Summary
Human dermal fibroblasts are generally considered to be restricted to a fibroblastic lineage. Although dermal fibroblasts do not typically express markers of osteoblastic differentiation, they have previously been shown to undergo osteoinduction when stimulated with bone morphogenetic proteins (BMPs) or vitamin D3. However, involvement of BMP signaling in vitamin D3-mediated osteoinduction has not been reported. In this study, human dermal fibroblasts were cultured in chemically-defined medium containing vitamin D3, in the presence of the BMP antagonist noggin or neutralizing antibodies specific for BMP-4 or BMP-6, and characterized for markers of osteoblastic differentiation. Treatment of dermal fibroblasts with vitamin D3 induced expression of BMP-4 (1.2±0.2, 1.7±0.2, and 1.8±0.2 relative fold increase) and BMP-6 (9.1±0.3, 23.3±2.1, and 30.4±3.0 relative fold increase) at 3, 14, and 21 days, respectively. Vitamin D3 was also shown to induce the expression of the osteoblast-specific markers, alkaline phosphatase and osteocalcin, in a dose-dependent manner in human dermal fibroblasts. Addition of noggin, BMP-4 antibodies, and BMP-6 antibodies resulted in a down-regulation of alkaline phosphatase activity (by 42, 22, and 20%, respectively) and secreted osteocalcin (by 20, 31, and 49%, respectively) after 21 days in culture. However, blocking BMP signaling did not result in complete recovery of a fibroblastic phenotype. Taken together, these results suggest that BMP signaling plays a role in the induction of an osteoblastic phenotype in human dermal fibroblasts in response to vitamin D3 stimulation.
Keywords: Dermal Fibroblast, Osteoblast, Vitamin D3, Gene Expression, Bone Morphogenetic Protein
Introduction
Cells of fibroblastic lineage have the potential to serve as an alternate cell source for engineering of specialized connective tissues, such as cartilage or bone. Although fibroblasts do not typically express markers of osteoblastic differentiation, they have previously been reported to undergo osteoinduction when stimulated with bone morphogenetic proteins (BMPs) or vitamin D3. For instance, dermal and gingival fibroblasts have been shown to exhibit an osteoblastic phenotype when transduced with vectors that drive the expression of BMP-2(1) or BMP-7(2-5). Additionally, murine NIH/3T3 cells(6) and human dermal fibroblasts(7) treated with vitamin D3 were able to differentiate along the osteoblastic lineage.
Individually, vitamin D3 and BMPs are known to enhance osteoblastic differentiation in stromal cells and osteoblasts. Vitamin D3 acts primarily through nuclear receptors that bind vitamin D response elements in the promoters of osteoblast-specific genes, such as alkaline phosphatase and osteocalcin(8, 9). Vitamin D3 is known to enhance differentiation and maturation of osteoblasts, although it has also been used for the osteoblastic differentiation of other cell types, including fibroblasts(6, 7) and stromal cells(10, 11). BMP family members, including BMP-2, -4, and -6, act as potent stimulators of osteoblast differentiation, mainly signaling through cell surface receptors and the intracellular SMAD pathway to effect changes in gene expression(12, 13). Interactions between the vitamin D3 and BMP pathways have also been observed. For example, expression of BMP-2, -3, -4, -5, and -6, have been shown to be regulated by vitamin D3 or its analogs in a variety of cell types, including osteosarcoma, bone marrow stromal, squamous carcinoma, and breast and prostatic epithelial cells(14-20). The mechanism by which vitamin D3 induces BMP expression is not known, though potential vitamin D response elements have been identified bioinformatically (21). Still, the effects of vitamin D3 on BMP signaling and the expression of osteoblast-specific proteins in dermal fibroblasts have not been reported.
Based on the known interaction between vitamin D3, alkaline phosphatase, and osteocalcin, it is unclear as to whether the observed expression of osteoblast-specific markers in human dermal fibroblasts(7) is due solely to direct binding of the vitamin D receptor to the promoter of these genes. The regulation of BMP expression by vitamin D3 in multiple cell types suggests that activation of the BMP signaling pathway may also play a role in the induction of osteoblastic differentiation in human dermal fibroblasts by vitamin D3. Therefore, the objectives of this study were to characterize the induction of BMP gene expression in dermal fibroblasts in response to vitamin D3 treatment and to determine the effect of the BMP antagonist noggin, and BMP-4 and BMP-6 neutralizing antibodies on the expression of osteogenic markers in human dermal fibroblasts cultured with vitamin D3.
Materials and Methods
Cell Culture
Human neonatal foreskin fibroblasts (Passage 4, Cascade Biologics, Portland, OR) were plated in 6-well plates at 1 × 104 cells/cm2 in serum-containing medium consisting of minimum essential medium (MEM, Invitrogen, Carlsbad, CA), 10% fetal bovine serum (Hyclone, Logan, UT), and antibiotics (Invitrogen). Additional supplements (described below) were added at the initial plating. After 24 hours, the monolayers were rinsed with Dulbecco's phosphate buffered saline (DPBS) and the medium was replaced with chemically-defined medium with 1% Insulin-Transferrin-Selenium (ITS, Invitrogen) substituted for serum.
