Abstract
Background
Factor analyses suggest that the structure underlying Metabolic Syndrome (MetS) is similar in adolescents and adults. However, adolescence is a period of intense physiological change, so stability of the underlying metabolic structure and clinical categorization based on metabolic risk is uncertain.
Methods and Results
We analyzed data from 1098 participants in the PSD Study, a school-based study begun in 2001-2002, who were followed for 3 years. We performed factor analyses of 8 metabolic risks at baseline and follow-up to assess stability of factor patterns and clinical categorization of MetS. MetS was defined using the current AHA/NHLBI definition for adults (AHA), a modified AHA definition used in prior pediatric MetS studies (pedsAHA) and the International Diabetes Federation (IDF) guidelines. We found that factor structures were essentially identical at both time points. However, clinical categorization was not stable. About half of adolescents with baseline MetS lost the diagnosis at follow-up regardless of the definitions used: pedsAHA=56% (95% CI: 42%, 69%), AHA=49% (95% CI: 32%, 66% ), IDF=53% (95% CI: 38%, 68%). In addition to loss of the diagnosis, new cases were identified. Cumulative incidence rates were: pedsAHA=3.8% (95% CI: 2.8%, 5.2%); AHA=4.4% (95% CI: 3.3%, 5.9%); IDF=5.2% (95% CI: 4.0%, 6.8%)].
Conclusions
During adolescence, metabolic risk factor clustering is consistent. However, there is marked instability in the categorical diagnosis of MetS. This instability, which includes both gain and loss of the diagnosis, suggests that the syndrome has reduced clinical utility in adolescence and that MetS-specific pharmacotherapy for youth may be premature.
Keywords: syndrome X, obesity, insulin resistance, adolescence
Introduction
Clustering of metabolic and clinical risk factors that predict subsequent development of disease is well established in adolescents and adults.1-3 Previous work in adults suggests that metabolic syndrome (MetS)-the clustering of hyperglycemia, hypertriglyceridemia, hypertension, low HDL-C, and abdominal obesity-is independently associated with future risk of developing both Type 2 diabetes and cardiovascular disease.4-6 This diagnosis is felt to be particularly valuable for identifying overweight or obese individuals at higher likelihood for developing disease and helping motivate these individuals to address their risks.7-9
Among children and adolescents, interest in MetS has been driven by soaring rates of overweight and obesity, particularly among youth.10 Current estimates suggest that more than 2 million adolescents, most of whom are overweight, have a metabolic syndrome phenotype.11 This number is expected to rise along with the prevalence of overweight and obesity in this age group, leading to fears of increasing prevalence and earlier onset of morbidity and mortality.12, 13 Despite these growing concerns, application of the MetS concept to children is more controversial than in adults. At present, there is no consensus regarding how to define pediatric MetS.14-18 Lack of consensus is in part due to our evolving understanding of normal developmental changes associated with childhood and puberty. These changes in metabolic and clinical characteristics impede agreement on criteria to define pediatric MetS.
The difficulties in creating a pediatric MetS definition highlight the differences between MetS as a concept, and MetS as a diagnostic category. The concept is based on clustering along an entire physiological spectrum, while the diagnostic category is based on dichotomies. Clustering of metabolic risk appears constant across development,19 but less is known about the stability of clinical diagnosis of MetS, especially in pediatric populations.16, 20, 21 The potential instability in the diagnosis of MetS is perhaps highest in adolescence, when pubertal growth and development, which influences a number of the metabolic traits used to define MetS, may cause the levels of these risks to cross the thresholds used in defining MetS. Such threshold crossings can be independent of the clustering phenomenon, but no studies to date in either adult or adolescent populations have assessed the stability of both metabolic risk factor clustering and the clinical diagnosis.
