Abstract
Circulating monocytes, as dendritic cell and macrophage precursors, exhibit several functions usually associated with antigen-presenting cells, such as phagocytosis and presence of endosomal/lysosomal degradative compartments particularly enriched in Lamp-1, MHC class II molecules, and other proteins related to antigen processing and MHC class II loading [MHC class II compartments (MIICs)]. Ultrastructural analysis of these organelles indicates that, differently from the multivesicular bodies present in dendritic cells, in monocytes the MIICs are characterized by a single perimetral membrane surrounding an electron-dense core. Analysis of their content reveals enrichment in myeloperoxidase, an enzyme classically associated with azurophilic granules in granulocytes and mast cell secretory lysosomes. Elevation in intracellular free calcium levels in monocytes induced secretion of β-hexosaminidase, cathepsins, and myeloperoxidase in the extracellular milieu; surface up-regulation of MHC class II molecules; and appearance of lysosomal resident proteins. The Ca2+-regulated surface transport mechanism of MHC class II molecules observed in monocytes is different from the tubulovesicular organization of the multivesicular bodies previously reported in dendritic cells and macrophages. Hence, in monocytes, MHC class II-enriched organelles combine degradative functions typical of lysosomes and regulated secretion typical of secretory lysosomes. More important, Ca2+-mediated up-regulation of surface MHC class II molecules is accompanied by extracellular release of lysosomal resident enzymes.—Bunbury, A., Potolicchio, I., Maitra, R., Santambrogio, L. Functional analysis of monocyte MHC class II compartments.
Keywords: MHCII trafficking, calcium flux, MIIC, lysosomes, cathepsins
Peripheral blood monocytes comprise a heterogeneous population of nondividing circulating myeloid precursors. Two principal subsets of monocytes have been described in humans: the CD14+ CD16+ cells that localize to noninflamed peripheral tissue to further differentiate into myeloid dendritic cells (DCs) and macrophages (1, 2), and the CD14+ CD16− population that is only recruited to inflamed tissue and that rapidly differentiates into DCs (1).
Circulating monocytes, as DC precursors, have different functions normally associated with antigen-presenting cells: phagocytosis of extracellular proteins and the presence of endosomal/lysosomal degradative compartments particularly enriched in MHC class II molecules and other proteins important for antigen processing, loading, and peptide editing [invariant chain, cathepsins, human leukocyte antigen (HLA) -DM] (3). These specialized organelles are commonly referred to as MHC class II compartments (MIICs) (4, 5). Ultrastructural analysis of monocyte MIICs, which we previously termed electron-dense bodies (EDBs), indicate that the majority of these compartments are delimited by a single perimetral membrane and are occupied by a core electron-dense material surrounded by few vesicles (3). In contrast, immature DCs derived from monocytes differentiated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL) -4 MIICs are organized as multivesicular bodies (MVBs), which are formed by a limiting membrane enclosing a few to several internal vesicles or as multilamellar bodies (MLBs) (6, 7), which are organized in a series of concentric lamellae (8). We previously reported that monocyte maturation into immature DCs is accompanied by an increase in the number of late endosomal MVBs and a morphological transformation of the lysosomal EDBs into MLBs (3). These qualitative and quantitative changes in the MIIC populations likely reflect increased influx of newly synthesized proteases, MHC class II molecules, and related chaperones that occurs on monocyte differentiation by GM-CSF and IL-4. It was previously reported that induced synthesis of MHC class II molecules is sufficient for the formation of MIIC-like multilamellar structures even in nonprofessional antigen-presenting cells (9).
The modality of MHC class II surface egress in relation to MIIC morphology has only begun to be investigated. Either multivesicular endosomes can fuse their limiting membrane with the plasma membrane and release the internal vesicle “exosomes” into the extracellular milieu (10, 11), or for a much more efficient surface MHC II transport, the vesicles fuse back onto the limiting membrane, allowing the formation of long tubular structures (12). Similar tubular structures were also observed originating from MLBs (13), even though it is still unclear whether only the more external membrane or also the inner ones can tubulate for surface protein transport (14). The modality of MHC class II transport from the monocyte EDBs is currently unknown.
Structurally, EDBs closely resemble secretory lysosomes, which are a combination of lysosomes and secretory granules because they are Lamp+ compartments that contain both protein hydrolases and specialized secretory products (15, 16). In T cells and mast cells, there is a high degree of overlap between secretory and lysosomal markers. On the other hand, in granulocytes both true lysosomes and secretory lysosomes are present (17,18,19). Secretory granules have been previously identified in monocytes, some containing transforming growth factor α (TGF-α) and others positive for myeloperoxidase, cathepsin G, and elastase (20). Whether any of these subtypes are also MHC class II positive is still unknown.
Exocytosis of secretory lysosomes is regulated by the intracellular level of Ca2+. Elevation in the level of intracellular free calcium induces fusion of the lysosomes with the plasma membrane, with subsequent secretion of β-hexosaminidase and serine and aspartic proteases into the extracellular milieu and appearance of lysosomal resident proteins at the plasma membrane (16). Herein we investigated whether monocyte EDBs are indeed secretory granules enriched in MHC class II molecules and whether their surface trafficking involves calcium-mediated fusion at the plasma membrane.