Vitamin D3 Dose-Dependence
Dermal fibroblasts were cultured in serum-free medium containing 1% ITS with concentrations of 0 (DMSO carrier control, 0.02%), 1, 10, 100, and 1000 nM 1α,25 dihydroxyvitamin D3 (Sigma), 50 μg/ml L-ascorbic acid, and 5 mM β-glycerophosphate (βGP, Sigma).
BMP Signaling
Experimental cultures were supplemented with 50 μg/ml L-ascorbic acid, 5 mM βGP, and 100 nM 1α,25 dihydroxyvitamin D3. Recombinant mouse noggin (100 ng/ml, R & D Systems, Minneapolis, MN), monoclonal anti-human BMP-4 neutralizing antibodies (1 μg/ml, R & D Systems), and monoclonal anti-human BMP-6 neutralizing antibodies (1 μg/ml, R & D Systems) were added to additional vitamin D3-supplemented cultures (ITS+vitD, ITS+vitD+noggin, ITS+vitD+BMP4Ab, or ITS+vitD+BMP6Ab). Control cultures (ITS) were supplemented with 50 μg/ml L-ascorbic acid (Sigma, St. Louis, MO). Culture medium was changed 3 times per week and samples were analyzed at 3, 14, and 21 days.
Real Time-PCR
At each time point, total cellular RNA was extracted using the TRIZOL isolation system (Invitrogen). Total RNA was treated with DNase I (Invitrogen) and reverse transcription was performed on 1 μg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time PCR was conducted with SYBR Green or TAQMAN master mixes (Applied Biosystems) using an Applied Biosystems 7300 Real Time PCR System. Primers and probes (Table 1) specific for alkaline phosphatase (ALP, GenBank accession no. NM_000478), BMP-6 (GenBank accession no. NM_001718), osteocalcin (OC, GenBank accession no. NM_199173), and Runx2 (GenBank accession no. NM_001015051) were designed using Applied Biosystems' Custom TAQMAN Assay service. Primers and probes for 18S RNA were as published(22). Primers for human BMP-2 (GenBank accession no. NM_001200) and human BMP-4 (GenBank accession no. NM_001202) were designed using Primer Express 3.0 software (Applied Biosystems). Gene expression was normalized to 18S RNA and is shown as relative fold induction (using the ΔΔCt method, Applied Biosystems User Bulletin #2). Alkaline phosphatase, BMP-2, BMP-4, BMP-6, and Runx2 fold induction is expressed relative to the day 3 ITS control cultures. Due to a lack of expression in ITS control cultures, osteocalcin gene expression is shown relative to day 3 cultures containing 100 nM vitamin D3.
Table 1. RT-PCR Primer and Probe Sequences.
| Gene | Forward and reverse primers (5′→3′) | Primer Conc. (nM) | Probe (5′→3′) | Probe Conc. (nM) |
|---|---|---|---|---|
| ALP | CGGAACTCCTGACCCTTGAC
TGTTCAGCTCGTACTGCATGTC |
300 | TCGAAGAGACCCAATAGGT | 150 |
| BMP-2 | TGCCCCCCTACATGCTAGAC
TCCAAAGATTCTTCATGGTGGAA |
300 | SYBR Green | 150 |
| BMP-4 | ACCACGAAGAACATCTGGAGAAC
GCTGAGGTTAAAGAGGAAACGAAA |
300 | SYBR Green | 150 |
| BMP-6 | AACCAAACTTTTCTTATCAGCATTTATCAAGTC
ATACTACACGGGTGTCCAACAAAA |
300 | ATCAGCACAGAGACTCT | 150 |
| OC | CTGGCCGCACTTTGCAT
CTGCACCTTTGCTGGACTCT |
300 | CACCTGCCTGGCCAGC | 150 |
| Runx2 | TGGACCTCGGGAACCCA
GCGGTCAGAGAACAAACTAGGTT |
300 | AAGGCACAGACAGAAGC | 150 |
| 18S RNA (22) | CGGCTACCACATCCAAGGAA
GCTGGAATTACCGCGGCT |
150 | CACCAGACTTGCCCTC | 100 |
The underlined base pairs indicate an exon-exon junction in the mRNA sequence
Biochemistry
Protein and DNA from the cell layer were extracted in 0.5% Triton X-100 in DPBS. ALP activity was determined using a p-nitrophenol phosphate colorimetric assay (Sigma)(7) and DNA content was quantified with the PicoGreen DNA assay (Invitrogen)(23). Osteocalcin protein secreted into the medium was detected using a human Osteocalcin Enzyme Amplified Sensitivity Immunoassay (Biosource, Camarillo, CA) according to manufacturer instructions.
