Abstract
Influenza virus infection is recognized by the innate immune system through Toll like receptor (TLR) 7 and retinoic acid inducible gene I. These two recognition pathways lead to the activation of type I interferons and resistance to infection. In addition, TLR signals are required for the CD4 T cell and IgG2a, but not cytotoxic T lymphocyte, responses to influenza virus infection. In contrast, the role of NOD-like receptors (NLRs) in viral recognition and induction of adaptive immunity to influenza virus is unknown. We demonstrate that respiratory infection with influenza virus results in the activation of NLR inflammasomes in the lung. Although NLRP3 was required for inflammasome activation in certain cell types, CD4 and CD8 T cell responses, as well as mucosal IgA secretion and systemic IgG responses, required ASC and caspase-1 but not NLRP3. Consequently, ASC, caspase-1, and IL-1R, but not NLRP3, were required for protective immunity against flu challenge. Furthermore, we show that caspase-1 inflammasome activation in the hematopoietic, but not stromal, compartment was required to induce protective antiviral immunity. These results demonstrate that in addition to the TLR pathways, ASC inflammasomes play a central role in adaptive immunity to influenza virus.
Influenza virus is responsible for annual epidemics that cause severe morbidity and death involving approximately five million people worldwide. Lethal pneumonia and encephalopathy caused by influenza virus have now become a serious problem, especially among the elderly and children, respectively (1). Furthermore, the H5N1 highly pathogenic avian influenza viruses that are associated with a high fatality rate (>60%) have been reported in Southeast Asia, Europe, and Africa. Therefore, there is an urgent and important public health need to develop effective vaccines against not only annual seasonal influenza viruses but also against highly pathogenic H5N1 avian influenza viruses. Influenza virus is recognized through at least two viral sensors. First, the cytosolic sensor retinoic acid inducible gene I (RIG-I) detects influenza after fusion and replication in infected cells (2). Second, influenza genomic RNA, upon release in late endosomes, is recognized by Toll-like receptor (TLR) 7 (3, 4). The RIG-I pathway is used by most cells to respond to virus infection, whereas the latter is used by plasmacytoid DCs (pDCs) (2). Signaling through both RIG-I and TLR7 results in the production of type I IFNs, which limit viral replication and increasing resistance to infection.
In addition to type I IFNs, proinflammatory cytokines such as IL-1 play a crucial role in protection against influenza. Influenza virus infection is accompanied by IL-1β production in bronchoalveolar lavage (BAL) of mice (5). Influenza virus infection activates IL-1β and IL-18 production in human macrophages (6). IL-1 is responsible for acute lung immunopathology and is required to promote survival of the mice after influenza virus infection (7). Influenza virus–specific CD4 T cell responses and IgM levels were reduced in IL-1R–deficient mice (7). Not surprisingly, influenza virus has evolved strategies to inhibit the activation of inflammasomes. NS1 protein of influenza virus suppressed caspase-1 activation, maturation of pro–IL-1β and pro–IL-18, and caspase-1–dependent apoptosis in infected primary human macrophages (8). However, the mechanism by which IL-1β and IL-18 are activated during influenza infection in vivo is unknown.
Inflammasomes are molecular platforms that allow activation of caspase-1 (9). Caspase-1 is an essential regulator of inflammatory response through its capacity to process and activate proIL-1β, proIL-18, and proIL-33 (10). NOD-like receptors (NLRs) comprise a large family of intracellular PRRs that play an important role in innate immunity in response to recognition of various “damaged” self (11) and nonself molecules (9, 12). NLR protein (NLRP) 3, also known as NALP3/Cryopyrin/CIAS1/PYPAF1 (13), forms a caspase-1–activating inflammasome. Mature IL-1β secretion requires at least two steps: first, transcriptional and translational up-regulation of pro–IL-1β through TLR stimulation; and second, the activation of caspase-1 by inflammasomes (9, 12). Recent reports indicate that infection by certain viruses also results in inflammasome activation (14–16). Kanneganti et al. (15) showed that Sendai and influenza viruses activated the NLRP3 inflammasome in macrophages pulsed transiently with ATP for 30 min in vitro. Muruve et al. (16) demonstrated that adenovirus infection activates IL-1β processing in NLRP3-, ASC-, and caspase-1–dependent manners. However, inflammasomes were not activated by transfection of RNA, Poly I:C, or infection with reovirus (double-stranded RNA virus) or vesicular stomatitis virus (single-stranded RNA virus). In another seminal study, Johnston et al. (14) reported that Myxoma virus carries a protein that inhibits ASC/caspase-1 activation and subsequent cell death after virus infection. Such an evasion mechanism supports the idea that inflammasomes might play a vital role in antiviral defense.
Although the proinflammatory role of inflammasomes is well known, less is understood with respect to the requirement for inflammasomes in the generation of adaptive immune responses. Uric acid, which triggers NLRP3 inflammasomes (17), has been shown to stimulate DC maturation and, when coinjected with antigen in vivo, significantly enhances the generation of responses from CD8+ T cells (18). NLRP3, as well as its adaptor molecule ASC, are required for contact hypersensitivity responses in vivo (19). More recently, the Th2-inducing adjuvant activity of alum was shown to be mediated through NLRP3/ASC inflammasomes (20). Thus, inflammasomes appear to play an important role in certain types of autoimmune diseases, hyperresponsiveness, and immunization. However, the role of inflammasomes in the recognition of viral infection in vivo and generation of protective adaptive immunity is unknown.
