Abstract
Transcription of the rearranged immunoglobulin gene and expression of the enzyme activation-induced deaminase (AID) are essential for somatic hypermutations of this gene during antibody maturation. While AID acts as a single-strand DNA-cytosine deaminase creating U · G mispairs that lead to mutations, the role played by transcription in this process is less clear. We have used in vitro transcription of the kan gene by the T7 RNA polymerase (RNAP) in the presence of AID and a genetic reversion assay for kanamycin-resistance to investigate the causes of multiple clustered mutations (MCMs) during somatic hypermutations. We find that, depending on transcription conditions, AID can cause single-base substitutions or MCMs. When wild-type RNAP is used for transcription at physiologically relevant concentrations of ribonucleoside triphosphates (NTPs), few MCMs are found. In contrast, slowing the rate of elongation by reducing the NTP concentration or using a mutant RNAP increases several-fold the percent of revertants containing MCMs. Arresting the elongation complexes by a quick removal of NTPs leads to formation of RNA-DNA hybrids (R-loops). Treatment of these structures with AID results in a high percentage of KanR revertants with MCMs. Furthermore, selecting for transcription elongation complexes stalled near the codon that suffers mutations during acquisition of kanamycin-resistance results in an overwhelming majority of revertants with MCMs. These results show that if RNAP II pauses or stalls during transcription of immunoglobulin gene, AID is likely to promote MCMs. As changes in physiological conditions such as occurrence of certain DNA primary or secondary structures or DNA adducts are known to cause transcriptional pausing and stalling in mammalian cells, this process may cause MCMs during somatic hypermutation.—Canugovi, C., Samaranayake, M., Bhagwat, A. S. Transcriptional pausing and stalling causes multiple clustered mutations by human activation-induced deaminase.
Keywords: hypermutations, antibody maturation, cytosine deamination, uracils in DNA
Activation-induced deaminase (AID) is a key enzyme expressed in germinal center B lymphocytes and is required for the maturation of antibodies (1). It is a single-strand-specific DNA-cytosine deaminase (see ref. 2 for a recent review). It converts cytosines in DNA to uracil, and when these are not excised by the uracil-DNA glycosylase (UDG), C to T mutations result (3,4,5,6,7). These properties of AID partly explain its essential role in somatic hypermutation (SHM) of the immunoglobulin (Ig) gene (2).
A key feature of the action of AID in SHM and class-switch recombination (CSR) is the requirement for transcription of the target Ig gene (8). Initially, transcriptional enhancers within introns downstream of the Ig genes were thought to be essential for SHM (9, 10), but subsequent work has shown that this phenomenon is not promoter-specific. When a defective GFP allele was expressed from a tetracycline-controlled promoter in a hypermutation-active pre-B cell line (11) or NIH 3T3 fibroblast cells expressing AID (12), it accumulated mutations in a transcription-dependent manner. Thus, transcription of the target gene may be sufficient condition for AID generated hypermutations.
The ability of AID in Escherichia coli or in vitro to convert cytosines in DNA to uracil also depends on transcription of the target gene. In E. coli, when kanamycin-resistant (KanR) revertants from a mutant kan gene were obtained in the presence of AID, transcription of the kan gene increased the revertant frequency 20-fold (4) to 50-fold (3). When the same kan allele was transcribed from a T7 promoter in vitro in the presence of AID, ∼300-fold increase in cytosine deaminations was observed (3). A smaller increase in the KanR frequency was obtained when a similar kan allele was transcribed in vitro by the E. coli RNA polymerase (RNAP; ref. 13). These results showed that DNA sequences and protein factors associated with Ig genes are not required for the transcription-dependence of mutagenic action of AID.
At least two areas of uncertainty remain regarding the action of AID on transcribing DNA. The first is whether AID can target only the nontranscribed strand (NTS) or both DNA strands (2). Initial results from the KanR reversion assay indicated that AID targeted only the NTS both in vivo (3, 4) and in vitro (3). Similar results were obtained when a biochemical assay was used to detect uracils generated by AID during transcription of a DNA by T7 RNAP in vitro (14) and by E. coli RNAP in vivo (15). However, some studies in which other gene targets were transcribed in vitro have not found a strong bias in favor of the NTS (13, 16). Finally, some recent work suggests (17, 18) that at least parts of both coding and noncoding DNA strands of Ig genes may be transcribed in vivo. This would mean that in B cells, AID would cause C to U deaminations in both DNA strands even if it attacks only NTS.
The second area of uncertainty is how AID may create multiple clustered mutations. SHM sometimes causes tandem base substitutions (19), but the source of these mutations is unclear (20). One possible explanation is that AID acts processively on single-stranded as well as transcribing double-stranded DNA (14, 21). The processive nature of AID action on transcribing DNA was supported by another study that used similar experimental conditions (16). On the other hand, Coker et al. (22) concluded that AID acts distributively on single-stranded DNA. Subsequent clarification of what constitutes processivity (22, 23) suggested that AID may dissociate following a turnover and perform intramolecular transfer to other sites in the same DNA molecule. Such transfers should lead to multiple cytosine deaminations during the same binding event in the same way as protein sliding along the DNA and this is still considered processive action. However, these experiments were done using single-stranded DNA, and it is not known whether similar jumping events also affect AID action on transcribing DNA.
To understand the basis of multiple clustered mutations (MCMs) caused by AID, we have studied its interaction with DNA under different conditions of transcription driven by the T7 RNAP. We find that AID can cause either single or multiple C to T mutations depending on the experimental conditions. We describe these results below.
MATERIALS AND METHODS
Bacterial strains and plasmids
Most of the strains used in this study were derived from GM31 (K-12 dcm6 thr1 hisG4 leuB6 rpsl ara14 supE44 lacY1 tonA31 tsx78 galK2 galE2 xyl5 thi1 mtl1). Strain BH156 (=GM31 ung-1 tyrA::Tn10) and BH214 (=BH156 mug::Tn10 with λ DE3 lysogen) have been described previously (3, 24). BH155 was constructed by introducing recA56 srlC::Tn10 markers into BH152 (=GM31 ung-1) by P1 transduction. For the expression and purification of GST-AID or T7 RNA polymerase (RNAP), E. coli strain BL21 (GOLD; E. coli B F− ompT hsdS TetR gal endA Hte) was used as host.