Histology
Cell layers were rinsed with DPBS and fixed in 4% paraformaldehyde. BMP-4 and BMP-6 were localized using monoclonal antibodies (R & D Systems) and visualized with AlexaFluor 568-conjugated anti-mouse secondary antibodies (Invitrogen). Control staining was conducted on cultures without primary antibodies. F-actin was stained overnight at 4° C with AlexaFluor 488-conjugated phalloidin (Invitrogen). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen). Samples were viewed with a Zeiss AxioVert 200 inverted microscope and images were captured using Axiovision software.
Statistical Analysis
A one-way ANOVA with a Tukey post-hoc test was performed to determine the effect of vitamin D3 concentration on alkaline phosphatase activity and osteocalcin gene expression. A two-way ANOVA with a Tukey post-hoc test was conducted to determine the effect of culture time and media supplements (vitamin D, noggin, BMP-4 Ab, BMP-6 Ab) on gene expression and biochemistry. A one-way ANOVA with repeated measures was carried out to determine differences in the osteocalcin release rate and cumulative osteocalcin secreted. Significance was determined at p<0.05. Data represent mean ± standard deviation (n=3).
Results
Vitamin D Dose Dependence
The dose-dependent effect of vitamin D3 on the expression of osteoblastic markers by human dermal fibroblasts was determined using concentrations of 0, 1, 10, 100, and 1000 nM of 1α,25 dihydroxyvitamin D3. There was a significant increase in normalized alkaline phosphatase activity (Figure 1A) in cultures containing 100 and 1000 nM vitamin D3 compared to those with 0, 1, and 10 nM at 14 days. There was no significant difference between the cultures maintained in 100 and 1000 nM vitamin D3. Osteocalcin gene expression (Figure 1C) was not detected in the absence of vitamin D3. At 14 days, osteocalcin was significantly up-regulated in both the 100 nM (140.1±4.8 fold) and 1000 nM (74.5±20.8 fold) concentrations of vitamin D3. Changes in cell shape (Figure 1B, D) were also observed in cells cultured in the presence of vitamin D3. Cytoskeletal staining showed that treatment with 100 nM vitamin D3 resulted in a change to a spread, polygonal morphology. Based on these findings, a concentration of 100 nM vitamin D3 was selected for further studies.
Figure 1.
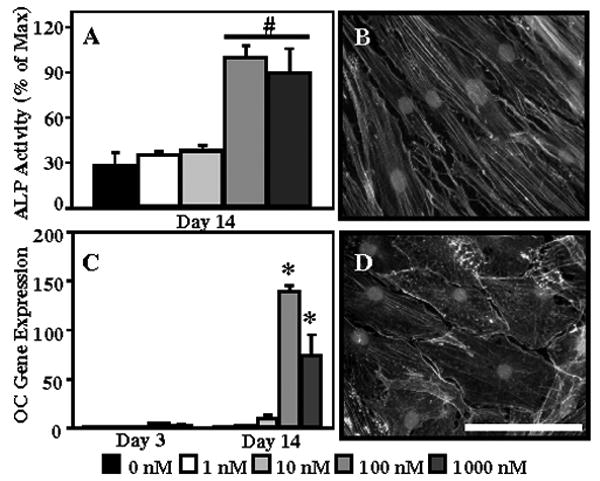
Human dermal fibroblasts were cultured in ITS medium in the presence of 0 (DMSO carrier control, 0.02%), 1, 10, 100, and 1000 nM vitamin D3 for 14 days to determine the dose-dependent effect of vitamin D3 on alkaline phosphatase activity (A) and osteocalcin (C) gene expression. F-actin was stained with phalloidin in order to visualize changes in cell morphology in ITS (B) and ITS+vitD (100 nM, D) medium. Nuclei are counterstained with DAPI. Scale bar = 100 μm. *: p<0.05 compared to all other groups; #: p<0.05 compared to 0, 1, 10 nM
BMP Signaling
Noggin, a BMP antagonist, and BMP-4 and -6 neutralizing antibodies were used to investigate the role of BMP signaling in the vitamin D3-induced expression of osteoblast-specific markers in human dermal fibroblasts. Induction of BMP-2, -4, and -6 gene expression was measured in dermal fibroblasts cultured in the presence of vitamin D3, with or without the addition of noggin, BMP-4, and BMP-6 antibodies. BMP-2 gene expression (Figure 2A) significantly decreased over time, with the highest expression seen in control cultures. BMP-4 gene expression (Figure 2B) was significantly up-regulated in samples cultured with vitamin D3 at days 14 and 21 (1.7±0.2 and 1.8±0.2 fold, respectively). Addition of noggin, BMP-4, and BMP-6 antibodies resulted in BMP-4 gene expression levels similar to controls. Treatment with vitamin D3 resulted in a robust up-regulation in BMP-6 expression (Figure 2C), with 9.1±0.3, 23.3±2.1, and 30.4±3.1 fold increases observed in cells cultured in ITS+vitD medium at 3, 14, and 21 days, respectively. Supplementation with noggin, BMP-4 antibodies, and BMP-6 antibodies produced a significant down-regulation of BMP-6 gene expression compared to ITS+vitD medium at all three time points.
