Abstract
TR3, also known as NGFI-B or nur77, is an immediate-early response gene and an orphan member of the steroid/thyroid/retinoid receptor superfamily. We previously reported that TR3 expression was induced by apoptotic stimuli and was required for their apoptotic effect in lung cancer cells. Here, we present evidence that TR3 was also induced by epidermal growth factor (EGF) and serum and was required for their mitogenic effect in lung cancer cells. Ectopic expression of TR3 in both H460 and Calu-6 lung cancer cell lines promoted their cell cycle progression and BrdU incorporation, while inhibition of TR3 expression by the small interfering RNA approach suppressed the mitogenic effect of EGF and serum. Analysis of TR3 mutants showed that both TR3 DNA binding and transactivation were required for its mitogenic effect. In contrast, they were dispensable for its apoptotic activity. Furthermore, confocal microscopy analysis demonstrated that TR3 functioned in the nucleus to induce cell proliferation, whereas it acted on mitochondria to induce apoptosis. In examining the signaling that regulates the mitogenic function of TR3, we observed that coexpression of constitutive-active MEKK1 inhibited TR3 transcriptional activity and TR3-induced proliferation. The inhibitory effect of MEKK1 was mediated through activation of Jun N-terminal kinase, which efficiently phosphorylated TR3, resulting in loss of its DNA binding. Together, our results demonstrate that TR3 is capable of inducing both proliferation and apoptosis in the same cells depending on the stimuli and its cellular localization.
TR3 (also known as NGFI-B and nur77) (6, 17, 44), an immediate-early response gene, is an orphan member of the steroid/thyroid/retinoid receptor superfamily, whose members mainly act as transcriptional factors to positively or negatively regulate gene expression (22, 41, 67). The role of TR3 in cell proliferation was suggested by numerous observations showing that its expression is rapidly induced by several mitogenic inducers, including serum growth factor, epidermal growth factor (EGF), and fibroblast growth factor (6, 9, 13, 17, 35, 44, 58). However, whether TR3 expression has a causal role in promoting cell proliferation remains to be illustrated. Recent evidence also indicates that the expression of TR3 is required for apoptosis. TR3 was rapidly induced by T-cell receptor signaling in immature thymocytes and T-cell hybridomas (39, 60). Overexpression of a dominant-negative TR3 protein (60) or inhibition of TR3 expression by antisense TR3 mRNA (39) inhibited T-cell-receptor-induced apoptosis, whereas constitutive expression of TR3 resulted in massive cell death (57, 64). The involvement of TR3 in the apoptotic process was also observed in cancer cells. Apoptosis of lung cancer cells was accompanied by a rapid induction of TR3, which was effectively inhibited by antisense TR3 mRNA expression (34). Rapid induction of TR3 also occurred in prostate cancer (56, 65), ovarian cancer (20), and gastric cancer (63) cells after stimulation of apoptosis by a variety of apoptosis-inducing agents. TR3 expression was also required for the apoptotic effect of Sindbis virus in NIH 3T3 cells (31).
A disturbance in the balance between cell proliferation and cell death may predispose to tumor development or progression (12, 15). Because of its involvement in regulating both processes, the abnormal expression and/or function of TR3 has been observed in cancer cells. TR3 is highly expressed in many different cancer cell lines (56, 62). Levels of TR3 expression were higher in cancerous tissue than in adjacent normal or benign prostate hypertrophic tissue (56). One member of the TR3 subfamily, Nor-1, was overexpressed in diffuse large B-cell lymphoma (54). The involvement of TR3 in cancer development is further suggested by the finding that a member of the TR3 subfamily is involved in a chromosomal translocation identified in extraskeletal myxoid chondrosarcoma (8, 27, 28). Interestingly, the receptor fusion protein is about 270-fold more active than the native receptor in transcriptionally activating a reporter containing the TR3 response element (27). This suggests that the fusion receptor may exert its oncogenic effects by acting as a transcriptional factor to regulate expression of genes involved in promoting tumor development.
The mechanism by which TR3 exerts its biological functions remains largely unknown. Similar to other members of the steroid/thyroid/retinoid receptor superfamily, it was believed that TR3 functioned as a transcriptional factor to regulate gene expression necessary to alter the cellular phenotype in response to various stimuli. Consistent with this idea, TR3 response elements (NBRE or NurRE) have been identified (49, 59). In addition, TR3 can heterodimerize with retinoid X receptor (RXR) (14, 48) and orphan receptor COUP-TF (62), both of which are known to positively or negatively regulate transactivation of many nuclear receptors, such as retinoic acid receptors, vitamin D receptor, and thyroid hormone receptor (TR) (22, 36, 41, 67). Through its interaction with RXR and COUP-TF, TR3 may alter gene expression and subsequently the growth response of cells to vitamins and hormones (66).
Recently we demonstrated that TR3, in response to apoptotic stimuli, translocates from the nucleus to the cytoplasm, where it targets mitochondria to induce cytochrome c release and apoptosis (33). The apoptotic effect of TR3 does not require its transcriptional activity or DNA binding (4, 33). Recent studies also demonstrated that another transcription factor, p53, targets mitochondria to initiate the apoptotic process (40). Mitochondrial targeting of TR3 is also responsible for apoptosis of NIH 3T3 cells by Sindbis virus (31). NGFI-B translocated from the nucleus to the cytoplasm in response to nerve growth factor (NGF) treatment in PC12 pheochromocytoma cells (23). These observations indicate that some important TR3 functions are mediated by transcription-independent mechanism(s). Cytoplasmic action appears to be important for other nuclear receptors, including glucocorticoid receptor (GR) (52), estrogen receptor (ER), and androgen receptor (25, 26, 43, 55).
Another level of regulation of TR3 activities involves phosphorylation. TR3 is heavily phosphorylated in vivo on multiple sites in the amino terminus, which is primarily responsible for its transactivation activity, whereas its carboxyl terminus is devoid of phosphorylation sites (10). Phosphorylation of TR3 at Ser350, a site within the A box downstream of the DNA-binding domain, inhibited its DNA binding and transactivation (19). Interestingly, Akt was found to be responsible for the phosphorylation (42, 47). In PC12 pheochromocytoma cells, NGFI-B is differentially phosphorylated upon treatment with NGF and membrane depolarization (16). Phosphorylation of Ser105 of NGFI-B by the TrKA/Ras/mitogen-activated protein (MAP) kinase pathway upon treatment with NGF resulted in the translocation of NGFI-B from the nucleus to the cytoplasm in PC-12 cells (23).
We previously reported that TR3 expression was rapidly induced by the retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid (AHPN), a potent apoptosis inducer, in lung cancer cells (34). In the present study, we found that TR3 was also induced by EGF and serum in the same lung cancer cell lines. Ectopic expression of TR3 in lung cancer cells stimulated their cell cycle progression and proliferation, whereas inhibition of endogenous TR3 expression suppressed proliferation induced by growth factors. We also observed that the mitogenic effect of TR3 required its DNA binding and transactivation functions, whereas both were dispensable for its apoptotic effect. In addition, we showed that the mitogenic effect of TR3 is associated with its presence in the nucleus, whereas its apoptotic effect was associated with its mitochondrial localization in the same cells. Furthermore, we found that MEKK1 strongly inhibited the transactivation and mitogenic effects of TR3 through activation of c-Jun N-terminal kinase (JNK), which in turn phosphorylated TR3, resulting in loss of its DNA binding activity.
MATERIALS AND METHODS
Cell culture.
Calu-6 lung cells were grown in minimal essential medium supplemented with 10% fetal bovine serum (FBS), CV-1 and HEK293T cells were grown in Dulbecco’s modified Eagel medium with 10% FBS, and H460 lung cancer cells were maintained in RPMI 1640 medium supplemented with 10% FBS.
Plasmid constructs.
The cloning of GFP-TR3, GFP-TR3/ΔDBD (33), and glutathione S-transferase (GST)-TR3 (62) have been described. TR3 cDNA fragments (154 to 583 and 123 to 583) were amplified by PCR using the forward primers 5′-GAA GAT CTT CAT GTG GGA TGG CTC CTT CGG CCA CTT C-3′ (for amplification of 154 to 583), 5′-GAA GAT CTT CAT GGC CCT GTC CTC CAG TGG CTC TGA C-3′ (for amplification of 123 to 583), and a common reverse primer 5′-CGG AAT TCC GGC ACC AAG TCC TCC AGC TTG AGG TAG-3′. The fragments were digested with BglII and EcoR1 and ligated into BglII- and EcoR1-digested pGFP-N2 vector (Clontech) to generate GFP-TR3/Δ153 and GFP-TR3/Δ122, respectively. TR3 cDNA (1 to 151) was amplified using 5′-GAA GAT CTT CAT GCC CTG TAT CCA AGC CCA ATA TG-3′ (forward primer) and 5′-CGG AAT TCC GGA GCT GGG GCG GCT GGA AGC TGG G-3′ (reverse primer), digested with BglII and EcoR1, and ligated into BglII- and EcoR1-digested pGFP-N2 vector or pGEX-3X vector to generate GFP-TR3-1-151 and pGEX-TR3-1-151, respectively. The construction of the reporter plasmids NurRE-tk-CAT, ERE-tk-CAT, TREpal-tk-CAT, GRE-tk-CAT, and −73-Col-CAT and the receptor expression plasmids pECE-ER, pECE-TR, and pECE-GR were described previously (33, 34, 36, 37, 38, 62). NBRE-tk-CAT was obtained by inserting two copies of the NBRE oligonucleotide (AAAGGTCA) into the BamHI site of pBLCAT2.
Confocal microscopy.
Cells were seeded onto coverslips in six-well plates overnight and then transiently transfected with green fluorescent protein (GFP) fusion expression plasmids. After 16 h, cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde. For mitochondrial staining, cells were then incubated with anti-heat shock protein 60 (Hsp60) goat immunoglobulin G (Santa Cruz Biotech) followed by anti-goat immunoglobulin G conjugated with Cy3 (Sigma). Cells were treated with 3-Cl-AHPC (MM002) (10−6 M) (68) for 3 h to study its effect on subcellular localization of endogenous TR3. Anti-TR3 antibody used for immunostaining was custom made by Abgent.
Cell proliferation assay.
Cells were seeded at 1,000 cells per well in 96-well plates and maintained in medium containing 0.5% FBS. After 24 h, they were treated with EGF or medium containing 10% FBS for 2 days. The control cells received vehicle (PBS). Viable cell number was determined by addition of 20 μl of 2-mg/ml MTS [3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]-phenozine methosulfate solution (46 μg/ml) (Promega) to each well as per the manufacturer's recommendation, and incubation was continued for 2 to 4 h at 37°C in the dark. Absorbance (490 nm) was measured on a Bio-Rad 550 microplate reader.