The plasmid constructs used here contain the kan allele kanS94D. A substitution of the cytosine at either the first or the second position of codon 94 of this allele (Fig. 1A) confers kanamycin-resistance on its host (25). Plasmid pAB7 is a pMB1-based plasmid and contains the kanS94D under T7 promoter control. (Fig. 1A; ref. 3), and pAB6 is similar to pAB7 except the replicon is a mini-F (26). Plasmid pAB71 was constructed by introducing a recognition site for the restriction enzyme SexAI (ACCNGGT), overlapping codon 94 of kanS94D (Fig. 1A). This was accomplished by PCR-based site-directed mutagenesis (Stratagene, La Jolla, CA, USA). The mutagenic primers used for this purpose were 5′-GCACTTCACCTGGTAGCAGCCAGTCC-3′ and 5′-GGACTGGCTGCTACCAGGTGAAGTGC-3′.
Figure 1.
Structure of plasmids and the genetic system. A) Plasmid constructs used in this study are schematically represented. Both plasmids contain kanamycin-sensitive allele under the T7 promoter control. Plasmids also contain ampicillin-resistance gene and lac IQ (not shown). Plasmid pAB71 is same as pAB7, except that a unique restriction recognition site for Sex AI (marked below the sequence) was introduced, overlapping codon 94. pAB6 (not shown) contains the same promoter and kan allele but is on a mini-F replicon. pUP21 (not shown) contains the same kan allele, but is transcribed from an E. coli promoter called UP-tac (3). B) KanR reversion assay. Deamination of one or both cytosines in codon 94 of inactive kan gene followed by DNA replication results in active Kan protein and restoration of KanR phenotype. The three possible mutations within KanR revertants and the amino acids they introduce instead of Proline in the inactive Kan are shown.
AID expression and purification
Recombinant AID was purified as described previously (3). Briefly, expression vector encoding AID fused to the carboxyl terminus of glutathione-S-transferase (GST) protein was introduced into BL21 (GOLD) cells. Protein expression was induced with the addition of 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) to the growth medium. Cells were harvested after 6 h of growth at 28°C by centrifugation at 1000 g. Cell pellet was resuspended in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH adjusted to 7.4) and lysed by sonication with 20 pulses of 15 s duration separated by an interval of 1 min on ice. Fusion protein was purified using a glutathione sepharose column according to the manufacturer’s recommendations (Amersham Biosciences, Piscataway, NJ, USA). Proteins from different fractions were separated on a 12% SDS-polyarylamide gel, and fractions containing GST-AID were identified. These fractions were pooled and concentrated using YM30 centricon filter (Millipore, Billerica, MA, USA) and equilibrated with the storage buffer [25 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, 1.0 mM dithiothreitol (DTT), and 10% glycerol] and kept at −70°C until use.
Purification of T7 RNAP and its mutant G645A
Quickchange site-directed mutagenesis kit (Stratagene) was used to change the codon 645 of the T7 RNAP gene from GGC to GCC. The wild-type (WT) polymerase gene was in plasmid pDL21 (27) (kindly provided by Dr. A. Nicholson, Temple University, Philadelphia, PA, USA), and the primers used for mutagenesis were 5′-GGGTCCAAAGAGTTCGCCTTCCGTCAACAAGTGC-3′ and 5′-GCACTTGTTGACGG AAGGCGAACTCTTTGGACCCG-3′. The resulting plasmid containing the mutation was called pDL21-T7G645A.
The following procedure was used to purify WT or G645 mutant of T7 RNAP. The appropriate plasmid was introduced into E. coli BL21 (GOLD) by transformation, and the expression of the mutant protein was induced by adding IPTG to 0.6 mM. The cells were grown at 37°C for 6 h and harvested. The cell pellet was resuspended in buffer A [50 mM Tris-HCl (pH 7.8), 20 mM NaCl, 200 μg lysozyme, 0.1% TritonX-100, 50 U DNase (New England Biolabs, Ipswich, MA, USA), 25 μg of RNase A] and kept on ice for 2 h. The cells were lysed by sonication, and WT or mutant protein was purified over an Ni-NTA column (Novagen, Madison, WI, USA) according to the manufacturer’s instructions. The eluted fractions were separated on a 12% SDS-PAGE to confirm the purity of the protein. The mutant polymerase was stored in 25 mM Tris-HCl (pH 7.8), 20 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1% Triton X-100, and 50% glycerol at −70°C.
DNA sequencing
Several revertants from the kanamycin reversion assay were picked and grown in liquid medium. The cells were harvested and stored at −70°C until use. Bio Robot 9600 (Qiagen, Valencia, CA, USA) was used to purify plasmid DNA from these cultures. The DNA was sequenced using T7 promoter-specific primer or 5′-CTGACCCCGGATGAATGTC-3′ (Sigma-Genosys, The Woodlands, TX, USA), which hybridizes midway between T7 promoter and the start of kan gene. The sequencing was performed at the University of Michigan Sequencing Core, Ann Arbor, MI, USA. The DNA sequences were aligned and analyzed by MacVector software (version 9.1; MacVector Inc., Cary, NC, USA). To resolve sequencing ambiguities, individual chromatograms were examined using Chromas software (Technelysium Pty. Ltd., Australia).
Kanamycin-resistance reversion assay
The general procedure for these experiments was as follows. The plasmid pAB7 or pAB71 were transcribed in vitro using a T7 RNAP and then introduced into E. coli strain BH156 by electroporation. KanR revertants were scored, and the frequency of the revertants was calculated by dividing the number of KanR revertants with the total number of viable cells.
The following conditions were used for in vitro transcription: 2 nM pAB7 plasmid DNA was transcribed in the transcription buffer (40 mM Tris-HCl, pH 7.8; 10 mM MgCl; 10 mM NaCl; 2 mM spermidine; 100 mM potassium glutamate; and 10 mM DTT) using 100 nM T7 RNAP in the presence or absence of all four ribonucleoside triphosphates (NTPs). Unless mentioned otherwise, the concentration of each NTP was 4 mM. AID (1.5 μg) was added to various reaction mixtures as indicated in the figure legends. The reaction mixtures were incubated at 37°C for the indicated length of time and were terminated by the addition of EDTA to 10 mM. Excess RNA was removed by adding 10 μg of RNase A (Fisher Scientific, Hampton, NH, USA) and incubating reaction mixtures for 15 min at 37°C. The DNA was deproteinated and further purified by ethanol precipitation prior to introduction into BH156 cells by electroporation. KanR revertants were selected and analyzed as described above.
To remove mRNA hybridized to template DNA during transcription 10 U of RNase H (Epicenter, Madison, WI, USA) was added to the transcription reaction mixtures, and incubation was continued for 25 min. The DNA from reaction mixtures with or without RNase H was deproteinated as described above, and a part of this DNA was used for the KanR assay. The remaining DNA was electrophoresed on agarose gels and stained with ethidium bromide to visualize the different forms of plasmid DNA.
