiency virus type 1 Vif determinants critical for interactions with human APOBEC3G and APOBEC3F. J Virol. 2007;81:8201–8210. doi: 10.1128/JVI.00395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhang W, Chen G, Xu R, Yu X F. Characterization of conserved motifs in HIV-1 Vif required for APOBEC3G and APOBEC3F interaction. J Mol Biol. 2008;381:1000–1011. doi: 10.1016/j.jmb.2008.06.061. [DOI] [PubMed] [Google Scholar]
- Conticello S G, Thomas C J, Petersen-Mahrt S K, Neuberger M S. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22:367–377. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- OhAinle M, Kerns J A, Malik H S, Emerman M. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J Virol. 2006;80:3853–3862. doi: 10.1128/JVI.80.8.3853-3862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S L, Emerman M, Malik H S. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol. 2004;2:E275. doi: 10.1371/journal.pbio.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Wang X, Esselman W J, Zheng Y H. Identification of APOBEC3DE as another antiretroviral factor from the human APOBEC family. J Virol. 2006;80:10522–10533. doi: 10.1128/JVI.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska A M, Tian C, Tan L, Wang T, Sarkis P T, Yu X F. Differential inhibition of long interspersed element 1 by APOBEC3 does not correlate with high-molecular-mass-complex formation or P-body association. J Virol. 2007;81:9577–9583. doi: 10.1128/JVI.02800-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Liu B, Xiao Z, Yu Y, Yu X, Gorelick R, Yu X F. Amino-terminal region of the human immunodeficiency virus type 1 nucleocapsid is required for human APOBEC3G packaging. J Virol. 2004;78:11841–11852. doi: 10.1128/JVI.78.21.11841-11852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applied Biosystems. Foster City, CA, USA: Applied Biosystems; ABI Prism 7700 User Bulletin Number 2. 1997:11–15. [Google Scholar]
- Ostertag E M, Prak E T, DeBerardinis R J, Moran J V, Kazazian H H., Jr Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res. 2000;28:1418–1423. doi: 10.1093/nar/28.6.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli P, Vianin S, Trono D. The innate antiretroviral factor APOBEC3G does not affect human LINE-1 retrotransposition in a cell culture assay. J Biol Chem. 2004;279:43371–43373. doi: 10.1074/jbc.C400334200. [DOI] [PubMed] [Google Scholar]
- Kinomoto M, Kanno T, Shimura M, Ishizaka Y, Kojima A, Kurata T, Sata T, Tokunaga K. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res. 2007;35:2955–2964. doi: 10.1093/nar/gkm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouha B, Schustak J, Badge R M, Lutz-Prigge S, Farley A H, Moran J V, Kazazian H H., Jr Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci U S A. 2003;100:5280–5285. doi: 10.1073/pnas.0831042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J V, Holmes S E, Naas T P, DeBerardinis R J, Boeke J D, Kazazian H H., Jr High frequency retrotransposition in cultured mammalian cells. Cell. 1996;87:917–927. doi: 10.1016/s0092-8674(00)81998-4. [DOI] [PubMed] [Google Scholar]
- Dewannieux M, Esnault C, Heidmann T. LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003;35:41–48. doi: 10.1038/ng1223. [DOI] [PubMed] [Google Scholar]
- Lander E S, Linton L M, Birren B, Nusbaum C, Zody M C, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov J P, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin J C, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston R H, Wilson R K, Hillier L W, McPherson J D, Marra M A, Mardis E R, Fulton L A, Chinwalla A T, Pepin K H, Gish W R, Chissoe S L, Wendl M C, Delehaunty K D, Miner T L, Delehaunty A, Kramer J B, Cook L L, Fulton R S, Johnson D L, Minx P J, Clifton S W, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng J F, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Chiu Y L, Soros V B, Kreisberg J F, Stopak K, Yonemoto W, Greene W C. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- Peng G, Lei K J, Jin W, Greenwell-Wild T, Wahl S M. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, Ikai I, Chiba T. Anti-viral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem Biophys Res Commun. 2006;341:314–319. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- Sarkis P T, Ying S, Xu R, Yu X F. STAT1-independent cell type-specific regulation of antiviral APOBEC3G by IFN-alpha. J Immunol. 2006;177:4530–4540. doi: 10.4049/jimmunol.177.7.4530. [DOI] [PubMed] [Google Scholar]
- Ying S, Zhang X, Sarkis P T, Xu R, Yu X. Cell-specific Regulation of APOBEC3F by Interferons. Acta Biochim Biophys Sin (Shanghai) 2007;39:297–304. doi: 10.1111/j.1745-7270.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Bonvin M, Achermann F, Greeve I, Stroka D, Keogh A, Inderbitzin D, Candinas D, Sommer P, Wain-Hobson S, Vartanian J P, Greeve J. Interferon-inducible expression of APOBEC3 editing enzymes in human hepatocytes and inhibition of hepatitis B virus replication. Hepatology. 2006;43:1364–1374. doi: 10.1002/hep.21187. [DOI] [PubMed] [Google Scholar]
- Dang Y, Siew L M, Wang X, Han Y, Lampen R, Zheng Y H. Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J Biol Chem. 2008;283:11606–11614. doi: 10.1074/jbc.M707586200. [DOI] [PMC free article] [PubMed] [Google Scholar]