Abstract
The endogenous cellular and molecular mechanisms that control acute inflammation and its resolution are of wide interest. Using self-resolving inflammatory exudates and lipidomics, we have identified a new pathway involving biosynthesis of potent antiinflammatory and proresolving mediators from the essential fatty acid docosahexaenoic acid (DHA) by macrophages (MΦs). During the resolution of mouse peritonitis, exudates accumulated both 17-hydroxydocosahexaenoic acid, a known marker of 17S-D series resolvin (Rv) and protectin biosynthesis, and 14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid from endogenous DHA. Addition of either DHA or 14S-hydroperoxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid to activated MΦs converted these substrates to novel dihydroxy-containing products that possessed potent antiinflammatory and proresolving activity with a potency similar to resolvin E1, 5S,12R,18R-trihydroxyeicosa-6Z,8E,10E,14Z,16E-pentaenoic acid, and protectin D1, 10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid. Stable isotope incorporation, intermediate trapping, and characterization of physical and biological properties of the products demonstrated a novel 14-lipoxygenase pathway, generating bioactive 7,14-dihydroxydocosa-4Z,8,10,12,16Z,19Z-hexaenoic acid, coined MΦ mediator in resolving inflammation (maresin), which enhances resolution. These findings suggest that maresins and this new metabolome may be involved in some of the beneficial actions of DHA and MΦs in tissue homeostasis, inflammation resolution, wound healing, and host defense.
Given the contribution of uncontrolled inflammation to many human diseases, the identification of endogenous control mechanisms in the acute inflammatory response is of wide interest (1). Classical lipid mediators such as the prostaglandins and leukotrienes are well appreciated for their important proinflammatory roles in inflammation (2). In recent years, the resolution of inflammation has emerged as an area with considerable potential to contain local mediators that may be useful for new therapeutic approaches (for reviews see references 3, 4). Using an unbiased systems approach using lipidomics, proteomics, and cell trafficking to study self-resolving inflammatory exudates revealed that the termination of acute inflammation involves active biosynthetic processes producing novel endogenous lipid mediators that are both antiinflammatory and proresolving (5–8). It is now clear that resolution of acute inflammation is an active rather than passive process, as previously understood (9), generating novel potent counterregulatory mediators termed resolvins (Rvs) and protectins (for review see reference 4).
Rvs and protectins are biosynthesized by exudates from essential omega-3 fatty acids (e.g., eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), and the structures are established for key members of these families (4). The immune regulatory actions of omega-3 fatty acids and their roles in human health and diseases such as cancer and neuroinflammation are widely appreciated (10–12). Although omega-3 fatty acids are in wide use as dietary supplements and potential therapeutics in many diseases, including inflammatory diseases, their mechanisms and connection to inflammation remain of interest. Rvs and protectins display potent multilevel antiinflammatory and proresolving actions (13) and are members of a new genus of endogenous mediators of resolution (4). For example, resolvin E1, 5S,12R,18R-trihydroxyeicosa-6Z,8E,10E,14Z,16E-pentaenoic acid (RvE1) is biosynthesized from EPA and interacts with specific receptors to control inflammatory cells (14, 15). Also, fat-1 transgenic mice, producing higher endogenous levels of omega-3, show reduced inflammatory status and elevated levels of Rvs and protectins, which when administered reduce inflammation and stimulate resolution (16–18). The main biosynthetic route with DHA for Rvs and protectins proceeds during resolution via a 17S-hydroperoxydocosahexaenoic intermediate produced by a lipoxygenase (LOX) mechanism. With aspirin therapy, acetylated cyclooxygenase-2 produces aspirin-triggered 17R-epimers of Rvs and protectins and also enhances their formation (6). Genetic deficiency or overexpression of mouse 12/15-LOX regulates production of Rvs and protectins, and alters their responses to both thermal injury and extent of atherosclerosis (17, 18).
In this report, we present evidence for a new pathway of mediators operative in resolution of acute inflammation that possess potent actions with polymorphonuclear neutrophils (PMNs) and macrophages (MΦs). Identification of these new mediators, coined MΦ mediators in resolving inflammation (maresins), provides evidence for autacoids produced from essential omega-3 fatty acids by a new pathway that may be linked to homeostasis, inflammation resolution, wound healing, and cancer.