Apoptosis analysis.
H460 cells were transfected with GFP-TR3/ΔDBD expression vector and maintained in 0.5% FBS for 36 h. Cells were then washed with PBS, fixed with 3.7% paraformaldehyde, and stained with 50 μg of 4,6-diamidino-2-phenylindole (DAPI) (Sigma)/ml containing 100 μg of DNase-free RNase A (Boehringer Mannheim)/ml to visualize the nuclei. Stained cells were examined by fluorescence microscopy as described previously (33, 34).
Cell cycle analysis.
Cells were trypsinized and collected by centrifugation at 1,000 rpm for 3 min. Cells were then washed with PBS, fixed with 4% formaldehyde, and permeabilized with 0.05% Tween 20 (Sigma). The fixed cells were then collected by centrifugation at 2,000 rpm (Eppendorf 5804R) for 5 min, and the cell pellets were washed twice with PBS and then resuspended in PBS containing 50 μg of propidium iodide (Sigma)/ml and 100 μg of DNase-free RNase A (Boehringer Mannheim)/ml. The cell suspension was incubated for 30 min at 37°C, protected against light, and analyzed using the FACScater-plus flow cytometer (Becton Dickinson) (24).
BrdU analysis.
Cells were incubated with 5-bromo-2′-deoxyuridine (5-BrdU) (20 μM) (Sigma) for 2 h before harvesting. Following trypsinization and two PBS washes, cells were fixed with 4% paraformaldehyde. After a 20-min incubation at room temperature, 0.1% saponin (Sigma) was added to the cell suspension, and the incubation was continued for another 10 min. The cells were then centrifuged, washed twice with PBS containing 0.1% saponin, and resuspended in PBS containing 30 μg of DNase I (Roche Diagnostics). After a 1-h incubation with either an anti-BrdU fluorescent antibody or an isotope control antibody (Pharmingen), cells were given a final PBS wash before being analyzed by the FACScater-Plus flow cytometer (Becton Dickinson).
Antibodies and Western blotting.
Cells were lysed in 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100 containing protease inhibitors, phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin, and sonicated on ice. Alternatively, cells were directly lysed on the plates with warm 2× Laemmli sample buffer, immediately after complete removal of medium from the cells. Cell lysates were then collected by scraping and sonicated on ice. Equal amounts of lysates (50 μg) or equal volumes (when cell lysates were made by direct addition of sample buffer) of cell lysates were boiled in 2× Laemmli sample buffer, resolved by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred onto Immobilon-P transfer membranes (Millipore). After transfer, the membranes were blocked with 5% nonfat milk in TBST (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20) and then incubated with primary antibody in TBST. The membranes were washed three times with TBST and then incubated for 1 h at room temperature in TBST containing horseradish peroxidase-linked anti-rabbit immunoglobulin (Sigma). After three washes in TBST, immunoreactive products were detected by chemiluminescence using an enhanced chemiluminescence system (ECL; Amersham). Anti-TR3 antibody used for Western blotting was obtained from Geneka (now Active Motif). Anti-α-tubulin was obtained from Sigma Biochemicals. Anti-GFP antibody was obtained from Santa Cruz Biotechnology.
Transient-transfection assay.
Cells (105) were seeded in 24-well culture plates. A modified calcium phosphate precipitation procedure was used for transient transfection as described elsewhere (36). Typically, 200 ng of reporter plasmid, 100 ng of β-galactosidase expression vector (pCH 110; Pharmacia), various amounts of receptor expression vector, and vector expressing either MEKK1-DA, MEKK1-DN, or JNK were mixed with carrier DNA (pBluescript) to give 1.0 μg of total DNA per well. Chloramphenicol acetyltransferase (CAT) activity was normalized for transfection efficiency on the basis of cotransfected β-galactosidase (β-Gal) gene activity. For cell cycle and Brdu incorporation analysis, cells were seeded in 90-mm-diameter dishes and transfected.
siRNA transfections.
Small interfering RNAs (siRNAs) used in these experiments were from Dharmacon Research Inc. The following siRNA sequences were used: TR3 siRNA, 5′-CAG UCC AGC CAU GCU CCU C dTdT-3′; scrambled siRNA, 5′-GCG CGC TTT GTA GGA TTC G dTdT-3′. A 10-μl aliquot of 20 μM siRNA/well was transfected into cells in six-well plates using Oligofectamine reagent (Invitrogen) as per the manufacturer's recommendations.
Bacterial expression of proteins.
A single colony of Escherichia coli (BL21) transformed with pGEX, pGEX-TR3, or pGEX-TR3-1-151 was grown in 3 ml of Luria-Bertani medium at 37°C overnight, diluted to 200 ml, and grown at 37°C until the optical density at 600 nm reached 0.8 to 1. Isopropyl-β-d-thiogalactopyranoside (1 mM) was added, and the bacterial culture was grown at room temperature for 3 h. The bacteria were harvested, resuspended in PBS containing a protease inhibitor cocktail (Sigma), and lysed by sonication on ice. The lysate was centrifuged at 10,000 × g for 30 min to remove cell debris. The supernatant was incubated with glutathione Sepharose 4B beads overnight (Pharmacia). The beads were washed three times with PBS containing protease inhibitors and eluted by reduced glutathione. GST-c-Jun was commercially supplied by Stratagene.
In vitro phosphorylation assay.
Bacterially expressed and purified GST-c-Jun or GST-TR3 protein was incubated with 2 μCi of [γ-32P]ATP (Amersham) and 0.4 μg of JNK (Stratagene) in JNK reaction buffer (25 μM HEPES [pH 7.5], 10 μM magnesium acetate, 50 μM ATP) at 30°C for 30 min and electrophoresed on a 10% polyacrylamide gel. The gel was dried and exposed to X-ray film.
Gel retardation assays.
For the protein-DNA binding assay, bacterially expressed and purified TR3 protein (approximately 100 μg) or TR3 protein subjected to in vitro phosphorylation by JNK was incubated with the 32P-labeled NurRE oligonucleotides (33) in a 20-μl reaction mixture containing 10 mM HEPES buffer (pH 7.9), 50 mM KCl, 1 mM dithiothreitol, 2.5 mM MgCl2, 10% glycerol, and 1 μg of poly(dI-dC) at 25°C for 20 min. Bovine serum albumin was used to maintain equal protein concentrations in all reaction mixtures. Each reaction mixture was then loaded onto a 5% nondenaturing polyacrylamide gel containing 0.5% TBE (1% TBE contains 0.089 M Tris-borate, 0.088 M boric acid, and 0.002 M EDTA). The gel was dried and exposed to X-ray film.
RESULTS
TR3 is induced by serum and EGF in lung cancer cells.
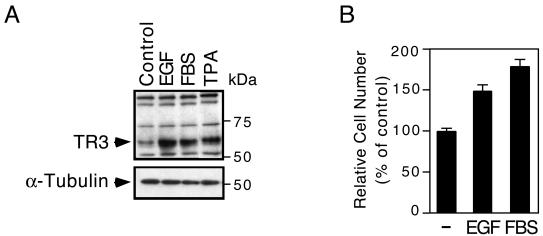
TR3 expression can be induced by both apoptotic and mitogenic agents (6, 9, 13, 17, 35, 44, 58). We previously reported that TR3 expression was induced by the apoptosis-inducing retinoid AHPN in H460 lung cancer cells (34). Here, we examined whether TR3 expression was also induced by mitogenic factors in these cells. H460 cells grown in the absence of serum were treated with EGF or serum. Cell extracts were then prepared and analyzed for TR3 expression by Western blotting. Treatment with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate, which is a strong inducer of TR3 expression (33), was used for comparison. TR3 was expressed at very low levels when cells were cultured in serum-free medium. However, higher levels of TR3 were detected when cells were cultured in medium containing either serum or EGF (Fig. 1A), indicating that both serum and EGF induce TR3 expression. To study the mitogenic effect of serum and EGF in lung cancer cells, we examined the growth of H460 cells cultured in serum or EGF-containing medium. As shown in Fig. 1B, both serum and EGF strongly promoted the growth of H460 cells. Thus, TR3 expression induced by mitogenic factors is associated with the growth of H460 lung cancer cells.
FIG. 1.
Induction of TR3 by serum and EGF. (A) H460 cells were serum starved for 16 h, replaced with medium containing 10% FBS or treated EGF (200 ng/ml) for 3 h, and then analyzed for TR3 expression by immunoblotting. α-Tubulin expression served as a control for similar loading of proteins in each lane. One of three similar experiments is shown. (B) Effect of EGF and serum on lung cancer cell proliferation. H460 cells were seeded (1,000 cells/well) in 96-well plates and maintained in serum-free medium for 24 h. The cells were then grown in serum-free medium or medium containing 10% FBS or treated with EGF (200 ng/ml) for 48 h. Viable cell numbers in quadruplicate were then determined by the MTS assay. Cells maintained in serum-free medium were set to 100%. The increase in cell numbers after EGF and FBS stimulation was normalized relative to cell numbers maintained in serum-free medium. The bars represent averages ± mean from two experiments.
Mitogenic effects of TR3.