Figure 2.
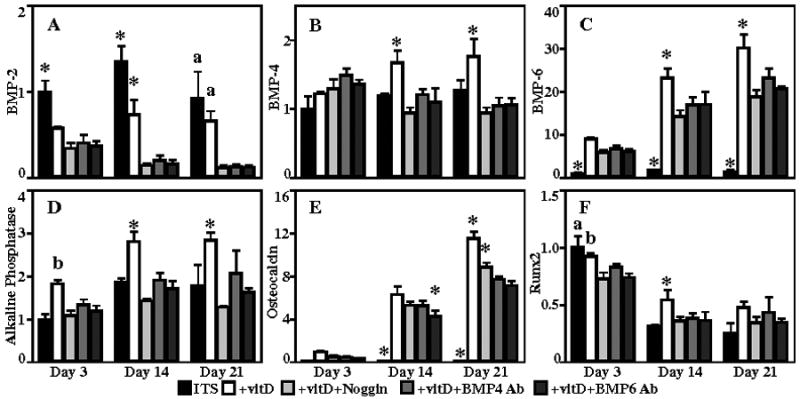
Human dermal fibroblasts were cultured in ITS, ITS+vitD, ITS+vitD+Noggin, ITS+vitD+BMP4 Ab, and ITS+vitD+BMP6 Ab medium. Gene expression for the bone differentiation factors BMP-2 (A), BMP-4 (B), BMP-6 (C) and osteoblast markers alkaline phosphatase (D), osteocalcin (E), Runx2 (F), are shown. *: p<0.05 compared to all other groups, a: p<0.05 compared to antagonist groups, b: p<0.05 compared to all but ITS+vitD+BMP4Ab.
The effect of blocking BMP signaling on the expression of osteoblast-specific markers was also examined. Alkaline phosphatase gene expression (Figure 2D) was significantly up-regulated in ITS+vitD medium (1.8±0.1, 2.8±0.2, 2.8±0.2, respectively) at all time points compared to controls. Addition of noggin, BMP-4, and BMP-6 antibodies for 14 and 21 days resulted in alkaline phosphatase gene expression similar to control levels. Osteocalcin gene expression (Figure 2E) was detected at low levels in cultures containing vitamin D3 at 3 days and increased over the 21 day experiment. Controls exhibited no osteocalcin expression at any time point. Samples cultured in medium containing vitamin D3 had the highest up-regulation of osteocalcin gene expression at 14 and 21 days (6.3±0.7 and 11.6±0.6 fold relative to day 3). Addition of noggin resulted in a significant decrease in expression at 14 and 21 days (by 16% and 23%, respectively). Further down-regulation of osteocalcin gene expression was observed at 14 and 21 days in cultures containing BMP-4 (by 16% and 33%) and BMP-6 (by 32% and 38%) antibodies. Runx2 (Figure 2F), a transcription factor involved in osteoblast differentiation(24), exhibited the highest level of gene expression at the day 3 time point in ITS and ITS+vitD cultures, with a decrease in expression over time.
DNA content (Figure 3A) significantly increased between day 3 and day 14, from 0.51±0.03 to 1.07±0.06 μg, and remained elevated at day 21. At all three time points, cells cultured in ITS+vitD medium had the highest alkaline phosphatase activity (Figure 3B), normalized to DNA content, of any group, while ITS controls had the lowest activity. Addition of noggin for 14 and 21 days resulted in a significant 44% and 42% decrease in activity, respectively, compared to the ITS+vitD cultures. Alkaline phosphatase activity at days 14 and 21 was significantly decreased in cultures supplemented with BMP-4 (by 29% and 22%, respectively) and BMP-6 (by 25% and 20%, respectively) antibodies compared to ITS+vitD, but this activity was significantly higher than cultures treated with noggin. In terms of the production of osteocalcin, no protein was detected in ITS control cultures at any time point. The osteocalcin release rate (Figure 4A) increased over time in all samples treated with vitamin D3. The rate of secretion in cells cultured in ITS+vitD medium was significantly greater than all other groups at all time points. Blocking BMP signaling with noggin, BMP-4 antibodies, or BMP-6 antibodies resulted in a decrease in the osteocalcin release rate, with the largest decrease in ITS+vitD+BMP6 Ab medium. Cells cultured in ITS+vitD medium had the most total secreted osteocalcin of any group, with 97.1±8.7 ng released over the 21 day duration. Samples cultured in ITS+vitD+noggin (77.9±9.0 ng) and ITS+vitD+BMP4Ab (66.9±2.6) secreted significantly less osteocalcin than the ITS+vitD group, but were not significantly different from each other at any time point. Addition of BMP-6 neutralizing antibodies resulted in a total osteocalcin secretion (49.9±3.2 ng) that was significantly less than all other vitamin D3 supplemented groups after 21 days in culture.