RESULTS AND DISCUSSION
Targeted lipidomics of resolving inflammatory exudates
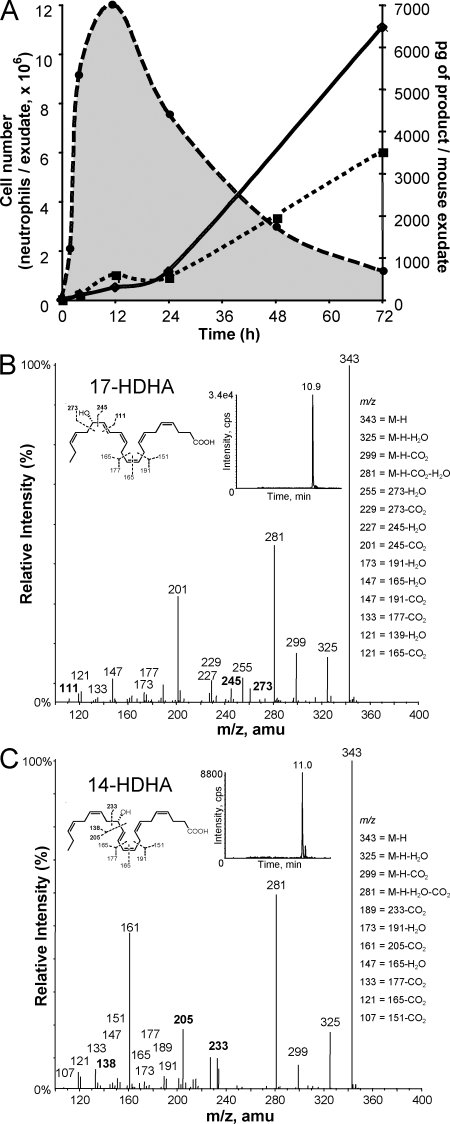
In view of the actions of specialized chemical mediators in resolution (6, 7), we monitored 17-hydroxydocosahexaenoic acid (17-HDHA) as a biomarker of activation and conversion of endogenous DHA, and also used targeted lipidomics to query whether other pathways were operative (Fig. 1). During this course of peritonitis, PMNs rapidly entered, reaching maximum within 12 h. As expected in this self-resolving system (19), PMNs declined and were lost from exudates, thus defining resolution (Fig. 1). Unbiased targeted mediator lipidomics using liquid chromatography–tandem mass spectrometry (LC/MS/MS)–based analyses were performed with these exudates. In addition to 17S-hydroxydocosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid, a marker of Rv and protectin biosynthesis (6), endogenous DHA was converted to 14S-hydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S-HDHA). Neither product was identified in exudates obtained with the LOX inhibitor esculetin (n = 3), and both were substantially reduced in peritonitis lavages from 12/15-LOX–deficient mice (n = 2, d = 4). The appearance of 14S-HDHA in this system accompanied 17-HDHA throughout the 72-h course, indicating that 14S-HDHA accumulated within the resolution phase.
Figure 1.
Self-resolving acute inflammatory exudates. (A) Time course of PMN (dashed line; n = 4) accumulation, resolution, and hydroxydocosahexaenoic acid formation during zymosan-initiated peritonitis. Exudates were extracted for targeted lipidomics using LC/MS/MS. Hydroxydocosahexaenoic acids 17-HDHA (dotted line) and 14S-HDHA (continuous line) identified using MRM results are representative (n = 3). (B and C) Representative mass spectra for 17-HDHA (B) and 14S-HDHA (C; n = 3). amu, atomic mass unit.
These two LOX products were identified by characteristic diagnostic ions in their respective mass spectra. Fig. 1 B shows a representative spectrum of 17-HDHA formed in the time course, and Fig. 1 C shows the spectrum of 14S-HDHA, as well as diagnostic ions for identification. These included mass-to-charge ratio (m/z) 205, 138, and 233, specific for 14S-HDHA. The m/z 343 [M-H], and m/z 325, 299, and 281 ions are shared between 14- and 17-HDHA (Fig. 1, B and C, insets). Both 14S-HDHA and 17-HDHA were also generated from endogenous DHA with isolated mouse MΦs activated with Ca2+ ionophore A23187 (5 μM, pH 7.45, n = 4; representative values: 14S-HDHA, 325 pg/106 cells, and 17-HDHA, 439 pg/106 cells). These results demonstrate that during the time course of acute inflammation and its resolution, a sustained production of endogenous LOX products was present in the initial acute phase at 2–4 h that accumulated during resolution with the highest levels of 14S-HDHA > 17-HDHA at 72 h.
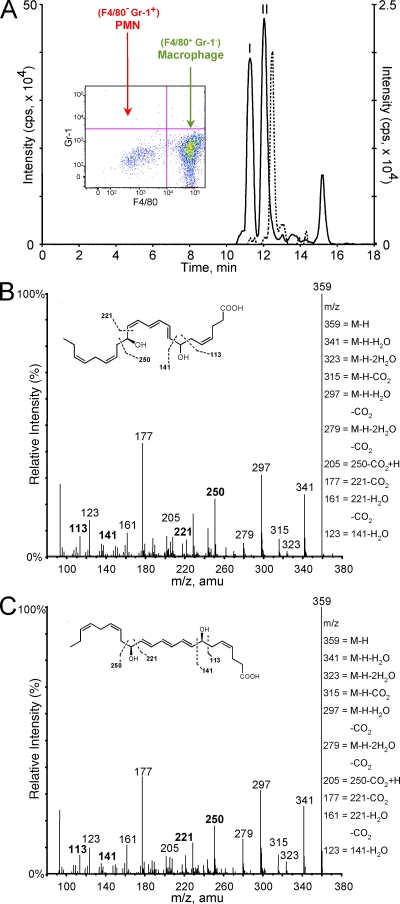
We reasoned that 14S-HDHA might be a marker reflecting activation of a novel DHA (C22:6) carbon 14–lipoxygenation pathway. This could lead to production of bioactive mediators via DHA, because monohydroxy products of polyunsaturated fatty acids are biomarkers of pathways leading to potent bioactive molecules, as in the case of 17-HDHA and 17-HpDHA, precursors to Rvs and protectins (6, 20, 21). Also, 5-hydroxyeicosatetraenoic acid is a well-appreciated marker of arachidonic acid conversion to leukotrienes (2). To this end, resident peritoneal MΦs were isolated (Fig. 2 A, FACS inset), containing ∼85–90% MΦs and 10–15% lymphocytes, and 14S-hydroperoxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S-HpDHA) was prepared via biogenic synthesis and incubated with these cells to determine whether it was a precursor to new bioactive products. 14S-HpDHA was isolated from 12-LOX incubated with HPLC-purified DHA (n = 7) and was >98% S configuration determined by chiral LC/MS/MS. Both 14S-HpDHA (10 μM) and DHA (10 μM) were converted by resident MΦs to new products identified via LC/MS/MS-based mediator lipidomics (Fig. 2 A) labeled I and II. Each had conjugated triene-containing UV chromophores, chromatographic behavior, and mass spectra consistent with 7,14-dihydroxy–containing products with a C22 backbone originating from DHA. Both gave essentially the same mass spectrum yet different retention times, indicating that they were very likely isomers (Fig. 2, B and C). These were isolated and subject to gas chromatography–mass spectrometry (GC-MS) to further identify and confirm fragment assignments and positional sites of oxygenation, e.g., carbon positions of alcohol groups. GC-MS analyses confirmed assigned ions from LC/MS/MS and were consistent with 7,14-dihydroxy–containing products biosynthesized from DHA (Table I). Isolated human MΦs incubated with 14S-HpDHA also gave the novel 7,14-dihydroxy–containing product and matched the compound from mouse MΦs (n = 3).
