Abstract
Pregnane × receptor (PXR; NR1I2) is a ligand-activated transcription factor that plays a role not only in drug metabolism and transport but also in various other biological processes. Ginkgo biloba is a herbal medicine commonly used to manage memory impairment. Treatment of primary cultures of rat hepatocytes with G. biloba extract increases the mRNA expression of CYP3A23, which is a target gene for rat PXR. The present study was conducted to test the hypothesis that G. biloba extract activates PXR. Treatment of mouse PXR (mPXR) or human PXR (hPXR)-transfected HepG2 cells with G. biloba extract at 200 μg/ml increased mPXR and hPXR activation by 3.2- and 9.5-fold, respectively. Dose-response analysis showed a log-linear increase in hPXR activation by the extract over the range of 200 to 800 μg/ml. To determine whether G. biloba extract induces hPXR target gene expression, cultured LS180 human colon adenocarcinoma cells were treated for 72 h with the extract. G. biloba extract at 200, 400, and 800 μg/ml increased CYP3A4 mRNA expression by 1.7-, 2.4-, and 2.5-fold, respectively. The same concentrations of the extract increased CYP3A5 (1.3−3.6-fold) and P-glycoprotein (ABCB) 1 (2.7−6.4-fold) mRNA expression. At concentrations (5 and 10 μM) that did not down-regulate PXR gene expression and were not cytotoxic, l-sulforaphane (an hPXR antagonist) decreased CYP3A4, CYP3A5, and ABCB1 gene expression in cells treated with G. biloba extract. In summary, G. biloba extract activated mPXR and hPXR in a cell-based reporter gene assay and induced CYP3A4, CYP3A5, and ABCB1 gene expression in hPXR-expressing LS180 cells.
Pregnane × receptor (PXR; gene designation NR1I2) is a ligand-activated transcription factor that belongs to the superfamily of nuclear receptors (Kliewer et al., 1998). It plays an important role in the regulation of numerous genes involved not only in drug metabolism and transport but also in various other biological processes, including lipid metabolism, glucose homeostasis, thyroid hormone synthesis, and inflammatory response (Nakamura et al., 2007; Moreau et al., 2008). Upon binding to a ligand, the activated PXR translocates from the cytoplasm to the nucleus, forms a heterodimer with retinoic acid receptor α, recruits coactivators, and binds to the promoter region of target genes, resulting in increased gene transcription (Squires et al., 2004).
Numerous structurally diverse chemicals have been shown to activate PXR; for example, endogenous substances, such as certain vitamins and hormones, and other chemicals, such as drugs, naturally occurring compounds, and herbal medicines (Chang and Waxman, 2006). Examples of herbal medicines identified to activate PXR include St. John's wort (Moore et al., 2000), gugulipid (Brobst et al., 2004), traditional Chinese medicines, such as wu wei zi (Schisandra chinensis Baill) (Mu et al., 2006), gan cao (Glycyrrhiza uralensis Fisch) (Mu et al., 2006), and tian xian (also known as tien hsien, which is a cocktail of 14 medicinal herbs, including Panax ginseng and Radix glycyrrhizae) (Lichti-Kaiser and Staudinger, 2008), and various Tanzanian plant extracts (e.g., Jatropha multifida) with traditional medicinal application (van den Bout-van den Beukel et al., 2008).
Ginkgo biloba is one of the most popular herbal medicines. A common use of this herbal medicine is to improve age-related memory impairment and cognitive function; for example, in the management of primary degenerative dementia in patients with Alzheimer's disease (Luo, 2006). The dietary ingestion of G. biloba extract by rats has been reported to alter the pharmacokinetics [i.e., decreases the area under the plasma concentration-time curve (AUC)] of an orally administered CYP3A substrate, nicardipine, and this was accompanied by decreased hypotensive action of this calcium channel blocker (Shinozuka et al., 2002). The extract also increases rat hepatic CYP3A expression (Shinozuka et al., 2002) and its associated microsomal testosterone 6β-hydroxylation activity (Umegaki et al., 2002). As demonstrated in primary cultures of rat hepatocytes, G. biloba extract increases CYP3A2, CYP3A18, and CYP3A23 mRNA levels (Chang et al., 2006). The in vivo administration of G. biloba extract has also been shown to increase CYP3A-mediated testosterone 6β-hydroxylation activity in mouse hepatic microsomes (Umegaki et al., 2007). Given that PXR controls the transcriptional regulation of CYP3A genes (Kliewer et al., 1998), our hypothesis is that G. biloba extract activates PXR.
In the present study, we determined the effect of G. biloba extract on rodent and human PXR activity and extended the findings by investigating the effect of the extract on the expression of various PXR target genes (CYP3A4, CYP3A5, and ABCB1) in PXR-expressing LS180 human colon adenocarcinoma cells in culture. Our results show G. biloba extract as a novel activator of mouse and human PXR.