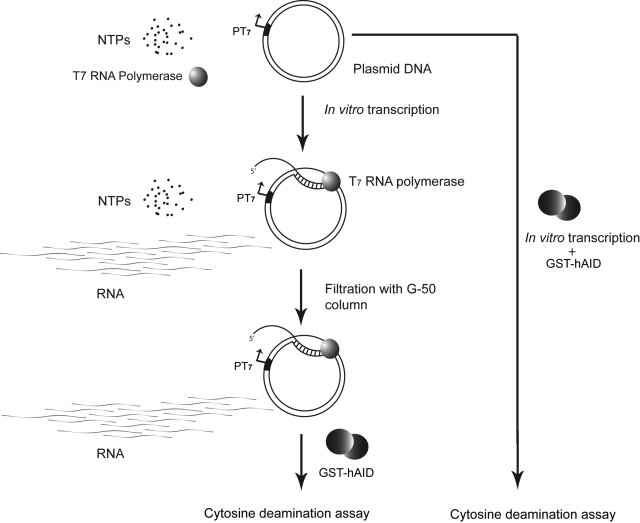
To stall the RNAP during transcription, plasmid pAB7 was transcribed for 1 h as described above but without AID. The reaction mixture was filtered through a G-50 spin-column (1100 g for 1 min) to remove NTPs, and AID was added to the reaction mixture. Following a 1 h incubation at 37°C, the DNA was deproteinated, ethanol precipitated, and used for the KanR assay.
Similar stalled transcription complexes were also created with plasmid pAB71. One-half of this reaction mixture was digested with 5 U of SexAI enzyme (New England Biolabs) for 2 h at 37°C to linearize DNA molecules containing duplex SexAI sites. The resulting DNAs were treated with AID for 1 h at 37°C. The digested and undigested DNAs were transformed into BH156 cells to obtain KanR revertants.
RESULTS
Multiple mutations during complete transcription reactions
When the plasmid pAB7 (Fig. 1) was incubated with AID in the absence of transcription, KanR revertant frequency was ∼6-fold higher than the untreated plasmid. The magnitude of increase was as much as ∼600-fold when AID treatment was performed simultaneously with transcription of the plasmid from its T7 promoter for 1 h (Fig. 2A). Increasing the incubation time to 6 h increased the revertant frequency by 1900-fold compared to untreated plasmid (Fig. 2A). When several independent revertants obtained in these experiments were sequenced, a couple of patterns emerged from the sequences.
Figure 2.
KanR revertant frequencies. A) Revertant frequency for pAB7. KanR revertant frequencies following in vitro of pAB7 for the indicated length of time. Data are means ± sd from 4 experiments. B) Revertant frequency for pAB8. KanR revertant frequencies following in vitro of pAB7 for the indicated length of time. Data are means ± sd from 3 experiments. Note the difference in the y-axis scale in A and B.
First, all the mutations were C to T, and an overwhelming majority of the mutations were within codon 94 of the kan gene. This finding is consistent with the ability of AID to convert C to U and the inability of host cells to repair uracils. Second, an overwhelming strand bias was found in the mutations. All the mutated cytosines lay in the nontranscribed strand (NTS). This finding was also consistent with our previously reported results (3) and is attributed to the greater access of the NTS to molecules during transcription (28). Third, we found sequence selectivity in the mutations. A vast majority of the mutations in the revertants occurred at the first C in CCA (Fig. 3A). Codon 94 contains two mutable Cs, only the first of which is within the WRCY motif that is preferentially targeted for SHMs (Fig. 1; ref. 29). Thus, AID targeted the same cytosine in this in vitro transcription system as would be targeted during SHM. Fourth, revertants isolated from 0.5 and 1 h incubations, contained only single C to T mutations; whereas at 2 and 6 h a significant fraction of revertants contained multiple mutations (6 and 11%, respectively). In other experiments, an occasional revertant isolated after 1 h of incubation contained more than one mutation, but the number of such revertants was larger after longer incubations (Fig. 3A and data not shown). These results suggest that AID can create single or multiple C to U deaminations in the same DNA molecule depending on the conditions of transcription.
Figure 3.
Distribution of mutations within codon 94 of kan. A) pAB7 mutations. Mutations at the first position, second position, or both and at other sites are shown. When mutations were at both first and second position and/or were outside codon 94, they were classified as multiple mutations. B) pAB8 mutations. Mutations at the first or second position are shown.
We wondered whether the direction of transcription within the plasmid had a significant effect on revertant frequencies and mutation distribution within kan gene. To study this, a plasmid in which the T7 promoter transcribed the coding strand of kan gene, pAB8 (Fig. 1), was used in experiments similar to those described for pAB7. Treatment of pAB8 with AID did increase the KanR revertant frequency, but concurrent transcription of kan did not increase it any further (Fig. 2B). When the distribution of mutations in pAB8 was examined, all the mutations were either at the first or second position in codon 94 of kan (Fig. 3B). At shorter time intervals of incubation (0.5 and 1.0 h), they were predominantly at the first position, while after longer incubation times they were at the two positions with roughly equal frequency (Fig. 3B). Furthermore, as there were no pAB8 revertants with multiple mutations, it appeared that changing the direction of transcription within kan did affect the distribution of mutations caused by AID. Although these differences in the pattern of mutations between pAB7 and pAB8 were interesting, we focused mainly on mutations promoted by AID during transcription, and the revertant frequency of pAB8 was not enhanced by transcription (Fig. 2B). Consequently, to understand the mechanism by which multiple mutations arise during transcription, several reaction parameters were changed and the effects of these changes on the occurrence of multiple mutations in pAB7 were studied.
Effect of NTP concentration on multiple mutations
It seemed likely that during prolonged transcription reactions such as the 2 and 6 h reactions described above, NTPs will become limiting. This should affect transcription because lowering the concentration of NTPs reduces the rate of polymerase elongation and causes the polymerase to pause along DNA (30). We speculated that this may alter the access of AID to DNA and hence studied the effects of lowering NTP concentration below the 4 mM used in the experiments described above on the distribution of mutations caused by AID.
Plasmid pAB7 was transcribed using different NTP concentrations in the presence of AID, and KanR revertants were isolated. We found that even at the lowest concentration of NTPs used, 50 μM, the revertant frequency was higher than in the reaction mixture lacking NTPs. Moreover, increasing the NTP concentration from 50 to 250 μM or higher increased significantly the revertant frequency (Fig. 4A). However, occurrence of MCMs did not directly correlate with high-mutation frequencies. Revertants with multiple mutations were most frequently found at NTP concentrations of 1 mM and lower, but rarely at 4 mM (Fig. 4B; Table 1).