The purpose of this study was to address this gap in the literature. First, we determine whether the metabolic risk factor relationships assessed through factor analysis are consistent during adolescence. Second, we explore changes in the metabolic risks associated with MetS over three years and evaluate the stability of the clinical classification of MetS within individual youth. We address these goals in a community-based sample of adolescents using MetS definitions from the current American Heart Association (AHA) guidelines and the International Diabetes Federation (IDF) guidelines. 8, 22
Methods
Study sample
Data were drawn from 1098 participants in The Princeton School District (PSD) Study, a longitudinal cohort study situated in a public school district near Cincinnati, Ohio which began in the 2001-2002 school year.23 The sample (51.6% non-Hispanic white, 46.9% non-Hispanic black, and 1.5% Hispanic; 50.5% female; mean baseline age=15.0years, SD=1.6 years, range 12.2 - 19.3 years) included those who had a baseline physical exam and useable fasting morning blood sample and who returned for re-assessment three years later (73% retention rate, mean length of follow up = 2.74yrs). The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Study Protocol and Measures
Study visits, including a physical exam and phlebotomy23, 24 took place in the morning after a verified minimum ten hour fast. The study protocol was approved the IRBs of the local Children's Hospital and participating university. Eight metabolic risks were assessed: waist circumference, body mass index (BMI), systolic (SBP) and diastolic (DBP) blood pressure, glucose, high density lipoprotein cholesterol (HDL-C), triglycerides, and insulin. Both student assent and parental consent were obtained. BMI was calculated as BMI = weight(kg)/height(m)2. BMI percentiles and z scores were based on the CDC growth charts data.25 Obesity was defined as a BMI >=95% or BMI>=30, overweight as a BMI between the 85% to less than the 95%, and normal weight as a BMI <85%. Although these categories are often referred to as “at risk for overweight” for the 85% to the 95% and “overweight” for those with BMI >=95% when referring to children, we use the terms “overweight” and “obesity”, respectively, because many of these young people are 18 or over, and because we feel these terms are better suited to developmental studies assessing the transition to adulthood. At baseline, SBP and DBP were assessed per protocol26 on a convenience subsample of 165/433 youth with BMI>=85% due to time restrictions in the school setting. Because MetS is rare in normal weight youth, BMI represented a logical screening factor to obtain this initial blood pressure sample. At follow-up, blood pressure was obtained on all subjects through use of a DynaPulse Pathway instrument (Pulse Metric, Inc., San Diego, California).27 Following a five minute rest, three blood pressure recordings were obtained averaged for analyses. Laboratory assays assessed glucose, insulin, HDL-C, and triglycerides.23, 28
Definition of Metabolic Syndrome and Group Categorization
We term the presence of a particular metabolic risk above the cut point used in defining MetS a “constituent risk.” For the AHA definition, a participant was categorized as having MetS if he/she had any three of the following five constituent risks: glucose>=100 mg/dl, triglycerides>=150 mg/dl, HDL-C <=40mg/dl for boys and 50 mg/dl for girls, waist circumference>= 102 cm for boys and 88 cm for girls, blood pressure >= 85mmHg diastolic, 130 mmHg systolic.8 The waist circumference cut points recommended for Euorpids and Sub-Saharan African populations were employed in the IDF definition (94cm for boys and 80cm for girls), as the cohort was 98.5% non-Hispanic black and white. Other cut points were the same as the AHA definition. Elevated waist circumference plus two of the other four constituent risks were required to be classified as MetS-positive per the IDF definition.22 In addition, we created a pediatric MetS definition similar to that used in prior pediatric MetS studies.15, 17 The pediatric definition (pedsAHA), was based on the adult AHA definition but used updated pediatric reference standards for the blood pressure, waist circumference, triglycerides, and HDL-C. The glucose cut point was identical to that in the adult definitions (100mg/dl). The pedsAHA cut points for the other constituent risks were: 90% for blood pressure adjusted for age, sex, and height;29 90% for waist circumference, adjusted for age, sex, and race/ethnicity;30 10% for HDL-C adjusted for race and sex; 31 midpoint for the borderline high triglycerides range (110 mg/dl).15 Thresholds for the oldest age group were applied to subjects older than the highest age category for the blood pressure (>17 yrs) and waist circumference (>18 yrs) criteria.
Subjects were further classified based on the number of times AHA-defined MetS was present into one of three groups: 1) baseline only, 2) incident (MetS at follow-up but not at baseline), 3) persistent (MetS at both time points). We identified a fourth group of all non-Hispanic black and white subjects who were baseline overweight and Met-S free at both time points as a comparison group to assess whether the metabolic changes in the MetS groups differed from a group of those who remained MetS-free. The comparison group was comprised of 291 youth (74 white boys, 81 black boys, 49 white girls and 87 black girls). Eighty-three individuals who, at baseline, were missing information on blood pressure and had two AHA-defined constituent risks, and therefore, may have been baseline AHA-MetS+, were excluded from the MetS group comparisons.
Statistical Analyses
Analyses were performed with SPSS for Windows.32 To identify the factor structure at baseline and follow-up, we performed principal components analysis, which is a type of exploratory factor analysis, using an Eigenvalue of one as the extraction method and Varimax rotation. Variables with factor loading>=0.4 were used in interpreting factors. Instability was defined as the % baseline MetS+ youth who were MetS- at follow-up. Cumulative incidence was defined as the proportion of new cases from those who had been MetS- at baseline. Instability and cumulative incidence were calculated for each definition. Because most MetS+ youth are obese, MetS prevalence at baseline and follow-up, instability, and cumulative incidence , along with their 95% confidence intervals, are reported for the total and the subgroup of those who were obese at baseline.