MATERIALS AND METHODS
Peripheral blood mononuclear cell preparation
Lymphomononuclear cells were separated over a Ficoll gradient, and the monocyte population was separated using CD14-conjugated MicroBeads (Miltenyi Biotec, Auburn, CA, USA). In some experiments, purified CD14+ cells were differentiated in the presence of GM-CSF (30 ng/ml) in complete RPMI 1640 (Gibco, Grand Island, NY, USA) (4).
Ionomycin and Ca2+ treatment
Cells (10×106) were incubated with either PBS or 1, 10, or 30 μM ionomycin for the indicated times. To observe lysosomal secretion, cells (5–10×106) were washed twice in ice-cold buffer A (20 mM Hepes, 110 mM NaCl, 5.4 mM KCl, 0.9 mM Na2HPO4, 10 mM MgCl2, 2 mM CaCl2, 11 mM glucose). Cells were then incubated at 4°C with Streptolysin O (0.5 U/ml; Sigma, St. Louis, MO, USA) in buffer A for 10 min and subsequently washed in buffer B (20 mM Hepes, 100 mM K-glutamate, 40 mM KCl, 5 mM EGTA). Mg-ATP (2 mM), 5 mM MgCl2, and 1 mM CaCl2 were then added to cells in buffer B to induce lysosomal secretion.
Flow cytometry
Cells were labeled for 30 min on ice with saturating amounts of primary monoclonal antibody (mAb; fluorescein isothiocyanate or phycoerythrin conjugated) in staining buffer (PBS, 0.1% BSA, 0.01% NaN3). The following anti-human mAbs were used: CD107a (clone H4A3), HLA-DR (clone L243), empty HLA-DR (clone MEM-265) (22), CD1a (clone 10D12.2), and CD63 (clone MX-49). The samples were analyzed with a flow cytometer (FACSCalibur™; BD Biosciences, San Jose, CA, USA).
Measurement of β-hexosaminidase, myeloperoxidase, and cathepsin S in the supernatant
Ten microliters of incubation buffer from ionomycin-treated or untreated cells was added to 100 μl of reaction mixture (5 ml 0.4 M sodium acetate, pH 4.4; 5 ml 8 mM 4-methylumbelliferyl-N-acetyl-B-d-glucopyranoside in water; 0.25 ml Triton X-100; 9.75 ml water) in a 96-well plate incubated at 37°C for 40 min. The reaction was stopped by addition of 75 μl of 2 M Na2CO3, and fluorescence was measured in a spectrofluorimeter (FluorStar Optima, BMG Labtechnologies, Durham, NC, USA) at excitation 370 nm, emission 450 nm.
To assay cathepsin S and myeloperoxidase in the culture supernatant, monocytes (5–10×106) were treated with either PBS or 10 or 30 μM ionomycin. The incubation buffer was collected, concentrated, and normalized for protein content. Samples (100 μg total protein) were electrophoresed through a 10% SDS-PAGE and electroblotted to a nitrocellulose membrane. The membrane was probed with anti-cathepsin S (clone C-19) or myeloperoxidase (clone C-16) primary antibody, followed by a secondary antibody coupled with peroxidase and using a chemiluminescence (ECL) detection kit (Pierce, Rockford, IL, USA).
Subcellular fractionation
Cells (100×106) were lysed in homogenization buffer (250 mM sucrose, 1 mM EDTA, pH 7.4). Early and late endosomes and lysosomes were prepared over consecutive Percoll gradients (27 and 10%). Each 1 ml fraction was tested for β-hexosaminidase to locate lysosomes and late endosomes. The late endocytic marker Lamp-1 (clone 1D4B; BD Pharmingen, San Diego, CA, USA) and the early endosomes and plasma membrane marker transferrin receptor (TrfR) (clone M-A712, BD Pharmingen) were used to assess the purity of the endosomal preparations. Pulled fractions 6–7 from the 27% Percoll gradient (lysosomes) and 3–4 from the 10% Percoll gradient (late endosomes) were lysed in Nonident P-40 (NP-40) lysis buffer (150 mM NaCl, 50 mM Tris HCl, 5 mM EDTA, 1% NP-40). Proteins were resolved through a 12% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with anti-HLA-DR (Champ; gift from L. J. Stern, University of Massachusetts, Worcester, MA, USA), Lamp-1 (clone H4A3), cathepsin S (clone C-19), invariant chain (clone Pin 1), and myeloperoxidase (clone C-16).
Confocal microscopy
Monocytes cultured onto collagen-treated glass coverslips for 2 h were fixed for 10 min in 1% paraformaldehyde, washed twice in 10 mM glycine, and permeabilized for 30 min in 0.05% saponin, 0.2% BSA, and 0.1% sodium azide. The following primary antibodies were used at 10 μg/ml: anti-human HLA-DR, DQ, and DP (BD Pharmingen); anti-cathepsin G (Abcam, Cambridge, MA, USA), and anti-human myeloperoxidase (clone C-16; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Secondary antibodies used were anti-mouse IgG-Alexa 488 and anti-rabbit-Alexa 568 (Invitrogen, Carlsbad, CA, USA). Fluorescence was viewed using a Leica AOBS scanning confocal microscope (Leica Microsystems, Wetzlar, Germany).
