Abstract
Adequate numbers and functional maturity are needed for leukocytes to exhibit a protective role in host defense. During intrauterine life, the skin immune system has to acquire these prerequisites to protect the newborn from infection in the hostile external environment after birth. We investigated the quantitative, phenotypic, and functional development of skin leukocytes and analyzed the factors controlling their proliferation and trafficking during skin development. We show that CD45+ leukocytes are scattered in embryonic human skin and that their numbers continuously increase as the developing skin generates an environment that promotes proliferation of skin resident leukocytes as well as the influx of leukocytes from the circulation. We also found that CD45+HLA-DRhighCD1c+ dendritic cells (DCs) are already present in the epidermis and dermis at 9 wk estimated gestational age (EGA) and that transforming growth factor β1 production precedes Langerin and CD1a expression on CD45+CD1c+ Langerhans cell (LC) precursors. Functionally, embryonic antigen-presenting cells (APCs) are able to phagocytose antigen, to up-regulate costimulatory molecules upon culture, and to efficiently stimulate T cells in a mixed lymphocyte reaction. Collectively, our data provide insight into skin DC biology and the mechanisms through which skin DCs presumably populate the skin during development.
The skin is a highly active immunological organ that contains a multitude of specialized immune cells. Among them, epidermal Langerhans cells (LCs), dermal DCs, and macrophages play a key role in the initiation and regulation of immune responses (1). A unifying feature is their hematopoietic origin and expression of HLA-DR. LCs and dermal DCs are professional APCs that express high levels of HLA-DR and CD1c (2). In humans, LCs can be identified by the expression of Langerin (CD207) (3–6), whereas the heterogeneous dermal DCs can be classified according to the expression of CD1a and various monocyte/macrophage markers (4, 7, 8). Skin macrophages, in contrast, express low levels of HLA-DR molecules and lack CD1c (6, 9). To date, various blood-borne and skin-resident precursors for LCs, dermal DCs, and macrophages have been described (10–15). Despite a considerable number of in vitro studies, the relationship among these cells, as well as the factors that control their immigration, differentiation, and proliferation under homeostatic and inflammatory conditions, remains unclear in humans. During skin development, LC, dermal DC, and macrophage precursors are believed to colonize the embryonic skin, showing a primitive surface marker pattern that subsequently develops into the profile of resident cells found in adult skin (16, 17). Thus, the study of the ontogeny of APCs during human intrauterine development provides a unique opportunity to evaluate these processes as well as their relationship in this human in vivo model.
During human embryogenesis, leukocytes have been first described in the yolk sac by around 5 wk estimated gestational age (EGA) on the basis of staining reactivity for various DC and macrophage markers, including HLA-DR (18). The few studies investigating the development of leukocytes in human skin focused primarily on LCs (16, 19, 20); therefore, our knowledge about the ontogeny of dermal DCs, macrophages and other skin leukocytes is scarce (17, 21). HLA-DR+ cells have been described in skin at 7 wk EGA. Apart from their rare occurrence, they also differ morphologically and phenotypically from leukocytes in adult skin. At 7 wk EGA, some epidermal cells already express HLA-DR, but not CD1a or Lag antigen, a component of Birbeck granules, which appear at ∼12 wk EGA (16, 19). To better understand the development of professional skin APCs and to gain information about the organization of the skin immune system in utero, we investigated the quantitative, phenotypic, and functional development of leukocytes and analyzed the factors that might control their proliferation and trafficking during human skin development.