Figure 2.
MΦs generate novel products. (A) Mouse-resident MΦs (5 × 106 cells/ml) incubated with DHA or 14S-HpDHA showing targeted LC/MS/MS-based mediator lipidomics. Selected ion chromatogram (m/z 359/250) of 7,14-diHDHA (II) and its transconjugated isomer (I) is shown. Selected ion chromatogram (dashed overlay; m/z 359/250) shows double dioxygenation product 7S,14S-diHDHA. (inset) FACS plot of isolated resident MΦs. (B and C) Lipid mediator lipidomics. (B) Mass spectra for 7,14-diHDHA (m/z 359; B) and corresponding isomer (C). See inset and Results for diagnostic ions (n = 3). amu, atomic mass unit.
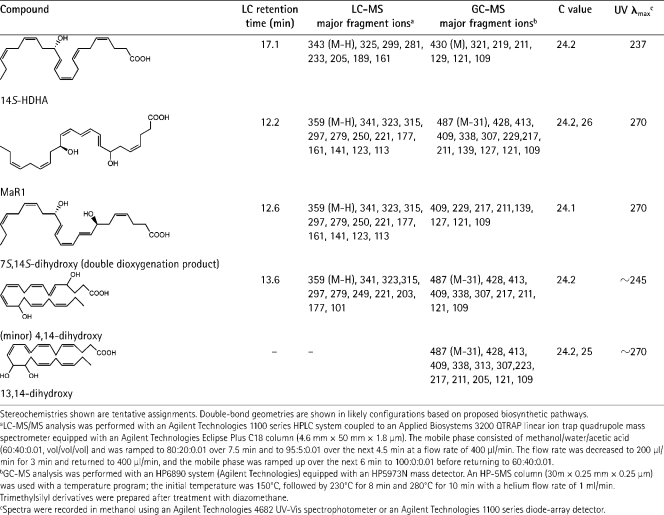
Table I.
Structures and LC-MS and GC-MS fragmentation for novel 14 series compounds identified using mediator-based lipidomics
Novel antiinflammatory and proresolving mediators
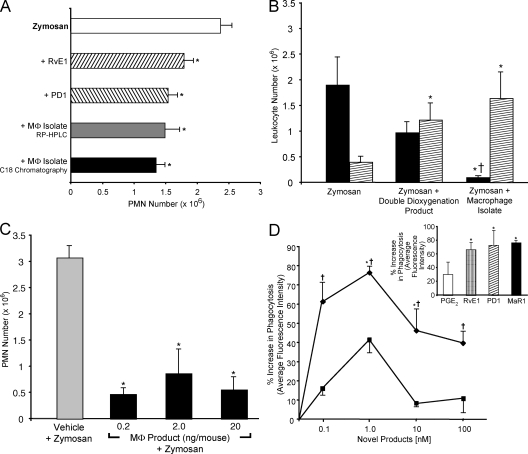
In parallel with these determinations, materials obtained from mouse MΦs were assessed for potential bioactivity after extraction and C18 solid-phase chromatography. MΦ-derived material showed remarkable antiinflammatory properties (Fig. 3 A) regulating PMN entry into zymosan-induced peritonitis when directly compared with other omega-3 fatty acid–derived mediators such as neuroprotectin/protectin D1, 10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (PD1) (20) and RvE1 (5, 6, 14). Thus, these findings suggested that within MΦ isolates obtained from cells incubated with 14S-HpDHA, potent bioactive materials were biosynthesized from this precursor that regulated PMNs, preventing their infiltration. These substances were likely to be very potent because only nanogram quantities given per mouse elicited antiinflammatory actions.
Figure 3.
