Abstract
Adiponectin is a major insulin-sensitizing, multimeric hormone derived from adipose tissue that acts on muscle and liver to regulate whole-body glucose and lipid metabolism. Here, we describe a novel and highly conserved paralog of adiponectin designated as C1q/TNF-related protein (CTRP) 9. Of all the CTRP paralogs, CTRP9 shows the highest degree of amino acid identity to adiponectin in its globular C1q domain. CTRP9 is expressed predominantly in adipose tissue and females expresses higher levels of the transcript than males. Moreover, its expression levels in ob/ob mice changed in an age-dependent manner, with significant up-regulation in younger mice. CTRP9 is a secreted glycoprotein with multiple post-translational modifications in its collagen domain that include hydroxylated prolines and hydroxylated and glycosylated lysines. It is secreted as multimers (predominantly trimers) from transfected cells and circulates in the mouse serum with levels varying according to sex and metabolic state of mice. Furthermore, CTRP9 and adiponectin can be secreted as heterooligomers when cotransfected into mammalian cells, and in vivo, adiponectin/CTRP9 complexes can be reciprocally coimmunoprecipitated from the serum of adiponectin and CTRP9 transgenic mice. Biochemical analysis demonstrates that adiponectin and CTRP9 associate via their globular C1q domain, and this interaction does not require their conserved N-terminal cysteines or their collagen domains. Furthermore, we show that adiponectin and CTRP9 form heterotrimers. In cultured myotubes, CTRP9 specifically activates AMPK, Akt, and p44/42 MAPK signaling pathways. Adenovirus-mediated overexpression of CTRP9 in obese (ob/ob) mice significantly lowered serum glucose levels. Collectively, these results suggest that CTRP9 is a novel adipokine, and further study of CTRP9 will yield novel mechanistic insights into its physiological and metabolic function.—Wong, G. W., Krawczyk, S. A., Kitidis-Mitrokostas, C., Ge, G., Spooner, E., Hug, C., Gimeno, R., Lodish, H. F. Identification and characterization of CTRP9, a novel secreted glycoprotein from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin.
Keywords: C1q/TNF family, adipokine, diabetes, obesity
Adipose tissue actively regulates whole-body glucose and lipid metabolism by secreting a variety of proteins collectively termed adipokines that act on muscle, liver, and the hypothalamus (1). Acrp30/adiponectin is one such adipokine whose expression is restricted to the adipose tissue, and it is dramatically induced during adipocyte differentiation in culture (2,3,4). Expression of adiponectin is significantly down-regulated in adipose tissue derived from genetically obese (ob/ob) and diabetic (db/db) mice (4). Similarly, mRNA and serum levels of adiponectin are significantly reduced in obese humans (5) and further reduced in diabetic humans with coronary artery disease (6). Reduction in serum levels of adiponectin is associated with insulin resistance (7), while increased levels of serum adiponectin due to weight loss, calorie restriction, or thiazolidinedione treatment correlates with increased insulin sensitivity (8,9,10). Several mutations and polymorphisms in the adiponectin gene found in diabetic patients are associated with reduction in serum levels of adiponectin, in part, due to their effects on protein secretion, multimerization, and/or stability (11,12,13).
A 2-fold increase in the serum levels of adiponectin by transgenic or adenovirus-based overexpression, or supplementation with purified recombinant adiponectin, is sufficient to ameliorate many metabolic abnormalities associated with insulin resistance and/or diabetes in various animal models (14,15,16,17,18,19,20). Mechanistically, adiponectin exerts its metabolic effects by activating the 5′-AMP activated protein kinase (AMPK), which results in increased glucose uptake, glycogen deposition, and enhanced fatty acids oxidation in muscle and reduced glucose output in liver (18, 21).
In serum, adiponectin exists in 3 oligomeric forms: trimers, hexamers, and high molecular weight (HMW) oligomers of 12–18 U (13, 22,23,24,25). Hexamer and HMW oligomers are assembled from the basic trimeric unit of adiponectin via disulfide bonding, involving Cys-39 located at the N-terminal variable region (13, 23, 24). Additional post-translational modifications involving lysine hydroxylation and glycosylation within the collagen domain of adiponectin is also important for the protein to form HMW oligomers (26, 27). Mutations of the 4 conserved lysine residues within the consensus GXKG(E/D) motif abrogate the formation of HMW oligomers and functionally impaired their ability to inhibit hepatic gluconeogenesis (26, 28). The proportion of the 3 oligomeric forms of adiponectin in serum varies according to metabolic status and disease states (29, 30). The amount of HMW, as well as the ratio of HMW to total adiponectin, decreases in diabetic humans (30). In some instances, the ratio of HMW to total adiponectin correlates better with insulin sensitivity (31). In terms of biological activities, hexameric and HMW forms of adiponectin induce NF-κB activation in C2C12 myocytes (23), whereas the trimeric form of adiponectin induces AMPK activation and enhanced fatty acid oxidation in muscle (21). In contrast, only the HMW oligomeric but not the trimeric or hexameric form of adiponectin activates AMPK in liver to reduce glucose production (32).
Recently, other physiological functions have also been ascribed to adiponectin that include its antiatherogenic, anti-inflammatory, antiproliferative, and antisteatotic properties (33,34,35,36). Overexpression of adiponectin attenuates the size of atherosclerotic lesions in apolipoprotein E-deficient mice by multiple effects that include decreasing the expression of inflammatory adhesion molecule ICAM-1, blocking macrophage to foam cell transformation, and inhibiting aortic smooth muscle cell proliferation (37). Administration of recombinant adiponectin to mice can significantly ameliorate alcohol-induced hepatic steatosis (36). Activation of AMPK by adiponectin in the myocardium also protects mice against cardiac hypertrophy and infarction induced by ischemia-reperfusion injury (38).
Given the central role adipose tissue plays in regulating systemic energy balance, intense efforts have been made to understand how secreted factors such as adiponectin and leptin produced by adipose tissue affect glucose and lipid metabolism. Here, we describe a novel paralog of adiponectin designated as C1q/TNF-related protein (CTRP) 9. We show that CTRP9 transcripts are predominantly expressed by adipose tissue and that its expression levels change in ob/ob mice. We demonstrate that CTRP9 is a secreted glycoprotein with multiple post-translational modifications. CTRP9 forms predominantly trimers and furthermore forms heterooligomers with adiponectin. In cultured differentiated myotubes, CTRP9 specifically activates AMPK, Akt, and p44/42 MAPK signaling pathways. In ob/ob mice, adenovirus-mediated overexpression of CTRP9 lowered serum glucose levels. Hence, CTRP9 represents a novel adipokine.
MATERIALS AND METHODS
Identification and cloning of CTRP9
In a search for adiponectin-like proteins in the NCBI GenBank databases, we identified several mouse expressed sequence tags (ESTs) that encode a novel protein with a significant homology to the globular C1q domain of adiponectin. These ESTs were different from the 7 recently identified adiponectin paralogs designated as CTRP1 to CTRP7. Because human CTRP8/C1qTNF8 has recently been identified (39), we designated our novel adiponectin paralog as CTRP9. The GenBank accession numbers for mouse and human CTRP9 are AAY21933 and NP_848635, respectively. On the basis of the sequences of overlapping EST clones corresponding to CTRP9, a polymerase chain reaction (PCR) method was used to clone the entire coding region of CTRP9.
Quantitative real-time PCR analysis of CTRP9 expression in mouse tissues
A quantitative real-time PCR approach was used to screen mouse multiple tissue cDNA panels (Clontech, Mountain View, CA, USA), RNA isolated from the adipose tissue of obese (ob/ob) mice and their lean control littermates, and RNA isolated from primary adipocytes and stromal cells for the presence of CTRP9 and adiponectin transcripts. ABI Prism Primer Express 2.0 software (Applied Biosystems, Foster City, CA, USA) was used to design the following PCR primers: adiponectin forward, 5′-ACTGCAACTACCCATAGCCCAT-3′; adiponectin reverse, 5′-TGTCGACTGTTCCATGATTCTCC-3′; CTRP9 forward, 5′-TGGTGAACGTGGTGCCTACA-3′; CTRP9 reverse, 5′-TGCAGTCACATCCCACCCT-3′; 18S RNA forward, 5′-GCAATTATTCCCCATGAACG-3′; 18S RNA reverse, 5′-GGCCTCACTAAACCATCCAA-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GGAGCGAGACCCCACTAACA-3′; GAPDH reverse, 5′-ACATACTCAGCACCGGCCTC-3′. The default PCR protocol was used on an Applied Biosystems Prism 7000 Sequence Detection System. Epididymal fat pad cDNA from ob/ob and their lean control littermates (8 and 12 wk old), and cDNA from primary adipocytes and stromal cells were synthesized from 2 μg of total RNA and 200 ng of random hexamers using Superscript II RNase H-Reverse Transcriptase protocol (Invitrogen, Carlsbad, CA, USA). For quantitative PCR, samples were analyzed in triplicate 25-μl reactions (10 ng of cDNA, 900 nmol of primer, 12.5 μl of Master-mix and water), according to the standard protocol provided in SYBR Green PCR Master Mix protocol (Applied Biosystems).