Materials and Methods
Extracts, Chemicals, and Reagents
G. biloba extract (isolated from leaves and supplied in a powder form) was obtained as three individual lots from Indena S.A. (Milan, Italy). The total amount of terpene trilactones [i.e., ginkgolide A, ginkgolide B, ginkgolide C, ginkgolide J, and bilobalide; see van Beek (2002) for the chemical structures] in lots A to C was 6.2, 6.2, and 6.8% (w/w), respectively, as quantified by gas chromatography (ChromaDex, Irvine, CA). The total amount of flavonols [i.e., quercetin, kaempferol, and isorhamnetin as the aglycone and glycosides; see van Beek (2002) for the chemical structures] in lots A to C was 21.0, 24.4, and 24.4% (w/w), respectively, as quantified by liquid chromatography-mass spectrometry (ChromaDex). The levels of terpene trilactones and flavonol glycosides contained in the extract used in the current study are similar to those present in a commonly studied G. biloba extract known as EGb 761 (van Beek, 2002). HepG2 human hepatoma cells and LS180 human colon adenocarcinoma cells were purchased from the American Type Culture Collection (Manassas, VA). FuGENE 6 transfection reagent was purchased from Roche Diagnostics (Mannheim, Germany). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI). Minimum essential medium, Opti-MEM, heat-inactivated fetal bovine serum, l-glutamine, penicillin G, streptomycin, and trypsin-EDTA were purchased from Invitrogen Canada Inc. (Burlington, ON, Canada). Triton X-100, dextran, and l-sulforaphane were purchased from Sigma-Aldrich (St. Louis, MO). The suppliers for the chemical and reagents used in the isolation of total RNA, reverse transcription, real-time polymerase chain reaction, and the lactate dehydrogenase (LDH) assay are detailed elsewhere (Chang et al., 2006; Rajaraman et al., 2006). Human CYP3A4, CYP3A5, ABCB1, and PXR gene-specific primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Plasmid Constructs
hPXR and mPXR full-length coding cDNAs were cloned into BamHI and XhoI sites of pCR3. XREM-CYP3A4-Luc, where XREM is the xenobiotic-responsive enhancer module originally named as p3A4−362(7836/7208ins) (Goodwin et al., 1999), was a kind gift from Bryan Goodwin (GlaxoSmithKline, Research Triangle Park, NC). The phRL-TK Renilla luciferase plasmid was purchased from Promega.
Cell Culture
HepG2 human hepatoma cells and LS180 human colon adenocarcinoma cells were cultured in T-75 flasks at 37°C in a humidified, 5% CO2 incubator. Cells were grown in minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin. Culture medium was changed once every 2 to 3 days, and cells were subcultured once weekly.
Transient Transfection
HepG2 cells were plated in 24-well microplates at a density of 100,000 cells/well. At 1 day after plating, cells were transfected for 24 h with XREM-CYP3A4-Luc (50 ng/well) and pCR3-mPXR (100 ng/well), pCR3-hPXR (100 ng/well), or pCR3 (100 ng/well; empty vector control) using FuGENE 6 (0.6 μl diluted in 20 μl of Opti-MEM/well), according to the manufacturer's instructions. Cells were also transfected with phRL-TK (50 ng/well), which was used as an internal control to normalize the firefly luciferase activity in each sample.
Treatment of Transfected HepG2 Cells and Luciferase Reporter Assay
Transfected cells were treated for 24 h with G. biloba extract (30, 100, 200, 400, or 800 μg/ml) or culture medium (vehicle control for G. biloba). As controls, cells were treated with rifampin (10 μM), pregnenolone 16α-carbonitrile (PCN; 10 μM), or dimethyl sulfoxide (DMSO) (0.1% v/v, vehicle control for rifampin and PCN). Firefly luciferase and Renilla luciferase levels were determined using the Dual-Luciferase Reporter Assay System (Promega), according to the manufacturer's instructions. Luminescence was measured using a Glomax 96 luminescence microplate reader (Promega).
Treatment of LS180 Cells
Cultured LS180 cells were plated at a density of 1 × 106 cells in T-25 flasks. Drug treatment was initiated 3 days later. LS180 cells were treated once every 24 h for a total of 72 h with varying concentrations of G. biloba extract or l-sulforaphane, as indicated in each figure legend. Control cells were treated with DMSO [0.1% (v/v); vehicle for l-sulforaphane] or culture medium (vehicle for G. biloba extract).
Isolation of Total RNA, Reverse Transcription, and Real-Time Polymerase Chain Reaction
At the end of the treatment period, total RNA was isolated from LS180 cells using TRIzol and reverse transcribed using Superscript II reverse transcriptase, as described previously (Chang et al., 2006). Total RNA concentration and total cDNA concentration were quantified by the RiboGreen RNA Quantitation Kit and PicoGreen dsDNA Quantitation Kit, respectively (Chang et al., 2006). Polymerase chain reaction (PCR) was performed in a real-time DNA thermal cycler (LightCycler; Roche Diagnostics). Each 20-μl PCR reaction contained 1 U of Platinum Taq DNA polymerase in 1× PCR II buffer (20 mM Tris-HCl, pH 8.4, and 50 mM KCl), 3 mM MgCl2 (except that 4 mM was used in the amplification of CYP3A4 cDNA), 0.25 mg/ml bovine serum albumin, 0.2 mM dNTP, 0.2 μM forward and reverse primers specific for CYP3A4, CYP3A5, and ABCB1, 1:30,000 SYBR Green I, and 10 ng of total cDNA. The sequences of the forward and reverse primers to amplify human CYP3A4 cDNA were 5′-CCT-TAC-ACA-TAC-ACA-CCC-TTT-GGA-AGT-3′ and 5′-AGC-TCA-ATG-CAT-GTA-CAG-AAT-CCC-CGG-TTA-3′, respectively (Schuetz et al., 1996). The sequences of the forward and reverse primers to amplify human CYP3A5 cDNA were 5′-CCT-TAC-ATA-TAC-ACA-CCC-TTT-GGA-AC-3′ and 5′-GTT-GAA-GAA-GTC-CTT-GCG-TGT-C-3′, respectively (Yamaori et al., 2005). The sequences of the forward and reverse primers to amplify human ABCB1 cDNA were 5′-GGC-CTA-ATG-CCG-AAC-ACA-TT-3′ and 5′-CAG-CGT-CTG-GCC-CTT-CTT-C-3′, respectively (Liu et al., 2002). The sequences of the forward and reverse primers to amplify human PXR cDNA were 5′-CAA-GCG-GAA-GAA-AAG-TGA-ACG-3′ and 5′-CAC-AGA-TCT-TTC-CGG-ACC-TG-3′, respectively (Chang et al., 2003). The cycling conditions for the real-time amplification (LightCycler; Roche Diagnostics) were 94°C for 5 s (denaturation), 60°C for 10 s (annealing), and 72°C for 15 s (extension) for CYP3A4, 95°C for 1 s (denaturation), 65°C for 6 s (annealing), and 72°C for 10 s (extension) for CYP3A5, 95°C for 1 s (denaturation), 61°C for 6 s (annealing), and 72°C for 10 s (extension) for ABCB1, and 95°C for 5 s (denaturation), 65°C for 10 s (annealing), and 72°C for 15 s (extension) for PXR. The initial denaturation was performed at 95°C for 5 min. Calibration curves were constructed by plotting the cross-point against known amounts of purified amplicon, as determined by the PicoGreen dsDNA Quantitation Kit. Initial experiments were performed to verify the specificity of the primers by purifying and sequencing the amplicons. Purification was carried out using the QIAquick Gel Extraction Kit, according to the manufacturer's instructions (QIAGEN, Valencia, CA). Purified amplicons were sequenced using the Applied Biosystems 377 DNA sequencer (Applied Biosystems, Inc., Foster City, CA) at the Nucleic Acid and Protein Service Unit at the University of British Columbia. The identity of the purified amplicons was confirmed using the BLAST program (http://www.ncbi.nlm.nih.gov).