Figure 4.
Effect of NTP concentration on AID mutagenesis. A) KanR revertant frequencies following in vitro of pAB7 using different NTP concentrations. Data are means ± sd from 3 experiments. B) Data are percentages of KanR revertant clones with multiple mutations resulting from transcription with different NTP concentrations.
TABLE 1.
Frequency of multiple clustered mutations
| Experimenta | Condition | Clones sequenced | Multiple mutations (%) | Clustersb | Ratioc |
|---|---|---|---|---|---|
| Different time intervals of transcription | Time (h) | ||||
| pAB7 | |||||
| 0.5 | 15 | 0 | 0 | 0.00 | |
| 1.0 | 16 | 0 | 0 | 0.00 | |
| 2.0 | 16 | 6 | 1 | 0.06 | |
| 6.0 | 45 | 11 | 1 | 0.02 | |
| pAB8 | |||||
| 0.5 | 16 | 0 | 0 | 0.00 | |
| 1.0 | 16 | 0 | 0 | 0.00 | |
| 2.0 | 16 | 0 | 0 | 0.00 | |
| 6.0 | 20 | 0 | 0 | 0.00 | |
| Different NTP concentrations | Concentration (mM) | ||||
| 0 | 41 | 24 | 6 | 0.15 | |
| 0.05 | 45 | 40 | 11 | 0.24 | |
| 0.25 | 41 | 37 | 7 | 0.17 | |
| 1.0 | 45 | 42 | 5 | 0.11 | |
| 4.0 | 48 | 2 | 1 | 0.02 | |
| Stalled transcription complexes | No NTP removal | 48 | 4 | 0 | 0.00 |
| NTP Removal | 55 | 35 | 12 | 0.22 | |
| SexAI selection | No SexAI | 22 | 46 | 7 | 0.32 |
| With SexAI | 26 | 77 | 13 | 0.50 | |
| T7 RNAP variants | Wild-type | 36 | 8 | 0 | 0.00 |
| G645A | 33 | 30 | 7 | 0.21 |
See text.
Ocurrence of 2 or more mutations within a 10 bp span is referred to as a cluster.
Ratio: clusters/clones sequenced.
When two or more mutations found within 10 bp were designated as “clustered mutations,” the ratio, number of mutational clusters/number of revertants sequenced, was inversely related to NTP concentration. At 50 μM NTP concentration, several clusters with 2 or more mutations (Supplemental Fig. S1) were found. In contrast, at the highest concentration, 4 mM, only 1 in 48 revertants had multiple mutations, and these were two tandem mutations in CCA (i.e., CCA to TTA; Supplemental Table S1). Cellular NTP levels have been variously reported to be between ∼1 to 3 mM (31) and ∼3 to 7 mM (32). These data show that MCMs created by AID occur more frequently at submillimolar NTP concentration than at the physiologically relevant concentrations above 1 mM.
T7 RNAP mutant with slower elongation rate and multiple mutations
The G645A mutation in T7 RNAP reduces the rate of polymerase elongation by 3- to 4-fold, without changing the rate of transcription initiation (33, 34). We reasoned that use of this mutant would slow down the transcription elongation complex in a manner analogous to reducing NTP concentrations and hence promote clustered mutations by AID. When this mutant was used for in vitro transcription of pAB7 instead of WT protein, the KanR reversion frequency was 2.7-fold lower. However, the percentage of revertants containing multiple mutations was ∼4-fold higher for the mutant RNAP compared to WT (Table 1; Supplemental Fig. S2). Furthermore, while many of the mutations created in the presence of mutant polymerase were clustered, none of the revertants isolated after incubation with WT enzyme contained clustered mutations (Table 1; Supplemental Fig. S2). It is likely that this increase in multiple clustered mutations in the presence of T7 G645A mutant is due to the longer length of time a transcription bubble resides at any one base or increased pausing by the enzyme at some sequences.
Action of AID on stalled transcription complexes
One way to understand the role of paused transcription complexes on AID-promoted mutations is to study them in the context of stalled polymerase complexes. If NTPs are limiting in the reaction mixture, the RNAP may stall at various points along the DNA waiting for the correct NTP to arrive. This should create access for AID to DNA within the stalled transcription bubble. The advantage of using transcriptional stalling instead of pausing to study AID action is that stalled complexes can be created for a high percentage of the elongation complexes by a quick filtration of NTPs. The procedure for this experiment is outlined in Fig. 5.
Figure 5.
Scheme for creating stalled transcription complexes. Steps in creating stalled transcription complexes by the filtration of NTPs are shown at left. Subsequently, the DNA was treated with AID for 1 h. The control DNA was treated with AID during transcription (right).
We found that AID treatment of transcribed DNA following NTP removal resulted in a substantial increase in KanR revertant frequency compared to nontranscribing DNA, but this number was significantly lower than when AID was included during transcription (Fig. 6). However, removal of NTPs prior to AID treatment resulted in high frequency of multiple mutations (35%) as well as more clustered mutations compared to the condition where transcription and AID treatment were done together. In the latter case, only 4% of clones contained multiple mutations and none had MCMs (Table 1; Supplemental Fig. S3; Supplemental Table S2). This shows that AID creates MCMs on stalled transcriptional complex and rarely creates them on continuously transcribing DNA. To confirm this conclusion we developed a way to enrich the stalled transcription complexes prior to treatment with AID.
Figure 6.
Effect of AID on stalled transcription complexes. KanR revertant frequencies obtained following treatment of stalled transcription complexes with AID and various control reactions are shown. Data are means ± sd from 3 experiments.
Plasmid pAB7 was mutated to introduce a unique SexAI restriction site (ACCNGGT) overlapping codon 94 of kan gene (Fig. 1; Supplemental Fig. S4). The resulting plasmid, pAB71, was transcribed with T7 RNAP, the NTPs were filtered out and half the DNA was digested with SexAI. One the one hand, this was expected to linearize the DNA fraction in which SexAI site was within duplex DNA and prevent it from being transformed into E. coli in a subsequent step. On the other hand, DNA that contained stalled transcription complexes at codon 94 would be resistant to SexAI and would give rise to transformants. If this DNA suffers more multiple mutations than the plasmid DNA as a whole, then this procedure should increase the fraction of KanR revertants that contain multiple clustered mutations.