For statistical testing, alpha was set at 0.05. Because the distribution of most of the metabolic risks were skewed, Kruskal Wallis tests were used to determine if within-person changes in the eight metabolic risks differed by MetS group assignment. These tests for group differences were also preformed on height and weight. In addition, we assessed whether the proportion of subjects with threshold crossings differed among the four groups. Chi square analyses for each of the six possible pairwise combinations among the four groups were run. Because multiple comparisons were involved in the tests for group differences of both continuous and categorical variables, P values were adjusted using Hochberg's method.33
Results
Metabolic Risks and Weight Status
Table 1 describes the distribution of metabolic risks in these 1098 adolescents. Baseline prevalence of overweight was 19.8% and of obesity was 19.7%. At follow-up, 20.1% were overweight and 20.4% obese. 14.3% of normal weight youth, 21.4% of youth with a BMI >=85%, and 77.9% of baseline obese youth were obese at follow-up.
Table 1.
Description of metabolic risks in 1098 PSD Study participants. Risks in bold were used in defining MetS.
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQ range | Mean | SD | Median | IQ range | |
| Age (yrs) | 15.0 | 1.6 | 14.8 | 2.4 | 17.7 | 1.5 | 17.6 | 2.2 |
| Insulin (pM/L) | 131.5 | 114.6 | 100.0 | 84.8 | 120.9 | 125.4 | 85.0 | 76.4 |
| BMI | 23.8 | 5.9 | 22.4 | 6 | 25.5 | 6.5 | 23.8 | 6.9 |
| BMI Z score | .73 | 1.00 | .72 | 1.45 | .71 | 1.05 | .70 | 1.51 |
| Glucose (mg/dl) | 85.9 | 9.2 | 85.7 | 11.8 | 76.3 | 10.3 | 76.1 | 11.5 |
| Waist circumference (cm) | 79.6 | 13.9 | 76.1 | 15.4 | 85.6 | 15.3 | 81.6 | 16.8 |
| SBP (mmHg) | 114.9 | 10.3 | 114 | 14 | 116.0 | 10.8 | 115.3 | 14.7 |
| DBP (mmHg) | 70.3 | 8.8 | 70 | 12 | 69.6 | 7.6 | 69.0 | 10.3 |
| HDL-C (mg/dl) | 45.9 | 11.0 | 45 | 14 | 48.0 | 11.7 | 46 | 14 |
| Triglycerides (mg/dl) | 77.2 | 42.6 | 67.5 | 35 | 82.6 | 51.7 | 69 | 42 |
Factor Structure Stability
Three factors (Table 2) were extracted at both baseline and follow-up. These included an adiposity factor (insulin, BMI, waist circumference), a metabolic factor (triglycerides, HDL-C), and a blood pressure factor (SBP, DBP). Factor loadings and the amount of variance explained were very similar at both time points. The major difference was that glucose loaded on the metabolic factor at baseline and on the adiposity factor at follow-up. Also of note was that insulin loaded on the metabolic factor and the adiposity factor at baseline, but only on the adiposity factor at follow-up. Overall, these differences were minor, and suggest that the factor structure is stable.
Table 2.
Rotated factor loadings from exploratory factor analysis. Bold indicates factor loading used in interpretation of the factor structure (loading >0.4).
| Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|
| Fat | Metabolic | Blood pressure | Fat | Metabolic | Blood pressure | |
| Insulin | .52 | .43 | -.06 | .66 | .29 | -.02 |
| BMI | .92 | .06 | .05 | .86 | .007 | .18 |
| Waist | .93 | .13 | .12 | .85 | .13 | .23 |
| HDL | -.36 | -.46 | -.007 | -.15 | -.76 | -.07 |
| Triglycerides | .21 | .66 | .09 | .16 | .79 | .07 |
| Glucose | -.06 | .73 | .004 | .44 | .11 | .07 |
| DBP | .06 | -.08 | .83 | .06 | .06 | .92 |
| SBP | .03 | .16 | .82 | .26 | .09 | .87 |
| %Variance Explained | 32.5 | 16.7 | 12.9 | 36.9 | 12.4 | 16.1 |
| % Total Variance Explained | 62.1 | 65.4 | ||||
Stability of the MetS Diagnosis
Table 3 provides information on the baseline and follow-up prevalences, as well as proportion who lost or gained the diagnosis. At baseline, 37 adolescents fulfilled the AHA definition for MetS, 57 the pedsAHA definition, and 49 the IDF definition. At follow-up, these numbers had increased to 66 for the AHA definition, 65 for the pedsAHA definition, and 78 for the IDF definition. The number of new cases was greater than would have been expected from the prevalence data because the diagnosis was unstable in about half of those who were MetS+ at baseline (Table 3, Figure 1). The pediatric-specific definition had a higher degree of instability than the either of the two adult definitions, although confidence intervals overlapped considerably. This pattern was also found among baseline obese MetS+ youth, more than 90% of whom remained obese (93% AHA, 92% IDF, 90% pedsAHA, p=ns).
Table 3.