Figure 3.
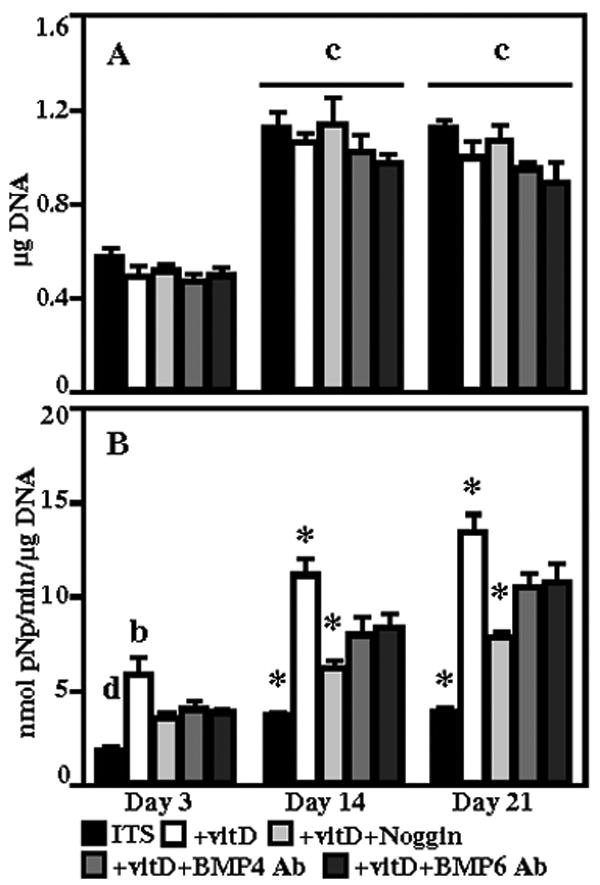
DNA content (A) and normalized alkaline phosphatase activity (B) for human dermal fibroblasts cultured in ITS, ITS+vitD, ITS+vitD+Noggin, ITS+vitD+BMP4 Ab, and ITS+vitD+BMP6 Ab media. *: p<0.05 compared to all other groups, b: p<0.05 compared to all but ITS+vitD+BMP4 Ab, c: p<0.05 compared to day 3, d: p<0.05 compared to all but ITS+vitD+Noggin.
Figure 4.
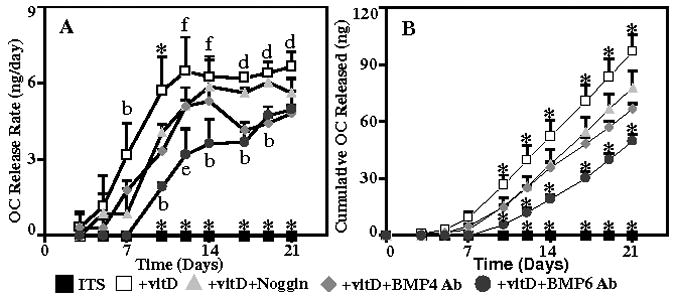
Osteocalcin release rate (A) and total osteocalcin protein released (B) into the medium by human dermal fibroblasts cultured in ITS, ITS+vitD, ITS+vitD+Noggin, ITS+vitD+BMP4 Ab, and ITS+vitD+BMP6 Ab media. *: p<0.05 compared to all other groups, b: p<0.05 compared to all but ITS+vitD+BMP4 Ab, d: p<0.05 compared to all but ITS+vitD+Noggin, e: p<0.05 compared to ITS+vitD, f: p<0.05 compared to ITS and ITS+vitD+BMP6Ab.
Secreted bone morphogenetic proteins and changes in cell morphology were detected using immunohistochemistry. BMP-4 staining was not detected in any of the samples. Accumulation of BMP-6 (Figure 5) was observed as punctate staining in the cell layer of all samples, with the most pronounced deposition in samples cultured with vitamin D3. No BMP-6 staining was observed in non-immune control samples. Dermal fibroblasts cultured in ITS control medium exhibited an elongated, spindle-shaped morphology consistent with fibroblastic cells (as shown in Figure 1). Cells cultured in the presence of vitamin D3 displayed a spread, polygonal morphology more consistent with an osteoblastic phenotype. Addition of noggin, BMP-4 antibodies, and BMP-6 antibodies to the cells cultured in the presence of vitamin D3 did not restore the elongated, fibroblastic morphology (data not shown).
Figure 5.