Isolation of primary adipocytes and stromal cells from adipose tissue
Epididymal fat pads from 10 male and 10 female C57BL/6 mice were removed, minced, and digested with collagenase (2 mg/g tissue) at 37°C for 1 h. Digestions were stopped by adding Dulbecco modified Eagle medium (DMEM) containing 10% FBS and filtered through a mesh (cell strainer) with 100-μm hole size (BD Falcon, San Jose, CA, USA) to remove undigested tissues. The collected cell suspensions were incubated for 10 min at room temperature or until adipocytes had floated to the top. The upper phase contained mature adipocytes, while the lower phase contained stromal and vascular cells, including preadipocytes, fibroblasts, mature endothelial cells, and smooth muscle cells. Primary adipocytes were collected from the floating cell layers, washed once with DMEM to remove collagenase, and pelleted at 180 g for 5–10 min. Total RNAs were extracted from primary adipocytes with TRI reagent (Molecular Research Center, Cincinnati, OH, USA). The lower phase layers containing stromal and vascular cells were centrifuged at 100–200 g for 5 min, resuspended in DMEM containing 10% FBS, and filtered through a 25- to 70-μm prewet cell strainer (BD Falcon). The filtrates were washed once with DMEM and resuspended in erythrocyte lysis buffer (ammonium chloride solution; Stem Cell Technologies, Seattle, WA, USA) at room temperature for 10 min. Cells were centrifuged at 100 g for 10 min, and the supernatants were removed. Total RNA from stromal and vascular cells was extracted with TRI reagent.
Generation of CTRP9 specific antibody
Recombinant mouse CTRP9 that corresponds to its C-terminal globular domain (residues 195 to 333) was produced by cloning the truncated form of the CTRP9 construct into pTrcHis TOPO vector (Invitrogen) and maintained in Escherichia coli strain BL21(DE3)pLysE. The N-terminal His6-tagged fusion protein was produced in E. coli, isolated from the lysed bacterial pellet by nickel affinity column with Probond resin (Invitrogen), eluted with imidazole-containing buffer, and dialyzed against PBS. Detoxi-Gel endotoxin-removing gel (Pierce, Rockford, IL, USA) was used to remove potential endotoxin contaminants. Rabbit polyclonal antibody directed at purified recombinant g-CTRP9 was produced by immunizing NZW rabbits as described previously (40). Sera were collected and tested for their ability to recognize CTRP9 found in the supernatant of transfected human embryonic kidney (HEK) 293T cells by Western blot analysis.
Site-directed mutagenesis and the generation of collagen domain-deleted CTRP9 constructs
Site-directed mutagenesis kit from Stratagene (La Jolla, CA, USA) was used to mutate Cys to Ala at amino acid position 39 and 23 in adiponectin and CTRP9, respectively. We used the following primers to perform mutagenesis: adiponectin forward primer, 5′-CCACCCAAGGGAACTGCTGCAGGTTGGATGGCA-3′; adiponectin reverse primer, 5′-TGCCATCCAACCTGCAGCAGTTCCCTTGGGTGG-3′; CTRP9 forward primer, 5′-TTCTCC CAGGACACCGCCCGGCAAGGGCACTCC-3′; CTRP9 reverse primer, 5′-GGAGTGCCCTTGCCGGGCGGTGTCCTGGGAGAA-3′. DNA templates used in the PCR mutagenesis were FLAG-tagged adiponectin and CTRP9 in pCRII TOPO vector. Once successful mutagenesis were verified by DNA sequencing, adiponectin and CTRP9 inserts were excised out with EcoRI and XhoI, respectively, from TOPO II vector and cloned into mammalian expression vector, pCDNA3.1.
A 3-step PCR approach was used to generate adiponectin (C39A) and CTRP9 (C23A) lacking the entire collagen domain (henceforth referred to as g-adiponectin and g-CTRP9, respectively). Adiponectin (C39A) and CTRP9 (C23A) constructs were used as DNA template in the PCR reactions. First, DNA sequences corresponding to the signal peptide and short variable region at the N termini of adiponectin and CTRP9 were amplified out, and the PCR products were purified. Second, DNA sequences corresponding to the entire globular domain of adiponectin and CTRP9 were amplified out, and the PCR products were purified. Third, using PCR products (having overlap at the short variable region) from the first and second steps as DNA template, we generated constructs encoding g-adiponectin and g-CTRP9. Deletion constructs were verified by DNA sequencing.
HEK293T cell transfection
Mammalian expression vector (pCDNA3.1) encoding C-terminal FLAG and HA-tagged adiponectin, adiponectin Cys mutant (C39A), g-adiponectin, CTRP9, CTRP9 Cys mutant (C23A), and g-CTRP9 constructs were used in our transfection studies. HEK293T cells were cultured in DMEM containing 10% fetal calf serum supplemented with 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Transient transfections were performed in HEK293T cells using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfections, cells were washed and then cultured in serum-free Opti-MEM I medium (Invitrogen) supplemented with vitamin C (0.1 mg/ml) for another 24–48 h before the conditioned medium was collected for Western blot analysis using anti-FLAG M2 (Sigma, St. Louis, MO, USA) or anti-HA (clone 3F10) (Roche, Nutley, NJ, USA) antibody. A sample of the supernatant from CTRP9 transfectant was also incubated with PNGaseF (New England Biolabs, Ipswich, MA, USA) to determine the presence of N-linked glycans.
Generation of retroviruses and their infection of 3T3-L1 adipocytes
C-terminal FLAG-tagged CTRP5 and CTRP9 cDNA were subcloned into the MSCV retroviral vector (XZ201) (41) upstream of an IRES-GFP sequence. Expression of green fluorescent protein (GFP) is, therefore, proportional to CTRP5 and CTRP9 cDNA (42). In addition, expression of GFP allows us to rapidly assess the infection rate. To generate retroviruses, MSCV-CTRP5-FLAG and MSCV-CTRP9-FLAG were cotransfected with pCL-ECO vector (expressing ecotropic retrovirus structural proteins) into HEK293T cells, and the supernatants containing infectious virions were collected and frozen in 1-ml aliquots until used. 3T3-L1 preadipocytes were grown in 10-cm tissue culture plates and infected with retrovirus encoding GFP, CTRP5-FLAG, or CTRP9-FLAG when they were ∼70% confluence. One to 2 days after the cells reached confluency, medium was replaced with differentiation cocktail (DMEM supplemented with 10% FBS, 0.25 pM dexamethasone, 0.5 mM methyl-1-isobutyl-methylxanthine, and 160 pM insulin) and allowed to differentiate for 2 days. On day 3, the medium was replaced with DMEM supplemented with 10% FBS and 160 pM insulin. After that, the medium was replaced every 2 days with DMEM supplemented with 10% FBS. On day 7, the medium was replaced with serum-free Opti-MEM, and the supernatants were collected on day 8 for Western blot analysis, immunoprecipitation, or gel filtration chromatographic [fast protein liquid chromatography (FPLC)] analysis.
Coimmunoprecipitation and Western blot analysis
C-terminal FLAG or HA-tagged adiponectin, adiponectin (C39A), g-adiponectin, CTRP9, CTRP9 (C23A), and g-CTRP9 were transfected individually or in combinations into HEK293T cells as described above. Aliquots of the collected supernatants (250–350 μl) combined with 500 μl of IP buffer (150 mM Tris-HCL, pH 7.4; 150 mM NaCl; 1 mM EDTA; and 1% Triton X-100) were subjected to immunoprecipitation using either 10 μl of EZview anti-FLAG affinity gel (Sigma) or an anti-HA affinity matrix (Roche). Samples were rotated for 4 h at 4°C, washed 4 times with IP buffer, resuspended in 60 μl NuPAGE Sample buffer (Invitrogen) containing reducing agent (dithiothreitol), heated at 90°C for 10 min, and separated on 10% NuPAGE gel (Invitrogen). Proteins from gels were transferred to 0.2 μm Protran BA83 nitrocellulose membrane (Whatman, Pistcataway, NJ, USA), blocked in 5% nonfat milk for 1 h, and probed with mouse anti-FLAG M2 (1:2000), or rat anti-HA (1:2000) antibody in the presence of 5% nonfat milk for 2 h or overnight. Immunoblots were washed 3 times (5–10 min each) in PBS containing 0.1% Tween 20 and incubated with sheep anti-mouse-horseradish peroxidase (HRP) or goat anti-rat-HRP (Amersham Biosciences, Piscataway, NJ, USA) (1:2000 to 1:5000) for 2 h. Blots were washed 3 times (10 min each) in PBS containing 0.1% Tween 20, developed in enhanced chemiluminescence (ECL) reagent (Millipore, Billerica, MA, USA) or Lumigen TMA-6 solution (GE Health Science, Piscataway, NJ, USA) for 2–5 min and exposed to Blue XB-1 film (Kodak, Rochester, NY, USA). Western blot analysis quantifications were carried out using Fujifilm LAS-3000 imaging system (Fuji, Elmsford, NY, USA). Similarly, aliquots (300–500 μl) of FPLC fractions or 20 μl of serum from adiponectin-FLAG or CTRP9-HA transgenic mice were subjected to coimmunoprecipitation as described above.
Gel filtration chromatographic analysis
Serum from CTRP9 transgenic mice (100 μl) or supernatants (500 μl) from transfected HEK293T cells or retrovirally infected adipocytes containing FLAG-tagged adiponectin, adiponectin (C39A), CTRP9, or CTRP9 (C23A) were sequentially loaded into an AKTA FPLC and fractionated through a 10/30 Superdex 200 column (GE Health Science) in PBS. The collected fractions were subjected to Western blot analysis using either anti-FLAG antibody, anti-adiponectin antibody, or anti-CTRP9 antibody.
Production and purification of recombinant adiponectin, g-adiponectin, CTRP9, and g-CTRP9
C-terminal FLAG-tagged adiponectin, globular head of adiponectin (g-adiponectin), CTRP9, and g-CTRP9 were produced and purified from the supernatants of transfected HEK293T cells. Briefly, 24 h after transfection, cell culture medium was replaced by serum-free Opti-MEM I supplemented with vitamin C (0.1 mg/ml). Supernatants were collected 3 times, every 48 h, pooled and purified using anti-FLAG affinity gel according to the manufacturer’s instructions (Sigma), and eluted with 150 μg/ml FLAG peptide (Sigma). Purified proteins were dialyzed against 20 mM HEPES buffer (pH 8.0) containing 135 mM NaCl in a 10-kDa cutoff Slide-A-Lyzer dialysis cassette (Pierce).