LDH Assay
Leakage of intracellular LDH into the culture medium was used to assess cellular toxicity in a method described previously (Rajaraman et al., 2006), but with the following modifications: 1) cells were plated at a density of 100,000 cells/well in 24-well plates; 2) 5 μl of the supernatant from each well containing cultured cells was transferred to a well in a 96-well microplate containing 95 μl of phosphate-buffered saline, pH 7.4, and 100 μl of the reaction mixture (provided in the Cytotoxicity Detection Kit; Roche Diagnostics); and 3) cells were lysed with 500 μl of lysis buffer (20 mM EDTA and 2% Triton X-100 in phosphate-buffered saline, pH 7.4), and the microplates were placed on an orbital shaker for 2 h. Results are expressed as a percentage of the total cellular LDH content (i.e., the level of LDH released to the culture medium divided by the sum of the LDH levels in the culture medium and in the cell lysate).
Data Analysis
Student's t test was used when analyzing data from two groups. In experiments where there were more than two groups, the data were analyzed by the Kruskal-Wallis one-way analysis of variance test and, if appropriate, was followed by the Newman-Keuls multiple comparison test. The level of statistical significance was set a priori at p < 0.05.
Results
Effect of G. biloba Extract, PCN, and Rifampin on Mouse and Human PXR Activity
The initial experiment was to determine whether G. biloba extract activates mouse and human PXR. Therefore, mouse PXR- and human PXR-transfected HepG2 cells were treated for 24 h with G. biloba extract (200 μg/ml) or culture medium (vehicle control). As shown in Fig. 1, the extract increased mouse PXR activation by 3.2 ± 0.3-fold, whereas it increased human PXR activation by 9.5 ± 0.9-fold. In comparison, PCN increased mouse PXR activation by 13.8 ± 4.0-fold, and rifampin increased human PXR activation by 31 ± 10-fold. Based on our initial findings, all subsequent experiments focused on human PXR because of the greater increase in human PXR activity by the extract.
Fig. 1.
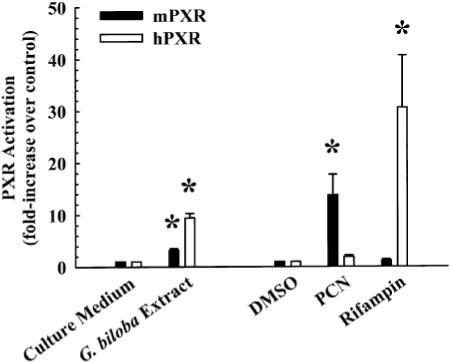
Effect of G. biloba extract, PCN, and rifampin on mouse and human PXR activation. Cultured HepG2 cells were transfected for 24 h with XREM-CYP3A4-Luc, phRL-TK along with pCR3-hPXR, pCR3-mPXR, or pCR3 (empty vector). Cells were then treated for 24 h with G. biloba extract (200 μg/ml), culture medium (vehicle control for G. biloba extract), PCN (10 μM), rifampin (10 μM), or DMSO [0.1% (v/v), vehicle control for PCN and rifampin]. Firefly and Renilla luciferase levels were quantified and normalized as described under Materials and Methods. Data are shown as mean ± S.E.M. for three independent experiments. *, significantly different from the vehicle-treated control group (p < 0.05).
Comparative Effect of Different Lots of G. biloba Extract on Activation of Human PXR
To compare the effect of different lots of G. biloba extract on human PXR activity, human PXR-transfected HepG2 cells were treated for 24 h with one of three lots of G. biloba extract at a concentration of 200 μg/ml. Each of the three lots increased human PXR activation, when compared with the vehicle-treated control group. Lots A to C increased human PXR activation by 10 ± 2.6-, 9.5 ± 0.9-, and 10.5 ± 3.6-fold, respectively, and the effects among the three lots were not significantly different. Based on this finding, lot A was used in all subsequent experiments.
Dose-Response Relationship in the Activation of Human PXR by G. biloba Extract
Human PXR-transfected HepG2 cells were treated for 24 h with varying concentrations of G. biloba extract (30, 100, 200, 400, or 800 μg/ml) or culture medium (vehicle control). As shown in Fig. 2, the 30 μg/ml concentration of the extract had no effect on human PXR activity, whereas the 100, 200, 400, and 800 μg/ml concentrations increased PXR activation by 2.9 ± 0.4-, 6.4 ± 1.4-, 14.4 ± 2.4-, and 32.2 ± 10.1-fold, respectively. A log-linear increase in PXR activation was obtained at concentrations of 200 to 800 μg/ml.
Fig. 2.