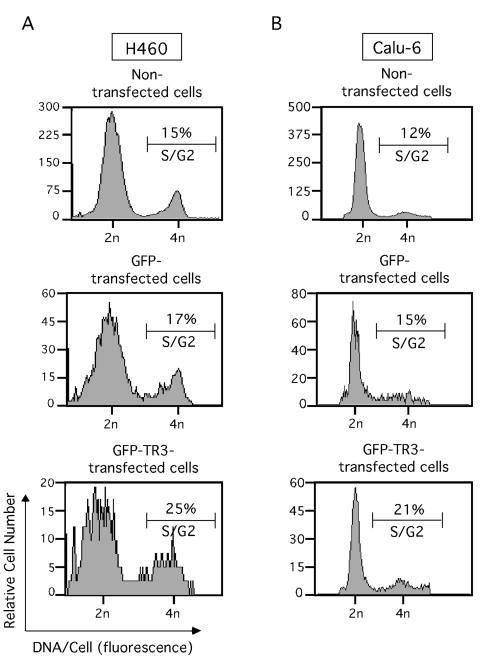
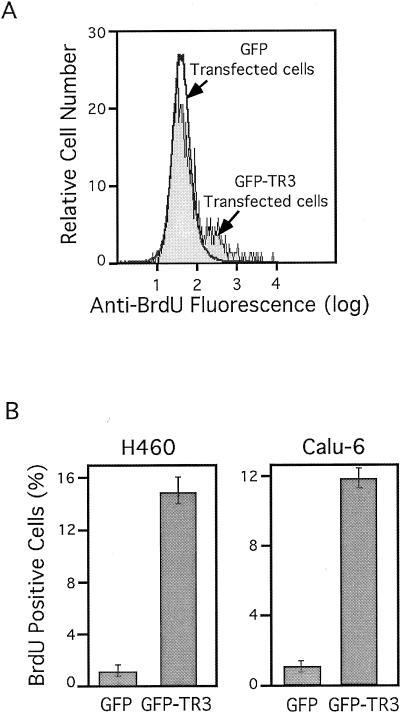
The above-described results suggested that TR3 expression might mediate the mitogenic effect of growth factors in lung cancer cells. We then tested whether ectopic expression of TR3 alone would confer proliferation of lung cancer cells. We transiently transfected an expression vector containing TR3 fused with GFP into both H460 and Calu-6 lung cancer cells. Fusion of TR3 with GFP allowed the identification of TR3-transfected cells by flow cytometry. As a control, cells were transfected with the GFP vector. TR3-transfected and nontransfected cells were identified by flow cytometry, and their cell cycle progression was determined (Fig. 2). Expression of GFP-TR3 in both H460 and Calu-6 cells resulted in enhanced S/G2-phase population. Fifteen percent of the nontransfected H460 cells were in S/G2 phase, which was increased to 25% upon GFP-TR3 expression. The effect of GFP-TR3 on induction of cell cycle progression was specific to TR3 expression, because expression of GFP control protein did not show a significant effect on the S/G2 cell population (Fig. 2A). Similarly, expression of GFP-TR3 in Calu-6 lung cancer cells led to a similar increase in the S/G2 cell population from 12 to 21% (Fig. 2B). These results demonstrated that the expression of TR3 promoted cell cycle progression in both H460 and Calu-6 lung cancer cells. We also confirmed the mitogenic effect of TR3 by BrdU labeling. Again, the GFP control vector or GFP-TR3 expression vector was transfected into lung cancer cells. GFP- or GFP-TR3-transfected cells were identified from nontransfected cells by flow cytometry, and both populations of cells were analyzed for BrdU incorporation (Fig. 3A). GFP-TR3-transfected H460 and Calu-6 cells exhibited much stronger BrdU incorporation (14.5 and 12%, respectively) than GFP-transfected cells, which when maintained in low serum displayed about 1% BrdU-positive cells (Fig. 3B). Together, these data demonstrate that ectopic TR3 expression promotes the cell cycle progression and proliferation of lung cancer cells.
FIG. 2.
TR3 expression promotes cell cycle progression in lung cancer cells. GFP-TR3 or GFP control expression vector (5 μg) was transfected into H460 (A) or Calu-6 (B) lung cancer cells which were seeded in 90-mm-diameter dishes and maintained in medium containing 0.5% FBS. The transfected and GFP-expressing subpopulation of cells was identified by the high level of green fluorescence compared to nontransfected cells using flow cytometry (24). The cell cycle distribution of the transfected cells was then determined by flow cytometry after 45 h of transfection. One of three similar experiments is shown.
FIG. 3.
TR3 expression stimulates cell proliferation. H460 or Calu-6 cells were seeded in 90-mm-diameter dishes, transfected with GFP-TR3 or GFP control expression vector (5 μg), and maintained in medium containing 0.5% FBS. Forty-five hours after transfection, cells were maintained in BrdU containing medium for 2 h. Transfected and nontransfected cells were identified by flow cytometry, and BrdU-positive cells in each population were identified by immunostaining, using anti-BrdU antibody conjugated to phycoerythrin (Pharmingen). (A) BrdU immunofluorescence of GFP-transfected cells is overlaid with that from GFP-TR3-transfected cells. (B) The bars represent increases in BrdU cells after GFP or GFP-TR3 transfection compared to the nontransfected cells from the same culture dish. The means ± standard deviations from three experiments are shown.
Requirement of TR3 for serum- or EGF-induced mitogenic effects in lung cancer cells.
To determine whether endogenous TR3 plays a role in serum- or EGF-induced mitogenic effects, we used the siRNA approach to inhibit the expression of endogenous TR3 and determined its effect on the mitogenic function of serum and EGF. Transfection of siRNA specific to TR3 was able to inhibit the expression of endogenous TR3 in H460 cells, compared to the transfection of control scrambled siRNA (Fig. 4A). A high-molecular-weight nonspecific band detected by anti-TR3 antibody and the expression of α-tubulin served as controls for specific inhibition of TR3 expression. We determined the mitogenic effects of EGF and serum in H460 cells transfected with either TR3 siRNA or the control siRNA. We observed a 45 or 31% decrease in the number of cells in the TR3 siRNA-transfected population (compared to the control scrambled siRNA transfected cells) when cells were cultured for 48 h in presence of EGF or serum, respectively (Fig. 4B). The effect of TR3 siRNA on cell proliferation was also studied by BrdU labeling. Fifteen and 12% of cells were BrdU positive after FBS and EGF stimulation of control cells, respectively. However, in TR3 siRNA-transfected cells, the BrdU-positive cells were reduced 5 and 2% after FBS and EGF stimulation, respectively. TR3 siRNA also suppressed the growth of H460 cells in the absence of EGF or FBS. This observation is likely due to the inhibition of mitogenic and survival function of endogenous TR3. Similar results were also obtained in H292 lung cancer cells (data not shown). Thus, TR3 plays a causal role in the proliferation of lung cancer cells in response to EGF and serum treatment.
FIG. 4.
TR3 is required for EGF- or serum-induced proliferation of lung cancer cells. (A) Suppression of endogenous TR3 expression by siRNA. H460 cells seeded in six-well plates were transfected with control scrambled or TR3 siRNA for 48 h. Cells were then treated with EGF or 10% FBS as described for Fig. 1A. Cell extracts were prepared and analyzed for TR3 expression by Western blotting. Two nonspecific bands around 105 kDa detected by anti-TR3 antibody and α-tubulin expression served as controls for similar loading of proteins in each lane. One out of four similar experiments is shown. (B) Suppression of proliferation by TR3 siRNA in H460 lung cancer cells. H460 cells were transfected with control scrambled or TR3 siRNA for 48 h in serum-free medium in 96-well plates and stimulated with EGF (200 ng/ml) or 10% FBS for 48 h or left untreated. Viable cell numbers in quadruplicate were then determined by the MTS assay. Absorbances (490 nM) representing the viable cells were plotted for nonstimulated cells maintained in serum-free medium (Control) and cells treated with EGF or FBS. The bars represent means ± standard deviation from four independent experiments. Similar results were also obtained by physically counting viable cells using trypan blue dye exclusion after transfection of H460 cells with control or TR3 siRNA in 24-well plates (n = 4).
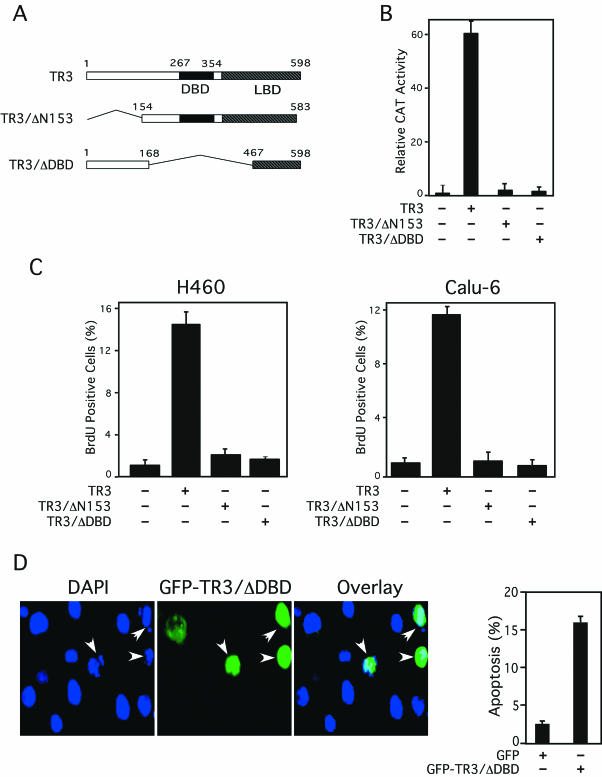
DNA binding and transactivation of TR3 are required for its mitogenic effect.
We previously showed that DNA-binding and transactivation of TR3 were not required for its apoptotic effect in LNCaP prostate cancer cells (33). To study whether DNA-binding and transactivation were required for the mitogenic effect of TR3 in lung cancer cells, TR3 mutants (Fig. 5A) lacking either the DNA-binding domain (DBD) (TR3/ΔDBD) or its N terminus (TR3/ΔN153), which contains the major transactivation function (1, 2, 10), were analyzed. The mutants were initially evaluated for their transactivation using the NurRE-tk-CAT reporter that contains a TR3 homodimer-binding site (NurRE) (49) fused with the tk promoter (Fig. 5B). Transfection of the TR3 expression vector strongly induced transcription of the reporter gene. As expected, cotransfection of TR3/ΔDBD did not show any induction of reporter gene activity. Deletion of the N-terminal region also impaired its transactivation function, since cotransfection of TR3/ΔN153 did not result in any induction of reporter gene transcription. TR3/ΔDBD and Tr3/ΔN153 were functionally expressed in the cells as they acted dominant negatively on TR3-dependent NurRE reporter gene activity (data not shown). The TR3 mutants fused to the GFP were then transfected into H460 and Calu-6 cells to determine their effect on cell proliferation. BrdU labeling and immunostaining of transfected cells showed that expression of both TR3 mutants failed to increase BrdU incorporation, while cells transfected with GFP-TR3 displayed strong BrdU incorporation (Fig. 5C). Thus, DNA binding and transactivation by TR3 are required for its mitogenic effect in lung cancer cells.
FIG. 5.