Detection of carbohydrate moiety
Approximately 50 ng of purified recombinant FLAG-tagged adiponectin, CTRP9, and g-CTRP9 was separated on duplicate SDS-PAGE gels and transferred to nitrocellulose membrane. One blot was immunoblotted with an anti-FLAG antibody to show the presence of FLAG-tagged proteins. A duplicate blot was used in an ECL glycoprotein detection protocol according to the manufacturer’s instructions (Amersham Bioscience). Briefly, any carbohydrate moiety on recombinant protein blotted onto the nitrocellulose membrane was oxidized with sodium metaperiodate and labeled with biotin hydrazide. The presence of carbohydrate moiety was then detected using streptavidin conjugated to HRP.
Mass spectrometry analysis
Purified recombinant CTRP9-FLAG protein was fractionated on SDS-PAGE gel, and the band that corresponded to CTRP9 protein on the Coomassie blue-stained gel was excised and subjected to trypsin digestion. Peptide fragments from the digest were loaded onto Waters Nano Acquity HPLC (Waters Corp., Milford, MA, USA) coupled to Thermo LTQ linear ion trap mass spectrometer (Thermo, Waltham, MA, USA) for MS/MS analysis. The resulting collision-induced-dissociation spectra were searched against a protein database using SEQUEST (Thermo) to identify the individual peptide and the modified residue.
Generation of adiponectin and CTRP9 transgenic mice
C-terminal FLAG-tagged adiponectin and HA-tagged CTRP9 were cloned into EcoRI and XhoI site of pCAGGS vector, respectively (43). Expressions of the transgenes were driven by the chicken β-actin promoter with a cytomegalovirus (CMV) enhancer. Pronuclear injections were performed at the transgenic mouse facility at the Massachusetts Institute of Technology (MIT) Cancer Center. All animal protocols were approved by the MIT Animal Care and Use Committee. Several founder lines produced high levels of CTRP9-HA proteins in their serum.
Myotube generation and stimulation
Mouse C2C12 myoblast cells were differentiated into myotubes in DMEM containing 2% horse serum. On day 6 or 7 after differentiation, myotubes were starved for 1–2 h in serum-free DMEM and then stimulated with recombinant CTRP9 (2–4 μg/ml) or control buffer (20 mM HEPES, pH 8, and 135 mM NaCl) for 15 min. Cells were lysed in RIPA buffer containing phosphatase inhibitors (Sigma), sodium fluoride, sodium orthovanadate, and protease inhibitor cocktail (Roche). Western blot analyses were carried out on the cleared lysates using rabbit antibodies recognizing phospho-AMPK-α (Thr-172), phospho-ACC (Ser-79, phopho-p44/42 MAPK (Thr-202 and Tyr-204), phospho-Akt (Thr308), phospho-mTOR (Ser-2448), and phospho-ΙκB (Ser-32) (all from Cell Signaling Technology, Danvers, MA, USA). Blots were stripped and reprobed with antibodies recognizing total AMPK, mTOR, Akt, ΙκB, p44/42-MAPK (all from Cell Signaling Technology), and ACC (Epitomics, Burlingame, CA, USA). Antibody conjugated to LI_COR IRdye800 (Rockland Immunochemicals, Gilbertsville, PA, USA) was used as our secondary antibody for the purpose of quantification using the LI-COR Odyssey infrared imaging system.
In vivo studies
Twelve-week-old male obese (ob/ob) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Baseline body weight and serum glucose and insulin levels were measured prior to any experimentation. Adenovirus-encoding GFP or adenovirus-encoding CTRP9 was injected intravenously at 0.5 × 109 plaque-forming units (pfu) in 0.1 ml/mouse (n=12–15). An equal volume of control buffer (10% glycerol in PBS) was also injected into ob/ob mice as an additional control group (n=15). Body weight of mice was measured at day 4 postinjection. Food intake was measured from 4 to 7 days postinjection. At day 7 postinjection, serum glucose and insulin were measured. At day 11 postinjection, mice were euthanized and serum samples were collected for measurements of glucose, insulin, free fatty acid, and triglyceride levels.
RESULTS
Identification and analysis of CTRP9 cDNA and gene
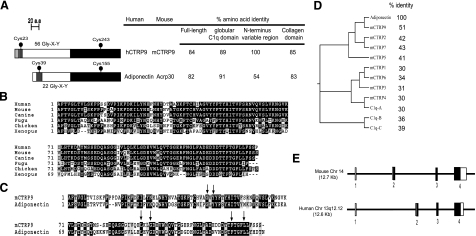
Using the globular C1q domain sequence of adiponectin to screen the NCBI GenBank databases, we identified a novel and highly conserved paralog of adiponectin (Fig. 1A). The novel cDNA is distinct from the 7 paralogs (CTRP1 to CTRP7) we recently identified (40). Because a different human cDNA has recently been designated as C1qTNF8/CTRP8 (39), we designated our novel cDNA and its encoded protein CTRP9. CTRP9 and adiponectin share a similar modular organization that consists of 4 distinct domains—a signal peptide (residues 1-19), a short N-terminal variable region (residues 20-28), a collagen domain with 56 Gly-X-Y repeats (residues 29-196), and a C-terminal globular domain that is homologous to immune complement protein C1q (residues 197-333).
Figure 1.
Identification and cloning of CTRP9 cDNA. A) The deduced amino acid sequences of CTRP9 consist of 4 distinct domains: a signal peptide (light gray), a short variable region (dark gray), a collagen domain with 56 Gly-X-Y repeats (white), and a C-terminal globular domain homologous to complement C1q (black). The predicted signal peptide, short variable region, collagen domain, and globular C1q domain of CTRP9 consist of 19, 9, 168, and 137 amino acids. Solid circles (•) indicate cysteine residues that are part of the mature protein. Numbers on the right refer to percentage of amino acid identity between human and mouse CTRP9. B) ClustalW alignments of CTRP9 globular C1q domain sequences of human (Homo sapiens; NP_848635), mouse (Mus musculus; AAY21933), canine (Canis familiaris; XP_851299), fugu (Fugu rubripes; CAAB01000512), chicken (Gallus gallus; XP_001234806), and Xenopus (Xenopus tropicalis; Ensembl peptide ID: ENSXETP00000021385). Identical amino acids are shaded; gaps are indicated by a dashed line. C) ClustalW alignment of the globular domain sequences between mouse CTRP9 and adiponectin. Arrows indicate amino acids that are conserved between C1q and TNF family of proteins, based on the crystal structure of adiponectin (44). D) Dendrogram based on the ClustalW alignments of the globular domain sequences of adiponectin and its paralogs. Percentage of amino acid identity between the globular C1q domain of adiponectin and its paralogs is indicated. E) Exon/intron structures of mouse and human CTRP9 genes. Sizes of mouse CTRP9 exons 1 to 4 are 35, 187, 63, and 1617 bp, respectively. Encoded mouse cDNA has a 46 bp 5′UTR and a 844 bp 3′UTR. Sizes of human CTRP9 exons 1 to 4 are 34, 188, 63, and 1194 bp, respectively. Encoded human cDNA has a 56 bp 5′UTR and a 421 bp 3′UTR. Open squares (white) refer to exons that encode the 5′ and 3′UTR; solid squares (black) refer to the exons that code for amino acids.
CTRP9 is highly conserved throughout evolution. Mouse CTRP9 and its corresponding human ortholog share 100, 85, and 89% amino acid identity in their short N-terminal variable regions, collagen domains, and C-terminal globular domains, respectively (Fig. 1A). Similarly, CTRP9 orthologs from the draft genome sequences of dogs (Canis familiaris), chickens (Gallus gallus), fish (Fugu rubripes), and frogs (Xenopus tropicalis) also show striking conservation in their globular domains (Fig. 1B), with amino acid identity of 93, 69, 71, and 51% to mouse CTRP9, respectively. Structure-based alignment between adiponectin, complement C1q, and TNF family members (TNF-α, TNF-β, and CD40L) reveals 4 highly conserved residues (Gly-159, Tyr-161, Phe-237, and Leu-241 in adiponectin) important in the packing of the protomer’s hydrophobic core (44). These residues are also conserved in CTRP9 (Fig. 1C, arrows). Of the 8 CTRPs and other C1q-domain containing proteins analyzed, CTRP9 is the closest paralog of adiponectin and shares the highest degree of amino acid identity (51%) to the globular domain of adiponectin (Fig. 1D). Both human and mouse CTRP9 genes have similar exon/intron structures (Fig. 1E). Mouse CTRP9 gene is 12.7 kb in size, consists of 4 exons, and is located on chromosome 14. Human CTRP9 gene is 12.6 kb in size, consists of 4 exons, and is located on chromosome 13q12.12.