In this paper, we examine the role of the NLRP3 inflammasomes in the initiation of adaptive immunity after physiological infection with influenza virus. We describe requirement for caspase-1 inflammasomes in the hematopoietic, but not stromal, cells in the establishment of Th1, CTL, and IgA responses to respiratory flu infection. Collectively, our data provide the first evidence of the in vivo requirement for the components of inflammasomes in adaptive immunity to a virus infection.
RESULTS AND DISCUSSION
NLRP3 inflammasome is activated in the lung after respiratory influenza infection
Although NLRP3-deficient macrophages were reported to be unable to activate IL-1β in response to influenza virus in the presence of ATP in vitro (21), the role of inflammasomes in influenza infection in vivo is unknown. In the lung, the primary targets of flu infection are the respiratory epithelial cells, which produce large amounts of virus that can subsequently infect alveolar macrophages (1) and DCs (22). To begin to dissect the importance of various components of the inflammasome complex in response to influenza virus infection, we first examined IL-1β secretion in candidate cell types including BM–derived macrophages (BMM), BM DCs and lung fibroblasts. Although BMM and BM DC exhibited significant reduction in IL-1β secretion in the absence of NLRP3 (Fig. 1, A and B), lung fibroblast cells activated inflammasomes independently of NLRP3 (Fig. 1 C). All cell types required caspase-1 for release of IL-1β (Fig. 1, A–C). Next, we examined the target of NLR recognition of influenza virus. Stimulation of cells with influenza virions showed that attachment (blocked by 65°C inactivation), fusion (blocked by 56°C inactivation), and replication (blocked by UV irradiation) of the virus were required to elicit inflammasome activation in BM DCs (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20081667/DC1) and in lung fibroblasts (Fig. S1 C). Purified genomic RNA, upon delivery by DOTAP, induced very low levels of IL-1β secretion in BM DC, which was enhanced by the addition of ATP (Fig. S1 B), indicating that flu genomic RNA, although insufficient to trigger inflammasomes, could serve as signal 1 for NLR-mediated IL-1β secretion. In contrast, flu genomic RNA and ATP treatment failed to elicit inflammasome activation in lung fibroblasts (Fig. S1 D). Collectively, these data indicated that influenza virus infection stimulates NLRP3-dependent and NLRP-3 independent inflammasomes in a cell autonomous and cell type–specific manner and that other factors, in addition to viral genomic RNA, are required to elicit inflammasome activation.
Figure 1.
The role of NLRP3, ASC, and caspase-1 in inflammasome activation and cellular recruitment to the lung after influenza infection. (A–G) BM macrophages (A), BMDCs (B), or primary lung fibroblast (C) prepared from WT, NLRP3-, and caspase-1–deficient mice were infected with A/PR8 virus at MOI 2.5. Supernatant was collected 12–24 h after stimulation and analyzed for IL-1β by ELISA. WT, NLRP3-, NLRC4-, ASC-, and caspase-1–deficient mice were infected intranasally with 103 PFU A/PR8 virus. BAL was collected by washing the trachea and lungs twice by injecting a total of 1 ml PBS containing 0.1% BSA (D and F). Lung homogenate was prepared in 2 ml PBS containing 0.1% BSA (E and G). IL-1β levels detected from the BAL or lung homogenate at different time points (D) or at 2 d p.i. (E–G) are shown. Horizontal lines show the mean. (H–I) Lung tissue was collected from the indicated groups of mice at 0 and 5 d p.i. Total lung leukocytes were enumerated. The values represent the mean of three mice per group and are expressed as the mean ± SD. Similar results were obtained from at least two separate experiments. *, P < 0.05; **, P < 0.01 versus a group of noninfected mice.
Next, to determine the role of NLRP3 inflammasomes in the secretion of IL-1β during physiologically relevant influenza virus infection in vivo, mice were challenged intranasally with influenza strain A/PR8. Active IL-1β was secreted into the BAL in a dose-dependent manner (Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20081667/DC1). Starting at 2 d post infection (p.i.), IL-1β secretion was evident in the BAL in WT mice infected with 103 PFU of influenza virus. However, mice deficient in NLRP3, ASC, or caspase-1, but not NLRC4, failed to secrete IL-1β (Fig. 1 D) or IL-18 (Fig. S2 B) into the alveolar space in response to influenza infection. Inflammasome-independent cytokines, such as IL-6, TNF-α, KC, and MIP-2, were secreted comparably from NLRP3, ASC, or caspase-1 KO mice (Fig. S2, C–F), indicating that the reduction in IL-1β and IL-18 secretion is not caused by a general immune deficiency in these mice. In contrast to the BAL fluid, IL-1β secretion in the lung tissue was found to be independent of NLRP3 (Fig. 1 E). To determine the cellular compartment responsible for the secretion of IL-1β in the lung, we generated BM chimeric mice in which only the hematopoietic (WT→caspase-1−/−) or the stromal cells (caspase-1−/−→WT) expressed caspase-1. Measurement of IL-1β in the BAL (Fig. 1 F) and lung tissue (Fig. 1 G) indicated that inflammasome activation in vivo occurs within the hematopoietic compartment and the stromal compartment. A previous study demonstrated the importance of type I IFNs in inflammasome activation after Francisella novicida infection (23). However, in response to influenza infection, although partly important in BM DC response, responsiveness through type I IFN receptor was dispensable for IL-1β secretion in the BAL (Fig. S3). Collectively, these data indicated that NLRP3 inflammasome is activated in the alveolar macrophages and DCs within the alveolar space after respiratory challenge in a type I IFN-independent manner, whereas the lung tissue resident hematopoietic cells activate caspase-1 through NLRP3-independent pathways (Fig. S4).