Changes in MetS prevalence in youth in the PSD Study followed for three years
| Total (N=1098) | Baseline Obese (N=216) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline Prevalence | Instability | Cumulative Incidence | FU Prevalence | Baseline Prevalence | Instability | Cumulative Incidence | FU Prevalence | |
| % | % | % | % | % | % | % | % | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| AHA | 3.4 | 48.6 | 4.4 | 6.0 | 14.8 | 46.9 | 16.8 | 22.2 |
| (2.4,4.7) | (31.9, 65.6) | (3.3, 5.9) | (4.7, 7.6) | (10.4, 20.3) | (29.1, 65.3) | (11.7,23.1) | (16.9, 28.4) | |
| IDF | 4.5 | 53.1 | 5.2 | 7.1 | 18.5 | 50.0 | 17.6 | 23.6 |
| (3.4, 5.9) | (38.3, 67.5) | (4.0, 6.8) | (5.7,8.8) | (13.6, 24.4) | (33.8, 66.2) | (12.3, 24.1) | (18.1,29.8) | |
| Peds AHA | 5.2 | 56.1 | 3.8 | 5.9 | 23.6 | 56.9 | 13.3 | 20.4 |
| (4.0, 6.7) | (42.4, 69.3) | (2.8, 5.2) | (4.6, 7.5) | (18.1,29.8) | (42.2, 70.7) | (8.5, 19.5) | (15.2, 26.4) | |
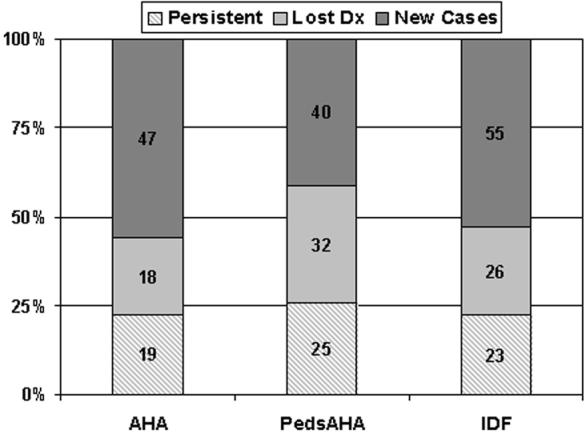
Categorization of MetS-positive youth in the PSD study into those with persistent MetS, baseline only MetS, and incident MetS according to three different MetS definitions. Numbers in the bar centers represent case numbers for each particular MetS group. The Y-axis represents percentage of total MetS cases identified.
Group Comparisons
Obesity was highly prevalent in all MetS groups, ranging from 63.3% at baseline in the incident group to 94.7% at follow-up in the persistent group. Table 4 describes group comparisons in the distribution of metabolic risks. Significant group differences were demonstrated for HDL, triglycerides, SBP, waist circumference, BMI, height, and weight. Insulin levels, glucose, and DBP were not significantly different between groups.
Table 4.
Distribution of metabolic risks and risk changes over three years in PSD Study youth who were AHA MetS+ during at least one study visit and a comparison group who were overweight at baseline but MetS-free at both study visits
| Baseline Overweight, MetS-free | Baseline Only MetS | Incident MetS | Persistent MetS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N=291) | (N=18) | (N=30) | (N=19) | |||||||||
| median (interquartile range) | median (interquartile range) | median (interquartile range) | median (interquartile range) | |||||||||
| Baseline | Follow-up | Within-person change |
Baseline | Follow-up | Within-person change |
Baseline | Follow-up | Within-person change |
Baseline | Follow-up | Within-person change |
|
| HDL-C* | 44.0 | 46.8 | 1.7 | 37.0 | 42.0 | 5.0 | 34.5 | 35.5 | 1.0 | 30.0 | 32.7 | 0 |
| (mg/dl) | (13.0) | (13.0) | (11.0) | (4.0) | (9.0) | (8.1) | (9.0) | (7.0) | (6.2) | (11.0) | (14.0) | (7.0) |
| Triglycerides* | 66.0 | 66.0 | 1.0 | 119.5 | 91.5 | 27.5 | 76.0 | 116.5 | 26.5 | 159.0 | 180.0 | 5.0 |
| (mg/dl) | (36.0) | (40.0) | (28.0) | (104.0) | (63.0) | (58.5) | (45.0) | (115.0) | (71.5) | (17.0) | (174.0) | (118.0) |
| Glucose | 86.7 | 76.6 | -9.8 | 95.9 | 85.3 | -8.4 | 91.0 | 78.9 | -10.5 | 91.7 | 81.3 | -9.8 |
| (mg/dl) | (10.4) | (11.2) | (13.6) | (16.4) | (13.8) | (19.7) | (12.7) | (16.2) | (12.3) | (19.5) | (22.3) | (16.7) |