Expression profile of CTRP9 transcript
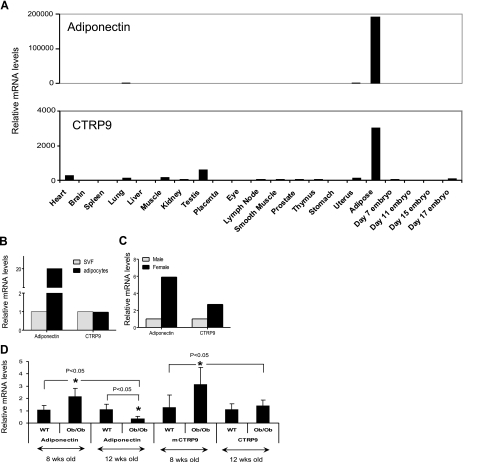
Adiponectin is expressed almost exclusively by adipose tissue (2). Similarly, the expression of CTRP9 transcripts is also relatively restricted to adipose tissue (Fig. 2A). Within the adipose tissue, we showed that both primary adipocytes and stromal cells expressed CTRP9 transcripts (Fig. 2B). Sex hormones influence the secretion and circulating serum levels of adiponectin (45, 46). We observed that both adiponectin and CTRP9 transcripts show a gender-biased expression pattern in which female mice expressed higher levels of adiponectin and CTRP9 transcripts relative to male mice (Fig. 2C).
Figure 2.
Expression profiles of CTRP9 transcript. A) Quantitative real time PCR analyses of adiponectin and CTRP9 expression in mouse tissues. Expression levels of adiponectin and CTRP9 transcripts in different tissues were normalized to their corresponding GAPDH transcript levels. B) Total RNA from primary adipocytes and stromal vascular fraction (SVF) were isolated from pooled epididymal fat pads of 8- to 10-week-old C57BL/6 mice (n=10) and subjected to quantitative real-time PCR analysis. All qPCR results were first normalized to their corresponding 18S RNA levels. The relative expression levels of adiponectin and CTRP9 in isolated adipocytes were normalized to their corresponding levels in the SVF. C) Expression levels of adiponectin in primary adipocytes (pooled from 10 mice) and CTRP9 transcripts in SVF (pooled from 10 mice) between males and females were compared. All qPCR results were first normalized to their corresponding 18S RNA levels. The relative expression levels of adiponectin and CTRP9 in males were normalized to their corresponding levels in female mice. D) Total RNA from epididymal fat pads of 8-wk (n=10) and 12-wk (n=8) old male obese (ob/ob) and their lean controls (WT, n=8–10) were isolated and subjected to real-time PCR analysis. All qPCR results were first normalized to their corresponding 18S RNA levels. The relative expression levels of adiponectin and CTRP9 transcript in ob/ob mice were normalized to their corresponding levels in the lean control mice (WT). *P < 0.05; t test.
Next, we examined the expression levels of CTRP9 transcript in adipose tissues (epididymal fat pads) harvested from obese (ob/ob) mice and their lean controls. As expected, the expression levels of adiponectin transcript were significantly down-regulated in adipose tissue derived from 12-wk-old ob/ob mice relative to their age-matched controls (Fig. 2D). Surprisingly, the expression levels of adiponectin transcript were significantly up-regulated in adipose tissue derived from 8-wk-old ob/ob mice relative to their age-matched controls (Fig. 2D). Consistent with the mRNA data, serum levels of adiponectin also increased in 8-wk-old ob/ob mice when compared to their age-matched controls (data not shown). We also observed a similar, statistically significant, up-regulation of CTRP9 transcript levels in adipose tissue derived from 8-wk-old ob/ob mice relative to their age-matched controls (Fig. 2D). However, in 12-wk-old mice, the expression levels of CTRP9 transcripts are similar between ob/ob and lean controls.
CTRP9 is a secreted glycoprotein with multiple post-translational modifications
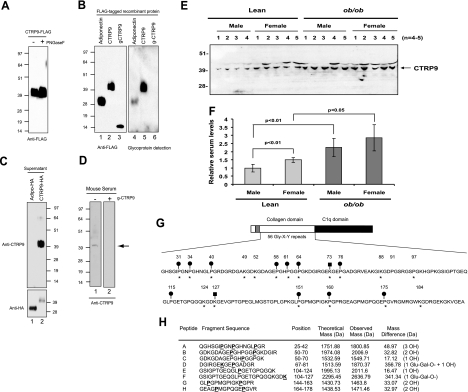
CTRP9 possesses a predicted signal peptide. When a full-length construct encoding C-terminal FLAG-tagged CTRP9 was transfected into HEK293T cells, the resultant protein was readily detected in the supernatant of transfected cells, demonstrating that CTRP9 protein is secreted (Fig. 3A). The deduced amino acid sequence of CTRP9 does not contain any potential N-linked glycosylation sites that conform to the consensus motif N-X-(S/T). When treated with N-glycanase (PNGase F), no shift in the apparent molecular weight of CTRP9 protein was observed on an SDS-PAGE immunoblot, thus confirming the absence of N-linked glycans (Fig. 3A). Although the predicted molecular mass of CTRP9 is ∼32 kDa, its apparent molecular mass on SDS-PAGE immunoblot is ∼40 kDa, suggesting the presence of carbohydrate moieties. We used a glycoprotein detection system that allowed us to demonstrate that CTRP9 is a secreted glycoprotein (Fig. 3B, lane 5). Because only full-length but not the collagen domain-deleted CTRP9 was labeled with biotin-hydrazide that specifically reacted with oxidized sugar groups, we conclude that the carbohydrate moieties in CTRP9 are localized to the collagen domain (Fig. 3B). We next investigated whether CTRP9 circulates in the serum and hence may function as an endocrine hormone. To do so, we generated a rabbit polyclonal antibody that specifically recognizes the globular domain of CTRP9 (Materials and Methods and Fig. 3C). This antibody did not recognize CTRP1 to CTRP7 proteins on immunoblot (data not shown). Using this CTRP9-specific antibody, we demonstrated the presence of CTRP9 protein in mouse serum (Fig. 3D, lane 1). Recombinant globular g-CTRP9 can compete out the binding of CTRP9 antibody to serum CTRP9 on immunoblots, thereby demonstrating the specificity of the CTRP9 antibody (Fig. 3D, lane 2). Consistent with our mRNA data, we observed that 8-wk-old C57BL/6 female mice have higher serum levels of CTRP9 relative to male mice (Fig. 3E, F). Moreover, 8-wk-old obese (ob/ob) male and female mice have higher serum levels of CTRP9 compared to their lean male and female counterparts.
Figure 3.
CTRP9 is a secreted glycoprotein with multiple post-translational modifications. A) Supernatant containing CTRP9-FLAG was incubated with (+) or without (−) peptide: N-glycosidase F (PNGaseF) to determine the presence of N-linked glycans. Proteins were subjected to Western blot analysis using an anti-FLAG antibody. B) Purified recombinant FLAG-tagged adiponectin, CTRP9, and g-CTRP9 (lacking the collagen domain) were analyzed by Western blot analysis. Left (lanes 1–3): blot was probed with an anti-FLAG antibody. Right (lanes 4–6): blot was subjected to glycoprotein detection analysis (see Materials and Methods) to reveal the presence of carbohydrates. C) Supernatants containing C-terminal HA-tagged adiponectin and CTRP9 were analyzed by Western blot analysis using an anti-CTRP9-specific antibody (top panel). Blot was reprobed for adiponectin and CTRP9 using an anti-HA antibody (bottom panel). D) Mouse serum was subjected to Western blot analysis using an anti-CTRP9 antibody. CTRP9 protein with an apparent molecular mass of ∼40 kDa was detected in the absence (−) but not the presence (+) of purified g-CTRP9 that can compete out the binding of antibody to CTRP9. E) Mouse sera from 8-wk-old lean (C57BL/6) and obese (ob/ob) male and female mice (n=4–5) were subjected to Western blot analysis using an anti-CTRP9 antibody. F) Quantification of results from E. G) Post-translational modifications of CTRP9. CTRP9 contains 56 Gly-X-Y repeats. Conserved proline residues (in the context of Gly-X-Pro) are indicated by asterisks (∗). Ten proline residues were found to be hydroxylated (•). CTRP9 has 7 potential lysine residues within the consensus sequence [GXKG(D/E)] that can be hydroxylated and further modified with a glucosyl-galactosyl group. Conserved lysine residues are indicated by asterisks. Lysine residues found to be modified with a glucosyl-galactosyl group are indicated by solid squares (▪). H) Peptide mass fingerprinting showing the modified tryptic peptides with their theoretical and observed mass. The mass of a hydroxyl (-OH) group attached to proline is 16 Da, whereas the mass of a glucosyl-galactosyl group (Glu-Gal-O-) is 340.3 Da. The mass differences represent different numbers of hydroxyl and glucosyl-galactosyl groups attached to the peptide. Peptides A through H demonstrate the presence of hydroxylated proline residues and the glycosylated lysine residues (bold and underlined) summarized in G.
Adiponectin contains multiple post-translational modifications in its collagen domain that affect its structure and function (26,27,28). Proline in the third position (Gly-X-Y, where Y is proline) within the collagen domain is often hydroxylated to enhance the stability of the collagen triple helical structure (47). Seven proline residues (at positions 44, 47, 53, 71, 76, 91, and 95) within the collagen domain of adiponectin have been shown to be hydroxylated (27). In addition, adiponectin has 4 conserved lysines (positions 68, 71, 80, and 104) in its collagen domain [within the consensus motif GXKG(E/D)] that are sequentially hydroxylated and glycosylated with a glucosyl-galactosyl group (26). CTRP9 has a longer collagen domain with 56 Gly-X-Y repeats. There are a total of 13 proline residues (in the context of Gly-X-Pro) that can be potentially hydroxylated. Eleven of the 13 proline residues are conserved in mice, rats, humans, chimpanzees, and dogs (Fig. 3G). Using mass spectrometry techniques, we showed that 10 of the 11 conserved proline residues (at position 31, 34, 40, 58, 61, 64, 76, 151, 160, and 175) are hydroxylated (Fig. 3G, H). A nonconserved proline residue at position 115 in mouse CTRP9 is also hydroxylated. Thus, a total of 11 hydroxyproline residues were detected by mass spectrometry. CTRP9 also has 7 lysine residues that conform to the consensus sequence [GXKG(D/E)] that can potentially be hydroxylated and glycosylated. All 7 of these lysine residues (at positions 49, 52, 73, 88, 124, 127, and 184) are conserved among mice, rats, humans, chimpanzees, and dogs. Two of the lysine residues (positions 73 and 127) are modified with a glucosyl-galactosyl group. Tryptic peptides that encompass lysine residues at positions 49, 52, 88, 124, and 184 were too small to be detected on the mass spectrometer used, and consequently their post-translational modification status remains to be determined.