Immunogold labeling
Monocytes grown in the presence or absence of GM-CSF were fixed with a mixture of 2% paraformaldehyde and 4% polyvinyl pyrrolidone (PVP) in 0.2 M phosphate buffer (pH 7.4) at 4°C and fixed for ultrathin cryosectioning (4). Immunogold labeling was performed using primary antibodies in combination with protein A coupled to either 10 or 15 nm gold particles. Contrast was obtained with a mixture of 2% methylcellulose (Sigma) and 0.4% uranyl acetate (pH 4; EMS, Hatfield, PA, USA). Samples were viewed under an electron microscope (CM120; Philips, Eindhoven, The Netherlands). The following antibodies were used: polyclonal anti-HLA-DR rabbit sera raised against purified HLA-DR1 (21), mouse mAb against human Lamp-1 (clone H4A3), MEM-265 mouse IgG2b specific for empty/open HLA-DR molecules (22), myeloperoxidase (clone C-16), and TGF-α (R&D Systems, Minneapolis, MN, USA).
Whole mount
Monocytes were cultured for 2 d on gold grids (200 mesh; EMS). Cells were allowed to endocytose horseradish peroxidase (HRP) for 1 h, after which lipopolysaccharide (LPS) or ionomycin (10 μM) was added for 2 h at 37°C. Grids were then incubated in 1.5 mg/ml 3,3′-diaminobenzidine (DAB) buffer (70 mM NaCl, 50 mM ascorbic acid, and 20 mM HEPES, pH 7.0, supplemented with H2O2 (0.6 μl/ml). Grids were dried using a critical-point dryer (Samdri-795; Tousimis Research Corporation, Rockville, MD, USA) and examined at 60–80 kV under a Philips CM120 electron microscope.
Scanning electron microscopy
Monocytes (5×106) were plated for 2 h on fibronectin-coated coverslips (BD BioCoat; BD Biosciences) and either left untreated or treated with ionomycin as described above. At the end of the treatment, cells were washed in PBS and fixed in 2.5% gluteraldehyde and 0.1 M cacodylate for 30 min at room temperature. Cells were dehydrated in ethanol and dried using a critical-point dryer (Samdri-795). Cells were then coated in gold-palladium alloy and examined under a JSM 6400 scanning microscope (Jeol Ltd., Akishima, Japan).
RESULTS
EDBs are a lysosomal MIIC present in monocytes
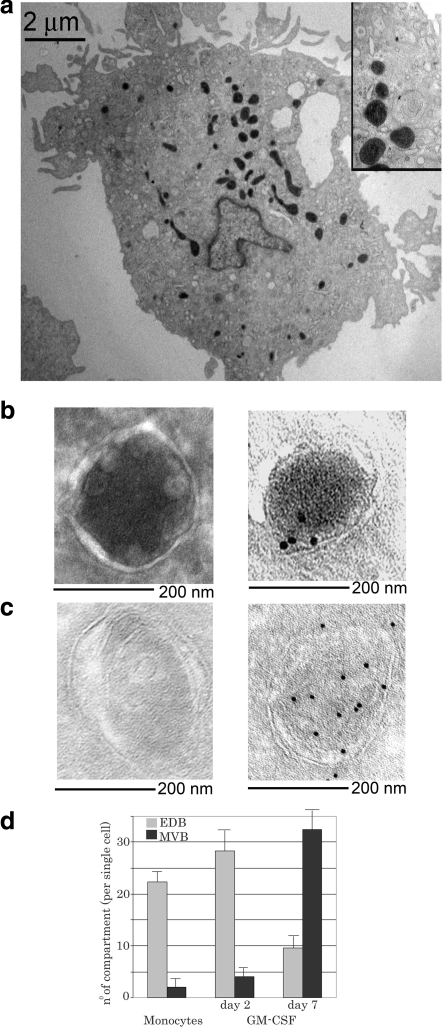
Ultrastructurally different MIICs are observed among different antigen-presenting cells. In circulating CD14+ monocytes, the majority of MIICs (20–25 compartments/cell) have an ultrastructural morphology characterized by an electron-dense core surrounded by membrane vesicles juxtaposed to the limiting membrane (Fig. 1A, B). We previously termed these MIICs EDBs (3). The few remaining MIICs (2–4/cell) are of the multivesicular type, formed by a limiting membrane enclosing several vesicles with an electron-lucent ultrastructure (Fig. 1C) (3, 5). A differentiation process initiated by GM-CSF increases the number of MVBs and decreases the number of EDBs. Monocytes differentiated with GM-CSF for 7 d increase the number of MVBs (>30 compartments/cell) and decrease the EDBs (8–12 compartments/cell) (Fig. 1D). These data indicate that in monocytes, EDBs are the most abundant MIICs.
Figure 1.
EDBs are the major Lamp-1+ compartments in human monocytes. A) Ultrathin cryosection of a monocyte. Endosomal compartments are visible following HRP endocytosis and 3,3′-diaminobenzidine staining. B, C) Images (×30,000) of a typical EDB (B) and a typical MVB (C) unlabeled (left panels) or immunogold labeled for MHC class II protein (right panels). D) Quantification of the number of EDBs vs. MVB late endosomal compartments in circulating CD14+ monocytes or monocytes treated with GM-CSF for 2 or 7 d. Monocyte preparations from 4 different subjects were analyzed.