Antiinflammatory and proresolving novel MΦ products. (A) Reduction in PMN in peritonitis. Activity in methyl formate fractions from C18 extraction of isolated MΦs and 20 ng/mouse of MΦ products isolated with RP-HPLC, 20 ng/mouse PD1, or 20 ng/mouse RvE1. Results are expressed as exudate PMN means ± SEM (n = 3; *, P < 0.05 compared with zymosan plus vehicle). (B) Differential PMN versus monocyte actions. Mice were injected with 0.1 ng/mouse of the double dioxygenation product, 0.1 ng/mouse of MΦ isolate, or vehicle alone (as in A), followed by i.p injection of 1 mg zymosan to evoke peritonitis. After 2 h, leukocytes were enumerated (black bar, PMNs; hatched bar, mononuclear cells). Results are means ± SEM (n = 3; *, P < 0.05 compared with zymosan plus vehicle; †, P < 0.05 for double dioxygenation vs. MΦ isolate). (C) Reduction in peritonitis showing dose response. MΦ product isolated after HPLC isolation was injected i.v. ∼2 min before i.p. zymosan. Results are means ± SEM (n = 3; *, P < 0.05 compared with zymosan plus vehicle). (D) MaR1 enhances phagocytosis. MΦs (24-well plate, 105 cells/well) were exposed to the indicated concentrations for 15 min followed by FITC-labeled zymosan for 30 min at 37°C. Results are means ± SEM expressed as the percent increase above vehicle (n = 3; *, P < 0.05 compared with vehicle; †, P < 0.05 for double dioxygenation vs. MaR1). The closed diamond represents MaR1, and the closed square represents the double dioxygenation product 7S,14S-diHDHA. (inset) Comparison of MaR1 with other mediators (1 nM).
Given the ability of DHA to serve as a substrate for LOX forming both double dioxygenation products related to PD1 as well as the carbon 17–hydroperoxide–containing precursor of epoxide (20), we reasoned that 14S-HpDHA might also be a substrate for double dioxygenation. This indeed proved to be the case: sequential actions of 12-LOX and 5-LOX with DHA generated 7S,14S-dihydroxydocosa-4Z,8E,10Z,12E,16Z,19Z-hexaenoic acid (7S,14S-diHDHA; n = 5; Fig. 2, dotted profile; and Table I). This is likely an isomer of the MΦ-isolated material (Figs. 2 and 3), because this reference compound (Fig. 2 A, dotted curve) did not coelute with MΦ-derived products beneath labels I and II. This double dioxygenation product at a 0.1-ng dose per mouse reduced PMN infiltration in zymosan-induced peritonitis but appeared to be less potent than the MΦ-isolated material (Fig. 3 B). Thus, the results in Fig. 3 clearly demonstrate potent bioactions of the novel 7,14-dihydroxy–containing product HPLC purified from resident MΦ incubations with zymosan and 14S-HpDHA.
Further experiments were performed to isolate material beneath peaks I and II (Fig. 2 A) and determine whether compound I is an isomer of II. Subjecting the isolated compounds to isomerization conditions (22) demonstrated that compound II likely contained a cis double bond–containing triene structure sensitive to conversion to an all-trans–containing conjugated triene (i.e., 8E,10E,12E) isomer I in bench and work-up conditions. In view of 18O incorporation and epoxide trapping, it is likely that the isomer I peak also contained R/S racemates at carbon 7. Further RP-HPLC purification of the 7,14-dihydroxy–containing product from MΦs was performed to assess its actions. The results in Fig. 3 C demonstrate that at doses as low as 0.2 ng/mouse, the new compound potently reduced infiltration of PMNs into sites of inflammation.
MΦ-enhanced phagocytosis
A key feature of a proresolving mediator, in addition to limiting PMN entry, is the dual action of stimulating MΦ uptake of apoptotic PMNs and/or zymosan to stimulate resolution and microbial clearance (4, 13, 23). We determined whether the new MΦ-derived compound enhanced phagocytosis and compared it with RvE1, PD1, and an eicosanoid, prostaglandin E2 (PGE2; Fig. 3 D, inset). RvE1 and PD1 are potent enhancers of MΦ phagocytosis at concentrations as low as 1 nM (13). For direct comparison, the double dioxygenation product (7S,14S-diHDHA) was active but less potent than the new product isolated from MΦs. Thus, given its potent actions and novel structure, the MΦ product was denoted maresin 1 (MaR1).
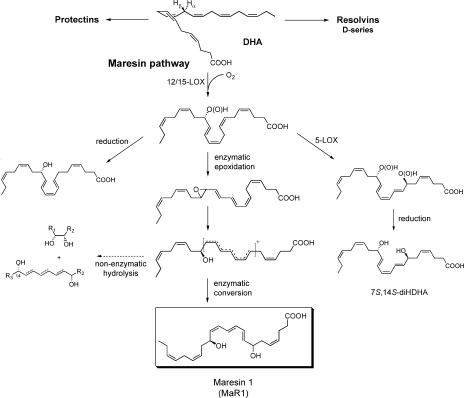
Maresin biosynthetic pathway
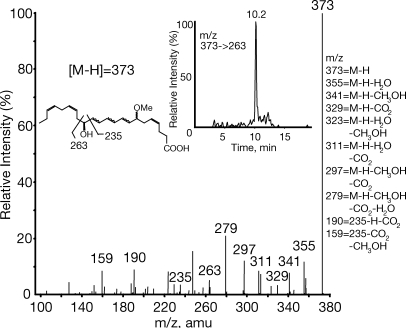
We present a hypothetical scheme for the maresin pathway (see Fig. 5). DHA is converted to 14-hydroperoxydocosahexaenoic acid, likely via 12-LOX in humans, as shown in incubations of DHA and 12-LOX, followed by either reduction to 14S-HDHA and/or, via double dioxygenation (e.g., sequential 12-LOX–5-LOX), to generate 7S,14S-diHDHA. In 12/15-LOX–deficient mice, 14S-HDHA generation in peritonitis was reduced >95%. The key 14S-hydroperoxide intermediate is enzymatically converted to a 13(14)-epoxide–containing intermediate that is then enzymatically hydrolyzed via a carbonium cation to bioactive 7,14S-diHDHA by creating a conjugated triene within three of the six double bonds. The results from 18O isotope incorporation using H218O demonstrated that >75% of the oxygen at the carbon 7 position was derived from H2O (see Supplemental materials and methods and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081880/DC1) and not from molecular oxygen, as would have been the case if this carbon 7 position alcohol group was generated by double lipoxygenation mechanism (compare with reference 20). In parallel, alcohol trapping with excess acidic methanol was performed with isolated MΦs that gave the methoxy-trapping product 7-methoxy-14-hydroxydocosa-4Z,8,10,12,16Z,19Z-hexaenoic acid, identified using LC/MS/MS targeted profiling. Its MS2 spectrum showed ions consistent with acid-assisted attack at the carbon 7 position and addition of the methoxy, giving the MS2 spectrum of m/z 373 at 10.2 min (Fig. 4, inset for ion assignments; and Fig. 5). It is also possible that a methoxy addition could have occurred at the carbon 13 position that could give essentially the same ions in MS2. It is more likely that methoxy addition was at carbon 7, because it is the least sterically hindered end of the conjugated carbonium cation.