CTRP9 forms higher-order oligomeric complexes
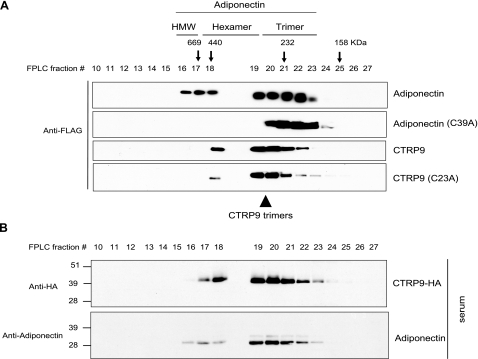
Adiponectin exists in 3 multimeric forms: trimers, hexamers, and HMW oligomers of 12–18 U that activate distinct signaling pathways (23). To address whether CTRP9 is also assembled into higher-order oligomeric complexes, we performed gel filtration (FPLC) analysis on supernatant collected from transiently transfected HEK293T cells (Fig. 4A). In contrast to the 3 multimeric forms of adiponectin, CTRP9 formed predominantly trimers and possibly hexamers and HMW oligomers (Fig. 4A). Because of the low resolution of FPLC separation, we cannot, at present, distinguish the hexamers from the HMW oligomers. Because the size of CTRP9 collagen domain is more than twice the length of adiponectin, it has a larger Stoke’s radius, and hence CTRP9 trimers elute from gel filtration column before adiponectin, with an apparent molecular size between that of adiponectin trimers and hexamers. Site-directed mutagenesis revealed that Cys-39 located at the N-terminal variable domain is required for adiponectin trimers to form hexamers and HMW oligomers via disulfide bonding (22, 24) (Fig. 4A). Unlike adiponectin, site-directed mutagenesis of Cys-23 located in the N-terminal variable region of CTRP9 resulted in only minor quantitative changes in its FPLC profile, suggesting that CTRP9 forms predominantly trimers. To investigate whether CTRP9 forms trimers and hexamers in vivo, we performed gel filtration analysis on serum obtained from C-terminal HA-tagged CTRP9 transgenic mice. As shown in Fig. 4B, serum CTRP9 also exists predominantly as trimers.
Figure 4.
CTRP9 protein forms higher-order oligomeric complexes. A) Gel filtration chromatographic analyses of FLAG-tagged adiponectin, adiponectin Cys mutant (C39A), CTRP9, or CTRP9 Cys mutant (C23A). Fractions 10 to 27 were subjected to Western blot analysis using an anti-FLAG antibody. Arrows with molecular mass markers of 669, 440, 232, and 158 kDa correspond to the peak elution fractions of molecular standards thyroglobulin, ferritin, catalase, and aldolase, respectively. FPLC fractions 16–17, 18–19, and 20–23, which correspond to adiponectin trimers, hexamers, and HMW oligomers, respectively, are indicated at top. Arrowhead at bottom indicates fractions 19–21, which correspond to CTRP9 trimers. B) Gel filtration analysis of serum obtained from CTRP9-HA transgenic mouse. Fractions 10 to 27 were subjected to Western blot analysis using an anti-HA antibody that recognizes CTRP9-HA (top panel). Duplicate blot was immunoblotted with an adiponectin-specific antibody (bottom panel).
Physical association between adiponectin and CTRP9
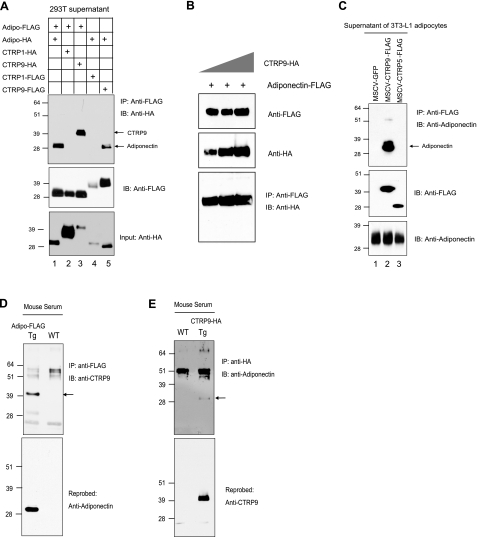
Several members of the C1q/TNF family of proteins are known to form heterooligomers; these include complement C1q (48), chipmunk hibernation proteins (49, 50), and cerebellins (51,52,53). Because both adiponectin and CTRP9 are expressed in the adipose tissue, we investigated whether they form heterooligomers. Using epitope-tagged (FLAG or HA) adiponectin and CTRP9 constructs in transiently transfected HEK293T cells, we were able to reciprocally coimmunoprecipitate both adiponectin and CTRP9 from the supernatants of cotransfected cells expressing both adiponectin and CTRP9 (Fig. 5A, lane 3 and 5). The association is specific because a related adiponectin paralog, CTRP1, did not coimmunoprecipitate with adiponectin when coexpressed in HEK293T cells, although both proteins were secreted in large quantities into the supernatants. Neither adiponectin nor CTRP9 proteins in the supernatants of transfected cells associate nonspecifically with the anti-FLAG or anti-HA resin (data not shown). By increasing the expression levels of CTRP9 protein, we were able to increase the amount of CTRP9 coimmunoprecipitated with adiponectin (Fig. 5B).
Figure 5.
Adiponectin and CTRP9 form heterooligomers. A) Coexpressed FLAG or HA-tagged adiponectin, CTRP1, and CTRP9 were subjected to coimmunoprecipitation using an anti-FLAG affinity gel and immunoblotted with an anti-HA antibody (top panel). Middle and bottom panels indicate the presence of FLAG and HA-tagged input proteins. B) Adiponectin-FLAG was coexpressed with increasing amounts of CTRP9-HA. Top and middle panels indicate the presence of adiponectin-FLAG and CTRP9-HA in the supernatants of transfected cells, respectively. Supernatants were subjected to coimmunoprecipitation using an anti-FLAG affinity gel and immunoblotted with an anti-HA antibody (bottom panel). C) Supernatants from 3T3-L1 adipocytes overexpressing GFP, CTRP9-FLAG, or CTRP5-FLAG were subjected to immunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting for adiponectin. The blot was reprobed with an anti-FLAG antibody (middle panel). Bottom panel shows the presence of adiponectin in each sample prior to immunoprecipitation. D) Twenty microliters of serum from wild-type (WT) and adiponectin-FLAG transgenic mice (Tg) was subjected to coimmunoprecipitation using an anti-FLAG affinity gel and immunoblotted with an anti-CTRP9 antibody (top panel). Arrow indicates the band that corresponds to CTRP9. Blot was reprobed with an anti-adiponectin antibody (bottom panel). E) Twenty microliters of serum from wild-type (WT) and CTRP9-HA transgenic mice (Tg) was subjected to coimmunoprecipitation using an anti-HA agarose gel and immunoblotted with an anti-adiponectin antibody (top panel). Arrow indicates the band that corresponds to adiponectin. Blot was reprobed with anti-CTRP9 antibody (bottom panel).
To rule out the possibility that adiponectin and CTRP9 association is unique to the transiently transfected HEK293T cells, we next investigated whether we could recapitulate this association in differentiated 3T3-L1 adipocytes. Because cultured 3T3-L1 adipocytes express very low levels of CTRP9 transcript, we overexpressed C-terminal FLAG-tagged CTRP9 using a retrovirus vector in 3T3-L1 preadipocytes prior to their differentiation into mature adipocytes in culture. In parallel, we also infected 3T3-L1 cells with retrovirus to overexpress GFP or CTRP5-FLAG as controls. Supernatants were harvested from fully differentiated 3T3-L1 adipocytes that contained high levels of endogenous adiponectin in combination with GFP, CTRP5-FLAG, or CTRP9-FLAG. As shown in Fig. 5C (lane 2), we were able to coimmunoprecipitate adiponectin and CTRP9 from the supernatant of 3T3-L1 adipocytes expressing endogenous adiponectin and CTRP9-FLAG. The association is specific because a related adiponectin paralog, CTRP5, did not coimmunoprecipitate with adiponectin, although it was expressed and secreted into the supernatant of cultured adipocytes (Fig. 5C, lane 3).