DNA binding and transactivation of TR3 are required for its mitogenic effect. (A) Schematic representation of TR3 mutants. (B) Transcriptional activity of TR3 mutants. (NurRE)2-tk-CAT (100 ng) and β-Gal gene expression vector (50 ng) were transiently transfected into CV-1 cells in 24-well plates with or without the expression vector for TR3 or its mutants (25 ng). CAT activity was determined and normalized relative to β-Gal activity. The bars represent averages ± mean from two experiments. (C) TR3 mutants with a deletion of the DNA-binding domain or the transactivation domain fail to promote lung cancer cell proliferation. H460 or Calu-6 cells were transfected with expression vectors for GFP-TR3 or its mutants. Transfected and nontransfected cells were identified by flow cytometry, and the number of BrdU-positive cells in each population was determined by immunostaining as described for Fig. 3. The bars represent the averages ± mean from two experiments. (D) TR3 with a deletion of its DNA-binding domain is capable of inducing apoptosis. The GFP-TR3/ΔDBD expression vector was transiently transfected into H460 cells. Nuclei were stained by DAPI 36 h after transfection. GFP-TR3/ΔDBD expression and nuclear morphology were visualized by fluorescence microscopy, and the two images were overlaid to show the effect of TR3/ΔDBD expression on nuclear condensation and fragmentation (shown by arrowheads). Apoptotic cells were scored by examining 300 transfected cells. The bars represent averages ± mean from two experiments.
To determine whether DNA binding and transactivation of TR3 were also required for its death activity in lung cancer cells, we studied the apoptotic effect of TR3/ΔDBD. TR3/ΔDBD was transiently transfected into H460 cells, and apoptosis of transfected cells was determined by DAPI staining. GFP-TR3/ΔDBD-expressing H460 cells, but not nonexpressing cells, displayed extensive nuclear fragmentation (Fig. 5D). Thus, TR3 retained its apoptotic activity in lung cancer cells despite the deletion of its DBD. Expression of TR3/ΔN153 in H460 cells did not exhibit any apoptotic effect, although it effectively suppressed TR3-induced transcription of the NurRE-tk-CAT reporter (data not shown). Together, our results demonstrate that the DNA-binding and transactivation functions of TR3 are required for its mitogenic effects, whereas they are dispensable for its apoptotic activity in the same cells.
Subcellular localization of mitogenic and apoptotic TR3.
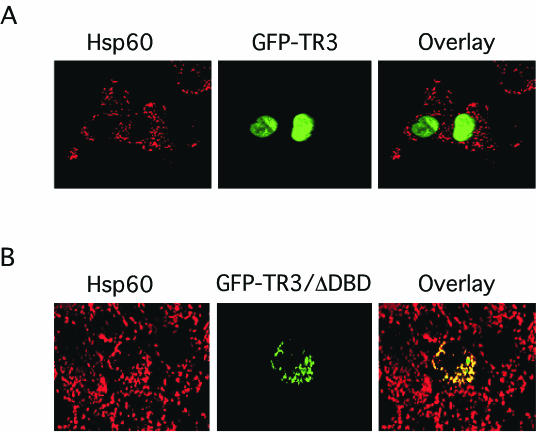
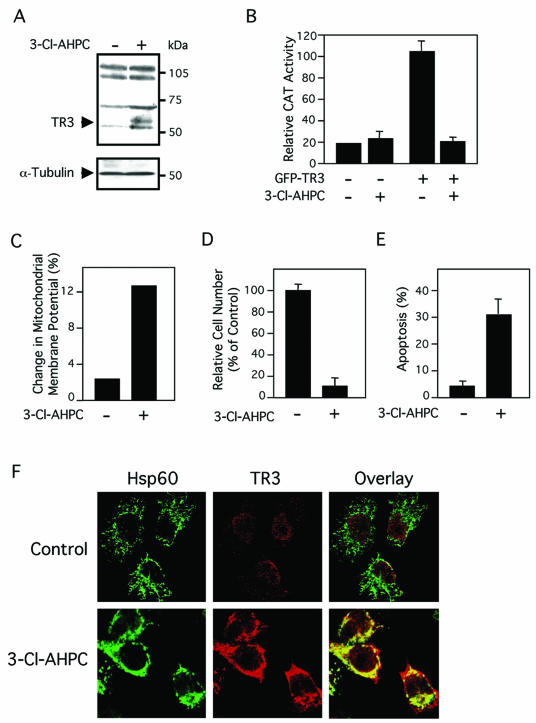
We previously reported that subcellular localization of TR3 determines its biological function in prostate cancer cells (33, 66). We next examined whether TR3 resided in the nucleus to confer its DNA binding, transactivation, and mitogenic effect in lung cancer cells. Subcellular localization of GFP-TR3, which induced proliferation of H460 cells (Fig. 2 and 3), was examined by confocal microscopy. Immunostaining of heat shock protein 60 (Hsp60), a mitochondrion-specific protein, was used as a “cytoplasmic” control. Our results demonstrated that GFP-TR3 was confined in the nucleus (Fig. 6A), suggesting that it exerts mitogenic effect via its nuclear action. We previously showed that TR3/ΔDBD acted on mitochondria to induce apoptosis of LNCaP prostate cancer cells (33). Since TR3/ΔDBD also induced apoptosis of H460 cells (Fig. 5D), we then investigated whether it also resided on the mitochondria in these cells. In Fig. 6B, we show that the distribution of transfected GFP-TR3/ΔDBD overlapped extensively with that of Hsp60, indicating the association of TR3/ΔDBD with mitochondria in H460 cells. We also examined whether apoptotic stimulus, such as an AHPN analog, could induce TR3 mitochondrial localization, as it does in LNCaP cells (33). Expression of TR3 was induced by the AHPN analog 3-Cl-AHPC (also called MM002) (68) (Fig. 7A), consistent with our previous observation (34). However, treatment of H460 cells with 3-Cl-AHPC did not activate the NurRE-tk-CAT reporter despite its induction of TR3 expression (Fig. 7B). In contrast, transfection of the GFP-TR3 expression vector into H460 cells strongly induced transcription of the reporter. Interestingly, transcriptional activity of GFP-TR3 was almost completely suppressed when H460 cells were treated with 3-Cl-AHPC (Fig. 7B). Similar to other AHPN analogs, 3-Cl-AHPC strongly induced mitochondrial membrane potential change (Fig. 7C), growth inhibition (Fig. 7D), and apoptosis (Fig. 7E) in H460 cells. These data suggest that transactivation of TR3 is not involved in apoptosis of H460 cells induced by 3-Cl-AHPC. To study the possible mechanism by which 3-Cl-AHPC induced apoptosis, we examined the subcellular localization of TR3 in H460 cells in the absence or presence of 3-Cl-AHPC. Although basal TR3 that was expressed in H460 cells resided in the nucleus (Fig. 7F), TR3 was found mainly in the cytoplasm and associated with mitochondria when cells were treated with 3-Cl-AHPC (Fig. 7F). Thus, TR3 exerts its mitogenic effect in the nucleus, consistent with our observations that both its DNA-binding and transactivation functions are required. In contrast, TR3 acts on mitochondria to initiate apoptosis in lung cancer cells, as observed previously in prostate cancer cells (33).
FIG. 6.
Cellular localization of GFP-TR3 and GFP-TR3/ΔDBD. The GFP-TR3 (A) or GFP-TR3/ΔDBD (B) expression vector was transiently transfected into H460 cells seeded onto coverslips. After 20 h, cells were immunostained with anti-Hsp60 antibody followed by Cy3-conjugated secondary antibody to detect mitochondria. GFP-TR3 or GFP-TR3/ΔDBD and mitochondria (Hsp60) were visualized using confocal microscopy, and the two images were overlaid (Overlay). About 80% of the transfected cells showed the pattern presented.
FIG. 7.
3-Cl-AHPC induces H460 cell apoptosis and TR3 mitochondrial targeting. (A) Induction of TR3 expression by AHPN analog 3-Cl-AHPC. H460 cells were treated with 3-Cl-AHPC (10−6 M) or not treated for 3 h and analyzed for TR3 expression by immunoblotting. (B) Regulation of transactivation activity of TR3 by 3-Cl-AHPC. (NurRE)2-tk-CAT (400 ng) and the β-Gal gene expression vector (100 ng) were transiently transfected into H460 cells in six-well plates by Lipofectamine Plus reagent (Invitrogen) together with or without the GFP-TR3 expression vector (50 ng). Cells were then treated with 3-Cl-AHPC (10−6 M) for 24 h. Reporter gene (CAT) activity was determined and normalized relative to the cotransfected β-Gal gene activity. The bars represent the averages ± mean from two experiments. (C) 3-Cl-AHPC disrupts mitochondrial membrane potential in H460 cells. H460 cells were treated with 10−6 M 3-Cl-AHPC or not treated for 18 h. Cells were then incubated with Rh123 for 30 min and analyzed by flow cytometry. Cells fluorescing within the range of Rh123 were considered depolarized. (D) 3-Cl-AHPC inhibits growth of H460 cells. H460 cells were treated with 10−6 M 3-Cl-AHPC for 48 h. Viable cell numbers in quadruplicate were determined by the 3-(4,5-dimethythiazol-2-yl)-2-5-diphenyl-tetrazolium assay. (E) 3-Cl-AHPC induces apoptosis in H460 cells. H460 cells were treated with 10−6 M 3-Cl-AHPC or not treated for 30 h. Nuclei were stained by DAPI. Apoptotic cells displaying nuclear fragmentation and/or condensation were scored by examining 600 cells. The bars represent the averages ± mean from two experiments. (F) 3-Cl-AHPC induces TR3 mitochondrial localization. H460 cells were treated with 10−6 M 3-Cl-AHPC or not treated (control) for 3 h, fixed, and then immunostained with mouse monoclonal anti-TR3 antibody followed by Cy3-conjugated secondary antibody or with anti-Hsp60 antibody followed by fluorescein isothiocyanate-conjugated secondary antibody. TR3 and Hsp60 were visualized using confocal microscopy, and images were overlaid (Overlay) as indicated. About 50% of the cells showed mitochondrial localization of endogenous TR3 after 3-Cl-AHPC treatment (n = 2).
MEKK1 downregulates TR3 transactivation.