Figure 5.
Biosynthetic scheme proposed for MaR1 and related products. Stereochemistries and double-bond geometries of the new dihydroxy-containing mediators are tentative assignments and depicted in likely configurations based on biogenic synthesis, trapping, and labeling (see Results).
Figure 4.
Identification of methoxy-trapping product from MΦs. MS/MS spectrum of m/z 373 product at 10.2 min. (inset) Extracted ion chromatogram of m/z 373→263 and deduced structure. amu, atomic mass unit.
To provide further confirmation of the maresin pathway and biosynthetic scheme, isolated mouse MΦs (15.5 × 106 cells/3 ml, 37°C, 30 min) were incubated with zymosan and 10 μM of deuterium-labeled DHA-d5 containing five deuterium atoms at the 21, 21, 22, 22, and 22 carbon positions. MΦs converted DHA-d5 to 14S-HDHA-d5, with diagnostic ions in its mass spectrum at m/z 348, 330, 304, 286, and 205, as well as further evidence for the 7,14S-dihydroxy–containing product. The dihydroxy structure carried d5, from precursor, and was confirmed with ions at m/z 364, 346, 320, and 302, and the hydroxy groups' 7 and 14 positions supported by m/z 251, 223 and 250, 221, respectively (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081880/DC1). Collectively, these results provide support for the biosynthesis of a 13(14) epoxide intermediate by MΦs from 14S-HpDHA that is enzymatically converted to the potent bioactive 7,14-dihydroxydocosa-4Z,8,10,12,16Z,19Z-hexaenoic acid MaR1 (Fig. 5).
DHA (C22:6) is an essential fatty acid and member of the omega-3 family of fatty acids, which are in high levels in marine oils. It is essential for mammalian systems in that it is not biosynthesized de novo and is therefore a nutritional requirement (10, 11, 21). In this report, we identified a novel pathway during resolution that converts DHA to new, potent bioactive products. This new pathway was identified using targeted mediator lipidomics of exudates from mouse peritonitis and was demonstrated with both mouse and human MΦs. Parallel biofunction and spectral analyses confirmed the new structures as 7,14-dihydroxy–containing products biosynthesized from DHA. One of the novel products characterized, MaR1, proved to be a potent mediator, stopping PMN infiltration and stimulating MΦ phagocytosis. An isomer of MaR1, 7S,14S-diHDHA, was less potent, indicating stereoselective actions in vitro and in vivo. These antiinflammatory and proresolving actions were evident in nanogram range both in vitro and in vivo, suggesting that this new mediator pathway could play a key role in regulating catabasis or the return of tissues from the inflammatory state to homeostasis (8, 13).
The new compounds isolated from MΦs showed distinct and separate actions on PMNs compared with mononuclear cells, such as those recently identified for multifunctional mediators. Specifically, to expedite resolution, members of this new genus of endogenous mediators carry multilevel actions limiting further PMN accumulation at tissue sites and stimulate clearance by enhancing MΦ nonphlogistic phagocytosis (4, 23). This type of selectivity places a spatial and temporal as well as a functional separation between these new mediators from, e.g., leukotrienes, which stimulate proinflammatory responses.
When compared with RvE1 derived from EPA and PD1/NPD1 from DHA that carries alcohol groups at carbons 10 and 17 (6, 7, 20, 21), MaR1 proved to be of comparable potency (Fig. 3). In contrast, PGE2 did not enhance phagocytosis, a finding consistent with PGE2 and PGD2 specifically reducing MΦ phagocytosis of apoptotic cells (24). Although D and E series Rvs as well as PD1 share proresolving and antiinflammatory actions for the genus, each member acts at specific receptors (4, 14) and, thus, given the stereospecific actions of the new pathway mediators, it is likely that they act on their own receptors separate from those for Rvs. These findings with novel chemical mediators suggest that enhancing MΦ capacity to remove apoptotic and necrotic cells at sites of inflammation and enhancing microbial particle containment along with down-regulating new PMN entry to the site may not only shorten the resolution interval (8, 13) but can also protect tissues from unwanted tissue injury damage and oxidative stress that can accompany inflammation and infection.