| Insulin (pM/L) | 118.8 | 98.0 | -17.6 | 261.5 | 177.9 | -8.4 | 138.6 | 152.5 | 36.9 | 299.4 | 236.8 | 3.9 |
| (98.9) | (82.8) | (87.6) | (176.9) | (188.6) | (214.3) | (105.9) | (171.8) | (142.8) | (219.8) | (279.0) | (176.5) | |
| SBP*,† | 114.0 | 116.3 | 3.0 | 127.0 | 122.5 | -2.0 | 118.0 | 132.0 | 11.3 | 124.0 | 130.3 | 6.3 |
| (mmHg) | (12.0) | (13.3) | (13.3) | (13.0) | (15.2) | (24.3) | (18.0) | (7.3) | (18.5) | (18.0) | (18.7) | (10.7) |
| DBP† | 70.0 | 70.0 | 1.0 | 80.0 | 73.2 | 0.5 | 77.0 | 78.3 | 2.2 | 72.0 | 77.7 | 4.0 |
| (mmHg) | (10.0) | (8.7) | (12.0) | (17.0) | (9.5) | (35.0) | (11.0) | (9.6) | (12.5) | (8.0) | (6.7) | (9.3) |
| Waist | 85.0 | 90.0 | 4.6 | 103.0 | 111.1 | 5.2 | 92.9 | 107.9 | 12.9 | 112.3 | 126.8 | 12.2 |
| Circumference (cm)* | (11.3) | (14.9) | (9.6) | (19.6) | (23.6) | (11.6) | (17.0) | (8.9) | (10.8) | (16.1) | (18.1) | (12.2) |
| BMI* | 26.2 | 27.8 | 1.3 | 33.3 | 34.3 | 1.9 | 30.4 | 35.6 | 4.4 | 34.1 | 40.5 | 2.8 |
| (kg/m2) | (4.4) | (5.1) | (3.9) | (11.1) | (14.2) | (3.7) | (9.6) | (6.4) | (4.1) | (7.0) | (9.0) | (4.8) |
| Weight* | 74.1 | 81.7 | 7.3 | 93.5 | 96.4 | 7.8 | 87.2 | 102.8 | 14.1 | 103.4 | 122.5 | 11.5 |
| (kg) | (16.6) | (19.4) | (12.1) | (31.5) | (42.2) | (11.6) | (21.0) | (20.4) | (12.9) | (27.6) | (35.5) | (21.0) |
| Height* | 1.65 | 1.71 | 0.02 | 1.65 | 1.66 | 0.01 | 1.71 | 1.73 | 0.007 | 1.72 | 1.76 | 0.01 |
| (m) | (0.13) | (0.14) | (0.05) | (0.07) | (0.13) | (0.02) | (0.12) | (0.14) | (0.02) | (0.14) | (0.12) | (0.04) |
| Weight Status | ||||||||||||
| Normal | -- | 59 (20.3) | 1 (5.6) | 2 (11.1) | 5 (16.7) | 1 (3.3) | 1 (5.3) | 1 (5.3) | ||||
| Overweight | 182 (62.5) | 120 (41.2) | 2 (11.1) | 3 (16.7) | 6 (20.0) | 3 (10.0) | 1 (5.3) | 0 | ||||
| Obese | 109 (37.5) | 111 (38.1) | 15 (83.3) | 13 (72.2) | 19 (63.3) | 26 (86.7) | 17 (89.5) | 18 (94.7) | ||||
differences across the 4 groups in within-person change in that particular metabolic risk significant based on Kruskal-Wallis test P value corrected for multiple comparisons33
measured at baseline on 123/291 in the MetS-free group, 10/18 in the baseline only group, 16/30 in the incident group, and 11/19 in the persistent group. All had blood pressure measured at follow-up.
The diagnosis of MetS is based on categorization of metabolic risks into constituent risks. Prevalence of each constituent risk is noted in Table 5. All those with MetS at baseline (persistent and baseline only groups) had low HDL-C. High waist circumference was the next most common risk, and hyperglycemia the rarest. This pattern of risks was also seen in the incident MetS group. In the MetS-free group, low HDL-C was also highly prevalent. High waist circumference was less prevalent in this group, perhaps due to the lower proportion of obese youth. Among the 109 obese youth in the MetS-free group, low HDL-C was the most prevalent baseline risk (45.9%) followed by high waist circumference (32.1%) and then high blood pressure (7.3%), hyperglycemia (4.6%), and hypertriglyceridemia (2.8%). At follow-up, high waist circumference (55.0%) had succeeded low HDL-C (38.5%) as the most common risk in the obese MetS-free subjects.
Table 5.