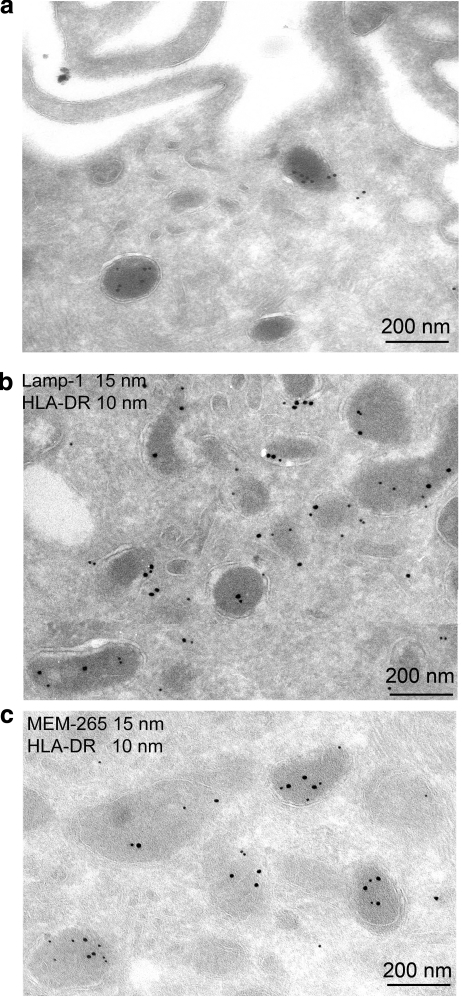
EDBs are defined as lysosomal MIICs because all EDBs positive for MHC class II molecules were also LAMP-1 positive, as determined by immunogold labeling (Fig. 2A, B) as well as processed invariant chain and HLA-DM (3). Also, empty HLA-DR molecules could be detected in these compartments by immunostaining using a polyclonal anti-HLA-DR antibody and MEM-265, a monoclonal antibody specific for empty HLA-DR molecules, together with 10 and 15 nm protein A gold particles, respectively (Fig. 2C).
Figure 2.
EDBs are HLA-DR+ compartments. Ultrathin cryosections of GM-CSF-treated monocytes. A) Ultrathin cryosection of GM-CSF-treated monocytes and immunogold labeling of EDBs with Lamp-1 (10 nm gold). B) Immunogold labeling with Lamp-1 (15 nm gold) and HLA-DR (10 nm gold). C) Immunogold labeling for empty HLA-DR (MEM-265; 15 nm gold) and total HLA-DR (10 nm gold). Monocyte preparations from 4 different subjects were analyzed.
EDBs are myeloperoxidase-positive MIICs
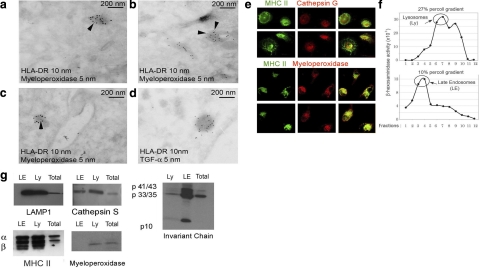
Ultrastructurally, the monocyte EDBs resemble the secretory lysosomes previously characterized in mast cells (19). In monocytes, EDBs have been previously subdivided as myeloperoxidase positive or negative (20). The former contains several proteolytic enzymes (cathepsins, elastase myeloperoxidase), whereas the latter have been characterized as storage granules for TGF-α (20). Thus, as a next step, the colocalization of HLA-DR with myeloperoxidase or TGF-α was examined. Immuno-electron microscopy using anti-HLA-DR1 and myeloperoxidase antibodies indicated that ∼80% of HLA-DR+ EDBs were also myeloperoxidase positive. However, it is possible that this is an underestimation, because the myeloperoxidase antibody did not provide a strong labeling (arrows in Fig. 3A–C indicate 5 nm gold myeloperoxidase labeling). On the other hand, both HLA-DR and TGF-α antibodies provided a strong labeling. Because colocalization of HLA-DR and TGF-α was never detected, we concluded that all TGF-α+ compartments were HLA-DR− (all gold particles in Fig. 3D are 5 nm in size). Colocalization between HLA-DR, cathepsin G, and myeloperoxidase was further validated by confocal microscopy (Fig. 3E). To confirm further the presence of myeloperoxidase in lysosomal compartments, a subcellular fractionation was performed. Purified CD14+ circulating monocytes (80×106) were lysed and fractionated over a two-step consecutive Percoll gradient (27 and 10%). Each fraction was tested for β-hexosaminidase to locate lysosomes and late endosomes (Fig. 3F). Pulled lysosomal fractions 6–7 from the 27% Percoll gradient (lysosomes) and 2–5 from the 10% Percoll gradient (late endosomes) were analyzed for endosomal resident proteins as well as myeloperoxidase. As expected, MHC class II proteins, cathepsin S, and Lamp-1 were enriched in late endosomal and lysosomal fractions as compared to total cell lysates (Fig. 3G) Invariant chains were mostly processed in lysosomes as compared to late endosomes, where both p33/35 and p41/43 forms were visible (Fig. 3G) Myeloperoxidase was mostly found in the high-density lysosomal fraction and barely evident in the late endosomal fraction (Fig. 3G). In conclusion, both electron and confocal microscopy indicated that myeloperoxidase-positive granules were also HLA-DR+, whereas MIIC subcellular fractionation revealed that most myeloperoxidase is found in the dense lysosomal fraction.
Figure 3.