ASC, caspase-1, and IL-1R, but not NLRP3, is required for cellular recruitment to the lung after flu infection
Next, we wished to determine the consequence of inflammasome activation in cellular recruitment to the infected lungs. In WT mice infected with A/PR8, accumulation of leukocytes became apparent starting around day 3 p.i. and peaking around day 5 p.i. (Fig. S5 A, available at http://www.jem.org/cgi/content/full/jem.20081667/DC1). Various leukocytes were detected including macrophages, DCs, pDCs, NK cells, neutrophils, and lymphocytes (Fig. S5 B). These cellular subsets were defined by flow cytometry as described in Fig. S6. In contrast, mice deficient in ASC and caspase-1, but not NLRP3, exhibited significant reduction in cellular recruitment of all subsets (Fig. S5 C). In addition, IL-1R–deficient mice were also impaired in their ability to induce leukocyte recruitment to the lung (Fig. 1 H). Furthermore, mice selectively defective in caspase-1 in the hematopoietic compartment (caspase-1−/−→WT) were impaired in their ability to recruit leukocytes to the lung (Fig. 1 I). Thus, these data indicated that although NLRP3 is required for IL-1β secretion into the alveolar space (Fig. 1 D), NLRP3-independent ASC/caspase-1–dependent pathways (Fig. 1 E) in the lung tissue hematopoietic cells (Fig. 1 I) result in IL-1β secretion leading to cellular infiltration in the lung.
IL-1β is a well known stimulator of neutrophil recruitment, which is triggered by the synthesis and display of chemokines and leukocyte adhesion molecules. Our data demonstrated that differential requirements for NLRP3–IL-1β secretion into the alveolar space depended on NLRP3 (Fig. 1 D), whereas IL-1β secretion in the lung interstitium (Fig. 1 E) and recruitment of leukocytes to the lung were independent of NLRP3 (Fig. 1 H). Furthermore, lung leukocyte infiltration depended on ASC, caspase-1, and IL-1R, indicating that inflammasome-released IL-1β is required to mediate this process. In contrast, NLRC4-deficient mice had normal IL-1β secretion as measured by BAL (Fig. 1 D). These data demonstrated a differential role of NLRP3; the cell types that secrete IL-1β in the alveolar space (such as alveolar macrophages and DCs) require NLRP3 for inflammasome activation, whereas those that secrete IL-1β in the lung interstitium (interstitial macrophages, DCs, and others) can trigger NLRP3-independent inflammasomes. Only the latter source of IL-1β is required to activate vascular endothelial cell to enable leukocyte migration into the lung (Fig. S4). Evidence exists for the NLRP3-independent ASC dimer–mediated pyroptosome that can activate caspase-1 directly (24). It is of interest that other studies have described NLRP3/NLRC4-independent ASC/caspase-1–dependent inflammasome activation in response to LPS (25) and cytosolic DNA (16).
Influenza-specific CD4 and CD8 T cell responses depend largely on ASC and Caspase-1 but not NLRP3
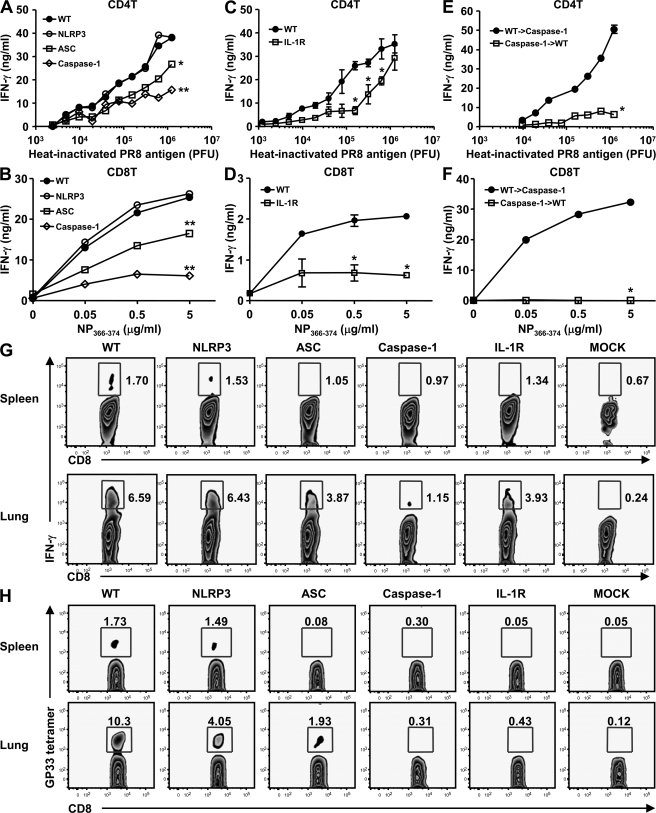
Next, to decipher the importance of inflammasomes in adaptive immune responses to influenza infection, mice deficient in NLRP3, ASC, caspase-1, or IL-1R were infected with a sublethal dose of A/PR8 (10 PFU or 0.4 LD50). 14 d later, CD4+ and CD8+ T cells were isolated from the spleen of flu-primed mice and restimulated with varying concentrations of inactivated virion or H-2Db–restricted nucleoprotein (NP) peptide, respectively. Although CD4+ and CD8+ T cells from infected NLRP3-deficient mice responded similarly to those from WT mice, ASC−/−, caspase-1−/− (Fig. 2 A), and IL-1R−/− (Fig. 2 C) mice failed to mount comparable CD4+ T cell responses. In addition, ASC−/− mice demonstrated a partially reduced CTL phenotype compared with caspase-1−/− or IL-1R−/− mice, in which CD8+ T cell responses were barely detectable after immunization with live flu infection (Fig. 2 B). Furthermore, comparable reduction in virus-specific CD8 T cell numbers in the spleen and lung was observed by intracellular IFN-γ staining (Fig. 2 G) and by tetramer staining (Fig. 2 H). These data indicated that NLRP3-independent ASC/caspase-1 inflammasomes and responsiveness through the IL-1R are required to elicit T cell responses to influenza virus in vivo. In addition, the intermediate phenotype of the defect in T cell responses in ASC−/− compared with caspase-1−/− mice suggests the presence of an ASC-independent caspase-1 inflammasome in the generation of T cell responses after influenza infection.