RESULTS
Embryonic and fetal skin, but not adult skin, contains high numbers of proliferating leukocytes
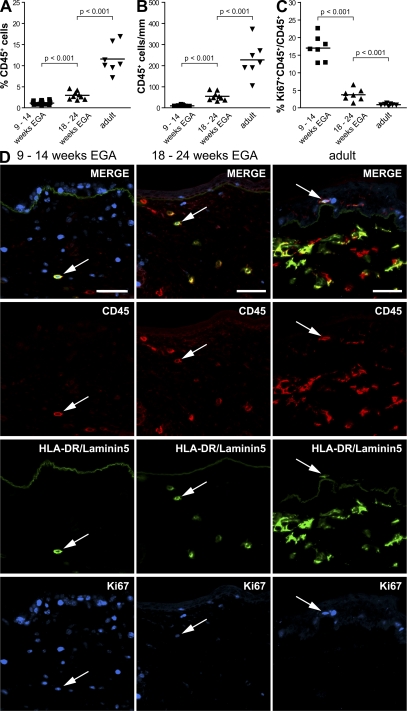
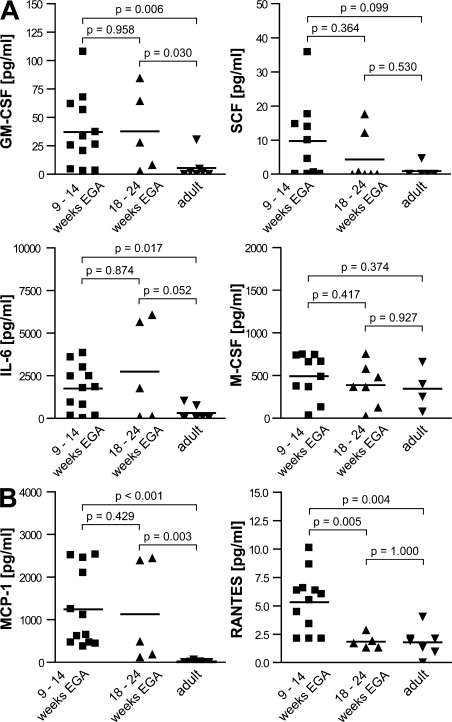
During intrauterine life, the skin immune system has to acquire the prerequisites to protect the newborn from infection in the hostile environment after birth, which includes the formation of a leukocyte network. To explore the kinetics of leukocyte numbers in developing and adult human skin, we enzymatically digested whole skin and analyzed the percentage of CD45+ leukocytes using flow cytometry. We found that relative leukocyte numbers among all skin cells in developing skin increase from 1.13% in embryonic skin (9–14 wk EGA) to 2.97% in fetal skin (18–24 wk EGA), which is significantly lower than in adult skin (11.59%; Fig. 1 A). To exclude skin digestion's influence on the quantity of released leukocytes, we analyzed in parallel the development of the absolute leukocyte numbers by counting CD45+ cells in skin sections. This confirmed that leukocyte numbers are significantly lower in embryonic and fetal skin than in adult skin (Fig. 1 B). To test whether the increase in leukocyte numbers in developing skin is caused by proliferating leukocytes, skin sections were counterstained with the nuclear proliferation marker Ki67. We found that 17.0% of CD45+ cells in embryonic skin, but only 3.7% in fetal skin and 0.9% in adult skin, expresses Ki67 (Fig. 1 C). Triple immunofluorescence staining of skin sections revealed that Ki67 is found in CD45+HLA-DR+ leukocytes in both the dermis and the epidermis (Fig. 1 D, arrows; and not depicted) and in dermal CD45+HLA-DR− leukocytes (not depicted) in all developmental stages as well as in adult skin. CD45−Ki67+ cells represent, among others, proliferating keratinocytes, fibroblasts and endothelial cells. We next asked whether the high rate of proliferating leukocytes in embryonic and fetal skin could be explained by higher concentrations of proliferation-inducing cytokines. Indeed, analysis of skin cell culture supernatants from embryonic, fetal, and adult single cells revealed that GM-CSF, SCF, and IL-6 concentrations are five- to sevenfold higher in supernatants of embryonic compared with adult skin (Fig. 2 A). No obvious differences in M-CSF concentrations among all investigated age groups were observed (Fig. 2 A), and IL-3 was not detectable (not depicted). In addition to the local proliferation of skin-resident leukocytes, their numerical increase may also be caused by the attraction of leukocytes from the circulation. As it is impossible to directly study the influx of leukocytes into skin in humans, we measured surrogate markers for leukocyte immigration such as chemokine levels. We found that MCP-1 (CCL2) concentrations are 80-fold higher and RANTES (CCL5) levels are twofold higher in supernatants from embryonic skin than from adult skin (Fig. 2 B). In contrast to MCP-1, RANTES reaches adult-like levels already at 18–24 wk EGA. We observed no significant differences in CCL20 and MDC (CCL22) concentrations among all age groups investigated (unpublished data).