We found that transcription of pAB71 DNA followed by NTP removal and AID treatment resulted in 46% of revertants with multiple mutations. Digestion of DNA with SexAI prior to AID treatment increased this number to 77% (Table 1). The distribution of mutations is shown in Supplemental Fig. S5. When clustered mutations in each revertant were counted, the ratio of number of clusters to revertants increased from 0.32 (no SexAI treatment) to 0.50 (with SexAI treatment, Table 1). This confirms our earlier conclusion that AID generates MCMs at stalled transcription complexes.
Structure of stalled polymerase complexes
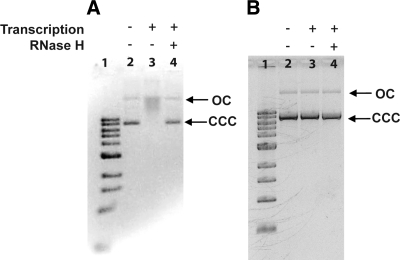
Preceding experiments assume that in vitro transcription with T7 RNAP creates RNA/DNA hybrid structures (R-loop-like structures), which can be trapped by removal of NTPs from transcription reaction mixture. To demonstrate this, pAB7 was transcribed in vitro for 1 h, the NTPs were removed by filtration, and the DNA was subjected to agarose gel electrophoresis. While the DNA that had not been subjected to transcription was predominantly in the covalently closed circle (CCC) form (Fig. 7A, lane 2), the transcribing DNA appeared as a smear with mobility between the CCC and open circle forms (Fig. 7A, lane 3). Subjecting the latter DNA to RNaseH digestion to remove RNA from RNA/DNA hybrids increased the mobility of this DNA, making it indistinguishable from untranscribed DNA (Fig. 7A, compare lanes 2 and 4). We conclude that transcriptional stalling creates RNA/DNA hybrids, which, when trapped by NTP removal, allow greater and longer access to AID promoting the occurrence of multiple clustered mutations.
Figure 7.
R-loop formation during transcription. A) In vitro transcription: ethidium bromide-stained agarose gel of pAB7 DNA is shown. The plasmid was transcribed, and NTPs were removed by filtration. The DNA was divided into two halves; one half was treated with RNase H (lane 4), and the other half was untreated (lane 3). Untranscribed DNA is in lane 2; 1 kbp size markers are in lane 1. Arrows indicate positions of covalently closed circular (CCC) and open circle (OC) of the plasmid. B) In vivo transcription: ethidium bromide-stained agarose gel of pAB7 DNA is shown. The DNA was extracted from cells induced for transcription, and half of the DNA was treated with RNase H (lane 4). Untreated DNA is in lane 3; DNA from uninduced cells is in lane 2; 1 kbp markers are in lane 1.
T7 RNAP transcription in vivo and clustered mutations
When we isolated DNA from cells that were induced with IPTG to transcribe kan gene in pAB7 and subjected this DNA to gel elctrophoresis, R-loop-like structures were not seen (Fig. 7B). This finding is likely to be due to millimolar levels of NTPs in cells and the existence of cellular proteins that help the cell overcome transcriptional pausing and arrest (35). If this theory is correct, then the fraction of transcription elongation complexes in vivo that are in a paused or stalled state should be quite small; hence, the occurrence of MCMs should also be small. We confirmed this by sequencing KanR revertants obtained after transcription of kan gene from a T7 promoter (Table 2, plasmid pAB6) or from a strong E. coli promoter (Table 2, plasmid pUP21). In either case, no revertants with multiple mutations were found and all the revertants contained single mutations at either the first or the second position in the CCA codon (Table 2).
TABLE 2.
Distribution of mutations obtained in vivo
| Mutation
|
pUP21
|
pAB6
|
||
|---|---|---|---|---|
| No IPTG | With IPTG | No IPTG | With IPTG | |
| CCA to TCA | 54.2 | 46.9 | 45.4 | 57.1 |
| CCA to CTA | 45.8 | 53.1 | 54.6 | 42.9 |
| Total sequenced | 24 | 32 | 11 | 14 |
pUP21 contains kan transcribed from E. coli UP-tac promoter; pAB6 contains the same gene transcribed from a T7 promoter. Values are percentages of mutants with a particular type of mutation.
DISCUSSION
We have used an in vitro transcription system that utilizes a bacterial phage-coded RNA polymerase to study the distribution of cytosine deaminations created by AID in the kan gene. Our results show that AID can cause single C to U conversions or clustered C to U conversions depending on the conditions of transcription. The C to T mutations resulting from the deaminations caused by AID during transcription were predominantly in a WRCY sequence, confirming that the preference for this sequence by AID is the likely explanation of why SHMs preferentially occur within this sequence (14). The occurrence of MCMs was not correlated with higher KanR reversion frequency; instead, it was correlated with transcription conditions that slowed down the RNAP. Stalling the RNAP during transcription also resulted in multiple clustered mutations. The clustering of mutations often involved tandem C to T mutations in codon 94 but sometimes included as many as 4 mutations within a 10 nucleotide (nt) stretch. As the transcription bubble of T7 RNAP is ∼10 nt in the NTS (36, 37), these mutations are likely to be due to attack of a single transcription bubble by AID. Slowing the RNAP or stalling it often resulted in mutations spreading across the ∼750 bp stretch of the kan gene, with some revertants suffering as many as 7 base substitutions. As pausing, stalling, and arrest are normal parts of transcript elongation (38), RNA polymerase II (Pol II) must also be subject to these changes during transcription of Ig genes. It is well recognized that several transcription factors exist in eukaryotic cells for the sole purpose of helping Pol II restart transcription following pausing or arrest (39). We propose that the changes in the access of AID to DNA during transcription should occasionally lead to multiple clustered and nonclustered C to U changes, which, following processing by UDG and other DNA repair processes, would result in multiple C to T and non-C to T base substitutions (2).
Our results also suggest that both elongating transcription complexes and stalled or paused transcription complexes are subject to attack by AID. In particular, they show that formation of stable RNA/DNA hybrids is not a required condition for cytosine deaminations by AID. We found that little of the plasmid DNA isolated from cells induced for transcription by the T7 RNAP contained R-loops (Fig. 7B), although these plasmids were targets for AID generated mutations (Table 2; ref. 3). While we did find that a significant fraction of plasmid molecules transcribed in vitro contained R-loops when NTPs were quickly removed from the reaction buffer (Fig. 7A), this was not essential for AID targeting. AID was mutagenic during in vitro transcription, regardless of whether NTPs were filtered out of the reaction mixture (Fig. 6). Additionally, we found that including RNaseH in the in vitro transcription buffer does not eliminate deaminations caused by AID (data not shown).