Figure 2.
Influenza-specific CD4 and CD8 T cell responses depend largely on ASC and Caspase-1 but not NLRP3. WT, NLRP3-, ASC-, and caspase-1–deficient mice (A and B), IL-1R−/− mice (C and D), or WT→caspase-1−/− and caspase-1−/−→WT BM chimeric mice (E and F) were infected intranasally with a sublethal dose (10 PFU) of A/PR8 virus. 14 d p.i., CD8 and CD4 T cells were isolated from spleen and stimulated with irradiated APCs with the indicated amount of heat-inactivated virion or NP peptide for 72 h, respectively. IFN-γ production from CD4+ T cells (A, C, and E) and CD8+ T cells (B, D, and F) was measured by ELISA or by intracellular IFN-γ staining (G). (H) Indicated groups of mice were infected with 10 PFU of A/PR8/GP-33 recombinant influenza virus, and GP33-specific CD8 T cells were detected in the spleen and the lung at 14 d p.i. using the Kb-GP33-41 tetramer. These figures are representative of three similar experiments. Error bars show SD. *, P < 0.05; **, P < 0.01 versus WT mice.
Next, we examined the cellular compartment responsible for the inflammasome-dependent generation of CD4 and CD8 T cell responses. T cell responses from flu-infected caspase-1−/−→WT, but not WT→caspase-1−/− mice, were diminished (Fig. 2, E and F), indicating that activation of caspase-1 inflammasomes by the hematopoietic, but not stromal, cells link NLR recognition of influenza virus to the activation of adaptive immunity in vivo.
Differential requirement for inflammasomes in immunoglobulin isotype responses to influenza virus
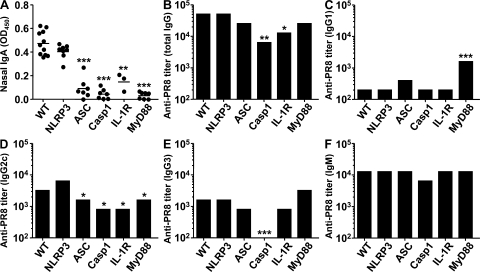
Antibody responses play a critical role in the clearance of many viral pathogens including influenza virus. Previous studies have indicated the role of MyD88 in IgG2a responses to influenza infection (26, 27). We determined the contributions of inflammasomes in this process. To this end, mice were immunized with a sublethal dose (10 PFU) of live A/PR8 virus. 2 wk p.i., influenza virion–specific nasal IgA and serum titers of IgM and IgG isotype levels were measured by serial dilution. Nasal IgA responses were found to be completely dependent on ASC, caspase-1, IL-1R, and MyD88 but not on NLRP3 (Fig. 3 A). In contrast, serum IgG1 levels were significantly elevated in MyD88-deficient mice compared with WT or other inflammasome KO groups (Fig. 3 C). A slight reduction in the IgG2c response was detected in ASC−/− and caspase-1−/− mice, which was similar to that seen in IL-1R−/− and MyD88−/− mice (Fig. 3 D). Only caspase-1−/− mice had a significant reduction in IgG3 responses but not IL-1R– or MyD88-deficient mice (Fig. 3 E), indicating that caspase-1–dependent IL-1β–, IL-18–, and IL-33 (all upstream of MyD88)–independent factors play a major role in the induction of this isotype response. In contrast, no significant differences were found in serum IgM responses (Fig. 3 F). Antibody secretion in the BM chimeric mice indicated that the requirement for caspase-1 was all attributable to its expression in the hematopoietic compartment alone (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20081667/DC1). These data indicated that at least one NLRP3-independent ASC- and caspase-1–dependent inflammasome is required for nasal IgA and, to some extent, serum IgG2c responses to influenza virus. In addition, IL-1R and MyD88, which is downstream of TLRs, IL-1R, IL-18R, and ST2, are required for the same process in vivo. In contrast, IgG3 responses appear to depend mainly on caspase-1 but not on IL-1R or MyD88, whereas MyD88 has a regulatory role for IgG1 responses.