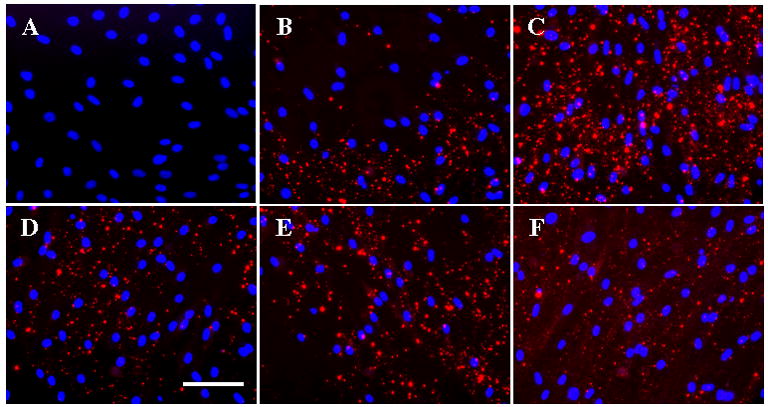
BMP-6 staining (red) of human dermal fibroblasts cultured for 21 days. Nuclei were counterstained with DAPI (Blue). Panels show representative staining of fibroblasts cultured in (B) ITS, (C) ITS+vitD, (D) ITS+vitD+Noggin, (E) ITS+vitD+BMP4 Ab, and (F) ITS+vitD+BMP6 Ab medium. (A) Non-immune control. (Scale bar = 100 μm)
Discussion
This study is the first to demonstrate that vitamin D3 is able to induce expression of BMP family members in human dermal fibroblasts and that inhibiting BMP signaling results in down-regulation of osteoblast-specific markers that are stimulated by vitamin D3. Vitamin D3 is known to up-regulate the expression of osteoblast-specific genes, such as alkaline phosphatase and osteocalcin, by binding directly to the promoter region of the respective genes(8, 9). Treatment of dermal fibroblasts with vitamin D3 resulted in a dose-dependent up-regulation of alkaline phosphatase activity and osteocalcin gene expression. Alkaline phosphatase (day 3-20) and osteocalcin (day 10-25) are markers that are expressed during the early and later stages of osteoblast differentiation in response to vitamin D3(25, 26). These markers of osteoblast differentiation are typically not expressed, or are expressed at low levels, in dermal fibroblasts. Peak osteocalcin expression and alkaline phosphatase activity was observed in cells cultured with 100 nM vitamin D3. This is in agreement with other studies that have investigated the role of vitamin D3 on fibroblastic cells(6, 7). Treatment of dermal fibroblasts with 100 nM vitamin D3 over a 21 day time course resulted in alkaline phosphatase activity and osteocalcin production levels that were consistent with those observed in differentiated osteoblasts(25, 26). Up-regulation of these markers was not surprising given that the vitamin D3 nuclear receptor is able to bind to the promoters of these genes(8, 9). However, a down-regulation in response to the inhibition of BMP signaling indicates that the osteoblastic differentiation of dermal fibroblasts by vitamin D3 is at least partially dependent on the activation of BMP signal transduction.
Since vitamin D3 has also recently been shown to induce BMP signaling(14-20), the expression of specific BMP family members were also investigated in response to vitamin D3 treatment. Specifically, BMP-4, and to a much greater extent, BMP-6 gene expression were elevated in human dermal fibroblasts supplemented with vitamin D3, while BMP-2 gene expression was down-regulated in these cultures. BMP-4 gene expression was increased 1.7-fold in cells cultured in the presence of vitamin D3, but BMP-4 protein was not detected in the cell layer. This inconsistency between BMP-4 gene and protein expression may be due to low levels of expression that do not give rise to appreciable accumulation in the cell layer. A 30-fold increase in BMP-6 gene expression was measured in samples cultured with vitamin D3 at 21 days, which corresponded with more intense staining in the cell layer. Inhibiting BMP activity using noggin or neutralizing antibodies resulted in a down-regulation of BMP-2, -4, and -6 gene expression in vitamin D3-treated dermal fibroblasts, which may be indicative of a regulatory feedback mechanism. The identification of potential vitamin D3 response elements in the promoters of BMP-4 and BMP-6 using bioinformatics(21) suggests that the observed induction of BMPs is driven by direct binding of the vitamin D3 receptor to the promoters, though experimental validation is necessary to confirm the presence of a vitamin D response element. Nevertheless, the induction of BMP expression by vitamin D3 treatment indicates that vitamin D3 may act on markers of osteoblast differentiation through both direct and indirect pathways.