Next, we determined whether adiponectin and CTRP9 associate in vivo. We performed reciprocal coimmunoprecipitations on serum obtained from a transgenic mouse overexpressing either C-terminal FLAG-tagged adiponectin or HA-tagged CTRP9. In reciprocal cases, CTRP9 only coimmunoprecipitated with adiponectin from transgenic but not wild-type mouse serum, thus confirming that adiponectin and CTRP9 associate in vivo and that the interaction is specific (Fig. 5D, E).,
Requirements for adiponectin and CTRP9 association
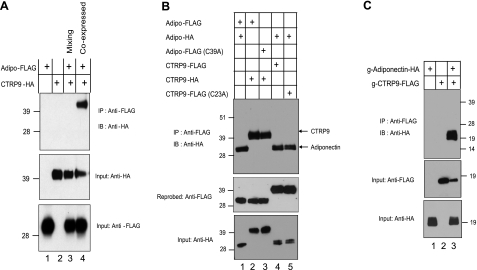
Proteomics approaches have uncovered several serum proteins and growth factors capable of interacting with adiponectin (54). These interactions can be demonstrated with recombinant proteins in vitro. These observations prompted us to address whether CTRP9 associates with adiponectin during their biosyntheses or whether they interact after proper folding and secretion. To address this, we performed mixing experiments using supernatants from transfected HEK293T cells expressing adiponectin-FLAG or CTRP9-HA alone or in combination. As shown in Fig. 6A (lane 4), adiponectin and CTRP9 associated with each other and coimmunoprecipitated from the supernatant only when coexpressed in the same cells. Mixing supernatants from cells that expressed adiponectin-FLAG or CTRP9-HA separately did not result in coimmunoprecipitation, demonstrating that adiponectin and CTRP9 associate during their biosyntheses in the endoplasmic reticulum or during transit through the Golgi compartment prior to their secretion into the culture medium. Prolonged mixing of supernatants containing adiponectin-FLAG with CTRP9-HA overnight did not result in coimmunoprecipitation, suggesting that once these proteins are properly folded and secreted, they are stable and do not interchange subunits (data not shown).
Figure 6.
Adiponectin and CTRP9 associate via their globular C1q domains during biosynthesis of the proteins. A) Supernatants containing adiponectin-FLAG and/or CTRP9-HA were subjected to coimmunoprecipitation. For the mixing experiment, equal amount of the supernatant containing adiponectin-FLAG was mixed with supernatant containing CTRP9-HA for 4 h before coimmunoprecipitation with an anti-FLAG affinity gel, followed by immunoblotting with an anti-HA antibody (top panel). Middle and bottom panels show the presence of all FLAG and HA-tagged input proteins in the supernatants. B) Coexpressed FLAG or HA-tagged adiponectin, adiponectin Cys mutant (C39A), CTRP9, and CTRP9 Cys mutant (C23A) were subjected to coimmunoprecipitation with an anti-FLAG affinity gel, followed by immunoblotting with an anti-HA antibody (top panel). Middle and bottom panels indicate the presence of FLAG and HA-tagged input proteins. C) Supernatants containing FLAG-tagged CTRP9ΔCollagen and/or HA-tagged adiponectinΔCollagen were subjected to coimmunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting with an anti-HA antibody (top panel). Middle and bottom panels indicate the presence of FLAG and HA-tagged input proteins.
One possibility is that adiponectin trimer associates with CTRP9 trimer via intermolecular disulfide bonding involving Cys 39 on adiponectin and Cys-23 on CTRP9. To address this possibility, we mutated Cys-39 and Cys-23 to Ala on adiponectin and CTRP9, respectively. As shown in Fig. 6B (lane 3), adiponectin (C39A) coimmunoprecipitated with CTRP9 from the supernatant of cotransfected HEK293T cells. Reciprocally, CTRP9 (C23A) also can be coimmunoprecipitated with adiponectin from the supernatant of cotransfected cells (Fig. 6B, lane 5). Thus, neither Cys-39 on adiponectin nor Cys-23 on CTRP9 was needed for their physical association, demonstrating that their association does not involve disulfide bond formation.
Next, we investigated whether adiponectin and CTRP9 association required the collagen domain. We generated epitope-tagged CTRP9 and adiponectin constructs lacking the entire collagen domain plus mutating their N-terminal Cys residues to Ala. As shown in Fig. 6C, g-adiponectin and g-CTRP9 were secreted into the conditioned medium of transfected cells, demonstrating that the collagen domains are not required for their proper folding and secretion. Importantly, g-adiponectin coimmunoprecipitated with g-CTRP9 from the supernatant of cotransfected cells (Fig. 6C, lane 3), demonstrating that adiponectin and CTRP9 associate via their C-terminal globular domains.
CTRP9 and adiponectin form heterotrimers
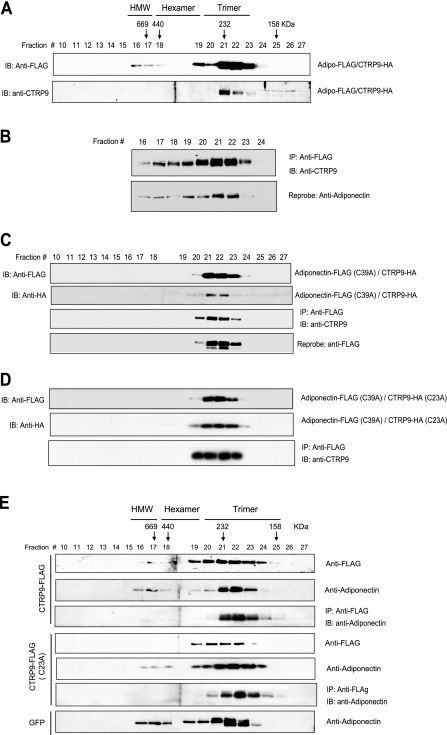
That CTRP9 and adiponectin associate via their globular domains suggests the possibility of forming adiponectin/CTRP9 heterotrimers and that this type of association may affect the proportion of different adiponectin and/or CTRP9 oligomeric forms. To address this issue, we performed gel filtration analysis on supernatant collected from HEK293T cells coexpressing adiponectin and CTRP9. Aliquots of the FPLC fractions were analyzed by Western blot analysis. As shown in Fig. 7A, when coexpressed in cells, both secreted adiponectin and CTRP9 formed predominantly trimers (compared to Fig. 4). To address the possibility that adiponectin and CTRP9 physically associate as heterotrimers, we performed coimmunoprecipitations on FPLC fractions 16–24 as shown in Fig. 7A and showed that CTRP9 coimmunoprecipitated with adiponectin from fractions 21–23 that contained predominantly the trimeric form of adiponectin (Fig. 7B). For further validation, we took advantage of the fact that adiponectin (C39A) forms exclusively trimers (22). We performed gel filtration analysis on supernatant harvested from HEK293T cells coexpressing adiponectin (C39A) and CTRP9. Aliquots of the FPLC fractions were analyzed by Western blot analysis. As expected, adiponectin (C39A) formed only trimers (Fig. 7C, top panel). When coexpressed with adiponectin (C39A), CTRP9 also formed trimers (Fig. 7C, second panel). We performed coimmunoprecipitations on the collected FPLC fractions and demonstrated that CTRP9 coimmunoprecipitated with the trimeric form of adiponectin, thus showing that CTRP9 and adiponectin formed heterotrimers. Similar results were obtained when adiponectin (C39A) was coexpressed with CTRP9 (C23A) (Fig. 7D), thus demonstrating that N-terminal Cys residues were not required for the formation of heterotrimers. However, the stoichiometry of adiponectin and CTRP9 in the heterotrimeric complex (1:2 or 2:1 or both) remained to be determined.
Figure 7.
CTRP9 heterotrimerizes with adiponectin. A) Supernatant from transfected cells coexpressing adiponectin-FLAG and CTRP9-HA was subjected to gel filtration chromatographic analysis. Fractions 10 to 27 were subjected to Western blot analysis using an anti-FLAG or an anti-CTRP9 antibody. B) FPLC fractions 16 to 24 were subjected to coimmunoprecipitation using an anti-FLAG affinity gel and immunoblotted with an anti-CTRP9 antibody (top panel). Blot was reprobed with an anti-adiponectin antibody (bottom panel). C) Gel filtration analyses of coexpressed FLAG-tagged adiponectin (C39A) and HA-tagged CTRP9. Fractions 10 to 27 were subjected to Western blot analysis using an anti-FLAG antibody to detect adiponectin (top panel) or an anti-HA antibody to detect CTRP9 (second panel). Fractions 10 to 27 were also subjected to coimmunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting with an anti-CTRP9 antibody (third panel). The blot was reprobed with an anti-FLAG antibody to show the presence of adiponectin (bottom panel). D) Gel filtration analyses of coexpressed FLAG-tagged adiponectin (C39A) and HA-tagged CTRP9 (C23A). Fractions 10 to 27 were subjected to Western blot analysis using an anti-FLAG antibody to detect adiponectin (top panel) or an anti-HA antibody to detect CTRP9 (middle panel). Fractions 10 to 27 were also subjected to coimmunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting with an anti-CTRP9 antibody (bottom panel). E) Gel filtration analyses of supernatants from 3T3-L1 adipocytes overexpressing GFP, CTRP9-FLAG, or CTRP9-FLAG (C23A). Fractions 10–27 were subjected to Western blot analysis using an anti-FLAG antibody (top panel) to detect CTRP9. A duplicate blot was probed with an anti-adiponectin antibody (second panel). Fractions 10–27 were also subjected to coimmunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting with an anti-adiponectin antibody (third panel). Fractions 10–27 of CTRP9-FLAG (C23A) were also subjected to immunoblot analysis using an anti-FLAG antibody (fourth panel) or an anti-adiponectin antibody (fifth panel). Fractions 10–27 were subjected to coimmunoprecipitation using an anti-FLAG affinity gel, followed by immunoblotting with an anti-CTRP9 antibody (sixth panel). Fractions 10–27 of GFP control were also subjected to immunoblot analysis using an anti-adiponectin antibody (bottom panel).