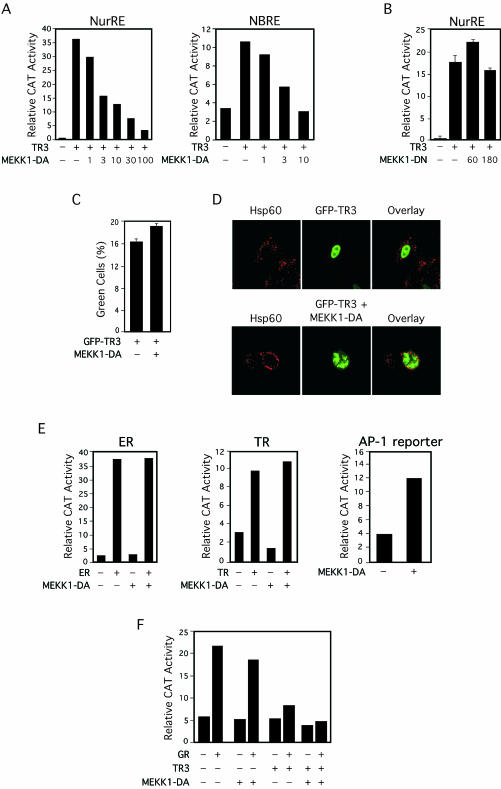
Phosphorylation of TR3 plays a role in modulating its transactivation function (10, 19). Since MEKK1 regulates activity of several nuclear receptors (3, 21, 53), we studied whether MEKK1 also modulated TR3 transactivation. A constitutively active MEKK1 (MEKK1-DA) expression vector (5) was cotransfected with TR3 expression vector into CV-1 cells. The effect of MEKK1-DA expression on TR3 transactivation was then evaluated by a reporter gene assay using either the reporter NurRE-tk-CAT or the reporter NBRE-tk-CAT that contains the TR3 monomer-binding site (59) fused with the tk promoter (Fig. 8A). Expression of TR3 strongly activated the transcription of both reporters. Expression of MEKK1-DA did not show any effect on tk promoter activity. However, upon cotransfection of the MEKK1-DA expression vector, the TR3-induced transcription of both reporters was suppressed in a MEKK1-DA concentration-dependent manner. Cotransfection of 100 ng of MEKK1-DA expression vector resulted in almost complete repression of TR3-induced NurRE-tk-CAT reporter transcription. In contrast, expression of a kinase-deficient dominant-negative MEKK1 mutant (MEKK1-DN) (5) did not show any effect on TR3 transactivation, suggesting that the kinase activity of MEKK1 was required (Fig. 8B). Inhibition of TR3 transactivation by MEKK1-DA expression was not due to inhibition of expression of TR3, because GFP-TR3 was equally expressed in the absence or presence of coexpressed MEKK1-DA (Fig. 8C). Confocal microscopy analysis revealed that the nuclear localization of GFP-TR3 was not apparently altered by MEKK1-DA coexpression (Fig. 8D), excluding the possibility that the inhibition of TR3 transactivation was due to relocalization of TR3 from the nucleus.
FIG. 8.
Effect of MEKK1 on transcriptional activity of TR3. (A) Inhibition of TR3 transcriptional activity by MEKK1. TR3 expression vector (25 ng), β-Gal expression vector (50 ng), and the indicated reporter gene (200 ng) were cotransfected into CV-1 cells in 24-well plates with or without the indicated amount (expressed in nanograms) of MEKK1-DA expression vector (5). Reporter gene activity was determined and normalized relative to cotransfected β-Gal gene activity. One of six (left panel) or three (right panel) similar experiments is shown. (B) TR3 transcriptional activity is unaffected by kinase-deficient MEKK1. TR3 expression vector (25 ng), (NurRE)2-tk-CAT (100 ng), β-Gal expression vector (50 ng) with or without the indicated amount (in nanograms) of MEKK1-DN expression vector (5) was transfected, and reporter gene activity was measured as described for panel A. The bars are averages ± mean from two experiments. (C) TR3 expression is not altered by MEKK1.GFP-TR3 expression vector (5 μg) was transfected into H460 cells with or without MEKK1-DA (5 μg) expression vector. Forty-five hours after transfection, amounts of GFP-TR3-expressing cells (percent green cells) were determined by flow cytometry. Similar results were observed in Calu-6 cells. The bars represent the averages ± mean from two experiments. (D) MEKK1 expression does not alter TR3 nuclear localization. GFP-TR3 alone (1.0 μg) or together with MEKK1-DA (1.0 μg) was transiently expressed in H460 cells. Fourteen hours later, cells were immunostained with anti-Hsp60 antibody followed by Cy3-conjugated secondary antibody to detect mitochondria. GFP-TR3 was visualized using confocal microscopy, and the two images were overlaid (Overlay). (E) Effect of MEKK1 expression on transactivation of other nuclear receptors and AP-1. The expression vector for ER or TR (25 ng) and the corresponding reporter gene (ERE-tk-CAT or TREpal-tk-CAT, respectively) were cotransfected into CV-1 cells with or without MEKK1-DA expression vector (30 ng). Twenty hours after transfection, cells were treated with estradiol (10−8 M) or T3 (10−7 M), and CAT activity was determined and normalized relative to cotransfected β-Gal gene activity. For measuring the effect of MEKK1 on AP-1 activity, the −73Col-CAT (250 ng) reporter (37) was transfected with or without MEKK1-DA expression vector (30 ng). Reporter gene activity was measured as described earlier. One of three similar experiments is shown. (F) MEKK1 does not affect the transrepression activity of TR3. TR3 expression vector (20 ng) with or without MEKK1-DA expression vector (30 ng) and/or GR expression vector (20 ng) was cotransfected into CV-1 cells with the GRE-tk-CAT reporter vector (50). Twenty hours after transfection, cells were treated with Dex (10−7 M), and CAT activity was determined and normalized relative to cotransfected β-Gal gene activity. One of two similar experiments is shown.
To determine whether the inhibition of TR3 transactivation by MEKK1-DA was specific to TR3, we examined the effect of MEKK1-DA on transactivation of ER and TR, using reporters containing their respective response elements fused to the tk promoter. As shown in Fig. 8E, cotransfection of the MEKK1-DA expression vector did not interfere with transcription induced by ER and TR. MEKK1 is a known activator of JNK, which in turn activates AP-1 (46). As expected, cotransfection of the MEKK1-DA expression vector strongly enhanced transcription of an AP-1 reporter gene, −73Col-CAT (Fig. 8E).
TR3 is known to inhibit transactivation of GR through their heterodimerization (50). To further characterize the effect of MEKK1-DA on TR3 activity, we examined whether expression of MEKK1-DA abolished transrepression of GR activity by TR3. Cotransfection of GR expression vector induced transcription of a GR-dependent reporter gene (GRE-tk-CAT) in cells treated with the GR ligand, dexamethasone (Dex) (Fig. 8F). GR-induced reporter transcription was repressed when TR3 expression vector was cotransfected, consistent with a previous observation (50). Cotransfection of the MEKK1-DA expression vector alone did not significantly regulate GR activity in the absence or presence of Dex. When it was cotransfected with TR3, TR3-mediated inhibition of GR activity was not prevented. Thus, MEKK1-DA specifically inhibits the transactivation function of TR3 but not its transrepression activity.
MEKK1 suppresses the mitogenic effect of TR3.
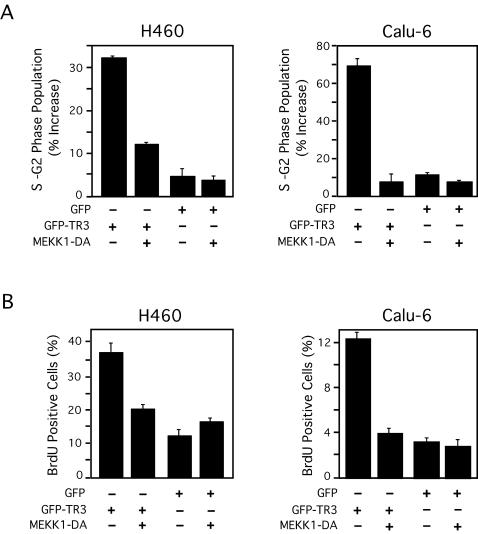
Since transactivation of TR3 was required for its mitogenic effect and MEKK1-DA inhibited TR3 transactivation, we examined the effect of MEKK1-DA expression on TR3-induced cell cycle progression in H460 and Calu-6 cells. As shown in Fig. 9A, the TR3-mediated increase in S/G2 cell population was largely abolished when MEKK1-DA was coexpressed, while the expression of MEKK1-DA alone did not have any effect on the cell cycle progression. In addition, the increase in incorporation of BrdU that resulted from TR3 expression was abrogated when cells were cotransfected with the MEKK1-DA expression vector (Fig. 9B). These data demonstrate that down-regulation of TR3 transactivation by MEKK1-DA is associated with inhibition of the mitogenic effect of TR3.
FIG. 9.
Effect of MEKK1 on TR3 mitogenic activity. (A) GFP-TR3 or GFP control expression vector (5 μg) and/or MEKK1-DA expression vector (5 μg) were transfected into H460 or Calu-6 cells, which were seeded in 9-mm-diameter dishes and then maintained in medium containing 0.5% FBS. The transfected and GFP-expressing subpopulations of cells were identified by their high level of green fluorescence compared to nontransfected cells using flow cytometry (24). The cell cycle distribution of the transfected cells was then determined as described for Fig. 2. The increase in S-G2 phase population was calculated by comparing the S-G2-phase cells from transfected cells to those from nontransfected cells in the same culture dish. (B) BrdU-positive cells were identified by immunostaining as described for Fig. 3, except that H460 cells were pulsed with BrdU for 6 h. The bars in panels A and B represent the averages ± mean from two experiments.
Activation of JNK is responsible for the effect of MEKK1.
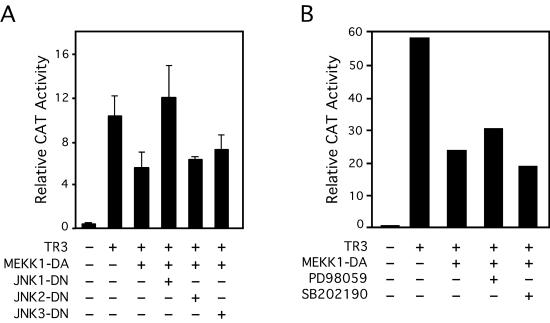
Because MEKK1 is a potent activator of JNK (46), we next examined whether activation of JNK mediated the MEKK1-induced inhibition of TR3 transactivation by analyzing the effect of dominant-negative JNK on MEKK1 activity (Fig. 10A). JNK consists of three different isoforms, JNK1, JNK2, and JNK3 (45). We therefore used dominant-negative mutants of the isoforms to determine the involvement of each isoform. Cotransfection of MEKK1-DA strongly inhibited TR3-induced NurRE-tk-CAT activity. When the expression vector for JNK1-DN was cotransfected, the inhibitory effect of MEKK1-DA on TR3-dependent transactivation was largely relieved. In contrast, cotransfection of JNK2-DN or JNK3-DN did not show any effect on MEKK1-DA activity. MEKK1 is also known to induce the activity of Erk and p38 (46). Therefore, we examined whether activation of Erk and p38 plays a role in mediating the effect of MEKK1, by using PD98059, a specific inhibitor of the Erk pathway, and SB202190, a p38 inhibitor. Treatment of cells with either inhibitor did not influence the inhibitory effect of MEKK1-DA on TR3 transactivation (Fig. 10B). Together, these results demonstrate that the inhibition of the TR3 transactivation function by MEKK1 is mainly mediated by the activation of JNK1 by MEKK1.