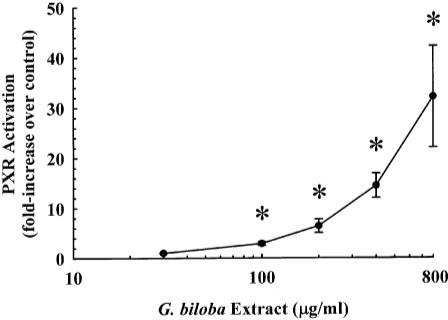
Dose-response curve for the activation of human PXR by G. biloba extract. Cultured HepG2 cells were transfected for 24 h with XREM-CYP3A4-Luc, phRL-TK, and either pCR3-hPXR or pCR3 (empty vector). Cells were then treated for 24 h with G. biloba extract (30, 100, 200, 400, or 800 μg/ml) or culture medium (vehicle control). Firefly and Renilla luciferase levels were quantified and normalized as described under Materials and Methods. Data are presented as mean ± S.E.M. for four independent experiments. *, significantly different from the vehicle-treated control group (p < 0.05).
Effect of G. biloba Extract on Human PXR Target Gene Expression in LS180 Human Colon Adenocarcinoma Cells in Culture
Human PXR regulates the expression of a broad array of genes, including CYP3A4, CYP3A5, and ABCB1 (Stanley et al., 2006). Therefore, to corroborate the findings obtained from the reporter gene assays (e.g., Figs. 1 and 2), we investigated the effect of G. biloba extract on the expression of PXR target genes in LS180 human colon adenocarcinoma cells previously shown to express PXR endogenously (Thummel et al., 2001) and where CYP3A4, CYP3A5, and ABCB1 are expressed constitutively and inducible in this cell line (Schuetz et al., 1996; Gupta et al., 2008). Cultured LS180 cells were treated for 72 h with G. biloba extract (200, 400, or 800 μg/ml) or culture medium (vehicle control). As shown in Fig. 3A, these concentrations of the extract increased CYP3A4 mRNA expression. The -fold increase (2.5 ± 0.1-fold) obtained at the highest concentration (800 μg/ml) of the extract was considerably less than the 49.4 ± 12.1-fold increase obtained with rifampin (10 μM). G. biloba extract at 200, 400, and 800 μg/ml also increased CYP3A5 (Fig. 3B) and ABCB1 mRNA levels (Fig. 3C), and the extent of induction by the extract at the highest concentrations tested was similar to or greater than that by rifampin.
Fig. 3.
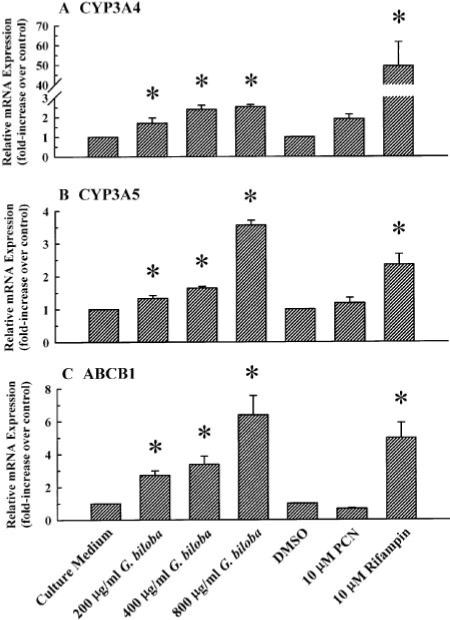
Effect of G. biloba extract on PXR target gene expression in cultured LS180 human colon adenocarcinoma cells. Cultured LS180 cells were treated once every 24 h for a total of 72 h with G. biloba extract (200, 400, or 800 μg/ml) or culture medium (vehicle control). As controls, cells were treated with PCN (10 μM), rifampin (10 μM), or DMSO [0.1% (v/v); vehicle for PCN and rifampin]. At the end of the treatment period, total RNA was isolated and reverse transcribed. CYP3A4 (A), CYP3A5 (B), and ABCB1 cDNA (C) were amplified by real-time PCR with gene-specific primers. Data are presented as mean ± S.E.M. for four independent experiments. *, significantly different from the vehicle-treated control group (p < 0.05).
Effect of G. biloba Extract on Viability of Cultured LS180 Cells
The LDH assay was performed to determine the viability of cultured LS180 cells treated with concentrations of G. biloba extract that were effective in modulating PXR target gene expression (Fig. 3). The results indicated that treatment of cultured LS180 cells for 72 h with G. biloba extract (200, 400, or 800 μg/ml) did not increase LDH release (data not shown). The positive [1% (v/v) Triton X-100] and negative [1% (w/v) dextran] controls produced the expected outcomes (data not shown).
PXR Expression in Cultured LS180 Cells Treated with G. biloba Extract
Next, we determined whether the increase in CYP3A4 (Fig. 3A), CYP3A5 (Fig. 3B), and ABCB1 (Fig. 3C) gene expression by G. biloba extract was associated with an increase in PXR gene expression. Therefore, real-time PCR analysis was performed to measure PXR mRNA levels in LS180 cells treated with G. biloba extract (200, 400, or 800 μg/ml) or culture medium (vehicle control). In agreement with previous data (Thummel et al., 2001), PXR mRNA was expressed in control LS180 cells (data not shown). Our finding indicated that the levels in G. biloba-treated cells were not significantly different from those in vehicle-treated cells (data not shown).
Effect of a PXR Antagonist, l-Sulforaphane, on the Induction of CYP3A4, CYP3A5, and ABCB1 Gene Expression by G. biloba Extract
As demonstrated in a recent study, sulforaphane is an antagonist of human PXR, whereas it does not affect the activity of retinoid × receptor, constitutive androstane receptor (CAR), vitamin D receptor, or peroxisome proliferator-activated receptors α and γ (Zhou et al., 2007). Therefore, to investigate the role of endogenous PXR in the induction of CYP3A4, CYP3A5, and ABCB1 gene expression by G. biloba extract, cultured LS180 cells were treated for 72 h with G. biloba extract (400 μg/ml) and l-sulforaphane (5, 10, or 20 μM) or DMS0 (0.1% v/v; vehicle control). Each of these concentrations of l-sulforaphane attenuated the increase in CYP3A4 (Fig. 4A), CYP3A5 (Fig. 4B), and ABCB1 (Fig. 4C) mRNA levels by G. biloba extract.