EDBs are myeloperoxidase positive and TGF-α negative. A–D) Ultrathin cryosections of monocytes and immunogold labeling: HLA-DR (10 nm gold) and myeloperoxidase (5 nm gold) (A–C); HLA-DR (10 nm gold) and TGF-α (5 nm gold) (D). E) Confocal immunostaining for MHC class II and cathepsin G (top panels) and myeloperoxidase (bottom panels). F) β-Hexosaminidase activity measured in each fraction (1 ml) of a 10/27% 2-step Percoll gradient to separate lysosomes and late endosomes. G) Western blot analysis of pulled fractions 6–7 of the 27% Percoll gradient (lysosomes, Ly), fractions 3–4 of the 10% Percoll gradient (late endosomes, LE) and total cell lysates (total). Membranes were probed using a rabbit serum recognizing both α and β subunits of HLA-DR1 or mAb specific for Lamp-1, cathepsin S, invariant chain, and myeloperoxidase. Monocyte preparations from 4 different subjects were analyzed.
EDBs behave as secretory lysosomes
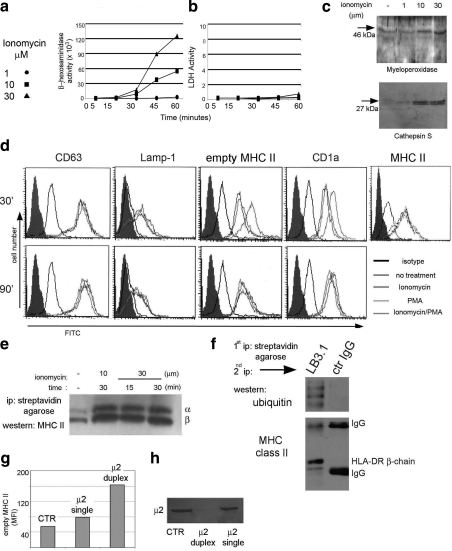
Extracellular release of lysosomal contents has been described in several cell types, such as macrophages and activated platelets (3,4,5). In these cells, lysosomal exocytosis is regulated by increases in Ca2+. To determine whether an increase in Ca2+ would stimulate exocytosis of EDBs, the calcium ionophore ionomycin was used. Human monocytes were incubated in the presence or absence of different concentrations of ionomycin at 37°C for different time points. Release of the lysosomal enzyme β-hexosamindase, frequently used as a marker for lysosomes, was then measured in the incubation buffer. A dose- and time-dependent release of β-hexasomindase was triggered by ionomycin (Fig. 4A). Similarly, secretion of cathepsin S and myeloperoxidase into the supernatant was observed after treatment with ionomycin. Again, the release was time and concentration dependent (Fig. 4C and data not shown). On the other hand, no release of cytosolic enzymes, such as lactic-dehydrogenase, was observed (Fig. 4B). Additional evidence for Ca2+-dependent EDB fusion with the plasma membrane was provided by the appearance on the plasma membrane of proteins normally expressed at the lysosomal limiting membrane. After treating monocytes with 10 or 30 μM ionomycin, surface staining was performed at 4°C with an mAb specific for Lamp-1, CD-1a, CD63, and MHC II. A gradual increase in these proteins was detected over time in ionomycin-treated cells, as compared to control buffer-treated cells (Fig. 4D). A similar increase in surface MHC class II proteins was observed on monocyte induction with ionomycin in the presence of non-cell permeable biotin. This treatment allows biotinylation of all surface proteins and their detection even after they have been internalized. Cells were then lysed, and biotinylated proteins were precipitated with streptavidin agarose and run on an SDS-PAGE after protein normalization. MHC II was then detected using a rabbit polyclonal that recognizes HLA-DR α and β chains. An increase in biotinylated MHC class II proteins was observed after ionomycin treatment in a time- and concentration-dependent manner (Fig. 4E).
Figure 4.
EDBs behave as secretory lysosomes. A) β-Hexosaminidase detection in the cell culture supernatant after treatment of monocytes with different concentrations of ionomycin for different times. B) Lactic-dehydrogenase detection in the cell culture supernatant after treatment of monocytes with different concentrations of ionomycin for different times. C) Western blot analysis for cathepsin S and myeloperoxidase secreted in the culture supernatant after ionomycin treatment of primary monocytes. D) Surface immunostaining of monocytes untreated or after ionomycin, phorbol-12-myristate-13-acetate (PMA), or ionomycin/PMA treatment. E) Western blot analysis of MHC class II proteins following ionomycin treatment, surface biotinilation, and streptavidin agarose pulldown. F) Western blot analysis of ubiquitinated surface biotinylated MHC class II proteins. G) Surface MHC class II protein staining of monocytes nontransfected (control) or transfected with single or duplex siRNA to the AP-2 adaptor complex. Data are reported as mean fluorescence intensity. H) Western blot analysis of monocytes nontransfected (control) or transfected with single or duplex siRNA. Membrane is probed with the μ2 mAb. Each reported experiment was performed between 3 and 5 times.