Figure 3.
Differential requirement for NLRP3, ASC, and caspase-1 for immunoglobulin isotype responses to influenza virus. WT, NLRP3-, ASC-, caspase-1–, IL-1R–, and MyD88-deficient mice were infected intranasally with a sublethal dose (10 PFU) of A/PR8 virus. Serum and nasal swab were collected at 2 wk p.i. A/PR8-specific nasal IgA levels were measured by ELISA (A). A/PR8-specific serum antibody titers were determined by serial dilution of serum total IgG (B), IgG1 (C), IgG2c (D), IgG3 (E), and IgM (F). These figures are representative of three similar experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus WT mice.
Our results indicated that ASC/caspase-1 inflammasomes and the secreted IL-1β play a key role in the generation of Th1, CTL, and IgA responses after influenza infection (Table S1, available at http://www.jem.org/cgi/content/full/jem.20081667/DC1). Previous studies have demonstrated the requirement for TLR7 and MyD88 in adaptive immunity to influenza virus infection with somewhat conflicting results. Lopez et al. (28) found no requirement for MyD88 on T cells or immunoglobulin responses to aerosolized influenza virus challenge. In contrast, Koyama et al. (27) showed that TLR7 or MyD88 is required for the induction of B cell secretion of anti-HA IgG2a and IgG2c but not CTL responses. Heer et al. (26) demonstrated that IgG2a and IgG2c responses to flu were impaired in MyD88−/− but not in TLR7−/− mice. All studies found IgG1 antibody levels to be elevated in MyD88−/− mice (26–28) as well as TLR7−/− mice (26). Together, these studies indicated that activation of CD8 T cells during antiinfluenza immune response relies on mechanisms other than the TLR7 and MyD88 (26–28), whereas IgG2a/c and CD4 T cell responses depend on MyD88 (26, 27). In light of the data we present here, inflammasome recognition of influenza virus infection plays a more dominant role in the establishment of CD8 T cell responses compared with TLR7. In addition, we found that serum IgG2c responses were largely dependent on both ASC-dependent caspase-1 inflammasome signals. These data suggested that caspase-1 inflammasomes, as well as TLR7/MyD88 (26, 27), play a key role in the formation of IgG2c-secreting B cells after flu infection. A pronounced defect in nasal IgA production in ASC and caspase-1–deficient mice and complete impairment in MyD88−/− mice suggest that inflammasomes and IL-1R signals are required to elicit B cell secretion of IgA in the nasal mucosa.
ASC, caspase-1, and IL-1R, but not NLRP3, are required for survival after sublethal influenza challenge
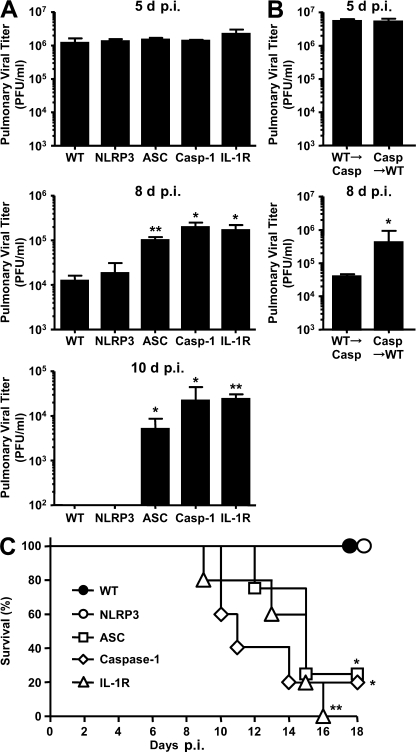
Finally, we examined the importance of inflammasomes in immune-mediated protection. To this end, we followed viral titer and survival of mice after intranasal challenge with a sublethal dose (10 PFU) of A/PR8. The virus titers in the lung of infected mice revealed that all mice sustained similar viral load at day 5 p.i., indicating that, with the low dose viral challenge, inflammasomes were not required for innate immune protection (Fig. 4, A and B). In contrast, at later stages of infection, viral clearance was severely impaired in mice deficient for ASC, caspase-1, or IL-1R, but not NLRP3 (Fig. 4 A). Specifically, caspase-1 in the hematopoietic but not stromal compartment was required for control of virus replication at this stage (Fig. 4 B). This effect became pronounced on day 10 p.i., when WT and NLRP3−/− mice had completely cleared the virus and those deficient in ASC, caspase-1, or IL-1R sustained high virus burden in the lung (Fig. 4 A). Consequently, although WT and NLRP3−/− mice recovered from sublethal infection with influenza virus, the majority of the flu-challenged ASC−/−, caspase-1−/−, and IL-1R−/− mice succumbed to death by 18 d p.i. (Fig. 4 C). These data indicated that NLRP3-independent ASC-dependent caspase-1 inflammasomes are required to provide IL-1R–dependent immune protection against respiratory influenza infection. It is unclear which NLRPs are responsible for the recognition of influenza virus in the initiation of ASC inflammasome-dependent immune responses. Future identification of NLRP responsible for influenza recognition will provide important insights into the molecular mechanism.
Figure 4.