Figure 1.
Proliferating CD45+ cells contribute to the increase of leukocyte numbers in developing skin. Dot graphs show the increase of relative (A) and absolute (B) numbers of CD45+ leukocytes in developing skin analyzed by flow cytometry and immunofluorescence, respectively. (C) The percentage of Ki67+CD45+ cells among total CD45+ skin cells during different stages of development was determined. 9-14 wk EGA: A, n = 18; B, n = 9; C, n = 7. 18–24 wk EGA: A and B, n = 9 each; C, n = 7. Adult: A–C, n = 7 each. Bars represent the mean of investigated groups. (D) Immunofluorescence triple labeling identified proliferating CD45+HLA-DR+Ki67+ cells (arrows) in all investigated age groups. Alexa Fluor 488–labeled Laminin5 mAb visualizes the dermo-epidermal junction. Shown is one representative experiment of three independent experiments per group with similar results. Bars, 50 μm.
Figure 2.
Cell culture supernatants of embryonic skin contain higher levels of proliferation-inducing growth factors and chemokines than adult skin. Single cell suspensions of embryonic (9–14 wk EGA), fetal (18–24 wk EGA), and adult skin were cultured for 48 h, and levels of proliferation-inducing growth factors (A) and chemokines (B) in supernatants were analyzed by ELISA and Luminex technology. Bars represent the mean of investigated groups. 9-14 wk EGA: GM-CSF, n = 12; SCF, n = 10; IL-6, n = 12; M-CSF, n = 10; MCP-1, n = 12; RANTES, n = 12. 18–24 wk EGA: GM-CSF, n = 5; SCF, n = 7; IL-6, n = 5; M-CSF, n = 7; MCP-1, n = 5; RANTES, n = 5. Adult GM-CSF, n = 7; SCF, n = 5; IL-6, n = 6; M-CSF, n = 4; MCP-1, n = 7; RANTES, n = 7.
To evaluate to what extent cells of the epidermal and the dermal compartment may contribute to the measured cytokines, we determined the percentage of keratinocytes, leukocytes, and CD45− dermal cells in single cell suspensions of whole embryonic and adult skin. The morphology of skin, including the number of cell layers in the epidermis and the overall thickness, changes markedly during development, and the cellular composition of single cell suspensions reflects this development (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1). Using CDCP1 (CD318), a cell surface marker for keratinocytes and hematopoietic stem cells (22, 23), we found that embryonic single cell suspensions contain 8.28% keratinocytes (i.e., CD318+ but CD45− cells), 1.13% CD45+ leukocytes, and, consequently, 90.59% dermal CD45− cells. In contrast, single cell suspensions of adult skin contain more keratinocytes (60.53%) and leukocytes (11.59%) and, thus, lower numbers of CD45− dermal cells (27.64%) than embryonic skin (Table S1, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1).