In addition to typical osteoblast markers and BMP expression, the effect of vitamin D3 on other indicators of osteoblastic differentiation was also investigated. The gene expression profile of Runx2, a transcription factor which mediates expression of osteoblast-specific genes(24, 27), demonstrated an early down-regulation of Runx2 compared to controls. This is consistent with other studies that show no change or a decrease in Runx2 expression in response to vitamin D3(6, 28), and that increased osteocalcin expression induced by vitamin D3 treatment does not appear to be mediated by Runx2(29). Furthermore, changes in cell shape typically observed during osteoblastic differentiation of preosteoblastic cells were examined. Vitamin D3 treatment induced a shift from an elongated, fibroblastic morphology to a spread, polygonal morphology consistent with cells undergoing osteoblastic differentiation(30-32). Inhibition of BMP signaling did not prevent the change in morphology caused by vitamin D3 treatment. The observation of a spread, osteoblast-like cell shape in vitamin D3-treated cells was not a result of a decrease in cell number, as shown by the DNA content data. The incomplete down-regulation of osteoblastic genes and lack of reversion to a fibroblastic morphology may be due to the concentration of BMP inhibitors used in this study. However, the concentrations were within the range of the effective doses (ED50) for BMP inhibition by noggin (0.06-0.3 μg/ml)(33), BMP-4 antibodies(1-3 μg/ml), and BMP-6 antibodies (0.5-2 μg/ml). While it is possible that higher doses of inhibitor may result in complete recovery of the fibroblastic phenotype, it is likely that direct binding of vitamin D3 to the respective promoters of alkaline phosphatase and osteocalcin(8, 9) is responsible for a baseline level of expression. Therefore, the inhibition of BMP signaling would only result in a partial down-regulation of osteoblast differentiation markers and not a complete recovery of a fibroblast phenotype, as was observed in this study.
While inhibition of BMP signaling had an effect on the osteoblastic differentiation of human dermal fibroblasts, the results of this study suggest that no specific BMP family member was solely responsible. BMP-6 was the most highly up-regulated BMP family member observed in this study, identifying BMP-6 as a possible key contributor to osteoblastic differentiation in dermal fibroblasts in response to vitamin D3. Specifically inhibiting BMP-6 resulted in the greatest down-regulation of osteocalcin expression. Conversely, general inhibition of BMP signaling by noggin produced a more robust down-regulation of alkaline phosphatase activity than blocking BMP-4 or BMP-6 alone. This may be due to the ability of noggin to inhibit, to varying degrees, multiple BMP family members by blocking the receptor binding epitopes(34). In particular, noggin exhibits the greatest affinity for BMP-2 and -4 and binds BMP-6 and -7 to a lesser degree(35, 36). The differential effects of noggin, BMP-4 antibodies, and BMP-6 antibodies on alkaline phosphatase activity and osteocalcin expression suggest that multiple BMP family members are responsible for the vitamin D3-induced expression of osteoblast markers in dermal fibroblasts, with BMP-6 possibly more involved in late differentiation events. BMP-2 signaling was not specifically investigated in this study due to a lack of induction of gene expression and to cross-reaction of BMP-2 antibodies with other BMP family members. Further studies will focus on the mechanisms involved in the induction of BMP signaling by vitamin D3. Taken together, the results of this study indicate that the osteoblastic differentiation of human dermal fibroblasts by vitamin D3 is regulated, in part, through BMP signaling.
Acknowledgments
The authors would like to acknowledge the Whitaker Foundation and NIH/NIDCR grant DE014228 for funding.
References
- 1.Hirata K, Tsukazaki T, Kadowaki A, et al. Transplantation of skin fibroblasts expressing BMP-2 promotes bone repair more effectively than those expressing Runx2. Bone. 2003;32:502–512. doi: 10.1016/s8756-3282(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 2.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: In vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78:476–486. doi: 10.1002/1097-4644(20000901)78:3<476::aid-jcb12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford RB, Moalli M, Franceschi RT, et al. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441–452. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford R, Gu K, Racenis P, Krebsbach P. Early events: the in vitro conversion of BMP transduced fibroblasts to chondroblasts. Connect Tiss Res. 2003;44:117–123. [PubMed] [Google Scholar]
- 6.Shui C, Scutt AM. Mouse embryo-derived NIH3T3 fibroblasts adopt an osteoblast-like phenotype when treated with 1alpha,25-dihydroxyvitamin D3 and dexamethasone in vitro. J Cell Physiol. 2002;193:164–172. doi: 10.1002/jcp.10157. [DOI] [PubMed] [Google Scholar]
- 7.Hee CK, Jonikas MA, Nicoll SB. Influence of three-dimensional scaffold on the expression of osteogenic differentiation markers by human dermal fibroblasts. Biomaterials. 2006;27:875–884. doi: 10.1016/j.biomaterials.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci USA. 1989;86:4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozono K, Liao J, Kerner S, et al. The vitamin D-responsive element in the human osteocalcin gene. Association with a nuclear proto-oncogene enhancer. J Biol Chem. 1990;265:21881–21888. [PubMed] [Google Scholar]