ASC-1–, caspase-1–, and IL-1R–deficient mice, but not NLRP3-deficient mice, are more susceptible to influenza infection and disease. WT, NLRP3-, ASC-, caspase-1–, and IL-1R–deficient mice (A) and WT→caspase-1−/− and caspase-1−/−→WT BM chimeric mice (B) were infected intranasally with a sublethal dose (10 PFU) of A/PR8 virus. The lungs of influenza-infected mice were harvested at 5, 8, and 10 d p.i., and viral titers were determined by plaque assay (A and B). The values are representative of three mice per group and are expressed as the mean ± SD. *, P < 0.05; **, P < 0.01 versus WT mice. (C) Survival of mice after infection is depicted. Similar results were obtained from at least two separate experiments.
Mortality of ASC−/−, caspase-1−/−, and IL-1R−/− mice after a low-dose influenza challenge was associated with reduced CD4 and CD8 T cell responses (Fig. 2) as well as impaired mucosal IgA and systemic IgG2 responses (Fig. 3) to influenza viral antigens (Table S1). It is well known that recovery from influenza infection requires CD8 T cells. The antiviral capacity of flu-specific CD8+ T cells is dependent on their ability to migrate to the lungs and come in contact with infected airway epithelium (29). Effector CD8 T cells begin to appear in the lung ∼5–7 d after infection (30). Thus, the inability of ASC- and caspase-1–deficient mice to clear virus in the lung at 8 and 10 d after influenza infection (Fig. 4 A) is likely the result of a combined effect of their failure to develop robust systemic CD8 T cell responses and to recruit effector CD8 T cells to the lung (Fig. 2 and Fig. S5 C). This recruitment and effector T cell induction are downstream of IL-1R signaling, as IL-1R−/− mice failed to elicit lung cellular infiltration (Fig. 1) or T cell responses (Fig. 2). These data provide important evidence for the requirements for inflammasomes in multiple stages of antiviral immune defense.
In conclusion, our study demonstrated the requirement for ASC/caspase-1 inflammasomes in the development of adaptive immune responses to respiratory influenza virus infection. Our data support the notion that influenza virus is recognized through an ASC-dependent pathway in addition to TLR7 and RIG-I. Both caspase-1 (this paper) and TLR7 (26, 27) recognition pathways, but not RIG-I (27), appear to be required for adaptive immune responses to influenza virus. These data have significant implications in the design of prophylactic vaccines and management of influenza infection in general. Stimulants of inflammasomes may provide an ideal adjuvant candidate in flu vaccines that will confer protective immune responses. Finally, clinical management of severe pathogenic influenza strains, such as H5N1 avian strains, which often cause immunopathology, may require blockade of inflammasome to attenuate pathology in addition to antiviral therapy.
MATERIALS AND METHODS
Animals.
The generation of mice deficient in NLRP3, ASC (19), NLRC4 (31), caspase-1 (10), and MyD88−/− (32) has been reported previously. All KO mice have been backcrossed at least nine generations onto the C57BL/6 background. Age- and sex-matched C57BL/6 mice from National Cancer Institute (Frederick, MD) were used as WT controls. IL-1R−/− mice were obtained from The Jackson Laboratory. All procedures used in this study complied with federal guidelines and were approved by the Yale Animal Care and Use Committee.
Influenza virus infection in vitro.
Lung fibroblasts were prepared according to published procedures (33). BMM, DCs (8 × 105 cells/24 wells), or lung fibroblasts (2 × 105 cells/96 wells) were stimulated with live or inactivated A/PR8 virus. 1 h after stimulation, cells were washed thoroughly and incubated in complete media for 24 h. Cell-free supernatant was collected and analyzed for IL-1β by ELISA.
Influenza virus infection in vivo.
A/PR8 virus (H1N1) used for all experiments was grown in allantoic cavities from 10–11-d-old fertile chicken eggs for 2 d at 37°C. This virus was stored at −80°C, and viral was quantified by a standard plaque assay using Madin-Darby canine kidney cells. For intranasal infection, mice were fully anesthetized by i.p. injection of ketamine and xylazine and then infected by intranasal application of 20 μl of virus suspension (10–1,000 PFU in PBS). This procedure leads to the upper and lower respiratory tract infection. Recombinant PR8 virus expressing the LCMV glycoprotein epitope GP33-41 (PR8-GP33) was a gift from S. Kaech (Yale University, New Haven, CT).
Preparation of lung single-cell suspensions.
To obtain single lung cell suspensions, lungs were perfused with 10 ml PBS through the right ventricle, minced using razor blades, and incubated in HBSS containing 2.5 mM Hepes and 1.3 mM EDTA at 37°C for 30 min. The cells were resuspended in RPMI containing 5% FBS, 1 mM CaCl2, 1 mM MgCl2, 2.5 mM Hepes, and 0.5 mg/ml collagenase d (Roche) and incubated at 37°C for 60 min. A single-cell suspension was prepared after RBC lysis. The resulting cells were filtered through a 70-μm cell strainer (BD) and used for FACS analysis.
Flow cytometry.