HLA-DR+ leukocytes are present in embryonic skin
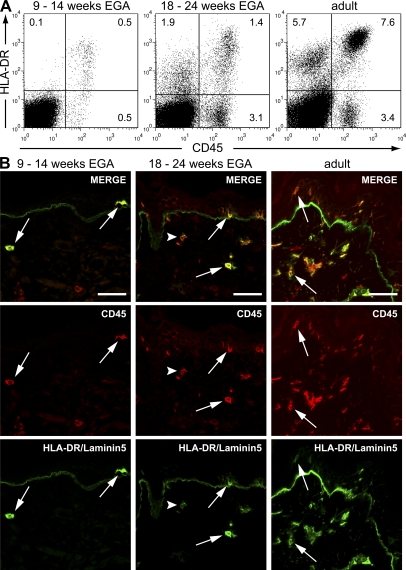
In healthy adult skin CD45+ LCs, dermal DCs, macrophages, and a fraction of CD45− endothelial cells express HLA-DR (24, 25). To explore the development of CD45+HLA-DR+ leukocytes, we analyzed embryonic, fetal, and, for comparison, adult skin by flow cytometry. We found that CD45+HLA-DR+ cells are already present in embryonic skin, although in lower frequency than in fetal or in adult skin (Fig. 3 A and Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1), thus confirming and extending findings of previous studies (16, 19, 21). In contrast to adult skin, where the majority of CD45+HLA-DR+ leukocytes express high levels of this marker, developing skin shows a continuum of HLA-DRlow to HLA-DRhigh leukocytes. Although HLA-DR expression is restricted to CD45+ leukocytes in embryonic skin, CD45−HLA-DR+ cells were first detected in fetal skin, although less frequently than in adult skin (Fig. 3 A; Fig. S2 B). Immunofluorescence locates CD45+HLA-DRhigh cells in the epidermis and the dermis, whereas CD45+HLA-DRlow cells are found exclusively in the dermis (Fig. 3 B, arrowheads). Using collagen type IV to depict the basement membrane of vessels, we detected CD45−HLA-DR+ cells in fetal and adult skin lining the luminal side of the basement membranes of vessels, thereby confirming their endothelial nature (Fig. S2 C). Keratinocytes show no expression of HLA-DR in all samples investigated.
Figure 3.
HLA-DR+ leukocytes are present in the skin already at 9 wk EGA. (A) Multiparameter flow cytometry of freshly isolated single cells of embryonic, fetal, and adult skin was performed by incubation with mAb against the cell surface markers indicated. Dead cells were excluded by 7-AAD uptake. Quadrants in dot plots were set according to isotype-matched control staining. Dot plots display 60,000 cells and are representative of 5–15 experiments (compare Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1). (B) Immunofluorescence double staining for the markers indicated was performed on cryostat sections of embryonic, fetal, and adult skin. One of at least three experiments per group is shown. Arrows denote CD45+HLA-DRhigh cells and arrowheads denote CD45+HLA-DRlow cells. Bars, 50 μm.
Some HLA-DRhigh leukocytes coexpress CD1c in embryonic skin
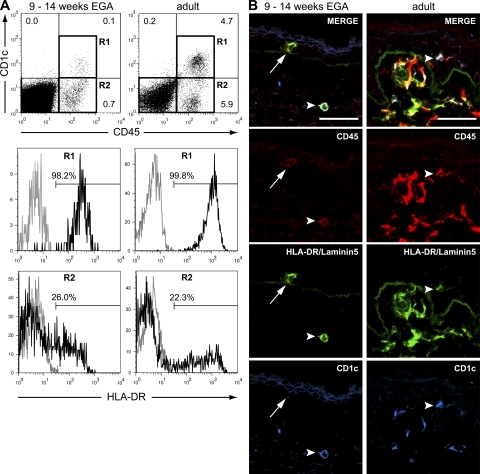
Skin DCs can be distinguished from macrophages by the expression of CD1 antigens (6, 9, 26). To address whether HLA-DR+ leukocytes in embryonic skin are already committed to the DC lineage, we analyzed whether they coexpress CD1c. Indeed, using flow cytometry, we identified a small CD45+CD1c+ population in embryonic skin that shows high expression of HLA-DR (Fig. 4 A, R1). The CD45+CD1c− population contains a small population expressing low levels of HLA-DR (Fig. 4 A, R2). Both populations are found in much higher frequencies in adult skin, where higher levels of HLA-DR expression are also found. Immunofluorescence revealed the presence of CD45+HLA-DR+CD1c+ cells in the dermis (Fig. 4 B, arrowheads) and the epidermis (not depicted; compare Fig. 5 B, left). Not all epidermal CD45+HLA-DR+ leukocytes in embryonic skin express CD1c, indicating that either immature or uncommitted epidermal LC precursors acquire this antigen in the epidermis (Fig. 4 B, arrows). However, CD1c may also be expressed on B cells (6, 27). To exclude the B cells contained by CD1c+ population, we analyzed developing skin for the presence of CD19+ B cells by flow cytometry. As we could not detect CD19+ cells in embryonic and fetal skin (unpublished data), our data indicate that CD1c+ cells in developing skin are exclusively DCs.