Several earlier publications have reported the occurrence of MCMs by AID acting on an in vitro transcribed gene (13, 16, 21). The results of these investigations relating to clustering of mutations are summarized in Table 3. All four studies used NTP concentrations of 250 μM or lower and reported a high instance of multiple clustered mutations. These results are generally consistent with our findings that when NTP concentrations are at 50 or 250 μM, ∼40% of the mutants contain multiple mutations and the ratio, number of mutational clusters/number of clones sequenced, is ∼0.2 (Table 1). The previous reports (13, 16, 21) contain somewhat higher values for these parameters than what we observed (Table 3), but these differences may be attributed to differences in the experimental system, such as use of different target genes and different amounts and qualities of AID preparations used in these experiments. The only report that differs substantially from our results is Shen et al. (16), in which the researchers studied the reversion of a mutation in bla gene due to the action of AID during transcription. They found that every bla+ revertant contained MCMs, and as a result, the ratio of mutational clusters to clones sequenced was >3.0 (Table 3, line 4). It is unclear why these investigators found such a high incidence of clustered mutations in this one experiment but not in some of the others (16).
TABLE 3.
Previous reports of multiple clustered mutations caused by AID
We used a genetic reversion assay for our study, with some initial concern that the requirement for selecting for a functional kanamycin-resistance mutant would severely restrict the mutation spectrum. The results show that this was not so, and the mutations (and hence targeting by AID) did not occur at a single base or a codon. In fact, the mutations were spread over much of the segment of kan gene sequenced (especially in the first 425 nt) and untranslated leader sequence (Fig. 8). While some clustering of mutations around codon 94 (CCA) occurred, which mutated to CTA, TCA, or TTA in every revertant, overall, 61 different cytosines of ∼200 in a ∼750 bp stretch of DNA were found mutated to thymines, and 21 of 40 WRCs were targeted by AID. These numbers compare well with the forward mutation assay, rifampicin-resistance, used in many studies of AID mutagenesis. In rifampicin-resistant mutants, only 24 cytosines in a ∼1600 bp stretch of rpoB gene have been found to be mutable, and of these only 13 cytosines may be mutated to thymines to acquire the antibiotic resistance (40).
Figure 8.
Distribution of mutations in KanR revertants. A) Distribution of cytosines in the kan allele. WRCs are indicated by an asterisk above, and cytosines in codon 94 of kan are marked with an arrow. B) Distribution of cytosines found mutated to thymine in various KanR revertants. WRCs that were mutated are indicated by asterisk above, and cytosines in codon 94 of kan are marked with an arrow. In each revertant, one or both cytosines in codon 94 were mutated to thymine.
A direct way to determine the distribution of cytosine deaminations caused by AID in vivo is to detect the generated uracils. To date, this has not been done. However, this may be determined indirectly in the same manner as done in this work. In cells and animals, two DNA repair pathways are thought to affect the distribution of hypermutations in the Ig gene. These are base-excision repair initiated by UDG and mismatch repair (MMR) (41). Consequently, hypermutation distribution in UDG-defective, MMR-defective mice has been used as proxy for sites of AID action within the Ig gene (42,43,44).
The results from the two major studies of this kind show significant differences. In one study (42), clones of VDJ-intron junction region DNA from Peyer’s patch cells from both WT and UDG−/− MSH2−/− mice showed high levels of mutations. In both genetic backgrounds, clones with single mutations were a small minority of all mutations, and the number of mutations per clone ranged from 1 to 41 (WT) or 1 to 33 (double mutant) with a fairly flat distribution (42). In contrast, Shen et al. (43) reported much lower mutation frequencies (between 2 and 3×10−3/base) in both WT and UDG−/− MSH6−/− in the same VDJ-intronic region from splenic cells and relatively few clones with 3 or more mutations in WT mice. In the double-knockout mice, the number of clones with multiple mutations did increase, but the distribution was still strongly skewed toward low mutation numbers per clone (43). Together, these studies show that AID generates multiple cytosine deaminations in a significant fraction of germination center B-cell clones in UDG-defective MMR-defective mice, but the extent to which this occurs is unclear. It is also unclear whether this depends in some way on the source of B cells (Peyer’s patches vs. spleen) and whether they represent independent single deamination events by AID or clones that have undergone deaminations during multiple deamination events in one or more cell generations (20).
Several years ago, the transcription dependence of SHM was explained by hypothesizing that a mutator factor (MuF) travels with RNAP II and transfers to DNA at pause sites to promote mutations (8, 45). MuF is now known to be AID and has been shown to be associated with RNAP II (46). However, that model may need some alterations. First, the requirement for single-stranded DNA by AID (3, 5,6,7) means that if the elongation complex “deposits” AID onto DNA, it is likely to be inactive. If it acts on DNA during its association with the elongation complex, it would cause single cytosine deaminations unless its kcat is equal to or better than ∼30 s−1, the rate of transcription elogation (20). Our results are consistent with the idea that AID can interact with an elongation complex to cause single C to U conversions. Furthermore, we show that if it does encounter a paused, stalled, or arrested complex, it is likely to cause MCMs.
Several different types of DNA structures have been proposed in the Ig gene when it undergoes SHM and CSR. The proposed structures include positively and negatively supercoiled domains (16), stable R-loops (47,48,49,50,51,52), and guanine quartet-containing loops (53). The last two structures have been proposed to occur in the switch regions of Ig genes (51, 53), while the supercoiled domains may occur at various points within the gene, or upstream or downstream of the gene. A common feature of most of these structures is that they contain non-B DNA that has significant single-strandedness and are likely to be quite stable once formed. This contrasts with a bubble in a transcription elongation complex, which moves along DNA at a rate of 15–30 nt/s (54), providing AID with only a brief access to single-stranded DNA. Thus, a simple model for why AID would cause single mutations within an elongation complex and MCMs within any of the specialized structures is based on time of access. The stability of the specialized structures would afford longer access for AID to single-stranded DNA, allowing it to deaminate multiple cytosines in a short stretch of DNA.
When we slowed down or stalled the T7 RNAP, we obtained several KanR revertants that had multiple mutations that were more than 10 nt apart (Supplemental Figs. S1–S5). In some cases, the mutations were spread over several hundred base pairs and cannot be explained by the access of AID to a single stalled or paused transcription complex. It is likely that slowing down the RNAP or a filtration of NTPs creates multiple paused or stalled complexes on DNAs, and this results in multiple AID molecules acting at different transcription complexes. Alternately, these deaminations may be caused by AID transferring from one transcription bubble to another (23). Our results cannot distinguish between one AID molecule acting on different transcription complexes in a single binding event (processive action) or one or more AID molecules acting on multiple transcription complexes during multiple DNA binding events (distributive action).