In immature DCs, it has been shown that the low amount of surface MHC class II proteins is due to rapid recycling of MHC class II proteins through either protein ubiquitination or AP-2-mediated internalization (23,24,25). To determine whether in a pre-DC population a similar mechanism could be controlling surface MHC class II protein expression, biotinylated surface proteins were immunoprecipitated with streptavidin agarose and after elution reprecipitated with an HLA-DR-specific antibody. Eluted proteins, analyzed by Western blotting, indicated that surface MHC class II molecules are ubiquitin bound (Fig. 4F). Thus ubiquitination could control MHC class II protein internalization in monocytes, as previously shown for immature DCs (24, 25). To determine a possible AP-2-dependent internalization pathway, we transfected monocytes with 5′-FITC-labeled siRNA specific for the μ2 subunit of AP2. Both μ2 and control siRNA exhibited similar levels of positively transfected cells (80 vs. 75% for μ2 and control siRNA, respectively; data not shown). Western blot analysis at 48 h postinfection showed a reduction of μ2 protein expression in μ2 siRNA-transfected cells as compared to nontransfected or single siRNA-transfected cells (Fig. 4H). In the same population, an increase in the amount of HLA-DR1 at the cell surface was observed in μ2 siRNA (Fig. 4G). These data indicate that the surface adaptor AP-2 is functionally involved in MHC class II protein internalization in monocytes (3, 26, 27).
EDB modification on ionomycin treatment
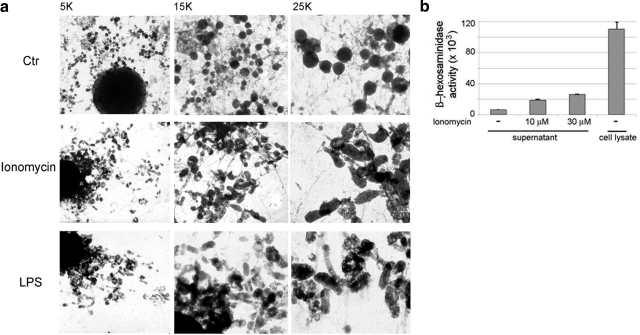
MHC class II protein surface trafficking from endosomal compartments in DCs is accompanied by profound modifications of the endosomal morphology in which multilamellar and multivesicular compartments assume a tubular morphology (12, 14, 28). Morphological modifications that accompany MHC class II protein egress from EDBs, the primary compartments in monocytes, are not known. A whole-mount electron microscopy technique was utilized to address EDB modification on ionomycin treatment. In all experiments, LPS treatment was used as a positive control. Morphological changes in endosomal compartments were observed in monocytes treated with ionomycin and LPS. In untreated monocytes, HRP-containing compartments were characterized by a round shape and were strongly electron dense (Fig. 5A). On ionomycin treatment, a shape change was observed. Elongated structures with a club-like morphology and much shorter than the tubules previously observed in maturing DCs (12) were identified in all cells. Also, a decrease in electron density was associated with the changes in morphology. This likely reflects that absorption of the internal limiting membrane had occurred. Similar morphological changes were observed in LPS-treated cells (Fig. 5A).
Figure 5.
Ionomycin-induced remodeling of the endocytic system. A) Overview of ionomycin-induced endosomal modification by whole-mount electron microscopy. B) β-Hexosaminidase detection in the cell culture supernatant of ionomycin-treated or untreated monocytes. β-Hexosaminidase detected in total cell lysate, after protein normalization between the different samples, is shown as control. Monocyte preparations from 3 different subjects were analyzed.
Analysis of the amount of β-hexosaminidase released extracellularly as compared to the total amount of cellular hexosaminidase indicated that ∼20% of the enzyme was secreted under the experimental conditions reported in the present study (Fig. 5B).
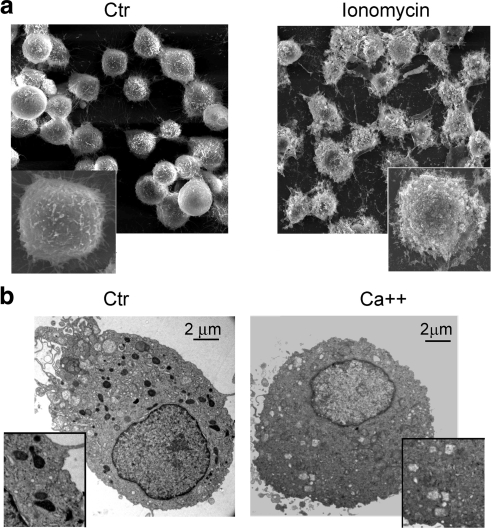
Low-magnification scanning microscopy identified ruffling of the plasma membrane on monocyte treatment with ionomycin (Fig. 6A). Finally, extracellular secretion could be visualized by loss of label in previously HRP-labeled EDB compartments following calcium-induced secretion (Fig. 6B).
Figure 6.
Surface lysosomal exocytosis following ionomycin treatment. A) Surface scanning microscopy of control or ionomycin-treated monocytes. B) Visualization of HRP-positive endosomal compartment before (control) and after (Ca2+) calcium-induced degranulation. Monocyte preparations from 3 different subjects were analyzed.
DISCUSSION
In the present study, several aspects of the MIICs present in CD14+ monocytes were characterized.
First, unlike more professional antigen-presenting cells, in which MIICs have a multivesicular and multilamellar morphology, in monocytes the majority of MIICs appear as classical lysosomal EDBs, with a few vesicles juxtaposed to the limiting membrane (3,4,5, 14, 28). The ultrastructure of these compartments closely resembles that of the type II MHC class II-positive compartments previously reported in mast cells, characterized by an electron-dense core surrounded by several vesicles juxtaposed to the limiting membrane (19). EDBs were previously described in monocytes as either myeloperoxidase-positive or -negative compartments (20). The former were reported to be particularly enriched in cathepsin G, elastase, and myeloperoxidase, whereas the latter were TGF-α positive but myeloperoxidase negative (20). We now report that the myeloperoxidase-positive EDBs are Lamp-1- and MHC class II-positive compartments, as determined by immunogold staining as well as analysis of endosomal organelles following subcellular fractionation.