We also found that the epoxide intermediate can undergo nonenzymatic hydrolysis to give 7R/S,14S-dihydroxy–containing products (which appear to coelute in this LC system) and corresponding vicinal diol, namely 13,14-diHDHA (which was isolated and identified; see Fig. 5, Table I, and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081880/DC1). The proposed biosynthetic sequence is also supported by results from 18O incorporation and deuterium (d5)-labeled tracking from d5-DHA to d5-labeled maresin pathway products. Collectively, these results provide evidence for a highly efficient pathway in isolated resident MΦs for the biosynthesis of potent new chemical mediators via 14-lipoxygenation of DHA and subsequent enzymatic steps (Fig. 5). We also identified 4,14-di-HDHA (Table I), a likely product of 5-LOX and 12-LOX interactions, as well as two other new products from DHA generated by MΦs, a 13(14)-epoxy-alcohol–containing docosanoid and 4S,13,14S-trihydroxydocosa-5,7,9,11,16Z,19Z-hexaenoic acid (their biosynthesis and actions will be reported separately).
The 14S-HDHA via arachidonate 12-LOX was first identified in the gills of fish (25) and in human platelets (26), neural systems (27), and many other mammalian and invertebrate marine organisms (28). Thus, although 14S-HDHA was identified in many systems, it remained to be determined that it can serve as a marker of DHA conversion to novel bioactive mediators. It should be noted that, in addition to 14S-HDHA production by cells with 12-LOX, human 15-LOX can also contribute to this 14-LOX pathway because MΦs and other human cells possess a prominent 12-LOX and 15-LOX (2, 10, 18), and 14S-HDHA was essentially absent in 12/15-LOX–deficient mice. Given the importance of platelet 12-LOX in transcellular biosynthesis of lipid mediators (4), it is likely that cell–cell interactions can also contribute to biosynthesis of maresins and related products.
Although marine omega-3 fatty acid supplements are in wide use in animals and humans because they are believed to possess therapeutic actions, convincing evidence from clinical trials supporting their actions in treating inflammatory disorders and reducing cancer risk are not without criticism (29–31). Possible sources of variation in their actions in clinical studies are (a) the very high doses used (milligram to gram doses), (b) the absence of appropriate biomarkers of omega-3 fatty acid utilization, and (c) specific mediator functions evoked in pico- to nanogram range. Thus, the new pathways and bioactive maresins documented in this report, along with Rvs, protectins, and related functional metabolome, might provide a new means to mark the impact of essential omega-3 fatty acids in health and disease, as well as provide new therapeutic approaches.
MATERIALS AND METHODS
Materials.
DHA, 12-LOX (porcine), 5-LOX (human), 17-HDHA-d5, and PGE2-d4 were purchased from Cayman Chemical. Zymosan A was purchased from Sigma-Aldrich. Solid-phase extraction materials, LC/MS/MS, and GC-MS solvents and reagents were obtained as previously described (32). Synthetic RvE1 and PD1 were prepared by total organic synthesis according to published matching criteria (14, 20, 32) and provided via the Synthesis Core (N.A. Petasis, University of Southern California, Los Angeles, CA), Specialized Research Center (C.N. Serhan).
14S-HpDHA was prepared from DHA (∼150 μM) incubated with 5.4 U/ml isolated 12-LOX (porcine) (0.05 M phosphate buffer, 0.02% Tween 20, pH 7.4). 14S-HpDHA was isolated via RP-HPLC (1100 Series; Agilent Technologies) using a C18 column (250 mm × 10 mm × 5 μm; Beckman Coulter) and a mobile phase consisting of methanol/water (80:20, vol/vol) at 4 ml/min for 20 min and was >98% (S) configuration (see Supplemental materials and methods). Reduction with NaBH4 yielded 14S-HDHA used for mass spectrometry standard. Biogenic synthesis of the double dioxygenation product (7S,14S-diHDHA) was performed with 5-LOX enzyme incubated with 14S-HDHA. The 7S,14S-diHDHA was scaled up for direct comparison of biological and physical properties with other novel compounds isolated from MΦs.
MΦ incubations.
Resident peritoneal MΦs were collected by lavage from naive mice (20–25 g, 6–8-wk-old FVB mice; Charles River Laboratories) with unlimited access to rodent diet 5001 (Laboratory Diet) containing EPA 1.5% and DHA 1.9% of total fatty acids (19). All animal studies were approved and performed in accordance with guidelines provided by the Harvard Medical Standing Committee on Animals (protocol no. 02570).
After centrifugation at 2,000 rpm and addition of DPBS+/+, MΦs (15.5 × 106 cells/3 ml) were incubated with zymosan A and 10 μM DHA or 14S-HpDHA (pH 7.45) at 37°C for 30 min. For assessing 18O incorporation, 0.45 ml H218O (Cambridge Isotopes) was added to 50 μl 10× DPBS+/+, mixed and adjusted to approximately pH 7.3 with 1 N HCl. 5 × 106 isolated peritoneal MΦs were suspended in H218O-containing buffer. After rapid freeze–thaw in liquid nitrogen, 5 μM of purified 14S-HpDHA was added with 2.5 μM A23187 for 30 min at 37°C. For alcohol trapping, isolated MΦs (5 × 106 cells/100 μl) were incubated at 37°C for 5 min with 100 μM 14S-HpDHA and 5 μM A23187, and incubations were stopped with 10× vol of cold methanol, with the apparent pH adjusted to approximately pH 3 (20). All other incubations were stopped with 2 vol of cold methanol and held at −80°C before extraction.