The single-cell suspensions of lung samples were stained with anti-B220, anti-CD3, anti-CD4, anti–CD8-α, anti–MHC class II, anti-CD19, anti-DX5, anti-F4/80, anti-CD11c, anti–mPDCA-1, anti–Siglec-H, anti-CD11b, or anti-CD45.2 antibodies. Leukocytes were gated based on forward and side scatter properties, and live cells were gated based on 7-aminoactinomycin d exclusion. Acquisition of eight color samples was performed on a cytometer (LSR II; BD). Leukocytes from spleen or lung of immunized mice were cultured in the presence of 10 μg/ml NP peptide (ASNENMETM; H-2Db) for 6 h, and 10 μg/ml of brefeldin A (Sigma-Aldrich) was added for an additional 12 h. Cells were then surface stained with anti–CD4-FITC, anti–CD44-PE, and anti–CD8-PerCP (eBioscience) and were fixed and permeabilized using a Cytofix/Cytoperm kit (BD) according to manufacturer's instructions. For intracellular staining, APC-labeled anti–mouse IFN-γ Ab (clone XMG1.2; eBioscience) was used. For tetramer staining, APC-labeled tetramers (Kb/LCMV GP33-41; gift of S. Kaech) were incubated in 0.1 ml of 1% FBS PBS for 30 min on ice. After washing, samples were resuspended in 1% paraformaldehyde in PBS. The frequency of IFN-γ–secreting or tetramer-positive CD8+ T cells was analyzed by FACS within the CD44+ cells. The final analysis and graphical output were performed using FlowJo software (Tree Star, Inc.).
CD4 and CD8 T cell responses.
CD4+ T cells and CD8+ T cells were isolated from the spleen of mice infected with 10 PFU A/PR8 at 14 d p.i. using anti-CD4 microbeads or anti-CD8 microbeads (Miltenyi Biotec) according to the manufacturer's instructions. 105 CD4+ T and CD8+ T cells were restimulated with 5 × 105 heat-inactivated virion- or NP peptide (ASNENMETM; H-2Db)–pulsed APCs for 72 h at 37°C and cultured in RPMI 1640 containing 10% FBS (Invitrogen), 50 μM 2-mercaptoethanol (Sigma-Aldrich), and 100 U/ml penicillin and streptomycin (Invitrogen) in 96-well U-bottom plates (BD). IFN-γ production in supernatants was measured by ELISA in triplicates.
Measurement of virus titers and anti-PR8 antibodies.
Serum, nasal swab, and BAL were collected for measurement of virus titer and antibodies against A/PR8 virus from mice. BAL was collected by washing the trachea and lungs twice by injecting a total of 2 ml PBS containing 0.1% BSA. The virus titer was measured as follows: aliquots of 200 μl of serial 10-fold dilutions of the lung wash by PBS containing 0.1% BSA were inoculated into Madin-Darby canine kidney cells in 6-well plates. After 1 h of incubation, each well was overlaid with 2 ml of agar medium. The number of plaques in each well was counted 2 d after inoculation.
The serum titers of IgM and IgG isotypes against A/PR8 viruses were determined by ELISA. In brief, a 96-well plate (EIA plates; Costar; Corning) was coated with formalin-inactivated A/PR8 virion with carbonate buffer. The reactions were detected by goat anti–mouse IgA (Invitrogen), goat anti–mouse IgG1 (Bethyl laboratories, Inc.), goat anti–mouse IgG2c (Bethyl laboratories, Inc.), goat anti–mouse IgG3 (SouthernBiotech), goat anti–mouse IgG (Jackson ImmunoResearch Laboratories), or goat anti–mouse IgM (SouthernBiotech) antibodies conjugated to horseradish peroxidase. Endpoint titers were considered positive for dilutions with OD values that were twofold higher than the background level (nonimmune serum).
Online supplemental material.
Fig. S1 shows the viral and cellular determinant for influenza recognition. Fig. S2 shows inflammasome-dependent and independent cytokine secretion in the BAL of influenza-infected mice. Fig. S3 examines the role of type I IFNs in inflammasome activation by influenza virus. Fig. S4 is a schematic representation of the role of inflammasomes in the generation of adaptive immunity against flu infection. Fig. S5 depicts leukocyte recruitment to the lung of influenza-infected mice. Fig. S6 describes the strategy for cell type identification by flow cytometry. Fig. S7 shows influenza virus specific antibody production in BM chimeric mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081667/DC1.
Supplementary Material
Acknowledgments
We thank Drs. S. Kaech and A. Chandele for critical reagents and technical support.
This study was supported by grants from the National Institutes of Health (AI054359, AI062428, and AI064705). T. Ichinohe is a recipient of the Japan Society for the Promotion of Science Postdoctoral Fellow for Research Abroad. H.K. Lee was supported by the Ministry of Science and Technology of Korea and is a recipient of Richard K. Gershon fellowship. A. Iwasaki is a recipient of the Burroughs Wellcome Investigators in Pathogenesis of Infectious Disease.
The authors have no conflicting financial interests.
Y. Ogura's present address is Division of Bacterial Pathogenesis, Graduate School of Medicine, University of the Ryukyus, 207 Uehara, Nishihara-cho Okinawa, Japan.