Fig. 4.
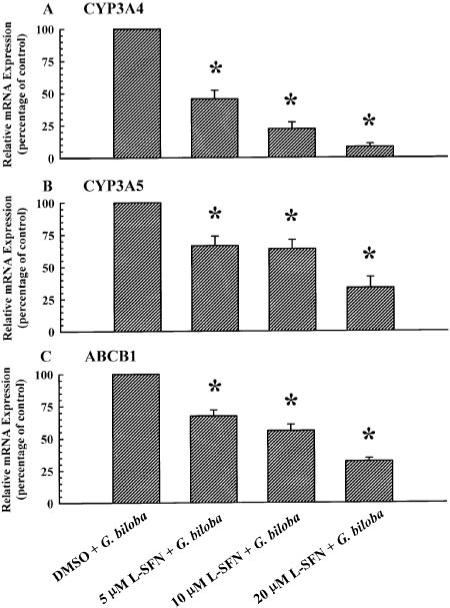
Effect of a PXR antagonist, l-sulforaphane, on CYP3A4, CYP3A5, and ABCB1 gene expression in cultured LS180 cells treated with G. biloba extract. Cultured LS180 cells were treated once every 24 h for a total of 72 h with G. biloba extract (400 μg/ml) and l-sulforaphane (5, 10, or 20 μM) or DMSO (0.1% v/v, vehicle control). At the end of the treatment period, total RNA was isolated and reverse transcribed. CYP3A4 (A), CYP3A5 cDNA (B), and ABCB1 (C) were amplified by real-time PCR with gene-specific primers. Data are presented as mean ± S.E.M. for four independent experiments. *, significantly different from the vehicle-treated control group (p < 0.05).
Assessment of Viability of Cultured LS180 Cells Treated with l-Sulforaphane and G. biloba Extract
We performed the LDH assay to determine whether treatment of LS180 cells with G. biloba extract and l-sulforaphane resulted in cytotoxicity and thereby led to the observed decrease in CYP3A4 (Fig. 4A), CYP3A5 (Fig. 4B), and ABCB1 (Fig. 4C) gene expression. As shown in Fig. 5, G. biloba extract (400 μg/ml) did not affect the extent of LDH release when compared with the vehicle-treated control group. Similarly, the combination of l-sulforaphane (5, 10, or 20 μM) and G. biloba extract (400 μg/ml) did not increase LDH release. Control analysis with 1% (v/v) Triton X-100 (positive control) and 1% (w/v) dextran (negative control) yielded the expected results.
Fig. 5.
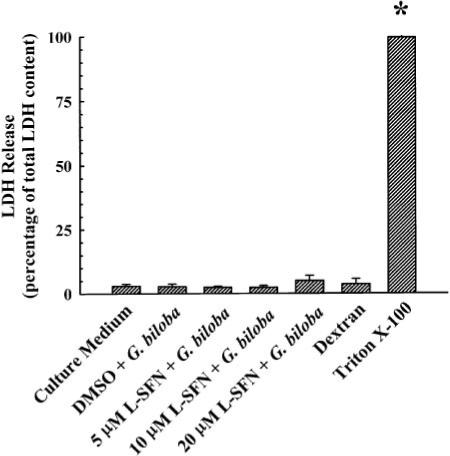
LDH release in cultured LS180 cells treated with l-sulforaphane and G. biloba extract. Cultured LS180 cells were treated once every 24 h for a total of 72 h with G. biloba extract (400 μg/ml) and l-sulforaphane (5, 10, or 20 μM) or DMSO [0.1% (v/v), vehicle control for l-sulforaphane]. In the same experiment, cells were treated for the same length of time with 1% (v/v) Triton X-100 (positive control), 1% (w/v) dextran (negative control), or culture medium (vehicle control for G. biloba extract, Triton X-100, and dextran). At the end of the treatment period, the LDH assay was performed as described under Materials and Methods. Data are presented as mean ± S.E.M. for four independent experiments. *, significantly different from the vehicle-treated control group (p < 0.05).
Effect ofl-Sulforaphane on PXR Expression in Cultured LS180 Cells Treated with G. biloba Extract
To rule out the possibility that decreased PXR expression is an explanation for the attenuating effect of l-sulforaphane on the induction of CYP3A4 (Fig. 4A), CYP3A5 (Fig. 4B), and ABCB1 (Fig. 4C) by G. biloba extract, real-time PCR analysis was conducted to determine PXR mRNA expression. As shown in Fig. 6, the administration of 5 or 10 μM l-sulforaphane did not affect PXR mRNA levels in LS180 cells treated with G. biloba extract (400 μg/ml). By comparison, PXR mRNA expression was decreased by 55% in cells cotreated with 20 μM l-sulforaphane and the extract. The extract alone did not affect PXR mRNA expression, when compared with the levels in vehicle-treated control cells (data not shown).
Fig. 6.
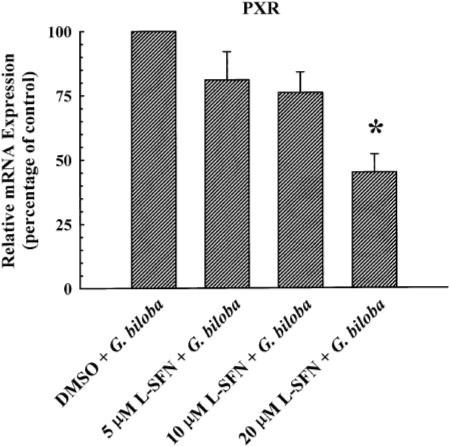
Human PXR mRNA expression in cultured LS180 cells treated with l-sulforaphane and G. biloba extract. Cultured LS180 cells were treated once every 24 h for a total of 72 h with G. biloba extract (400 μg/ml) and l-sulforaphane (5, 10, or 20 μM) or DMSO [0.1% (v/v), vehicle control for l-sulforaphane]. At the end of the treatment period, total RNA was isolated and reverse transcribed. Human PXR cDNA was amplified by real-time PCR with gene-specific primers. Data are presented as mean ± S.E.M. for four independent experiments.