FIG. 10.
Activation of JNK, but not Erk or p38, mediates the effect of MEKK1 on TR3 transcriptional activity. (A) Effect of JNK dominant-negative mutants on MEKK-1-induced inhibition of TR3 transactivation. The NurRE-tk-CAT reporter vector was cotransfected with TR3 (25 ng) and/or MEKK1-DA (10 ng) expression vector(s) into CV-1 cells together with or without the indicated JNK dominant-negative (DN) mutant expression vectors (50 ng). CAT activity was determined and normalized to cotransfected β-Gal gene activity. The bars represent means ± standard deviations from three experiments. (B) Effect of a MEK1 or p38 inhibitor on MEKK1-induced inhibition of TR3 transactivation. The NurRE-tk-CAT reporter vector(s) was cotransfected with TR3 (25 ng) and/or MEKK1-DA (10 ng) expression vector into CV-1 cells. Cells were treated with or without either PD98059 (20 μM) or SB202190 (20 μM). CAT activity was determined and normalized relative to cotransfected β-Gal gene activity. One of two similar experiments is shown.
JNK phosphorylates the N terminus of TR3 and inhibits binding of TR3 to DNA.
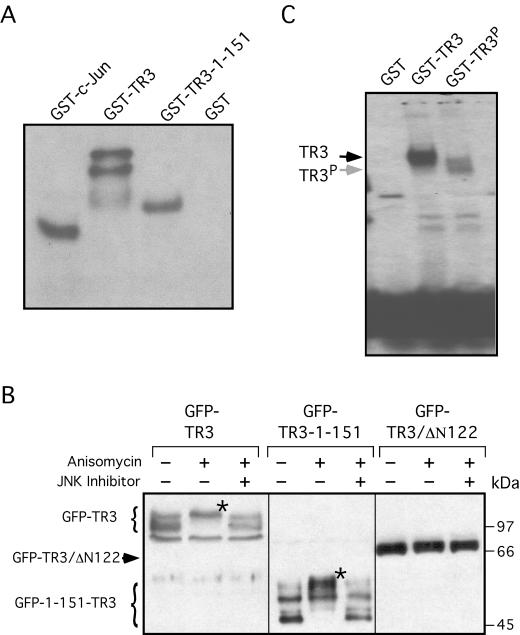
To study how JNK regulated TR3 activity, we examined whether JNK phosphorylated TR3. Bacterially expressed GST-TR3 fusion protein was incubated with JNK in vitro. As shown in Fig. 11A, GST-TR3 was strongly phosphorylated by JNK, whereas the similarly prepared GST control protein was not. For comparison, GST-c-Jun was found to be similarly phosphorylated by JNK. Interestingly, a TR3 fragment containing only the N terminus was also heavily phosphorylated. We also studied whether TR3 was phosphorylated by JNK in vivo. The TR3 expression vector was transfected into HEK293T cells, which were then treated with anisomycin, a potent inducer of JNK (18). Immunoblotting analysis revealed that anisomycin treatment resulted in the slow migration of the expressed GFP-TR3 protein, compared to the GFP-TR3 protein expressed in untreated cells (Fig. 11B). The anisomycin-induced retarded migration of the TR3 protein was prevented when cells were pretreated with a JNK inhibitor. These results suggest that phosphorylation of TR3 by JNK was responsible for the slower migration of the TR3 protein. Similar to full-length TR3, the N-terminal mutant of TR3 (TR3-1-151), which contained the first 151 amino acid residues, was modified by the anisomycin treatment, which was also prevented by the JNK inhibitor (Fig. 11B). In contrast, the migration of TR3/Δ122, which lacks the first 122 amino acid residues, was not affected by anisomycin treatment. These data strongly suggest that TR3 is mainly phosphorylated on the N terminus by JNK.
FIG. 11.
Phosphorylation of TR3 by JNK and its effect on TR3 DNA binding. (A) Phosphorylation of TR3 by JNK. Equal amounts of bacterially expressed and purified GST-TR3 (33), GST-TR3 mutant (1-151), or c-Jun were incubated with JNK in an in vitro kinase assay. One of four similar experiments is shown. (B) Phosphorylation of the TR3 N terminus. Expression vectors for GFP-TR3, GFP-TR3-1-151, and GFP-TR3/Δ122 (a TR3 mutant with a deletion of the first 122 amino acid residues) (2.5 μg) were transfected into HEK293T cells in six-well plates. Twenty-four hours after transfection, cells were treated for 2 h with 10 μM anisomycin (Sigma) with or without a 30-min pretreatment with 20 μM JNK inhibitor II (Cat-10-420119; CalBiochem). Cell lysates were prepared and subjected to Western blotting using anti-GFP antibody. * indicates the modification of TR3 induced by anisomycin. One of two similar experiments is shown. (C) Effect of phosphorylation on TR3 DNA binding. Bacterially expressed TR3 or TR3 that had been phosphorylated by JNK in vitro were incubated with 32P-labeled NurRE and analyzed by gel retardation assay as described previously (36). One of two similar experiments is shown.
To determine whether phosphorylation of TR3 by JNK affected its DNA binding, a gel retardation assay was conducted to determine the binding of TR3 protein to NurRE before and after its phosphorylation by JNK. As shown in Fig. 11C, bacterially expressed TR3 protein formed a strong complex with the NurRE. However, upon phosphorylation by JNK in vitro, binding of TR3 to the NurRE was largely impaired. In addition, the weak complex formed between TR3 and NurRE migrated slightly faster than that formed by unphosphorylated TR3, probably reflecting a change in TR3 conformation by phosphorylation. Thus, the effect of MEKK1 is likely mediated by its activation of JNK, which then phosphorylates TR3 and inhibits TR3 DNA binding and transactivation.
DISCUSSION
Numerous studies have demonstrated that TR3 expression is rapidly and strongly induced by a variety of growth factors (13, 17, 44). However, whether TR3 expression plays a causal role in mediating the growth-stimulating effect of these growth factors remains to be illustrated. In this study, we provide several pieces of evidence demonstrating that TR3 is directly involved in promoting cell growth. First, TR3 expression was strongly induced by such mitogenic stimuli as EGF or serum in lung cancer cells (Fig. 1), similar to that observed in other cell types (6, 9, 13, 17, 35, 44, 58). In addition, we show for the first time that ectopic expression of TR3 resulted in the acceleration of cell cycle progression and proliferation in both H460 and Calu-6 lung cancer cell lines (Fig. 2 and 3). Moreover, inhibition of endogenous TR3 expression by the siRNA approach suppressed the growth of H460 cells and also abrogated cell proliferation induced by EGF or serum (Fig. 4). These data clearly demonstrate that TR3 is directly involved in promoting lung cancer cell growth.
The results of our studies on how TR3 exerts its mitogenic effect in lung cancer cells indicate that both DNA binding and transactivation of TR3 are required. TR3 lacking the DBD (TR3/ΔDBD) failed to promote the growth of H460 cells (Fig. 5). In addition, deletion of the N-terminal sequence, which contributes to the major transactivation function of TR3, impaired TR3-mediated proliferative effects (Fig. 5). Furthermore, inhibition of TR3 transactivation by constitutively active MEKK1 (MEKK1-DA) suppressed its mitogenic effect (Fig. 8 and 9). Consistent with the notion that TR3 transactivation is associated with cell proliferation, both basal endogenous TR3 and ectopically expressed TR3 resided in the nuclei of H460 lung cancer cells (Fig. 6 and 7). Thus, TR3 confers its growth-promoting activities through its transcriptional regulation of genes in the nucleus. How TR3 acts in the nucleus to exert its mitogenic effects remains unknown. Recently we found that TR3 expression was associated with retinoid resistance (62). In addition, we observed that TR3 could inhibit transcriptional activation induced by retinoids (7). TR3 can heterodimerize with RXR (14, 48, 61) and COUP-TF (62), important regulators of retinoid responses. Thus, modulation of retinoid signaling by TR3 may represent a mechanism by which TR3 acts in the nucleus to promote cancer cell growth.
TR3 is recognized by Western blot analysis as three bands ranging from 55 to 70 kDa (Fig. 4). All the three bands were induced by the AHPN analog 3-Cl-AHPC (Fig. 7A), whereas serum or EGF specifically induced only one form of TR3 (Fig. 1A). We consistently observed that the ectopically expressed GFP-TR3 or Myc-tagged TR3 also exists as multiple forms (Fig. 11B and data not shown). The multiple bands may correspond to different phosphorylation states of TR3. The N terminus (amino acids 1 to 151) of TR3 is composed of 29 serines and is heavily phosphorylated. In support of this, GFP-TR3-1-151 exists as multiple forms in the cell (Fig. 11B), whereas a TR3 mutant lacking the N-terminal sequences (GFP-TR3/ΔN122) is recognized as a single band on the Western blot (Fig. 11B). Thus, differential induction of TR3 expression reflects different modification of TR3 protein in response to an apoptotic stimulus or a mitogenic stimulus. This is consistent with the notion that TR3 function is regulated by modification of the TR3 protein, most likely through phosphorylation.