References
- 1.La Gruta, N.L., K. Kedzierska, J. Stambas, and P.C. Doherty. 2007. A question of self-preservation: Immunopathology in influenza virus infection. Immunol. Cell Biol. 85:85–92. [DOI] [PubMed] [Google Scholar]
- 2.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 23:19–28. [DOI] [PubMed] [Google Scholar]
- 3.Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 4.Lund, J.M., L. Alexopoulou, A. Sato, M. Karow, N.C. Adams, N.W. Gale, A. Iwasaki, and R.A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA. 101:5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennet, T., H.J. Ziltener, K. Frei, and E. Peterhans. 1992. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 149:932–939. [PubMed] [Google Scholar]
- 6.Pirhonen, J., T. Sareneva, M. Kurimoto, I. Julkunen, and S. Matikainen. 1999. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J. Immunol. 162:7322–7329. [PubMed] [Google Scholar]
- 7.Schmitz, N., M. Kurrer, M.F. Bachmann, and M. Kopf. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79:6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasakova, J., B. Ferko, C. Kittel, S. Sereinig, J. Romanova, H. Katinger, and A. Egorov. 2005. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1beta and 18. J. Gen. Virol. 86:185–195. [DOI] [PubMed] [Google Scholar]
- 9.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature. 442:39–44. [DOI] [PubMed] [Google Scholar]
- 10.Kuida, K., J.A. Lippke, G. Ku, M.W. Harding, D.J. Livingston, M.S. Su, and R.A. Flavell. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 267:2000–2003. [DOI] [PubMed] [Google Scholar]
- 11.Kono, H., and K.L. Rock. 2008. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura, Y., F.S. Sutterwala, and R.A. Flavell. 2006. The inflammasome: first line of the immune response to cell stress. Cell. 126:659–662. [DOI] [PubMed] [Google Scholar]
- 13.Ting, J.P., R.C. Lovering, E.S. Alnemri, J. Bertin, J.M. Boss, B.K. Davis, R.A. Flavell, S.E. Girardin, A. Godzik, J.A. Harton, et al. 2008. The NLR gene family: a standard nomenclature. Immunity. 28:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston, J.B., J.W. Barrett, S.H. Nazarian, M. Goodwin, D. Ricciuto, G. Wang, and G. McFadden. 2005. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 23:587–598. [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti, T.D., N. Ozoren, M. Body-Malapel, A. Amer, J.H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, et al. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 440:233–236. [DOI] [PubMed] [Google Scholar]
- 16.Muruve, D.A., V. Petrilli, A.K. Zaiss, L.R. White, S.A. Clark, P.J. Ross, R.J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 452:103–107. [DOI] [PubMed] [Google Scholar]
- 17.Martinon, F., V. Petrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 440:237–241. [DOI] [PubMed] [Google Scholar]
- 18.Shi, Y., J.E. Evans, and K.L. Rock. 2003. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 425:516–521. [DOI] [PubMed] [Google Scholar]
- 19.Sutterwala, F.S., Y. Ogura, M. Szczepanik, M. Lara-Tejero, G.S. Lichtenberger, E.P. Grant, J. Bertin, A.J. Coyle, J.E. Galan, P.W. Askenase, and R.A. Flavell. 2006. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 24:317–327. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbarth, S.C., O.R. Colegio, W. O'Connor, F.S. Sutterwala, and R.A. Flavell. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 453:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanneganti, T.D., M. Body-Malapel, A. Amer, J.H. Park, J. Whitfield, L. Franchi, Z.F. Taraporewala, D. Miller, J.T. Patton, N. Inohara, and G. Nunez. 2006. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560–36568. [DOI] [PubMed] [Google Scholar]
- 22.GeurtsvanKessel, C.H., M.A. Willart, L.S. van Rijt, F. Muskens, M. Kool, C. Baas, K. Thielemans, C. Bennett, B.E. Clausen, H.C. Hoogsteden, A.D. Osterhaus, G.F. Rimmelzwaan, and B.N. Lambrecht. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry, T., A. Brotcke, D.S. Weiss, L.J. Thompson, and D.M. Monack. 2007. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri, T., J. Wu, J.W. Yu, P. Datta, B. Miller, W. Jankowski, S. Rosenberg, J. Zhang, and E.S. Alnemri. 2007. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14:1590–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, M., K. Yaginuma, H. Tsutsui, J. Sagara, X. Guan, E. Seki, K. Yasuda, M. Yamamoto, S. Akira, K. Nakanishi, et al. 2004. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 9:1055–1067. [DOI] [PubMed] [Google Scholar]
- 26.Heer, A.K., A. Shamshiev, A. Donda, S. Uematsu, S. Akira, M. Kopf, and B.J. Marsland. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178:2182–2191. [DOI] [PubMed] [Google Scholar]
- 27.Koyama, S., K.J. Ishii, H. Kumar, T. Tanimoto, C. Coban, S. Uematsu, T. Kawai, and S. Akira. 2007. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179:4711–4720. [DOI] [PubMed] [Google Scholar]
- 28.Lopez, C.B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R.A. Flavell, and T.M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882–6889. [DOI] [PubMed] [Google Scholar]
- 29.Cerwenka, A., T.M. Morgan, and R.W. Dutton. 1999. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J. Immunol. 163:5535–5543. [PubMed] [Google Scholar]
- 30.Lawrence, C.W., R.M. Ream, and T.J. Braciale. 2005. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 174:5332–5340. [DOI] [PubMed] [Google Scholar]
- 31.Franchi, L., A. Amer, M. Body-Malapel, T.D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, et al. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7:576–582. [DOI] [PubMed] [Google Scholar]
- 32.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.