Discussion
Although many studies conducted to date have investigated the role of individual chemicals, particularly synthetic drugs, as agonists or antagonists of PXR, considerably less is known as to which herbal medicine is capable of activating this receptor (Chang and Waxman, 2006). The present study provides the first demonstration that a novel action of G. biloba extract is the activation of mouse and human PXR, as assessed by an in vitro cell-based reporter gene assay. The activation of mouse PXR by G. biloba extract is consistent with the previous finding that the in vivo administration of this herbal medicine to mice increases the hepatic microsomal enzyme activity of CYP3A, which is a PXR target gene product (Umegaki et al., 2007). However, G. biloba extract was more effective in activating human PXR than in activating mouse PXR. When analyzed at the same concentration (200 μg/ml), G. biloba extract increased human PXR activation by 9.5-fold, whereas it increased mouse PXR activation by only 3.2-fold. It is important to assess not only rodent PXR activity, but also human PXR activity, because of the well established species-dependent chemical activation of PXR. For example, as demonstrated previously (Jones et al., 2000) and confirmed in our control experiments, rifampin activates human PXR, but not mouse PXR, whereas PCN activates mouse PXR, but not human PXR. These differences in PXR activation reflect the relatively modest degree (77%) of sequence identity in the ligand binding domain of mouse and human PXR (Moore et al., 2002).
As shown in our dose-response experiments, a log-linear increase in human PXR activation was obtained with G. biloba extract at a concentration range of 200 to 800 μg/ml. A complete dose-response experiment was not performed because of solubility problems. As a consequence, it was not possible to calculate the EC50 (concentration required to achieve half of the maximal activity) or Emax (maximal activity) values for the activation of human PXR by G. biloba extract. However, the magnitude of the increase (32-fold) in human PXR activation by the 800 μg/ml concentration of the extract was comparable with the 31-fold increased by 10 μM rifampin. This concentration of rifampin was previously shown to be a near-maximal concentration in the in vitro activation of human PXR (El-Sankary et al., 2001).
Our data obtained from the reporter gene assay were corroborated by the finding that G. biloba extract increased the expression of PXR target genes, namely CYP3A4, CYP3A5, and ABCB1, as determined in LS180 human colon adenocarcinoma cells. LS180 cells were chosen because of their: 1) endogenous expression of PXR (Thummel et al., 2001); 2) constitutive and inducible expression of CYP3A4, CYP3A5, and ABCB1 (Schuetz et al., 1996; Gupta et al., 2008); and 3) lack of CAR expression (Gupta et al., 2008). The absence of CAR in the LS180 cell line renders it a useful model to study PXR-mediated gene transcription because the expression of some of the PXR target genes, including CYP3A4, CYP3A5, and ABCB1 (Stanley et al., 2006), is also controlled, at least in part, by CAR. As shown in the present study, PXR plays a role in the induction of CYP3A4, CYP3A5, and ABCB1 expression by G. biloba extract in LS180 cells. This conclusion is based on the results obtained from our gene expression experiment conducted with l-sulforaphane, which is an antagonist of human PXR (Zhou et al., 2007). At 5 and 10 μM, which was not cytotoxic and did not alter PXR gene expression, l-sulforaphane was effective in attenuating the increase in CYP3A4, CYP3A5, and ABCB1 gene expression in LS180 cells treated with G. biloba extract.
The human CYP3A subfamily consists of CYP3A4, CYP3A5, CYP3A7, and CYP3A43 (Plant, 2007). Among these enzymes, CYP3A4 and CYP3A5 are the most important in adult human tissues, such as liver and small intestine. Our findings in cultured LS180 cells that G. biloba extract increases CYP3A4 and CYP3A5 expression are consistent with those reported in a recent study conducted with primary cultures of human hepatocytes. In that study, treatment of cultured hepatocytes with an extract of G. biloba (reported to contain 24% flavonol glycosides and 6% terpene trilactones) was shown to increase the levels of CYP3A4 mRNA, CYP3A protein, and CYP3A-mediated testosterone 6β-hydroxylation activity (Deng et al., 2008). Collectively, results from the cell culture studies with LS180 cells and human hepatocytes indicate that G. biloba extract is an inducer of CYP3A. Consistent with the cell culture data, a study involving healthy human volunteers demonstrated that the oral ingestion of G. biloba extract at a dosage of 120 mg twice daily for 28 days led to a decrease in the AUC for midazolam, which was employed as a CYP3A-selective substrate (Robertson et al., 2008). In another human study, G. biloba extract (120 mg twice daily for 14 days) decreased the AUC for another CYP3A substrate, alprazolam (Markowitz et al., 2003). In contrast, a study suggested inhibition of CYP3A-catalyzed drug metabolism, based on the finding that G. biloba extract (120 mg three times daily for 28 days) decreased the apparent oral clearance of midazolam (Uchida et al., 2006). Another study reported that G. biloba extract (60 mg four times daily) did not affect the serum 1′-hydroxymidazolam/midazolam ratio (Gurley et al., 2002), which was used as an in vivo index of CYP3A-mediated drug metabolism. The reasons for the conflicting data from the human studies are not known, but G. biloba extract is able to inhibit the in vitro catalytic activity of CYP3A4 (Gaudineau et al., 2004). Therefore, the lack of inductive effect of G. biloba extract reported previously (Gurley et al., 2002; Uchida et al., 2006) may relate to the specific experimental design employed. In those human studies, the inhibitory effect of CYP3A4 may have masked any inductive effect of the extract on the pharmacokinetics of the CYP3A drug substrate.