Mediator lipidomics: product isolation and extractions.
Deuterated internal standard (17-HDHA-d5, PGE2-d4; ∼3 ng) was added to each incubation after protein precipitation >30 min. Samples were extracted (32) and methyl formate fractions were taken for LC/MS/MS-based mediator lipidomics. UV spectra were recorded in methanol using an Agilent Technologies 4682 system for quantitation and assessment of structural integrity of known mediators using appropriate extinction coefficients (32).
LC/MS/MS-based analysis was performed with an HPLC system (Agilent Technologies 1100 series) with a linear ion trap quadrupole mass spectrometer (3200 QTRAP; Applied Biosystems) equipped with an Eclipse Plus C18 column (4.6 mm × 50 mm × 1.8 μm; Agilent Technologies). The instrument was run in negative ionization mode, and for enhanced product ion (EPI) mode, the mobile phase consisted of methanol/water/acetic acid (60:40:0.01, vol/vol/vol) and ramped to 80:20:0.01 over 7.5 min and to 95:5:0.01 in the next 4.5 min at a flow rate of 400 μl/min. The flow rate was decreased to 200 μl/min for 3 min and returned to 400 μl/min, and the mobile phase was ramped up over the next 6 min to 100:0:0.01. For multiple reaction monitoring (MRM) data acquisition, the mobile phase was methanol/water/acetic acid (60:40:0.01) ramped to 80:20:0.01 after 5 min, 95:5:0.01 after 8 min, and 100:0:0.01 after 14 min to wash the column.
The novel dihydroxy-containing products from DHA were monitored in EPI mode (359.2). Ion pairs from reported MRM methods were used for profiling and quantitation of 17-HDHA, 14S-HDHA, and internal standards. Ion pair 359.2/250.2 was used to identify 7,14-dihydroxy–containing products. Criteria for matching retention time and greater than or equal to six diagnostic ions to those of synthetic references were used for identification (32). Quantitation was performed using calibration curves constructed for each compound, and recoveries were monitored using added deuterated internal standards.
Mouse peritonitis and phagocytosis.
The 7,14-dihydroxy–containing products were isolated from methyl formate fractions obtained from mouse MΦs via RP-HPLC (Agilent Technologies 1100 series) using a Beckman Coulter C18 column (250 mm × 10 mm × 5 μm) and methanol/water (65:35, vol/vol) ramped to 85:15 for 30 min. Their actions were assessed in mouse zymosan A–induced peritonitis (19). Peritonitis was initiated by i.p. administration of 1 mg zymosan A in 1 ml of sterile saline, and each compound was administered i.v. 5 min before zymosan. At 2 h, mice were killed, and peritoneal exudates were harvested (5 ml DPBS−/− without calcium and magnesium), identified, and enumerated by light microscopy and FACS. Resident MΦs were identified by FACS (8). To assess proresolving actions, peritoneal MΦs (24-well plate, 105 cells/well) from naive mice were incubated with each compound (15 min, pH 7.45), followed by the addition of FITC-labeled zymosan A for 30 min at 37°C. Trypan blue was used to quench extracellular zymosan particles for 1 min at 37°C, followed by DPBS+/+ (pH 7.45), and phagocytosis was quantified using a Victor3 (PerkinElmer).
Statistical analysis.
Results are expressed as means ± SEM. Statistical significance was determined using a two-tailed Student's t test.
Online supplemental material.
Fig. S1 shows the MS-MS spectrum of the novel product obtained with H218O and MΦ incubations. Fig. S2 is the representative mass spectrum of d5-MaR1 from MΦ incubation with d5-DHA. Fig. S3 is the GC-MS spectrum obtained for the 13,14-dihydroxy vicinal diol from DHA and MΦs. This product derivative was obtained after treatment with diazomethane and BSTFA to give the methyl ester, OTMS derivative. Supplemental materials and methods describes human MΦ incubations, GC-MS analysis, and chiral HPLC-MS/MS. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081880/DC1.
Supplementary Material
Acknowledgments
We thank M.H. Small for expert assistance in manuscript preparation, Dr. N. Chiang for FACS analysis, and Dr. K. Gronert for carrying out peritonitis with 12/15-LOX KO mice.
We acknowledge National Institutes of Health grants P50-DE016191 and R37-GM038765.
C.N. Serhan is an inventor on U.S. patents for RvE1, Rv composition, and related compounds, as well as their clinical uses assigned to Brigham and Women's Hospital that are licensed and subject for consultantship. The authors have no other conflicting financial interests.