Figure 4.
Not all HLA-DR+ epidermal leukocytes express CD1c at 9 wk EGA. (A) Flow cytometric analysis revealed the presence of CD45+CD1c+ cells in embryonic skin. Histograms display HLA-DR staining (black line) of CD45+CD1c+ (R1) and CD45+CD1c− (R2). Gray line, isotype control. Shown are representative dot plots of three to five experiments per group. (B) Immunofluorescence triple labeling identified HLA-DR+CD1c− (arrows) and HLA-DR+CD1c+ (not depicted) leukocytes in embryonic epidermis. Arrowheads denote CD45+HLA-DR+CD1c+ cells. Data are representative of at least three experiments per group. Bars, 50 μm.
Figure 5.
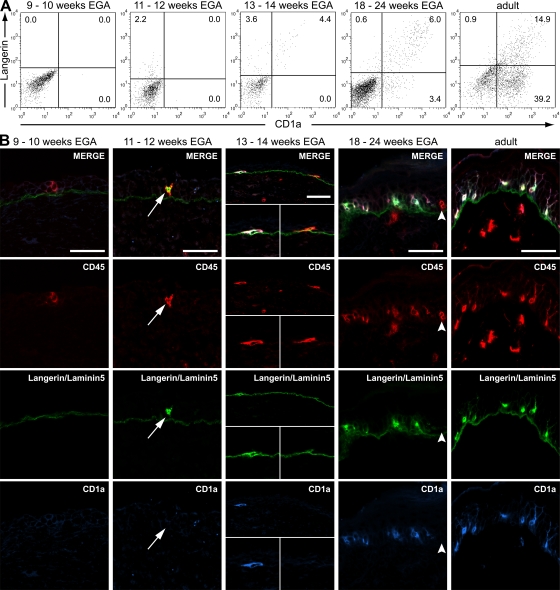
Epidermal leukocytes sequentially acquire CD1c and Langerin. CD1c and Langerin expression in developing human skin was assessed by flow cytometry (A) and in situ by immunofluorescence staining (B). (A) Dot plots show the development of CD45+ cells with regard to the expression of CD1c and Langerin (2,500 CD45+ cells displayed) and are representative of three to five experiments per group. (B) Immunofluorescence triple labeling identifies CD45+CD1c+Langerin+ cells (middle, arrows) at 11 wk EGA. Arrowheads denote a CD45+CD1c−Langerin− LC precursor. Data are representative of at least three experiments per group. Bars, 50 μm.