The ABCB1 gene encodes P-glycoprotein, which is an efflux pump that controls the bioavailability of orally administered drugs and plays an important role in anticancer drug resistance (Sharom, 2008). Recent data from in vitro experiments indicate that G. biloba extract inhibits the efflux activity of P-glycoprotein (Hellum and Nilsen, 2008). The present study shows a novel action of G. biloba extract. It up-regulates ABCB1 gene expression, as demonstrated in cultured LS180 cells. A human study reported that the administration of G. biloba extract does not affect the pharmacokinetics of fexofenadine (Robertson et al., 2008). However, fexofenadine is transported not only by P-glycoprotein but also by various other transporters, including multidrug resistance-associated protein 2 (Matsushima et al., 2008). Overall, further studies will be required to determine the consequences of the increase in ABCB1 expression by G. biloba extract.
G. biloba extracts contain terpene trilactones (e.g., ginkgolide A, ginkgolide B, ginkgolide C, and ginkgolide J) and flavonols (e.g., aglycones and glycosides of quercetin, kaempferol, and isorhamnetin) (van Beek, 2002). In a previous study, we reported that ginkgolide A accounted for part of the observed increase in the expression of a PXR target gene, CYP3A23, by G. biloba extract in primary cultures of rat hepatocytes (Chang et al., 2006). Therefore, this compound may be capable of activating PXR. In a recent study, ginkgolide A and B at 10, 50, and 100 μM concentrations were shown to increase human PXR activity in an in vitro cell-based reporter gene assay (Satsu et al., 2008). By comparison, it is not clear whether bilobalide contributes to the activation of PXR by G. biloba extract. The in vivo administration of this compound to mice has been reported to increase hepatic microsomal enzyme activity of a PXR target gene product, CYP3A (Umegaki et al., 2007). However, as shown in primary cultures of rat hepatocytes, bilobalide does not increase CYP3A23 gene expression or its associated testosterone 6β-hydroxylation activity (Chang et al., 2006). In future investigations, detailed chemical analysis of G. biloba extract will be required to identify the specific chemical constituents responsible for the activation of human and rodent PXR by G. biloba extract. Studies are planned to address these issues.
In summary, G. biloba extract activated mouse and human PXR, as assessed by an in vitro cell-based assay. Our novel finding was corroborated by the observation that the extract increased the expression of CYP3A4, CYP3A5, and ABCB1 in PXR-expressing LS180 cells, and that the effects were attenuated by a PXR antagonist. Given that PXR regulates the expression of a broad array of genes that are of fundamental importance in mammalian biology (Stanley et al., 2006; Nakamura et al., 2007; Moreau et al., 2008), results from the present study will provide an impetus to conduct studies in the future to delineate novel physiological, pharmacological, and toxicological actions of G. biloba.
Acknowledgments
We thank Indena S.A. for the generous provision of G. biloba extracts, Bryan Goodwin (GlaxoSmithKline) for the kind gift of the XREM-CYP3A4-Luc, and Jie Chen for assistance with the PCR analyses.
This study was supported by the Canadian Institutes of Health Research (Grant MOP-84581 to T.K.H.C.), by the Dawson Endowment Fund in Pharmaceutical Sciences (a major equipment grant to T.K.H.C.), and by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences (to T.S., M.N.). E.Y.H.Y. received a Kam Li Ma Scholarship in Pharmaceutical Sciences from the University of British Columbia and a partial graduate student traineeship in drug metabolism from Merck Research Laboratories (United States). T.K.H.C. received an Izaak Walton Killam Faculty Research Fellowship and a Senior Scholar Award from the Michael Smith Foundation for Health Research.
ABBREVIATIONS
- PXR
pregnane × receptor
- AUC
area under the plasma concentration-time curve
- ABCB1
P-glycoprotein
- LDH
lactate dehydrogenase
- hPXR
human PXR
- mPXR
mouse PXR
- PCN
pregnenolone 16α-carbonitrile
- DMSO
dimethyl sulfoxide
- PCR
polymerase chain reaction
- CAR
constitutive androstane receptor
References
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther. 2004;310:528–535. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Bandiera SM, Chen J. Constitutive androstane receptor and pregnane X receptor gene expression in human liver: interindividual variability and correlation with CYP2B6 mRNA levels. Drug Metab Dispos. 2003;31:7–10. doi: 10.1124/dmd.31.1.7. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Chen J, Teng XW. Distinct role of bilobalide and ginkgolide A in the modulation of rat CYP2B1 and CYP3A23 gene expression by Ginkgo biloba extract in cultured hepatocytes. Drug Metab Dispos. 2006;34:234–242. doi: 10.1124/dmd.105.005751. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Waxman DJ. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR). Drug Metab Rev. 2006;38:51–73. doi: 10.1080/03602530600569828. [DOI] [PubMed] [Google Scholar]
- Deng Y, Bi H, Zhao L, Wang X, Chen J, Ou Z, Ding L, Xu L, Guan S, Chen X, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60–66. doi: 10.2174/187231208783478489. [DOI] [PubMed] [Google Scholar]
- El-Sankary W, Gibson GG, Ayrton A, Plant N. Use of a reporter gene assay to predict and rank the potency and efficacy of CYP3A4 inducers. Drug Metab Dispos. 2001;29:1499–1504. [PubMed] [Google Scholar]
- Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem Biophys Res Commun. 2004;318:1072–1078. doi: 10.1016/j.bbrc.2004.04.139. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36:1172–1180. doi: 10.1124/dmd.107.018689. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CYW. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in human. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- Hellum BH, Nilsen OG. In vitro inhibition of CYP3A4 metabolism and P-glycoprotein-mediated transport by trade herbal products. Basic Clin Pharmacol Toxicol. 2008;102:466–475. doi: 10.1111/j.1742-7843.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Lichti-Kaiser K, Staudinger JL. The traditional Chinese herbal remedy tian xian activates pregnane X receptor and induces CYP3A gene expression in hepatocytes. Drug Metab Dispos. 2008;36:1538–1545. doi: 10.1124/dmd.108.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZL, Onda K, Tanaka S, Toma T, Hirano T, Oka K. Induction of multidrug resistance in MOLT-4 cells by anticancer agents is closely related to increased expression of functional P-glycoprotein and MDR1 mRNA. Cancer Chemother Pharmacol. 2002;49:391–397. doi: 10.1007/s00280-001-0411-5. [DOI] [PubMed] [Google Scholar]
- Luo Y. Alzheimer's disease, the nematode Caenorhabditis elegans, and Ginkgo biloba leaf extract. Life Sci. 2006;78:2066–2072. doi: 10.1016/j.lfs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Donovan JL, Lindsay DeVane C, Sipkes L, Chavin KD. Multiple-dose administration of Ginkgo biloba did not affect cytochrome P-450 2D6 or 3A4 activity in normal volunteers. J Clin Psychopharmacol. 2003;23:576–581. doi: 10.1097/01.jcp.0000095340.32154.c6. [DOI] [PubMed] [Google Scholar]
- Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. Involvement of multiple efflux transporters in hepatic disposition of fexofenadine. Mol Pharmacol. 2008;73:1474–1483. doi: 10.1124/mol.107.041459. [DOI] [PubMed] [Google Scholar]
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 2008;5:35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, Huang L, Yaramus M, Baum A, Venkataramanan R, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increases warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–1377. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T. Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem. 2007;282:9768–9776. doi: 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant N. The human cytochrome P450 sub-family: transcriptional regulation, interindividual variation and interaction networks. Biochim Biophys Acta. 2007;1770:478–488. doi: 10.1016/j.bbagen.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Rajaraman G, Chen J, Chang TKH. Ginkgolide A contributes to the potentiation of acetaminophen toxicity by Ginkgo biloba extract in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 2006;217:225–233. doi: 10.1016/j.taap.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Davey RT, Voell J, Formentini E, Alfaro RM, Penzak SR. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr Med Res Opin. 2008;24:591–599. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- Satsu H, Hiura Y, Mochizuki K, Hamada M, Shimizu M. Activation of pregnane X receptor and induction of MDR1 by dietary phytochemicals. J Agric Food Chem. 2008;56:5366–5373. doi: 10.1021/jf073350e. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P450 3A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49:311–318. [PubMed] [Google Scholar]
- Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- Shinozuka K, Umegaki K, Kubota Y, Tanaka N, Mizuno H, Yamauchi J, Nakamura K, Kunitomo M. Feeding of Ginkgo biloba extract (GBE) enhances gene expression of hepatic cytochrome P-450 and attenuates the hypotensive effect of nicardipine in rats. Life Sci. 2002;70:2783–2792. doi: 10.1016/s0024-3205(02)01530-8. [DOI] [PubMed] [Google Scholar]
- Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004;279:49307–49314. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Horsburgh BC, Ross J, Scheer N, Wolf CR. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab Rev. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E. Transcriptional control of intestinal cytochrome P-450 3A by 1α,25-dihydroxy vitamin D3. Mol Pharmacol. 2001;60:1399–1406. doi: 10.1124/mol.60.6.1399. [DOI] [PubMed] [Google Scholar]
- Uchida S, Yamada H, Li XD, Maruyama S, Ohmori Y, Oki T, Watanabe H, Umegaki K, Ohashi K, Yamada S. Effects of Ginkgo biloba extract on pharmacokinetics and pharmacodynamics of tolbutamide and midazolam in healthy volunteers. J Clin Pharmacol. 2006;46:1290–1298. doi: 10.1177/0091270006292628. [DOI] [PubMed] [Google Scholar]
- Umegaki K, Saito K, Kubota Y, Sanada H, Yamada K, Shinozuka K. Ginkgo biloba extract markedly induces pentoxyresorufin O-dealkylase activity in rats. Jpn J Pharmacol. 2002;90:345–351. doi: 10.1254/jjp.90.345. [DOI] [PubMed] [Google Scholar]
- Umegaki K, Taki Y, Endoh K, Taku K, Tanabe H, Shinozuka K, Sugiyama T. Bilobalide in Ginkgo biloba extract is a major substance inducing hepatic CYPs. J Pharm Phamacol. 2007;59:871–877. doi: 10.1211/jpp.59.6.0014. [DOI] [PubMed] [Google Scholar]
- van Beek TA. Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr A. 2002;967:21–55. doi: 10.1016/s0021-9673(02)00172-3. [DOI] [PubMed] [Google Scholar]
- van den Bout-van den Beukel CJ, Hamza OJ, Moshi MJ, Matee MI, Mikx F, Burger DM, Koopmans PP, Verweij PE, Schoonen WG, van der Ven AJ. Evaluation of cytotoxic, genotoxic and CYP450 enzymatic competition effects of Tanzanian plant extracts traditionally used for treatment of fungal infections. Basic Clin Pharmacol Toxicol. 2008;102:515–526. doi: 10.1111/j.1742-7843.2008.00225.x. [DOI] [PubMed] [Google Scholar]
- Yamaori S, Yamazaki H, Iwano S, Kiyotani K, Matsumura K, Saito T, Parkinson A, Nakagawa K, Kamataki T. Ethnic differences between Japanese and Caucasians in the expression levels of mRNAs for CYP3A4, CYP3A5, and CYP3A7: lack of co-regulation of the expression of CYP3A in Japanese livers. Xenobiotica. 2005;35:69–83. doi: 10.1080/00498250400021796. [DOI] [PubMed] [Google Scholar]
- Zhou C, Poulton EJ, Grün F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]