- 10.Beresford JN, Joyner CJ, Devlin C, Triffitt JT. The effects of dexamethasone and 1,25-dihydroxyvitamin D3 on osteogenic differentiation of human marrow stromal cells in vitro. Arch Oral Biol. 1994;39:941–947. doi: 10.1016/0003-9969(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Leong DT, Bai HF, et al. Osteo-maturation of adipose-derived stem cells required the combined action of vitamin D3, β-glycerophosphate, and ascorbic acid. Biochem Biophys Res Comm. 2007;362:17–24. doi: 10.1016/j.bbrc.2007.07.112. [DOI] [PubMed] [Google Scholar]
- 12.Reddi AH. Regulation of cartilage and bone differentiation by bone morphogenetic proteins. Curr Opin Cell Biol. 1992;4:850–855. doi: 10.1016/0955-0674(92)90110-x. [DOI] [PubMed] [Google Scholar]
- 13.Wozney J. Overview of bone morphogenetic proteins. Spine. 2002;27:S2–S8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 14.Virdi AS, Cook LJ, Oreffo ROC, Triffitt JT. Modulation of bone morphogenetic protein-2 and bone morphogenetic protein-4 gene expression in osteoblastic cell lines. Cell Mol Biol. 1998;44:1237–1246. [PubMed] [Google Scholar]
- 15.Faucheux C, Bareille R, Amédée J, Triffitt JT. Effect of 1,25(OH)2D3 on bone morphogenetic protein-3 mRNA expression. J Cell Biochem. 1999;73:11–19. doi: 10.1002/(sici)1097-4644(19990401)73:1<11::aid-jcb2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, Nagai Y, Sladek R, et al. Expression profiling in squamous carcinoma cells reveals pleiotropic effects of vitamin D3 analog EB1089 signaling on cell proliferation, differentiation, and immune system regulation. Mol Endocrinol. 2002;16:1243–1256. doi: 10.1210/mend.16.6.0874. [DOI] [PubMed] [Google Scholar]
- 17.Swami S, Raghavachari N, Muller UR, et al. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 18.Peehl DM, Shinghal R, Nonn L, et al. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol. 2004;92:131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Wislocki A, Goodman C, et al. A novel vitamin D derivative activates bone morphogenetic protein signaling in MCF10 breast epithelial cells. Mol Pharmacol. 2006;69:1840–1848. doi: 10.1124/mol.105.022079. [DOI] [PubMed] [Google Scholar]
- 20.Lee HJ, Liu H, Goodman C, et al. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72:332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Wang TT, Tavera-Mendoza LE, Laperriere D, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 22.Lossos IS, Czerwinski DK, Wechser MA, Levy R. Optimization of quantitative real-time RT-PCR parameters for the study of lymphoid malignancies. Leukemia. 2003;17:789–795. doi: 10.1038/sj.leu.2402880. [DOI] [PubMed] [Google Scholar]
- 23.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228–38. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 24.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 25.Owen TA, Aronow MS, Barone LM, et al. Pleiotropic effects of vitamin D on osteoblast gene expression are related to the proliferative and differentiated state of the bone cell phenotype: dependency upon basal levels of gene expression, duration of exposure, and bone matrix competency in normal rat osteoblast cultures. Endocrinology. 1991;128:1496–1504. doi: 10.1210/endo-128-3-1496. [DOI] [PubMed] [Google Scholar]
- 26.Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGFBeta1) and hormones (vitamin D and glucocorticoids) J Oral Implantol. 1993;19:95–105. [PubMed] [Google Scholar]
- 27.Xiao ZS, Thomas R, Hinson TK, Quarles LD. Genomic structure and isoform expression of the mouse, rat and human Cbfa1/Osf2 transcription factor. Gene. 1998;214:187–197. doi: 10.1016/s0378-1119(98)00227-3. [DOI] [PubMed] [Google Scholar]
- 28.Drissi H, Pouliot A, Koolloos C, et al. 1,25-(OH)2-vitamin D3 suppresses the bone-related runx2/cbfa1 gene promoter. Exp Cell Res. 2002;274:323–333. doi: 10.1006/excr.2002.5474. [DOI] [PubMed] [Google Scholar]
- 29.Viereck V, Siggelkow H, Tauber S, et al. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86:348–356. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 30.Pockwinse SM, Stein JL, Lian JB, Stein GS. Developmental Stage-Specific Cellular Responses to Vitamin D and Glucocorticoids during Differentiation of the Osteoblast Phenotype: Interrelationship of Morphology and Gene Expression by in Situ Hybridization. Exp Cell Res. 1995;216:244–260. doi: 10.1006/excr.1995.1031. [DOI] [PubMed] [Google Scholar]
- 31.Lian JB, Stein GS, Canalis E, et al. Bone formation: Osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Fourth. Philadelphia: Lippencott Williams and Wilkens; 1999. pp. 14–29. [Google Scholar]
- 32.Bodine PVN, Komm BS. Tissue culture models for studies of hormone and vitamin action in bone cells. Vitam Horm. 2002;64:101–151. doi: 10.1016/s0083-6729(02)64004-x. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 34.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signaling inhibition by the cysteine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 35.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 36.Abe E. Function of BMPs and BMP antagonists in adult bone. Ann NY Acad Sci. 2006;1068:41–53. doi: 10.1196/annals.1346.007. [DOI] [PubMed] [Google Scholar]