Distribution of constituent MetS risks over three years in PSD Study youth who were AHA MetS+ during at least one study visit and a comparison group who were overweight at baseline but MetS-free at both baseline and follow-up
| Baseline Overweight, | Baseline Only MetS | Incident MetS | Persistent MetS | |||||
|---|---|---|---|---|---|---|---|---|
| MetS-free (N=291) | (N=18) | (N=30) | (N=19) | |||||
| N (%) | N (%) | N (%) | N (%) | |||||
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
| Waist ≥102cm♂, | 49 | 104 | 15 | 14 | 11 | 26 | 18 | 18 |
| 88cm♀ | (16.9) | (35.7) | (83.3) | (77.8) | (36.7) | (86.7) | (94.7) | (94.7) |
| HDL-C ≤ 40mg/dl ♂, | 130 | 106 | 18 | 9 | 27 | 28 | 19 | 19 |
| 50mg/dl ♀ | (44.7) | (36.4) | (100) | (50.0) | (90.0) | (93.3) | (100) | (100) |
| Triglycerides ≥ | 5 | 7 | 7 | 2 | 1 | 13 | 13 | 10 |
| 150mg/dl | (1.7) | (2.4) | (38.9%) | (11.1) | (3.3) | (43.3) | (68.4) | (52.6) |
| Glucose ≥ 100mg/dl | 10 | 5 | 7 | 0 | 1 | 3 | 6 | 2 |
| (3.4) | (1.7) | (38.9) | (0) | (3.3) | (10.0) | (31.6) | (10.5) | |
| DBP ≥ 85mmHg or | 6/123 | 25 | 8/10 | 1 | 1/17 | 24* | 3/11 | 11 |
| SBP≥ 130mmHg | (4.9) | (8.6) | (80) | (5.6) | (5.9) | (80) | (27.2) | (57.9) |
| Total threshold | 168/1287 | 33/82†‡§ | 30/137† | 12/87 | ||||
| crossings | 13.1% | 40.2% | 21.9% | 13.8% | ||||
| N(%)∥ | ||||||||
14/17 (82.3%) with baseline BP data.
significantly different from MetS-free group. P<0.001 for baseline only. P=0.013 for incident group.
significantly different from persistent group (P<0.001).
significantly difference from incident group (p=0.015).
Total within-group potential threshold crossings = number of subjects in group times number of constituent risks (5) minus the number who did not have blood pressure measured at baseline.
When the variability in a metabolic risk between baseline and follow-up caused its level to cross the threshold used in defining MetS, a change in that particular constituent risk occurred. The total number of such threshold crossings, which were demonstrated in all groups, is found in the bottom row of Table 5. In the baseline only MetS group, 61.1% lost one constituent risk, 22.2% lost 2 constituent risks, and 10.0% lost three constituent risks. Conversely, in the incident MetS group, 53.3% gained two constituent risk, 36.7% gained one constituent risks, 10.0% gained three constituent risks and none lost a constituent risk. In the persistent MetS, the proportion who gained or lost constituent risks was more equal (31.6% gained one constituent risk and 47.4% lost a constituent risk). In the MetS-free group, changes also occurred in both directions, but gain in risks was more common than loss of risks; 25.5% gained at least one constituent risk, while 8.2% lost one constituent risk. Differences in threshold crossings between the persistent group and both the incident MetS and MetS-free groups were not statistically significant. All other between pairwise group differences in threshold crossings were statistically significant.
Discussion
This study demonstrates two important points regarding MetS in youth. First, the replicability of the factor structure suggests that the overall clustering of metabolic risks, which provides the conceptual underpinnings for MetS, does not change during adolescence. Second, the frequent instability of the clinical diagnosis of MetS within individual youth suggests that the clinical utility of the syndrome is reduced among adolescents. Further, because the insulin changes in this observational study did not distinguish between MetS groups, our findings suggest that changes in fasting insulin during adolescence do not cause short term changes in metabolic risk. Whether such developmental changes cause alterations in metabolic risks over a longer time frame requires further investigation.
These findings have important implications for both investigators and clinicians. For researchers working to understand the developmental trajectory of cardiovascular risk clustering, the replication of the factor analysis results within this cohort demonstrates the robust nature of the linear relationships between these physiological factors. Additionally, our findings support the use of factor analysis, specifically principal components analysis, as a valuable analytic tool for investigators who want to assess dysregulation across multiple metabolic pathways. A MetS approach reduces such multiple pathway effects to a single, dichotomous outcome. In contrast, factor analysis allows investigators to expand their assessment of such multi-system dysregulation and can provide more detailed information on the natural history of the metabolic derangements. Factor scores from a principal components analysis may be more useful than MetS for developmental studies of heart disease risk.28
For clinicians, these data provide a note of caution. MetS was developed as a means to identify overweight adults at greatest risk for diabetes and cardiovascular disease.34 The application of this construct has not been thoroughly evaluated in children and adolescents. In addition, regardless of age, measurement variability is a critical issue when specific cut points are used to create the definition of a pathologic state such as MetS. These data demonstrate that, in adolescents, there is significant within-person variability across the diagnostic thresholds during growth and development. This variability led to loss of the diagnosis in about half of those with baseline MetS and gain of the diagnosis in others. The high degree of diagnostic instability, whether due to measurement variability or normal physiologic variation, suggests that MetS classification may not be an effective method for risk stratification in pediatrics.