To rule out the possibility that the formation of adiponectin and CTRP9 heterotrimers is unique to transiently transfected HEK293T cells, we investigated their association in differentiated 3T3-L1 adipocytes. Gel filtration analysis was carried out on supernatants harvested from fully differentiated 3T3-L1 adipocytes that expressed high levels of endogenous adiponectin in combination with CTRP9-FLAG, CTRP9-FLAG (C23A), or GFP. In 3T3-L1 adipocytes that expressed control GFP protein, the secreted adiponectin formed trimers, hexamers, and HMW oligomers (Fig. 7E, bottom panel) in proportions similar to adiponectin secreted from transfected HEK293T cells (Fig. 4A, top panel). In contrast, when 3T3-L1 adipocytes overexpressed CTRP9, the secreted adiponectin and CTRP9 formed predominantly trimers (Fig. 7E, first and second panels), suggesting that CTRP9 and adiponectin preferentially form heterotrimers. To confirm this, we performed coimmunoprecipitations on the collected FPLC fractions. As shown in Fig. 7E (third panel), most of the CTRP9 that coimmunoprecipitated with adiponectin were found in fractions 21–23 that contained adiponectin trimers. A similar result was also obtained from 3T3-L1 adipocytes that expressed CTRP9 (C23A) (Fig. 7E, panels 4–6). All together, these studies establish that adiponectin and CTRP9 form a heterotrimeric complex.
CTRP9 affects glucose and insulin metabolism
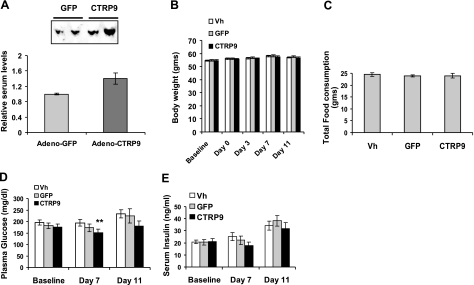
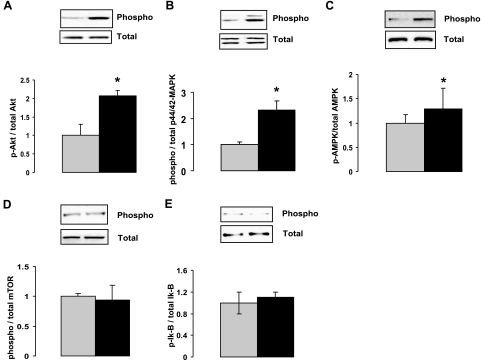
To investigate whether recombinant CTRP9 has any metabolic effect in vivo, we overexpressed the protein in mice using an adenovirus vector. This led to a modest increase (∼40%) in the serum levels of CTRP9 (Fig. 8A). Overexpression of CTRP9 in mice did not affect their body weight or the amount of food consumed during the course of the experiment (Fig. 8B, C). In contrast to ob/ob mice injected with control adenovirus encoding GFP or control buffer, we observed a modest but statistically significant reduction of serum glucose levels in ob/ob mice injected with adenovirus encoding CTRP9 (Fig. 8D). Overexpression of CTRP9 also lowered serum insulin levels in the ob/ob mice compared to controls (Fig. 8E). To address what signaling pathways are activated by CTRP9, we stimulated differentiated mouse C2C12 myotubes with purified recombinant CTRP9. At a concentration of 2–4 μg/ml, we observed specific phosphorylation and activation of AMPK, Akt, and p44/42 MAPK signaling pathways in myotubes (Fig. 9). In contrast, other signaling pathways, such as mTOR and NF-κB, were not activated. Together, these results highlight a potential important metabolic function for CTRP9.
Figure 8.
Adenovirus-mediated overexpression of CTRP9 reduces serum glucose levels in ob/ob mice. Adenovirus encoding GFP or CTRP9 and vehicle buffer were injected intravenously into 12-wk-old male obese (ob/ob) mice (n=12–15). A) Representative Western blot analysis of serum CTRP9 levels and their quantifications in mice overexpressing GFP or CTRP9. B) Body weight of mice was measured at day 4 postinjection. C) Food intake was measured from day 4 to 7 postinjection. D) Blood glucose level was measured prior to adenovirus injection (baseline) and at day 7 and 11 post injection. E) Serum insulin level was measured prior to adenovirus injection (baseline) and at days 7 and 11 postinjection. A modest but statistically significant difference (t test; *P<0.05) in serum glucose levels was observed between mice injected with vehicle buffer or adenovirus encoding GFP vs. CTRP9.
Figure 9.
Recombinant CTRP9 activates AMPK, Akt, and MAPK signaling pathways in differentiated C2C12 myotubes. A–E) Differentiated C2C12 myotubes were treated with control buffer or 4 μg/ml of recombinant CTRP9 for 15 min. Western blot analysis was carried out on cleared cell lysates using antibodies specific to phospho-Akt (Thr308), total Akt, phospho-AMPK (Thr 172), total AMPK-α, phospho-mTOR (Ser-2448), total mTOR, phospho-p44/42 MAPK (Thr202/Tyr204), total p44/42 MAPK, phospho-IκB (Ser-32), and total IκB. Ratios of phopho to total proteins were quantified. Results shown are fold difference between control buffer (value set to 1) vs. recombinant CTRP9 treated myotubes. All experiments were performed in triplicate and repeated at least twice. *P < 0.05 vs. control; t test.
DISCUSSION
By secreting a variety of adipokines that include adiponectin, adipose tissue plays an important role in regulating systemic glucose and lipid metabolism. Considerable efforts have been made to discover novel adipose tissue-secreted factors that affect whole-body insulin sensitivity and energy balance. In this study, we identified CTRP9 as a novel serum glycoprotein secreted by adipose tissue that can trimerize with adiponectin. Functional studies of CTRP9 showed that it activates AMPK and Akt signaling pathways in cultured myotubes and that it lowered serum glucose levels in obese (ob/ob) mice. Hence, CTRP9 represents a novel adipokine.
Type II diabetes is a complex metabolic disease that involves the contribution of many genes within the human genome. In recent years, many genome-wide scans have been carried out to identify relevant genes and their allelic variants that might give rise to or predispose an individual to developing insulin resistance and type II diabetes. The adiponectin gene, located on human chromosome 3q27, and its allelic variants are genetically linked to type II diabetes and the metabolic syndrome (11, 12, 55). The CTRP9 gene is relatively small (∼12.6 kb) and is located on human chromosome 13q12.12. Interestingly, a recent multipoint linkage analysis for insulin sensitivity phenotypes conducted on Mexican Americans showed a significant linkage signal on chromosome 13q between the two markers D13S787 and D13S252 (56). The CTRP9 gene is located between these two markers, in the vicinity of D13S787. As such, the CTRP9 gene and its allelic variants may be a candidate that confers risk factor for diabetes in Mexican Americans.
Formation of CTRP9/adiponectin trimers
All proteins with Gly-X-Y repeats form a characteristic triple helical structure (57). Adiponectin and CTRP9 have 22 and 56 Gly-X-Y repeats in their collagen domains, respectively. The unequal length of their collagen domains suggests two possible modes of association. First, the collagen domain of CTRP9 and adiponectin do not form a triple helical structure, and their association is strictly mediated by their respective C1q domain. However, such a heterooligomer configuration with an unstructured, “non-triple helical” collagen domain has never been documented. Alternatively, the collagen domains of CTRP9 and adiponectin could still form a triple helical structure with an overhang of extra Gly-X-Y repeats at their N termini. In support of this possibility, a survey of proteins with collagen domains showed that despite the unequal numbers of Gly-X-Y repeats, some collagen domain-containing proteins can still form heterotrimers in vivo. As an example, the basic structural unit of mouse complement C1q is a heterotrimer composed of A, B, and C chains (48). The A chain has 26 imperfect Gly-X-Y repeats interrupted once by 1 amino acid; the B chain has 29 perfect Gly-X-Y repeats; and the C chain has 26 imperfect Gly-X-Y repeats interrupted once by 3 amino acids. Despite these differences, complement C1q A, B, and C chains form heterotrimers. Chipmunk hibernating proteins are heterotrimers composed of HP20, HP25, and HP27 (49, 50). While HP20 and HP27 have 13 Gly-X-Y repeats, HP25 has 14 Gly-X-Y repeats. Type VI collagen is found ubiquitously in connective tissues. Its basic structural unit is that of a trimer composed of α1, α2, and α3 chains encoded by separate genes (58, 59). The α1 and α2 chains each possess 100 imperfect Gly-X-Y repeats interrupted once by 2 amino acids, while the α3 chain has 110 imperfect Gly-X-Y repeats interrupted twice by 2 and 4 amino acids. The positions of the interrupting amino acids do not correspond to each other on different subunits of the heterotrimer. Despite the extra 10 Gly-X-Y repeats and the asymmetric distributions of interrupting amino acids, the α1, α2, and α3 subunits of type VI collagen still come together to form a characteristic triple helical structure. Thus, the CTRP9/adiponectin complex is another example of heterotrimer composed of collagen domain-containing proteins with unequal numbers of Gly-X-Y repeats.
Possible implications of CTRP9/adiponectin heterotrimer
We and other groups have shown that the globular form of adiponectin is functionally active (14, 17). On the basis of these initial observations, it has been proposed that adiponectin undergoes a proteolytic cleavage event at the target tissue to generate the “active” globular head. Although it has been observed in SDS-PAGE immunoblot analysis, the truncated globular form of adiponectin has never been isolated from the serum. It may represent a transient species generated locally at the site of action. Nonetheless, neutrophil elastase is able to cleave adiponectin at 5 distinct sites, 2 of which will generate adiponectin with the size corresponding to its globular head (60). It is possible that adiponectin/CTRP9 heterotrimers are more susceptible to cleavage than adiponectin homotrimers. In support of this possibility, we often observed the presence of secreted adiponectin that corresponded to the globular head on SDS-PAGE immunoblots when we coexpressed with CTRP9 in HEK293T cells (data not shown). In contrast, we did not detect secreted adiponectin with the size of the globular head when it was expressed alone in HEK293T cells.