CD45+CD1c+ LC precursors acquire Langerin and CD1a in the epidermis
To test whether LC precursors acquire their surface marker profile in the skin, we analyzed the expression of the LC markers Langerin and CD1a during skin development. We found that Langerin is absent on CD45+CD1c+ cells between 9 and 10 wk EGA (Fig. 5, A and B, left). It exclusively appears on CD45+CD1c+ cells in the epidermis after 11 wk EGA (Fig. 5, A and B, middle, arrows). We demonstrate that embryonic (Fig. 5 B, arrowheads) and fetal (not depicted) epidermis contain LCs in different developmental stages with regard to CD1c and Langerin expression. In addition, Langerin expression precedes CD1a acquisition. CD1a is not found on CD45+HLA-DR+Langerin− and CD45+HLA-DR+Langerinlow cells until 13 wk EGA (Fig. 6 A). Thereafter, LangerinlowCD1a− and LangerinhighCD1a+ leukocytes can be identified. Immunofluorescence reveals that these changes take place in the epidermis, given that both Langerin+CD1a+ and Langerin+CD1a− epidermal leukocytes can be detected (Fig. 6 B, middle, insets). CD1a+Langerin− dermal DCs are not present in developing skin before 18–24 wk EGA. At this stage of development, the staining pattern of Langerin and CD1a resembles very much the pattern observed in adult skin, even though the frequency is lower. Surprisingly, dendritic Langerin−CD1a− cells are still found in the epidermis at the end of the second trimester (Fig. 6 B, arrowheads). To exclude death and replacement of these LC precursors, we analyzed developing skin for the presence of apoptotic CD45+ leukocytes. As we did not detect terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)+ leukocytes in embryonic and fetal skin (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1), our data strongly indicate that LC precursors acquire CD1c, Langerin, and CD1a in the epidermis during intrauterine development in a stepwise manner.
Figure 6.
Epidermal leukocytes sequentially acquire Langerin and CD1a. CD1a and Langerin expression in developing skin was assessed by flow cytometry (A) and immunofluorescence (B). (A) Dot plots are representative of three to five experiments per group and show the development of CD45+HLA-DR+ cells with regard to the expression of CD1a and Langerin (1,200–2,400 cells displayed). (B) The first Langerin+CD1a− LC precursors are identified by immunofluorescence at 11 wk EGA (second column, arrows). CD1a expression is found on some (middle, left insets) but not all (middle, right insets) CD45+Langerin+ cells at 13 wk EGA. Arrowheads denote a CD45+Langerin−CD1a− LC precursor. Data are representative of at least three experiments per group. Bars, 50 μm.
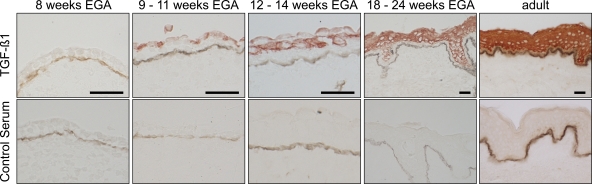
Several studies demonstrated that LC development is dependent on TGF-β1 (14, 15, 28–30). We therefore asked whether the acquisition of the LC phenotype correlates with the incipient production of this cytokine in the epidermis. Immunohistochemical analysis revealed that the TGF-β1 precursor protein LAP is already detectable at 8 wk EGA in the periderm, which is a protective development-specific cell layer covering the basal layer (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081747/DC1). Within the next weeks of development, production of LAP shifts to the basal epidermal layer but can still be found in the other layers, which is a staining pattern comparable to that of adult skin. The active form TGF-β1 is first detectable in the primitive epidermis at 9 wk EGA (Fig. 7). After the beginning of stratification, strong intracellular TGF-β1 expression is found in the intermediate cell layers between the periderm, which shows faint TGF-β1 staining reactivity until its disintegration at the end of the second trimester, and the basal layer, which never shows TGF-β1 production during development. Already at the end of the second trimester, the epidermal TGF-β1 staining pattern resembles the one observed in adult skin, apart from a general weaker staining intensity. As described in adult skin (31), throughout development TGF-β1 is primarily localized in the epidermis.
Figure 7.
Epidermal TGF-β1 production precedes the acquisition of the LC phenotype. Double immunohistochemical staining was performed on cryostat sections of embryonic, fetal, and adult human skin. TGF-β1 was visualized with AEC (red). The dermo-epidermal junction was identified both in the TGF-β1 staining and in the controls with mAb directed against collagen type IV until 14 wk EGA and Laminin5 in the remaining age groups and visualized with diamino-benzidine-tetrahydrochloride (DAB; brown line). Data are representative of at least three experiments per group. Bars, 50 μm.