In our study, the instability was greatest when a pediatric-specific, percentile-based definition of MetS was employed. The definition of pediatric MetS is currently being debated. At present, pediatric studies use a multitude of strategies to define MetS for children and youth. The application of an adult-based definition is rare.23 These findings suggest that, even if consensus is reached regarding definitional criteria for pediatric MetS, the problem of instability of the clinical syndrome will remain. Thus, these data call into question the utility of the concept of pediatric MetS.
The major limitation of this study is lack of baseline blood pressure data for most of the cohort. However, we did have baseline blood pressure for 38.1% of youth with BMI >=85%, who are at greatest risk for MetS, and for the entire cohort at follow-up. We were able to identify only 14 youth (1.5%) who were missing baseline blood pressure data and had high blood pressure at follow-up, ten of whom met criteria for MetS at follow-up. Thus, we may have slightly underestimated baseline MetS prevalence and overestimated cumulative incidence. In addition, this cohort does not provide adequate representation from racial/ethnic groups other than non-Hispanic blacks and whites and the number of MetS+ youth at baseline was relatively small, which limited our ability to determine predictors of instability using multivariable analyses. Balancing these limitations are the longitudinal design of our study, careful measurement of physiologic risks, and use of a community-based sample, which provides greater generalizability than clinic-based samples of overweight youth.16, 17
Since publication of the first paper on MetS in adolescents in 2003,15 there has been growing interest in and movement toward therapeutic intervention for this syndrome in children and adolescents.17, 35, 36 This drive toward treatment has been fueled, in part, by reports, that rising rates of obesity and concomitant MetS would lead to “epidemics” of Type 2 diabetes and early cardiovascular disease in the young.16, 37 These epidemics have not materialized,38 yet the focus on intervention, including potential use of pharmacologic agents, remains.9, 35, 36, 39-41 Our findings do not support the use of pharmacologic agents, such as insulin sensitizers,17 specifically for treatment of MetS in youth. The issues we raise for adolescents regarding MetS treatment echo the concerns of a number of MetS experts who have suggested that the syndrome's definition is not sensitive or specific enough to be used in treatment decisions in adults.42-45 Our data suggest that treatment of cardiovascular risk in adolescence should focus on treatment of established risks, rather than MetS. Such a focus might include emphasis on exercise promotion, prevention and treatment for teen smoking, and obesity prevention and treatment. Indeed, for growing youth, encouraging and supporting weight stability during the years of long bone growth, which can be a more readily attainable goal for many youth than weight loss, may provide the clinician with the most effective means to reduce cardiovascular risk factor clustering and long term risk of cardiovascular disease.
Acknowledgements
The authors thank Ralph B. D'Agostino, PhD, for statistical advice.
Funding Sources: Supported by NIH grants HD41527, DK59183 and M01RR 08084.
Footnotes
Conflict of Interest Disclosures: Dr Meigs has received research grants from GlaxoSmithKline and Wyeth, and has served on Merck's Advisory board. Dr. Daniels has been a consultant to Abbott Laboratories. Drs. Goodman and Dolan have no conflict of interest disclosures.
References
- 1.Smoak CG, Burke GL, Webber LS, Harsha DW, Srinivasan SR, Berenson GS. Relation of obesity to clustering of cardiovascular disease risk factors in children and young adults. The Bogalusa Heart Study. Am J Epidemiol. 1987;125:364–372. doi: 10.1093/oxfordjournals.aje.a114543. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. 2000;49:1042–1048. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, D'Agostino RB, Sr., Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997;46:1594–1600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Sr., Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52:2160–2167. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 5.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The Metabolic Syndrome and Total and Cardiovascular Disease Mortality in Middle-aged Men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA, for Conference Participants Clinical Management of Metabolic Syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association Conference on Scientific Issues Related to Management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM. Metabolic Syndrome Scientific Statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Arterioscler Thromb Vasc Biol. 2005;25:2243–2244. doi: 10.1161/01.ATV.0000189155.75833.c7. [DOI] [PubMed] [Google Scholar]
- 9.Smith SR. Importance of Diagnosing and Treating the Metabolic Syndrome in Reducing Cardiovascular Risk. Obesity. 2006;14:128S–134. doi: 10.1038/oby.2006.292. [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and Trends in Overweight Among US Children and Adolescents, 1999-2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 11.Duncan GE, Li SM, Zhou X-H. Prevalence and Trends of a Metabolic Syndrome Phenotype Among U.S. Adolescents, 1999-2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 12.Hannon TS, Rao G, Arslanian SA. Childhood Obesity and Type 2 Diabetes Mellitus. Pediatrics. 2005;116:473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- 13.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A Potential Decline in Life Expectancy in the United States in the 21st Century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 14.Raikkonen K, Matthews KA, Salomon K. Hostility predicts metabolic syndrome risk factors in children and adolescents. Health Psychol. 2003;22:279–286. doi: 10.1037/0278-6133.22.3.279. [DOI] [PubMed] [Google Scholar]