References
- 1.Nathan, C. 2002. Points of control in inflammation. Nature. 420:846–852. [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson, B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220:568–575. [DOI] [PubMed] [Google Scholar]
- 3.Gilroy, D.W., T. Lawrence, M. Perretti, and A.G. Rossi. 2004. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3:401–416. [DOI] [PubMed] [Google Scholar]
- 4.Serhan, C.N., N. Chiang, and T.E. Van Dyke. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan, C.N., C.B. Clish, J. Brannon, S.P. Colgan, N. Chiang, and K. Gronert. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2–nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan, C.N., S. Hong, K. Gronert, S.P. Colgan, P.R. Devchand, G. Mirick, and R.-L. Moussignac. 2002. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 196:1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong, S., K. Gronert, P. Devchand, R.-L. Moussignac, and C.N. Serhan. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 278:14677–14687. [DOI] [PubMed] [Google Scholar]
- 8.Bannenberg, G.L., N. Chiang, A. Ariel, M. Arita, E. Tjonahen, K.H. Gotlinger, S. Hong, and C.N. Serhan. 2005. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174:4345–4355. [DOI] [PubMed] [Google Scholar]
- 9.Cotran, R.S., V. Kumar, and T. Collins, editors. 1999. Robbins Pathologic Basis of Disease. W.B. Saunders Co., Philadelphia. 1425 pp.
- 10.Calder, P.C. 2007. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 77:327–335. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos, A.P. 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21:495–505. [DOI] [PubMed] [Google Scholar]
- 12.Orr, S.K., and R.P. Bazinet. 2008. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Investig. Drugs. 9:735–743. [PubMed] [Google Scholar]
- 13.Schwab, J.M., N. Chiang, M. Arita, and C.N. Serhan. 2007. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 447:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arita, M., F. Bianchini, J. Aliberti, A. Sher, N. Chiang, S. Hong, R. Yang, N.A. Petasis, and C.N. Serhan. 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 201:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cash, J.L., R. Hart, A. Russ, J.P.C. Dixon, W.H. Colledge, J. Doran, A.G. Hendrick, M.B.L. Carlton, and D.R. Greaves. 2008. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 205:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudert, C.A., K.H. Weylandt, J. Wang, Y. Lu, S. Hong, A. Dignass, C.N. Serhan, and J.X. Kang. 2006. Transgenic mice rich in endogenous n-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. USA. 103:11276–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronert, K., N. Maheshwari, N. Khan, I.R. Hassan, M. Dunn, and M.L. Schwartzman. 2005. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 280:15267–15278. [DOI] [PubMed] [Google Scholar]
- 18.Merched, A., K. Ko, K.H. Gotlinger, C.N. Serhan, and L. Chan. 2008. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 22:3595–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winyard, P.G., and D.A. Willoughby, editors. 2003. Inflammation Protocols. Humana, Totowa, NJ. 378 pp.
- 20.Serhan, C.N., K. Gotlinger, S. Hong, Y. Lu, J. Siegelman, T. Baer, R. Yang, S.P. Colgan, and N.A. Petasis. 2006. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 176:1848–1859. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee, P.K., V.L. Marcheselli, C.N. Serhan, and N.G. Bazan. 2004. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 101:8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox, M.A., and J.K. Whitesell. 1997. Organic Chemistry. Jones and Bartlett Publishers, Boston. 1248 pp.
- 23.Godson, C., S. Mitchell, K. Harvey, N.A. Petasis, N. Hogg, and H.R. Brady. 2000. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164:1663–1667. [DOI] [PubMed] [Google Scholar]
- 24.Rossi, A.G., J.C. McCutcheon, N. Roy, E.R. Chilvers, C. Haslett, and I. Dransfield. 1998. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J. Immunol. 160:3562–3568. [PubMed] [Google Scholar]
- 25.German, J.B., G.G. Bruckner, and J.E. Kinsella. 1986. Lipoxygenase in trout gill tissue acting on arachidonic, eicosapentaenoic and docosahexaenoic acids. Biochim. Biophys. Acta. 875:12–20. [DOI] [PubMed] [Google Scholar]
- 26.Lagarde, M., M. Croset, M. Guichardant, and M. Dechavanne. 1985. Role of lipoxygenase products in platelet function: relation to fatty acid modified phospholipids. Adv. Exp. Med. Biol. 192:327–335. [DOI] [PubMed] [Google Scholar]
- 27.Kim, H.Y., J.W. Karanian, T. Shingu, and N. Salem Jr. 1990. Stereochemical analysis of hydroxylated docosahexaenoates produced by human platelets and rat brain homogenate. Prostaglandins. 40:473–490. [DOI] [PubMed] [Google Scholar]
- 28.Rowley, A.F., H. Kühn, and T. Schewe, editors. 1998. Eicosanoids and Related Compounds in Plants and Animals. Portland Press, London. 240 pp.
- 29.MacLean, C.H., S.J. Newberry, W.A. Mojica, P. Khanna, A.M. Issa, M.J. Suttorp, Y.W. Lim, S.B. Traina, L. Hilton, R. Garland, and S.C. Morton. 2006. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 295:403–415. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer, J.H., H. Allayee, K.M. Dwyer, J. Fan, H. Wu, R. Mar, A.J. Lusis, and M. Mehrabian. 2004. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N. Engl. J. Med. 350:29–37. [DOI] [PubMed] [Google Scholar]
- 31.Colomer, R., J.M. Moreno-Nogueira, P.P. García-Luna, P. García-Peris, A. García-de-Lorenzo, A. Zarazaga, L. Quecedo, J. del Llano, L. Usán, and C. Casimiro. 2007. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br. J. Nutr. 97:823–831. [DOI] [PubMed] [Google Scholar]
- 32.Serhan, C.N., Y. Lu, S. Hong, and R. Yang. 2007. Mediator lipidomics: search algorithms for eicosanoids, resolvins and protectins. Methods Enzymol. 432:275–317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.