Second, we determined that the EDB MIICs behave as secretory lysosomes. To confirm the lysosomal nature of the secretion, different approaches were used: 1) measurement of the release into the extracellular medium of the enzymes β-hexosaminidase, cathepsin S, and myeloperoxidase (Figs. 4 and 5); 2) appearance on the cell surface of lysosomal resident proteins (Lamp-1, MHC II, CD63) (Fig. 4); and 3) a Ca2+-induced degranulation assay (Fig. 6).
Calcium-regulated exocytosis of lysosomal compartments has been reported in several cell types (15, 16). Granulocytes secrete the content of lysosomal-related azurophil granules as part of their innate immune response to pathogens (29, 30), and mast-cell lysosomes also secrete several inflammatory mediators in response to external stimuli (18). In osteoclasts, Ca2+-regulated lysosomal secretion is an important event for bone remodeling (31). Release of lysosomal content from platelet and pancreatic acinar cells is also a Ca2+-regulated process (32). Recently, it has also been reported that lysosomal exocytosis is a generalized process common to every cell type, triggered by damage as a means to repair the plasma membrane; such localized Ca2+ influx triggers the fusion of nearby lysosomes with the damaged membrane (33).
The Ca2+-regulated secretion observed in monocytes is different from the regulated retrograde transport described for MVBs and MLBs (12, 13). In DCs, on LPS treatment MHC class II molecules relocate onto the plasma membrane following a tubulovesicular transport (12), which requires an ultrastructural remodeling of the endosomal compartments, with retrograde fusion of the inner vesicles (for MVBs) or outer membrane (for MLBs) onto the limiting membrane. On the other hand, in monocyte MIICs, the long tubular extensions typical of maturing DCs (12) could not be observed following either ionomycin or LPS treatment. This is consistent with the very low number of MVBs (1–2/cell) present in monocytes compared to that observed in GM-CSF- and IL-4-differentiated DCs (Fig. 1C). More important, in monocytes, Ca2+-regulated exocytosis allows a rapid secretion of lysosomal enzymes, such as β-hexosaminidase, myeloperoxidase, and cathepsins, in the extracellular milieu, whereas in DCs, the exocytosis process appears to specifically single out MHC class II peptide complexes while retaining lysosomal enzymes and resident proteins (12, 34).
Monocytes are transitional cells, with a very short half-life due to rapid differentiation into macrophages or DCs. Several studies have investigated the cellular differentiation potential of monocytes (1, 2); however, the role of monocytes in innate or adaptive immune responses has not been thoroughly investigated. Herein we characterized the ultrastructure of monocyte EDBs and found that these organelles combine properties related to MHC class II presentation, as well as inducible secretion of myeloperoxidase, an enzyme classically associated with native immune responses of granulocytes. Monocytes are rapidly recruited to sites of inflammation (1); thus, secreted proteases could contribute to extracellular killing of microorganisms, as previously shown for granulocytes (35). Released serine proteases could also be implicated in noninfectious inflammatory processes, such as ischemia, reperfusion injury, and autoimmune diseases (36). Extracellular processing of chemokines and cytokines is a well-defined means to increase or decrease their biological activity. Serine proteases are major players in this process, as, for example, the increase in chemotactic activity noted for CXCL5, CCL15, and chemerin following N-terminal processing by cathepsin G. On the other hand, cathepsin G processing of IL-6 and TNF-α inactivates the biological activity of these cytokines. The ability of monocytes to secrete aspartic and serine proteases, which in turn modulates the activity of chemokines and cytokines, could influence cell recruitment at inflammatory sites.
Thus, besides their role as cellular precursors to dendritic cells and macrophages, monocytes may play an important role in innate immune responses through enzymes exocytosed during inflammatory processes.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (L.S.) and Irene Diamond Professorships in Immunology (L.S.). The authors declare no conflicts of interest.