While the overall mechanics of transcript elongation are similar in vertebrates and E. coli, some important differences remain. For example, it is not clear whether the presence of nucleosomes within the Ig locus affects distribution of mutations created by SHM. Eukaryotic transcription factor TFIIS (39) has bacterial equivalents, GreA and GreB (35, 38), and all these proteins act on arrested RNA polymerases to promote resumption of RNA synthesis. However, many eukaryotic factors such as ELL and Elongin that are thought to interact with elongation complex (39) have no homologs in E. coli. It is not known how the different vertebrate elongation factors change the structure of the elongation complex during polymerase progression or at pause sites, and consequently the access to single-stranded DNA for AID within a paused or arrested elongation complex in Ig gene may be significantly different from what we found in bacteria or in an in vitro transcription reaction.
We have shown here how AID can promote both single and multiple clustered mutations. The mechanism of these different types of mutations is intimately connected with the ability of AID to target transcribing DNA and changes that are known to occur in the elongation complex when it encounters NTP shortages, DNA secondary structures, or DNA adducts. It is not yet possible to study the progress of Pol II on chromatin in vivo, and hence we do not know where the polymerase pauses along the Ig gene and how this correlates with the distribution of SHMs. As technology for studying transcription in vivo improves or an authentic mammalian in vitro system for SHM becomes available, it should be possible to test the proposal presented here about how MCMs arise.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (GM 57200 and CA 97899).
References
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Samaranayake M, Bujnicki J M, Carpenter M, Bhagwat A S. Evaluation of molecular models for the affinity maturation of antibodies: roles of cytosine deamination by AID and DNA repair. Chem Rev. 2006;106:700–719. doi: 10.1021/cr040496t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail A, Klapacz J, Samaranayake M, Ullah A, Bhagwat A S. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Res. 2003;31:2990–2994. doi: 10.1093/nar/gkg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro A R, Stavropoulos P, Jankovic M, Nussenzweig M C. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- Dickerson S K, Market E, Besmer E, Papavasiliou F N. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt F W. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- Bransteitter R, Pham P, Scharff M D, Goodman M F. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U. The molecular basis of somatic hypermutation of immunoglobulin genes. Curr Opin Immunol. 1996;8:206–214. doi: 10.1016/s0952-7915(96)80059-8. [DOI] [PubMed] [Google Scholar]
- Storb U, Peters A, Klotz E, Kim N, Shen H M, Hackett J, Rogerson B, Martin T E. Cis-acting sequences that affect somatic hypermutation of Ig genes. Immunol Rev. 1998;162:153–160. doi: 10.1111/j.1600-065x.1998.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Klix N, Jolly C J, Davies S L, Bruggemann M, Williams G T, Neuberger M S. Multiple sequences from downstream of the J kappa cluster can combine to recruit somatic hypermutation to a heterologous, upstream mutation domain. Eur J Immunol. 1998;28:317–326. doi: 10.1002/(SICI)1521-4141(199801)28:01<317::AID-IMMU317>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. Increased transcription levels induce higher mutation rates in a hypermutating cell line. J Immunol. 2001;166:5051–5057. doi: 10.4049/jimmunol.166.8.5051. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Okazaki I M, Eto T, Kinoshita K, Muramatsu M, Nagaoka H, Honjo T. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- Besmer E, Market E, Papavasiliou F N. The transcription elongation complex directs activation-induced cytidine deaminase-mediated DNA deamination. Mol Cell Biol. 2006;26:4378–4385. doi: 10.1128/MCB.02375-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham P, Bransteitter R, Petruska J, Goodman M F. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424:103–107. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- Martomo S A, Fu D, Yang W W, Joshi N S, Gearhart P J. Deoxyuridine is generated preferentially in the nontranscribed strand of DNA from cells expressing activation-induced cytidine deaminase. J Immunol. 2005;174:7787–7791. doi: 10.4049/jimmunol.174.12.7787. [DOI] [PubMed] [Google Scholar]
- Shen H M, Ratnam S, Storb U. Targeting of the activation-induced cytosine deaminase is strongly influenced by the sequence and structure of the targeted DNA. Mol Cell Biol. 2005;25:10815–10821. doi: 10.1128/MCB.25.24.10815-10821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T, Li G, Alt F W. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proc Natl Acad Sci U S A. 2008;105:3843–3848. doi: 10.1073/pnas.0712291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronai D, Iglesias-Ussel M D, Fan M, Li Z, Martin A, Scharff M D. Detection of chromatin-associated single-stranded DNA in regions targeted for somatic hypermutation. J Exp Med. 2007;204:181–190. doi: 10.1084/jem.20062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D B, Phung Q H, Umar A, Baker S M, Tarone R E, Tanaka K, Liskay R M, Kunkel T A, Bohr V A, Gearhart P J. Altered spectra of hypermutation in antibodies from mice deficient for the DNA mismatch repair protein PMS2. Proc Natl Acad Sci U S A. 1998;95:6953–6958. doi: 10.1073/pnas.95.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat A S. DNA-cytosine deaminases: from antibody maturation to antiviral defense. DNA Repair. 2004;3:85–89. doi: 10.1016/j.dnarep.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bransteitter R, Pham P, Calabrese P, Goodman M F. Biochemical analysis of hypermutational targeting by wild type and mutant activation-induced cytidine deaminase. J Biol Chem. 2004;279:51612–51621. doi: 10.1074/jbc.M408135200. [DOI] [PubMed] [Google Scholar]
- Coker H A, Petersen-Mahrt S K. The nuclear DNA deaminase AID functions distributively whereas cytoplasmic APOBEC3G has a processive mode of action. DNA Repair. 2007;6:235–243. doi: 10.1016/j.dnarep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Pham P, Chelico L, Goodman M F. DNA deaminases AID and APOBEC3G act processively on single-stranded DNA. DNA Repair. 2007;6:689–692. doi: 10.1016/j.dnarep.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Lutsenko E, Bhagwat A S. The role of the Escherichia coli mug protein in the removal of uracil and 3, N(4)-ethenocytosine from DNA. J Biol Chem. 1999;274:31034–31038. doi: 10.1074/jbc.274.43.31034. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Gabbara S, Bhagwat A S. Cytosine deaminations catalyzed by DNA cytosine methyltransferases are unlikely to be the major cause of mutational hot spots at sites of cytosine methylation in Escherichia coli. Proc Natl Acad Sci U S A. 1994;91:1574–1578. doi: 10.1073/pnas.91.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletskii A, Grigoriev A, Joyce S, Bhagwat A S. Mutations induced by bacteriophage T7 RNA polymerase and their effects on the composition of the T7 genome. J Mol Biol. 2000;300:1057–1065. doi: 10.1006/jmbi.2000.3944. [DOI] [PubMed] [Google Scholar]