Previous studies demonstrated that phosphorylation plays a critical role in regulating TR3 functions. In PC12 cells, NGFI-B induced by NGF or fibroblast growth factor, which promoted PC12 cell differentiation, was found to be heavily phosphorylated (13). Notably, phosphorylated NGFI-B was diffusely distributed in both the nucleus and the cytoplasm, while the underphosphorylated NGFI-B was found only in the nucleus. These observations suggest that phosphorylation regulates biological function and cellular localization of NGFI-B. Katagiri et al. recently reported that NGF-induced phosphorylation of Ser105 of NGFI-B by the Trk/Ras/MAP kinase pathway was involved in controlling NGFI-B nuclear export in PC12 cells (23). Phosphorylation of nur77 at Ser350 inhibited its DNA binding and transactivation (19). Akt was subsequently found to be capable of phosphorylating Ser350 (42, 47). In the present study, we demonstrated that MEKK1 is involved in regulating TR3 activity. Expression of constitutively active MEKK1 strongly inhibited TR3 transactivation (Fig. 8A). The kinase activity of MEKK1 was required for inhibition, since expression of kinase-deficient MEKK1 (MEKK1-DN) failed to suppress TR3 transcriptional activity (Fig. 8B). The inhibition of TR3 transactivation by MEKK1 was not due to the change in TR3 cellular localization, since cotransfected MEKK1-DA did not cause TR3 relocalization (Fig. 8D), and was specific to TR3, since it did not inhibit transactivation of ER and TR (Fig. 8E). Interestingly, MEKK1 failed to regulate the transrepression of GR activity by TR3 (Fig. 8F). MEKK1 was shown to positively or negatively regulate the transcriptional activity of several nuclear receptors, including androgen receptor (3), progesterone receptor (53), ER (29), and RXR (21). However, the molecular basis for regulation of these receptors by MEKK1 was different and appeared to be complex. Enhancement of ER transcriptional activity was mediated through activation of JNK and p38 by MEKK1 (29), whereas enhancement of progesterone receptor activity is primarily due to its activation of the p42 and p44 MAP kinases (53). Our results demonstrate that the inhibition of TR3 transactivation by MEKK1 occurred in the presence of inhibitors of Erk and p38, but it was relieved when JNK1-DN was coexpressed (Fig. 10). Thus, inhibition of TR3 transactivation activity by MEKK1 is due to its activation of JNK1 but not Erk or p38. Modulation of transcriptional activity by JNK has also been demonstrated for other nuclear receptors, including RXR (30, 32) and GR (51). Our in vitro and in vivo phosphorylation assays (Fig. 11) revealed that JNK could effectively phosphorylate TR3 and that phosphorylation appeared to occur primarily at the N terminus, which is crucial for TR3 transactivation (Fig. 11). In studying how phosphorylation of TR3 by JNK regulated its biological functions, we observed that phosphorylation of TR3 by JNK resulted in loss of its DNA binding activity (Fig. 11C). This observation could explain the inhibition of TR3 transactivation (Fig. 8A) and suppression of TR3-induced proliferation (Fig. 9) by MEKK1. Because the TR3 DBD is devoid of phosphorylation by JNK, it is tempting to speculate that JNK phosphorylation of the N-terminal end or probably the C-terminal end may inhibit TR3 DNA binding through modulation of its dimerization function.
One unique function of TR3 is that it is required not only for cell proliferation but also for apoptosis. We previously reported that TR3 was induced by the apoptosis-inducing retinoid AHPN in H460 cells, with induction being essential for the apoptotic effect (34). Our present observation that TR3 was induced by survival factors in H460 cells, mediating cell proliferation, demonstrated that the opposing activities of TR3, survival and death, could occur in the same cells depending on the stimuli. The observation that TR3 expression confers differential biological effects in the same cells appears to be paradoxical. However, our results explain this dichotomy in that TR3 acts in the nucleus to confer its mitogenic effect but migrates to mitochondria to initiate apoptosis in response to the AHPN analog 3-Cl-AHPC (Fig. 7). In addition, TR3/ΔDBD effectively targeted mitochondria (Fig. 6B) and induced apoptosis (Fig. 5D) of H460 cells. Further, we show that different domains of TR3 are responsible for its survival and death effects, since the DBD of TR3 is essential for its mitogenic activity but dispensable for its apoptotic effect (Fig. 5). This observation, together with our finding that 3-Cl-AHPC suppressed TR3 transactivation (Fig. 7B), demonstrates that transcriptional activation by TR3 is not involved in its apoptotic effect in H460 lung cancer cells. Mitochondrial targeting of TR3 was also shown to mediate the apoptotic effect of Sindbis virus in NIH 3T3 cells (31). EBNA2, a transcription factor from Epstein-Barr virus, inhibited TR3-induced apoptosis by inhibiting TR3 nuclear export induced either by Sindbis virus or by treatment with 12-O-tetradecanoylphorbol-13-acetate (31). In PC12 cells, NGF-induced TR3 translocated from the nucleus to the cytoplasm, potentially to regulate cell differentiation (23). Thus, the cellular localization of TR3 plays a critical role in determining its biological function.
Our finding that the cellular localization of TR3 defines its biological function suggests that TR3 may be a molecular target for developing new anticancer agents. TR3 is often overexpressed in cancer cells, due to the uncontrolled expression of growth factors which induce its expression (56, 62), but normally it is not expressed in adult cells. TR3 overexpression might confer a proliferative advantage to tumor cells (28, 54, 66). Agents that induce translocation of TR3 from the nucleus to mitochondria may potently inhibit the growth and induce apoptosis of cancer cells. In fact, AHPN analogs induce the translocation of TR3 and effectively promote apoptosis in a variety of cancer cells (11, 68). Interestingly, overexpression of Nor-1, a family member of TR3, in diffuse large B-cell lymphoma was associated with favorable outcome in patients treated with chemotherapeutic agents (54). Thus, agents that inhibit TR3 transactivation and target TR3 to mitochondria to induce cell death may have therapeutic value by specifically eliminating tumor cells.
Acknowledgments
We thank Laura Frazer for preparation of the manuscript, Sharon James for reviewing the manuscript, John Kim and Hui Li for excellent technical assistance, Steven Frisch for providing MEKK1 expression plasmids, and Dan Mercola for providing JNK dominant negative expression plasmids.
This work was in part supported by grants to X.-K. Zhang from the National Institutes of Health (CA60988 and CA87000) and NCI grant P01 CA51993 (M.I.D and X.Z.). S. K. Kolluri and N. Bruey-Sedano were supported by fellowships from the U.S. Army Medical Research and Materiel Command (DAMD17-00-1-0173) and the California Breast Cancer Research Program, respectively.
REFERENCES
- 1.Aarnisalo, P., C. H. Kim, J. W. Lee, and T. Perlmann. 2002. Defining requirements for heterodimerization between the retinoid X receptor and the orphan nuclear receptor Nurr1. J. Biol. Chem. 277:35118-35123. [DOI] [PubMed] [Google Scholar]
- 2.Abayratna Wansa, K. D., J. M. Harris, and G. E. Muscat. 2002. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J. Biol. Chem. 277:33001-33011. [DOI] [PubMed] [Google Scholar]
- 3.Abreu-Martin, M. T., A. Chari, A. A. Palladino, N. A. Craft, and C. L. Sawyers. 1999. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol. 19:5143-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, C., and G. Kroemer. 2000. Apoptosis. Mitochondria—the death signal integrators. Science 289:1150-1151. [DOI] [PubMed] [Google Scholar]
- 5.Cardone, M. H., G. S. Salvesen, C. Widmann, G. Johnson, and S. M. Frisch. 1997. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell 90:315-323. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C., and J. Kokontis. 1988. Identification of a new member of the steroid receptor super-family by cloning and sequence analysis. Biochem. Biophys. Res. Commun. 155:971-977. [DOI] [PubMed] [Google Scholar]
- 7.Chen, G. Q., B. Lin, M. I. Dawson, and X. K. Zhang. 2002. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int. J. Cancer 99:171-178. [DOI] [PubMed] [Google Scholar]
- 8.Clark, J., H. Benjamin, S. Gill, S. Sidhar, G. Goodwin, J. Crew, B. A. Gusterson, J. Shipley, and C. S. Cooper. 1996. Fusion of the EWS gene to CHN, a member of the steroid/thyroid receptor gene superfamily, in a human myxoid chondrosarcoma. Oncogene 12:229-235. [PubMed] [Google Scholar]
- 9.Crawford, P. A., Y. Sadovsky, K. Woodson, S. L. Lee, and J. Milbrandt. 1995. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol. Cell. Biol. 15:4331-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, I. J., T. G. Hazel, R. H. Chen, J. Blenis, and L. F. Lau. 1993. Functional domains and phosphorylation of the orphan receptor Nur77. Mol. Endocrinol. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, M. I., P. D. Hobbs, V. J. Peterson, M. Leid, C. W. Lange, K. C. Feng, G. Chen, J. Gu, H. Li, S. K. Kolluri, X. Zhang, Y. Zhang, and J. A. Fontana. 2001. Apoptosis induction in cancer cells by a novel analogue of 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalenecarboxylic acid lacking retinoid receptor transcriptional activation activity. Cancer Res. 61:4723-4730. [PubMed] [Google Scholar]
- 12.Evan, G. I., and K. H. Vousden. 2001. Proliferation, cell cycle and apoptosis in cancer. Nature 411:342-348. [DOI] [PubMed] [Google Scholar]
- 13.Fahrner, T. J., S. L. Carroll, and J. Milbrandt. 1990. The NGFI-B protein, an inducible member of the thyroid/steroid receptor family, is rapidly modified posttranslationally. Mol. Cell. Biol. 10:6454-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman, B. M., K. Umesono, J. Chen, and R. M. Evans. 1995. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell 81:541-550. [DOI] [PubMed] [Google Scholar]
- 15.Green, D. R., and G. I. Evan. 2002. A matter of life and death. Cancer Cell 1:19-30. [DOI] [PubMed] [Google Scholar]
- 16.Hazel, T. G., R. Misra, I. J. Davis, M. E. Greenberg, and L. F. Lau. 1991. Nur77 is differentially modified in PC12 cells upon membrane depolarization and growth factor treatment. Mol. Cell. Biol. 11:3239-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazel, T. G., D. Nathans, and L. F. Lau. 1988. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc. Natl. Acad. Sci. USA 85:8444-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazzalin, C. A., R. Le Panse, E. Cano, and L. C. Mahadevan. 1998. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol. Cell. Biol. 18:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirata, Y., K. Kiuchi, H. C. Chen, J. Milbrandt, and G. Guroff. 1993. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J. Biol. Chem. 268:24808-24812. [PubMed] [Google Scholar]