The question of which is the most metabolically “active” form of adiponectin in vivo continues to be debated. In humans, the amount of HMW oligomeric form or the ratio of HMW to trimeric form of adiponectin correlates better with insulin sensitivity (30, 31, 61). In the rodent system, adiponectin trimers have been shown to exert a potent ability to reduce serum glucose (24). Scherer and coworkers (24) showed that adiponectin treated with a reducing agent, or an adiponectin (C39S) mutant that can only form trimers, is more bioactive than the higher-order oligomeric forms of the protein with respect to the reduction of serum glucose levels, leading to the speculation that a serum reductase is involved in generating the active form of adiponectin. Transgenic overexpression of just the globular head (presumably forming only trimers) of adiponectin in ob/ob mice was also able to ameliorate insulin resistance and diabetes (17). Our results showed that when adiponectin was coexpressed with CTRP9, it preferentially formed trimers. It remains to be determined whether adiponectin/CTRP9 heterooligomers induce distinct signaling pathway in cells and tissues compared to adiponectin and CTRP9 alone.
Three distinct adiponectin receptors were recently identified by expression-cloning strategies (62, 63). AdipoR1 and adipoR2 are unusual 7 transmembrane receptors with an inverse topology, having the N terminus residing in the cytoplasm (63). T-cadherin is a GPI-anchored member of the cadherin family that preferentially binds the HMW oligomeric form of adiponectin (62). Overexpression of T-cadherin in multiple cell types did not result in enhanced binding of CTRP9, suggesting that the putative CTRP9 receptors are distinct from those of adiponectin (data not shown).
Several groups have recently generated adiponectin-null mice (64,65,66). In all cases, the knockout mice show a mild or no metabolic phenotype when maintained with a normal chow diet. Under this condition in which mice are not being metabolically challenged, we propose that other serum proteins may compensate for the loss of serum adiponectin. In support of this notion, adenovirus-mediated overexpression of CTRP9 significantly lowered serum glucose levels in ob/ob mice compared to controls. Although the natural target tissues of CTRP9 in vivo are not known, the fact that CTRP9 stimulates AMPK, Akt, and p44/42 MAPK phosphorylation and activation in differentiated myotubes suggests that muscle may be among the target tissues where CTRP9 exerts its function.
In summary, we have identified, cloned, and characterized a novel serum glycoprotein with important metabolic functions and that can trimerize with adiponectin. Beyond its physiological function, circulating serum CTRP9 levels may have diagnostic value in various disease states. Future studies will uncover the physiological function and mechanisms of action of CTRP9 in both normal and pathological conditions.
Acknowledgments
We thank Drs. Prakash Rao, Joseph Marszalek, Alec Cheng Cheng Zhang, and Kelly Wong for helpful comments during the course of this work. This work was supported in part by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R37 DK47618–19 (to H.F.L.), NIH KO8 grant HL077499–01 (to C.H.), and NIH National Research Service Award postdoctoral fellowship F32DK067835 (to G.W.W.)
References
- Berg A H, Combs T P, Scherer P E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Scherer P E, Williams S, Fogliano M, Baldini G, Lodish H F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman B M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley R E, Tataranni P A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- Tonelli J, Li W, Kishore P, Pajvani U B, Kwon E, Weaver C, Scherer P E, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, Ouchi N, Kihara S, Kawamoto T, Sumitsuji S, Funahashi T, Matsuzawa Y. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–2328. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Lepretre F, Dupont S, Hara K, Clement K, Bihain B, Kadowaki T, Froguel P. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607–2614. doi: 10.1093/hmg/11.21.2607. [DOI] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao T S, Javorschi S, Ebbets-Reed D, Erickson M R, Yen F T, Bihain B E, Lodish H F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A H, Combs T P, Du X, Brownlee M, Scherer P E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman M L, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn B B, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Combs T P, Pajvani U B, Berg A H, Lin Y, Jelicks L A, Laplante M, Nawrocki A R, Rajala M W, Parlow A F, Cheeseboro L, Ding Y Y, Russell R G, Lindemann D, Hartley A, Baker G R, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer P E. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nguyen M T, Trujillo M, Imamura T, Usui I, Scherer P E, Olefsky J M. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao T S, Saha A K, Murrey H E, Zhang Cc C, Itani S I, Lodish H F, Ruderman N B. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao T S, Tomas E, Murrey H E, Hug C, Lee D H, Ruderman N B, Heuser J E, Lodish H F. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- Tsao T S, Murrey H E, Hug C, Lee D H, Lodish H F. Oligomerization state-dependent activation of NF-κB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- Pajvani U B, Du X, Combs T P, Berg A H, Rajala M W, Schulthess T, Engel J, Brownlee M, Scherer P E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Wilson-Kubalek E M, Wert D, Tsao T S, Lee D H. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–814. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu A, Knight C, Xu L Y, Cooper G J. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- Richards A A, Stephens T, Charlton H K, Jones A, Macdonald G A, Prins J B, Whitehead J P. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam K S, Chan L, Chan K W, Lam J B, Lam M C, Hoo R C, Mak W W, Cooper G J, Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert M O, Mohlig M, Pfeiffer A F, Spranger J. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- Pajvani U B, Hawkins M, Combs T P, Rajala M W, Doebber T, Berger J P, Wagner J A, Wu M, Knopps A, Xiang A H, Utzschneider K M, Kahn S E, Olefsky J M, Buchanan T A, Scherer P E. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Fisher F F, Trujillo M E, Hanif W, Barnett A H, McTernan P G, Scherer P E, Kumar S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- Combs T P, Berg A H, Obici S, Scherer P E, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Hara K, Kubota N, Terauchi Y, Tobe K, Froguel P, Nagai R, Kadowaki T. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:243–254. doi: 10.2174/1568008033340090. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel D R, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci W S, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu L Y, Lam K S, Cooper G J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A R, Kahn C R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel D R, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H F, Gurney A L, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass P E, Heldens S, Huang A, Kim H S, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie M H, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood W I, Godowski P, Gray A. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13:2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G W, Wang J, Hug C, Tsao T S, Lodish H F. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- Liu X, Constantinescu S N, Sun Y, Bogan J S, Hirsch D, Weinberg R A, Lodish H F. Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal Biochem. 2000;280:20–28. doi: 10.1006/abio.2000.4478. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Scherer P E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- Combs T P, Berg A H, Rajala M W, Klebanov S, Iyengar P, Jimenez-Chillaron J C, Patti M E, Klein S L, Weinstein R S, Scherer P E. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Xu A, Chan K W, Hoo R L, Wang Y, Tan K C, Zhang J, Chen B, Lam M C, Tse C, Cooper G J, Lam K S. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- Vitagliano L, Berisio R, Mazzarella L, Zagari A. Structural bases of collagen stabilization induced by proline hydroxylation. Biopolymers. 2001;58:459–464. doi: 10.1002/1097-0282(20010415)58:5<459::AID-BIP1021>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Kishore U, Reid K B. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Kondo J. Identification of novel blood proteins specific for mammalian hibernation. J Biol Chem. 1992;267:473–478. [PubMed] [Google Scholar]
- Takamatsu N, Ohba K, Kondo J, Kondo N, Shiba T. Hibernation-associated gene regulation of plasma proteins with a collagen-like domain in mammalian hibernators. Mol Cell Biol. 1993;13:1516–1521. doi: 10.1128/mcb.13.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Zuo J, Morgan J I. Cbln3, a novel member of the precerebellin family that binds specifically to Cbln1. J Neurosci. 2000;20:6333–6339. doi: 10.1523/JNEUROSCI.20-17-06333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao D, Pang Z, Morgan M A, Parris J, Rong Y, Li L, Morgan J I. Cbln1 is essential for interaction-dependent secretion of Cbln3. Mol Cell Biol. 2006;26:9327–9337. doi: 10.1128/MCB.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, Parris J, Rong Y, Watanabe M, Yuzaki M, Morgan J I. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L Y, Lam K S, Lu G, Cooper G J, Xu A. Proteomic characterization of human serum proteins associated with the fat-derived hormone adiponectin. Proteomics. 2006;6:3862–3870. doi: 10.1002/pmic.200500840. [DOI] [PubMed] [Google Scholar]
- Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, Kadowaki H, Hagura R, Akanuma Y, Yazaki Y, Nagai R, Taniyama M, Matsubara K, Yoda M, Nakano Y, Tomita M, Kimura S, Ito C, Froguel P, Kadowaki T. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536–540. doi: 10.2337/diabetes.51.2.536. [DOI] [PubMed] [Google Scholar]
- Cai G, Cole S A, Freeland-Graves J H, MacCluer J W, Blangero J, Comuzzie A G. Genome-wide scans reveal quantitative trait Loci on 8p and 13q related to insulin action and glucose metabolism: the San Antonio Family Heart Study. Diabetes. 2004;53:1369–1374. doi: 10.2337/diabetes.53.5.1369. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Cartailler J P, Alvares K, Veis A, Hudson B G. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- Stokes D G, Saitta B, Timpl R, Chu M L. Human alpha 3(VI) collagen gene. Characterization of exons coding for the amino-terminal globular domain and alternative splicing in normal and tumor cells. J Biol Chem. 1991;266:8626–8633. [PubMed] [Google Scholar]
- Chu M L, Pan T C, Conway D, Kuo H J, Glanville R W, Timpl R, Mann K, Deutzmann R. Sequence analysis of alpha 1(VI) and alpha 2(VI) chains of human type VI collagen reveals internal triplication of globular domains similar to the A domains of von Willebrand factor and two alpha 2(VI) chain variants that differ in the carboxy terminus. EMBO J. 1989;8:1939–1946. doi: 10.1002/j.1460-2075.1989.tb03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad N S, Bogan J S, Tsao T S, Lodish H F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno N H, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha P K, Kojima H, Li L, Chang B H, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]