- He B, Rong M, Lyakhov D, Gartenstein H, Diaz G, Castagna R, McAllister W T, Durbin R K. Rapid mutagenesis and purification of phage RNA polymerases. Protein Expr Purif. 1997;9:142–151. doi: 10.1006/prep.1996.0663. [DOI] [PubMed] [Google Scholar]
- Beletskii A, Bhagwat A S. Transcription-induced mutations: increase in C to T mutations in the nontranscribed strand during transcription in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:13919–13924. doi: 10.1073/pnas.93.24.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogozin I B, Pavlov Y I, Bebenek K, Matsuda T, Kunkel T A. Somatic mutation hotspots correlate with DNA polymerase η error spectrum. Nat Immunol. 2001;2:530–536. doi: 10.1038/88732. [DOI] [PubMed] [Google Scholar]
- Guajardo R, Lopez P, Dreyfus M, Sousa R. NTP concentration effects on initial transcription by T7 RNAP indicate that translocation occurs through passive sliding and reveal that divergent promoters have distinct NTP concentration requirements for productive initiation. J Mol Biol. 1998;281:777–792. doi: 10.1006/jmbi.1998.1988. [DOI] [PubMed] [Google Scholar]
- Mathews C K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972;247:7430–7438. [PubMed] [Google Scholar]
- Gallant J, Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969;244:3125–3132. [PubMed] [Google Scholar]
- Bonner G, Lafer E M, Sousa R. Characterization of a set of T7 RNA polymerase active site mutants. J Biol Chem. 1994;269:25120–25128. [PubMed] [Google Scholar]
- Bonner G, Patra D, Lafer E M, Sousa R. Mutations in T7 RNA polymerase that support the proposal for a common polymerase active site structure. EMBO J. 1992;11:3767–3775. doi: 10.1002/j.1460-2075.1992.tb05462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S, Lee J, Laptenko O. Bacterial transcription elongation factors: new insights into molecular mechanism of action. Mol Microbiol. 2005;55:1315–1324. doi: 10.1111/j.1365-2958.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- Sohn Y, Kang C. Sequential multiple functions of the conserved sequence in sequence-specific termination by T7 RNA polymerase. Proc Natl Acad Sci U S A. 2005;102:75–80. doi: 10.1073/pnas.0406581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Martin C T. Fluorescence characterization of the transcription bubble in elongation complexes of T7 RNA polymerase. J Mol Biol. 2001;308:465–475. doi: 10.1006/jmbi.2001.4601. [DOI] [PubMed] [Google Scholar]
- Landick R. The regulatory roles and mechanism of transcriptional pausing. Biochem Soc Trans. 2006;34:1062–1066. doi: 10.1042/BST0341062. [DOI] [PubMed] [Google Scholar]
- Shilatifard A, Conaway R C, Conaway J W. The RNA polymerase II elongation complex. Annu Rev Biochem. 2003;72:693–715. doi: 10.1146/annurev.biochem.72.121801.161551. [DOI] [PubMed] [Google Scholar]
- Garibyan L, Huang T, Kim M, Wolff E, Nguyen A, Nguyen T, Diep A, Hu K, Iverson A, Yang H, Miller J H. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amst) 2003;2:593–608. doi: 10.1016/s1568-7864(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Rada C, Ehrenstein M R, Neuberger M S, Milstein C. Hot spot focusing of somatic hypermutation in MSH2-deficient mice suggests two stages of mutational targeting. Immunity. 1998;9:135–141. doi: 10.1016/s1074-7613(00)80595-6. [DOI] [PubMed] [Google Scholar]
- Rada C, Di Noia J M, Neuberger M S. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Shen H M, Tanaka A, Bozek G, Nicolae D, Storb U. Somatic hypermutation and class switch recombination in Msh6(-/-)Ung(-/-) double-knockout mice. J Immunol. 2006;177:5386–5392. doi: 10.4049/jimmunol.177.8.5386. [DOI] [PubMed] [Google Scholar]
- Xue K, Rada C, Neuberger M S. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2-/- ung-/- mice. J Exp Med. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U, Klotz E L, Hackett J, Jr, Kage K, Bozek G, Martin T E. A hypermutable insert in an immunoglobulin transgene contains hotspots of somatic mutation and sequences predicting highly stable structures in the RNA transcript. J Exp Med. 1998;188:689–698. doi: 10.1084/jem.188.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu Y, Sugai M, Gonda H, Lee C G, Katakai T, Agata Y, Yokota Y, Shimizu A. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- Daniels G A, Lieber M R. RNA:DNA complex formation upon transcription of immunoglobulin switch regions: implications for the mechanism and regulation of class switch recombination. Nucleic Acids Res. 1995;23:5006–5011. doi: 10.1093/nar/23.24.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta R, Iwai K, Shigeno M, Mizuta M, Uemura T, Ushiki T, Kitamura D. Molecular visualization of immunoglobulin switch region RNA/DNA complex by atomic force microscope. J Biol Chem. 2003;278:4431–4434. doi: 10.1074/jbc.M209262200. [DOI] [PubMed] [Google Scholar]
- Reaban M E, Lebowitz J, Griffin J A. Transcription induces the formation of a stable RNA. DNA hybrid in the immunoglobulin alpha switch region. J Biol Chem. 1994;269:21850–21857. [PubMed] [Google Scholar]
- Yu K, Chedin F, Hsieh C L, Wilson T E, Lieber M R. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- Yu K, Roy D, Bayramyan M, Haworth I S, Lieber M R. Fine-structure analysis of activation-induced deaminase accessibility to class switch region R-loops. Mol Cell Biol. 2005;25:1730–1736. doi: 10.1128/MCB.25.5.1730-1736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F T, Yu K, Balter B B, Selsing E, Oruc Z, Khamlichi A A, Hsieh C L, Lieber M R. Sequence dependence of chromosomal R-loops at the immunoglobulin heavy-chain Smu class switch region. Mol Cell Biol. 2007;27:5921–5932. doi: 10.1128/MCB.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette M L, Handa P, Vincent J A, Taylor A F, Maizels N. Intracellular transcription of G-rich DNAs induces formation of G-loops, novel structures containing G4 DNA. Genes Dev. 2004;18:1618–1629. doi: 10.1101/gad.1200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilatifard A. Transcriptional elongation control by RNA polymerase II: a new frontier. Biochim Biophys Acta. 2004;1677:79–86. doi: 10.1016/j.bbaexp.2003.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.