References
- Geissmann F, Jung S, Littman D R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Randolph G J, Inaba K, Robbiani D F, Steinman R M, Muller W A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Potolicchio I, Chitta S, Xu X, Fonseca D, Crisi G, Horejsi V, Strominger J L, Stern L J, Raposo G, Santambrogio L. Conformational variation of surface class II MHC proteins during myeloid dendritic cell differentiation accompanies structural changes in lysosomal MIIC. J Immunol. 2005;175:4935–4947. doi: 10.4049/jimmunol.175.8.4935. [DOI] [PubMed] [Google Scholar]
- Kleijmeer M J, Raposo G, Geuze H J. Characterization of MHC class II compartments by immunoelectron microscopy. Methods. 1996;10:191–207. doi: 10.1006/meth.1996.0095. [DOI] [PubMed] [Google Scholar]
- Stern L J, Potolicchio I, Santambrogio L. MHC class II compartment subtypes: structure and function. Curr Opin Immunol. 2006;18:64–69. doi: 10.1016/j.coi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Peters P J, Neefjes J J, Oorschot V, Ploegh H L, Geuze H J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Peters P J, Raposo G, Neefjes J J, Oorschot V, Leijendekker R L, Geuze H J, Ploegh H L. Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes. J Exp Med. 1995;182:325–334. doi: 10.1084/jem.182.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk J L, Lebbink M N, Humbel B M, Geerts W J, Griffith J M, Langenberg D M, Verreck F A, Verkleij A J, Koster A J, Geuze H J, Kleijmeer M J. 3-D Structure of multilaminar lysosomes in antigen presenting cells reveals trapping of MHC II on the internal membranes. Traffic. 2004;5:936–945. doi: 10.1111/j.1600-0854.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Calafat J, Nijenhuis M, Janssen H, Tulp A, Dusseljee S, Wubbolts R, Neefjes J. Major histocompatibility complex class II molecules induce the formation of endocytic MIIC-like structures. J Cell Biol. 1994;126:967–977. doi: 10.1083/jcb.126.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer M J, Geuze H J, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky A Y, Ossendorp F, Melief C J, Stoorvogel W, Geuze H J. Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol. 2001;155:53–63. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barois N, de Saint-Vis B, Lebecque S, Geuze H J, Kleijmeer M J. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- Murk J L, Humbel B M, Ziese U, Griffith J M, Posthuma G, Slot J W, Koster A J, Verkleij A J, Geuze H J, Kleijmeer M J. Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc Natl Acad Sci U S A. 2003;100:13332–13337. doi: 10.1073/pnas.2232379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. What’s special about secretory lysosomes? Semin Cell Dev Biol. 2002;13:279–284. doi: 10.1016/s1084-9521(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Holt O J, Gallo F, Griffiths G M. Regulating secretory lysosomes. J Biochem (Tokyo) 2006;140:7–12. doi: 10.1093/jb/mvj126. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J C, Griffiths G M. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–305. doi: 10.1016/j.smim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Thery C, Mazzeo D, Tenza D, Raposo G, Bonnerot C. Secretory granules of mast cells accumulate mature and immature MHC class II molecules. J Cell Sci. 2001;114:323–334. doi: 10.1242/jcs.114.2.323. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J, Janssen H, Stahle-Backdahl M, Zuurbier A E, Knol E F, Egesten A. Human monocytes and neutrophils store transforming growth factor-alpha in a subpopulation of cytoplasmic granules. Blood. 1997;90:1255–1266. [PubMed] [Google Scholar]
- Neefjes J J, Stollorz V, Peters P J, Geuze H J, Ploegh H L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Carven G J, Chitta S, Hilgert I, Rushe M M, Baggio R F, Palmer M, Arenas J E, Strominger J L, Horejsi V, Santambrogio L, Stern L J. Monoclonal antibodies specific for the empty conformation of HLA-DR1 reveal aspects of the conformational change associated with peptide binding. J Biol Chem. 2004;279:16561–16570. doi: 10.1074/jbc.M314315200. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Strominger J L. The ins and outs of MHC class II proteins in dendritic cells. Immunity. 2006;25:857–859. doi: 10.1016/j.immuni.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ohmura-Hoshino M, Matsuki Y, Aoki M, Goto E, Mito M, Uematsu M, Kakiuchi T, Hotta H, Ishido S. Inhibition of MHC class II expression and immune responses by c-MIR. J Immunol. 2006;177:341–354. doi: 10.4049/jimmunol.177.1.341. [DOI] [PubMed] [Google Scholar]
- Van Niel G, Wubbolts R, Ten Broeke T, Buschow S I, Ossendorp F A, Melief C J, Raposo G, van Balkom B W, Stoorvogel W. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Dugast M, Toussaint H, Dousset C, Benaroch P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J Biol Chem. 2005;280:19656–19664. doi: 10.1074/jbc.M501357200. [DOI] [PubMed] [Google Scholar]
- McCormick P J, Martina J A, Bonifacino J S. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci U S A. 2005;102:7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murk J L, Stoorvogel W, Kleijmeer M J, Geuze H J. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin Cell Dev Biol. 2002;13:303–311. doi: 10.1016/s1084952102000605. [DOI] [PubMed] [Google Scholar]
- Tapper H. The secretion of preformed granules by macrophages and neutrophils. J Leukoc Biol. 1996;59:613–622. doi: 10.1002/jlb.59.5.613. [DOI] [PubMed] [Google Scholar]
- Fittschen C, Henson P M. Linkage of azurophil granule secretion in neutrophils to chloride ion transport and endosomal transcytosis. J Clin Invest. 1994;93:247–255. doi: 10.1172/JCI116952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaraniemi J, Halleen J M, Kaarlonen K, Ylipahkala H, Alatalo S L, Andersson G, Kaija H, Vihko P, Vaananen H K. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res. 2004;19:1432–1440. doi: 10.1359/JBMR.040603. [DOI] [PubMed] [Google Scholar]
- Polasek J. Platelet secretory granules or secretory lysosomes? Platelets. 2005;16:500–501. doi: 10.1080/09537100500169926. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler E V, Andrews N W. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Potolicchio I, Fessler S P, Wong S H, Raposo G, Strominger J L. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- Raptis S Z, Shapiro S D, Simmons P M, Cheng A M, Pham C T. Serine protease cathepsin G regulates adhesion-dependent neutrophil effector functions by modulating integrin clustering. Immunity. 2005;22:679–691. doi: 10.1016/j.immuni.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pham C T. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]