- 15.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a Metabolic Syndrome Phenotype in Adolescents: Findings From the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 16.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the Metabolic Syndrome in Children and Adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 17.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 18.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27:1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- 19.Pladevall M, Singal B, Williams LK, Brotons C, Guyer H, Sadurni J, Falces C, Serrano-Rios M, Gabriel R, Shaw JE, Zimmet PZ, Haffner S. A single factor underlies the metabolic syndrome: a confirmatory factor analysis. Diabetes Care. 2006;29:113–122. doi: 10.2337/diacare.29.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Balkau B, Vernay M, Mhamdi L, Novak M, Arondel D, Vol S, Tichet J, Eschwege E. The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome. The French D.E.S.I.R. study. Diabetes Metab. 2003;29:526–532. doi: 10.1016/s1262-3636(07)70067-8. [DOI] [PubMed] [Google Scholar]
- 21.Maumus S, Marie B, Siest G, Visvikis-Siest S. A prospective study on the prevalence of metabolic syndrome among healthy french families: two cardiovascular risk factors (HDL cholesterol and tumor necrosis factor-alpha) are revealed in the offspring of parents with metabolic syndrome. Diabetes Care. 2005;28:675–682. doi: 10.2337/diacare.28.3.675. [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 23.Goodman E, Daniels SR, Morrison JA, Huang B, Dolan LM. Contrasting prevalence of and demographic disparities in the World Health Organization and National Cholesterol Education Program Adult Treatment Panel III definitions of metabolic syndrome among adolescents. J Pediatr. 2004;145:445–451. doi: 10.1016/j.jpeds.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Goodman E, McEwen BS, Huang B, Dolan LM, Adler NE. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosom Med. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics CDC Growth Charts: United States. Available at: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm. Accessed July 2, 2001.
- 26.Morrison JA, James FW, Sprecher DL, Khoury PR, Daniels SR. Sex and race differences in cardiovascular disease risk factor changes in schoolchildren, 1975-1990: the Princeton School Study. Am J Public Health. 1999;89:1708–1714. doi: 10.2105/ajph.89.11.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18:767–771. doi: 10.1016/j.amjhyper.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Goodman E, Dolan LM, Morrison JA, Daniels SR. Factor Analysis of Clustered Cardiovascular Risks in Adolescence: Obesity Is the Predominant Correlate of Risk Among Youth. Circulation. 2005;111:1970–1977. doi: 10.1161/01.CIR.0000161957.34198.2B. [DOI] [PubMed] [Google Scholar]
- 29.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 30.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distributions and Trends of Serum Lipid Levels among United States Children and Adolescents Ages 4-19 Years: Data from the Third National Health and Nutrition Examination Survey. Preventive Medicine. 1998;27:879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 32.SPSS . SPSS Base 10.0 User's Guide. SPSS, Inc; Chicago: 1999. [Google Scholar]
- 33.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 34.Grundy SM. Point: the metabolic syndrome still lives. Clin Chem. 2005;51:1352–1354. doi: 10.1373/clinchem.2005.050989. [DOI] [PubMed] [Google Scholar]
- 35.Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. 2005;147:839–842. doi: 10.1016/j.jpeds.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Chi CH, Wang Y, Wilson DM, Robinson TN. Definition of metabolic syndrome in preadolescent girls. J Pediatr. 2006;148:788–792. doi: 10.1016/j.jpeds.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 37.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the Metabolic Syndrome in American Adolescents: Findings From the Third National Health and Nutrition Examination Survey. Circulation. 2004;110:2494–2497. doi: 10.1161/01.CIR.0000145117.40114.C7. [DOI] [PubMed] [Google Scholar]
- 38.Dolan LM, Bean J, D'Alessio D, Cohen RM, Morrison JA, Goodman E, Daniels SR. Frequency of Abnormal Carbohydrate Metabolism and Diabetes in a Population-based Screening of Adolescents. J Pediatr. 2005;146:751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 39.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105:2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 41.Pi-Sunyer FX. Use of Lifestyle Changes Treatment Plans and Drug Therapy in Controlling Cardiovascular and Metabolic Risk Factors. Obesity. 2006;14:135S–142. doi: 10.1038/oby.2006.293. [DOI] [PubMed] [Google Scholar]
- 42.Kahn R, Buse J, Ferrannini E, Stern M. The Metabolic Syndrome: Time for a Critical Appraisal: Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM. The Metabolic Syndrome: Requiescat in Pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 44.Gale EA. The myth of the metabolic syndrome. Diabetologia. 2005;48:1679–1683. doi: 10.1007/s00125-005-1873-5. [DOI] [PubMed] [Google Scholar]
- 45.Mitka M. Does the Metabolic Syndrome Really Exist? Diabetes and Heart Disease Groups Spar Over Issue. JAMA. 2005;294:2010–2011. doi: 10.1001/jama.294.16.2010. [DOI] [PubMed] [Google Scholar]