- 20.Holmes, W. F., D. R. Soprano, and K. J. Soprano. 2002. Elucidation of molecular events mediating induction of apoptosis by synthetic retinoids using a CD437-resistant ovarian carcinoma cell line. J. Biol. Chem. 277:45408-45419. [DOI] [PubMed] [Google Scholar]
- 21.Ishaq, M., M. Fan, and V. Natarajan. 2000. Accumulation of RXR alpha during activation of cycling human T lymphocytes: modulation of RXRE transactivation function by mitogen-activated protein kinase pathways. J. Immunol. 165:4217-4225. [DOI] [PubMed] [Google Scholar]
- 22.Kastner, P., M. Mark, and P. Chambon. 1995. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83:859-869. [DOI] [PubMed] [Google Scholar]
- 23.Katagiri, Y., K. Takeda, Z. X. Yu, V. J. Ferrans, K. Ozato, and G. Guroff. 2000. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat. Cell. Biol. 2:435-440. [DOI] [PubMed] [Google Scholar]
- 24.Kolluri, S. K., C. Weiss, A. Koff, and M. Gottlicher. 1999. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 13:1742-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kousteni, S., T. Bellido, L. I. Plotkin, C. A. O'Brien, D. L. Bodenner, L. Han, K. Han, G. B. DiGregorio, J. A. Katzenellenbogen, B. S. Katzenellenbogen, P. K. Roberson, R. S. Weinstein, R. L. Jilka, and S. C. Manolagas. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719-730. [PubMed] [Google Scholar]
- 26.Kumar, R., R. A. Wang, A. Mazumdar, A. H. Talukder, M. Mandal, Z. Yang, R. Bagheri-Yarmand, A. Sahin, G. Hortobagyi, L. Adam, C. J. Barnes, and R. K. Vadlamudi. 2002. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature 418:654-657. [DOI] [PubMed] [Google Scholar]
- 27.Labelle, Y., J. Bussieres, F. Courjal, and M. B. Goldring. 1999. The EWS/TEC fusion protein encoded by the t(9;22) chromosomal translocation in human chondrosarcomas is a highly potent transcriptional activator. Oncogene 18:3303-3308. [DOI] [PubMed] [Google Scholar]
- 28.Labelle, Y., J. Zucman, G. Stenman, L. G. Kindblom, J. Knight, C. Turc-Carel, B. Dockhorn-Dworniczak, N. Mandahl, C. Desmaze, M. Peter, et al. 1995. Oncogenic conversion of a novel orphan nuclear receptor by chromosome translocation. Hum. Mol. Genet. 4:2219-2226. [DOI] [PubMed] [Google Scholar]
- 29.Lee, H., F. Jiang, Q. Wang, S. V. Nicosia, J. Yang, B. Su, and W. Bai. 2000. MEKK1 activation of human estrogen receptor alpha and stimulation of the agonistic activity of 4-hydroxytamoxifen in endometrial and ovarian cancer cells. Mol. Endocrinol. 14:1882-1896. [DOI] [PubMed] [Google Scholar]
- 30.Lee, H. Y., Y. A. Suh, M. J. Robinson, J. L. Clifford, W. K. Hong, J. R. Woodgett, M. H. Cobb, D. J. Mangelsdorf, and J. M. Kurie. 2000. Stress pathway activation induces phosphorylation of retinoid X receptor. J. Biol. Chem. 275:32193-32199. [DOI] [PubMed] [Google Scholar]
- 31.Lee, J. M., K. H. Lee, M. Weidner, B. A. Osborne, and S. D. Hayward. 2002. Epstein-Barr virus EBNA2 blocks Nur77-mediated apoptosis. Proc. Natl. Acad. Sci. USA 99:11878-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, D., T. L. Zimmerman, S. Thevananther, H. Y. Lee, J. M. Kurie, and S. J. Karpen. 2002. Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J. Biol. Chem. 277:31416-31422. [DOI] [PubMed] [Google Scholar]
- 33.Li, H., S. K. Kolluri, J. Gu, M. I. Dawson, X. Cao, P. D. Hobbs, B. Lin, G. Chen, J. Lu, F. Lin, Z. Xie, J. A. Fontana, J. C. Reed, and X. Zhang. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159-1164. [DOI] [PubMed] [Google Scholar]
- 34.Li, Y., B. Lin, A. Agadir, R. Liu, M. I. Dawson, J. C. Reed, J. A. Fontana, F. Bost, P. D. Hobbs, Y. Zheng, G. Q. Chen, B. Shroot, D. Mercola, and X. K. Zhang. 1998. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol. Cell. Biol. 18:4719-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim, R. W., C. Y. Zhu, and B. Stringer. 1995. Differential regulation of primary response gene expression in skeletal muscle cells through multiple signal transduction pathways. Biochim. Biophys. Acta 1266:91-100. [DOI] [PubMed] [Google Scholar]
- 36.Lin, B., G. Q. Chen, D. Xiao, S. K. Kolluri, X. Cao, H. Su, and X. K. Zhang. 2000. Orphan receptor COUP-TF is required for induction of retinoic acid receptor beta, growth inhibition, and apoptosis by retinoic acid in cancer cells. Mol. Cell. Biol. 20:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, F., D. Xiao, S. K. Kolluri, and X. Zhang. 2000. Unique anti-activator protein-1 activity of retinoic acid receptor beta. Cancer Res. 60:3271-3280. [PubMed] [Google Scholar]
- 38.Liu, Y., M. O. Lee, H. G. Wang, Y. Li, Y. Hashimoto, M. Klaus, J. C. Reed, and X. Zhang. 1996. Retinoic acid receptor beta mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol. Cell. Biol. 16:1138-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, Z. G., S. W. Smith, K. A. McLaughlin, L. M. Schwartz, and B. A. Osborne. 1994. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature 367:281-284. [DOI] [PubMed] [Google Scholar]
- 40.Manfredi, J. J. 2003. p53 and apoptosis. It's not just in the nucleus anymore. Mol. Cell 11:552-554. [DOI] [PubMed] [Google Scholar]
- 41.Mangelsdorf, D. J., and R. M. Evans. 1995. The RXR heterodimers and orphan receptors. Cell 83:841-850. [DOI] [PubMed] [Google Scholar]
- 42.Masuyama, N., K. Oishi, Y. Mori, T. Ueno, Y. Takahama, and Y. Gotoh. 2001. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J. Biol. Chem. 276:32799-32805. [DOI] [PubMed] [Google Scholar]
- 43.Migliaccio, A., G. Castoria, M. Di Domenico, A. de Falco, A. Bilancio, M. Lombardi, M. V. Barone, D. Ametrano, M. S. Zannini, C. Abbondanza, and F. Auricchio. 2000. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 19:5406-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milbrandt, J. 1988. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1:183-188. [DOI] [PubMed] [Google Scholar]
- 45.Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta 1333:F85-F104. [DOI] [PubMed] [Google Scholar]
- 46.Minden, A., A. Lin, M. McMahon, C. Lange-Carter, B. Derijard, R. J. Davis, G. L. Johnson, and M. Karin. 1994. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266:1719-1723. [DOI] [PubMed] [Google Scholar]
- 47.Pekarsky, Y., C. Hallas, A. Palamarchuk, A. Koval, F. Bullrich, Y. Hirata, R. Bichi, J. Letofsky, and C. M. Croce. 2001. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc. Natl. Acad. Sci. USA 98:3690-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perlmann, T., and L. Jansson. 1995. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 9:769-782. [DOI] [PubMed] [Google Scholar]
- 49.Philips, A., S. Lesage, R. Gingras, M. H. Maira, Y. Gauthier, P. Hugo, and J. Drouin. 1997. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol. Cell. Biol. 17:5946-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philips, A., M. Maira, A. Mullick, M. Chamberland, S. Lesage, P. Hugo, and J. Drouin. 1997. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol. Cell. Biol. 17:5952-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogatsky, I., S. K. Logan, and M. J. Garabedian. 1998. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 95:2050-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheller, K., C. E. Sekeris, G. Krohne, R. Hock, I. A. Hansen, and U. Scheer. 2000. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol. 79:299-307. [DOI] [PubMed] [Google Scholar]
- 53.Shen, T., K. B. Horwitz, and C. A. Lange. 2001. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 21:6122-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shipp, M. A., K. N. Ross, P. Tamayo, A. P. Weng, J. L. Kutok, R. C. Aguiar, M. Gaasenbeek, M. Angelo, M. Reich, G. S. Pinkus, T. S. Ray, M. A. Koval, K. W. Last, A. Norton, T. A. Lister, J. Mesirov, D. S. Neuberg, E. S. Lander, J. C. Aster, and T. R. Golub. 2002. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat. Med. 8:68-74. [DOI] [PubMed] [Google Scholar]
- 55.Simoncini, T., A. Hafezi-Moghadam, D. P. Brazil, K. Ley, W. W. Chin, and J. K. Liao. 2000. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uemura, H., and C. Chang. 1998. Antisense TR3 orphan receptor can increase prostate cancer cell viability with etoposide treatment. Endocrinology 139:2329-2334. [DOI] [PubMed] [Google Scholar]
- 57.Weih, F., R. P. Ryseck, L. Chen, and R. Bravo. 1996. Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Proc. Natl. Acad. Sci. USA 93:5533-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams, G. T., and L. F. Lau. 1993. Activation of the inducible orphan receptor gene nur77 by serum growth factors: dissociation of immediate-early and delayed-early responses. Mol. Cell. Biol. 13:6124-6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson, T. E., T. J. Fahrner, M. Johnston, and J. Milbrandt. 1991. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252:1296-1300. [DOI] [PubMed] [Google Scholar]
- 60.Woronicz, J. D., B. Calnan, V. Ngo, and A. Winoto. 1994. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature 367:277-281. [DOI] [PubMed] [Google Scholar]
- 61.Wu, Q., M. I. Dawson, Y. Zheng, P. D. Hobbs, A. Agadir, L. Jong, Y. Li, R. Liu, B. Lin, and X. K. Zhang. 1997. Inhibition of trans-retinoic acid-resistant human breast cancer cell growth by retinoid X receptor-selective retinoids. Mol. Cell. Biol. 17:6598-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, Q., Y. Li, R. Liu, A. Agadir, M. O. Lee, Y. Liu, and X. Zhang. 1997. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J. 16:1656-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, Q., S. Liu, X. F. Ye, Z. W. Huang, and W. J. Su. 2002. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis 23:1583-1592. [DOI] [PubMed] [Google Scholar]
- 64.Xue, Y., P. Chomez, E. Castanos-Velez, P. Biberfeld, T. Perlmann, and M. Jondal. 1997. Positive and negative thymic selection in T cell receptor-transgenic mice correlate with Nur77 mRNA expression Eur. J. Immunol. 27:2048-2056. (Erratum, 27: 2748.) [DOI] [PubMed] [Google Scholar]
- 65.Young, C. Y., P. E. Murtha, and J. Zhang. 1994. Tumor-promoting phorbol ester-induced cell death and gene expression in a human prostate adenocarcinoma cell line. Oncol. Res. 6:203-210. [PubMed] [Google Scholar]
- 66.Zhang, X. K. 2002. Vitamin A and apoptosis in prostate cancer. Endocr. Relat. Cancer 9:87-102. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, X. K., and M. Pfahl. 1993. Hetero- and homodimeric receptors in thyroid hormone and vitamin A action. Receptor 3:183-191. [PubMed] [Google Scholar]
- 68.Zhang, Y., M. I. Dawson, R. Mohammad, A. K. Rishi, L. Farhana, K. C. Feng, M. Leid, V. Peterson, X. K. Zhang, M. Edelstein, D. Eilander, S. Biggar, N. Wall, U. Reichert, and J. A. Fontana. 2002. Induction of apoptosis of human B-CLL and ALL cells by a novel retinoid and its nonretinoidal analog. Blood 100:2917-2925. [DOI] [PubMed] [Google Scholar]