Embryonic HLA-DRhigh leukocytes are functional
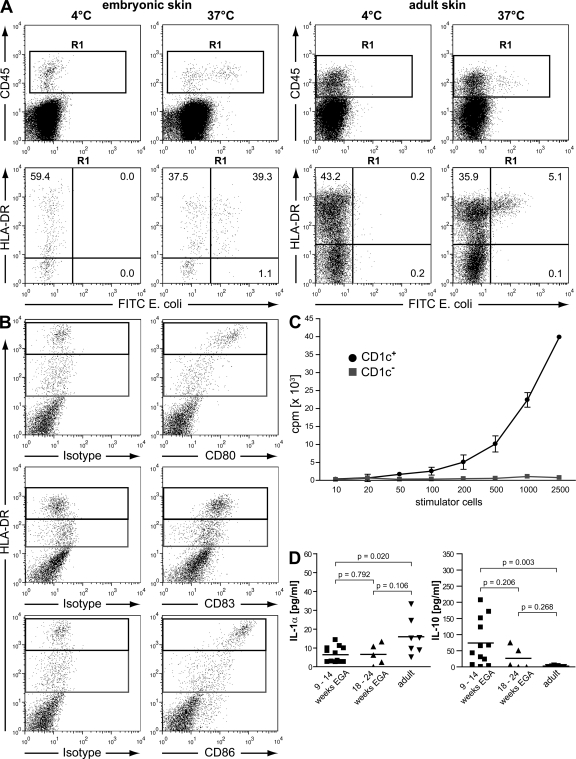
It has been speculated that embryonic and fetal leukocytes exhibit intrinsic functional deficiencies, a concept which may explain some deficits of the neonatal immune system (32–35). To address whether CD45+HLA-DR+ leukocytes from embryonic skin are functionally active, we analyzed their capacity to phagocytose bacteria, up-regulate costimulatory molecules during culture, and induce a primary T cell response in vitro. We found that a considerable fraction of embryonic skin leukocytes internalize opsonised FITC Escherichia coli at 37°C but not at 4°C (Fig. 8 A, left). Additional phenotypic characterization revealed that uptake of bacteria is restricted to HLA-DRlow and HLA-DRhigh leukocytes. In contrast, in single cell suspensions of adult skin, only HLA-DRhigh leukocytes take up FITC-labeled E. coli. Although 46.97% (SD 14.3%; n = 3) of HLA-DR+ leukocytes take up E. coli in embryonic skin, only 10.74% (SD 5.0%; n = 3) of HLA-DR+ leukocytes take up the labeled bacteria in adult skin (Fig. 8, right). The reason for this difference remains to be investigated.
Figure 8.
Embryonic DC precursors ingest E. coli, up-regulate costimulatory molecules during culture, and stimulate allogeneic T cells. (A) Phagocytic capacity of leukocytes from embryonic (9–14 wk EGA) and adult skin was assessed by the uptake of FITC-labeled E. coli (107 bacteria/2 × 106 cells). Flow cytometric analysis revealed exclusive uptake of bacteria in CD45+HLA-DR+ cells (R1). Data are representative of three experiments in embryonic skin and of three experiments in adult skin. (B) Single cells from embryonic skin were cultured for 48 h and expression of CD80, CD86 (donor 1), and CD83 (donor 2) on HLA-DR+ cells was analyzed (gated on CD45+ cells). Shown are two representative experiments out of three to five. (C) Allogeneic T cells (5 × 104/well) were cocultured with graded numbers of CD1c+ and CD1c− stimulator cells from embryonic skin. T cell proliferation was determined after 5 d by measuring the incorporation of [3H]thymidine. Data are displayed as the mean ± SD of triplicate cultures, and one representative of three independent experiments is shown. (D) Single cell suspensions of embryonic, fetal, and adult skin were cultured for 48 h, and IL-1α and IL-10 levels in supernatants were analyzed by Luminex technology. Bars represent the mean of investigated groups. 9-14 wk EGA: IL-1α and IL-10, n = 12 each. 18–24 wk EGA: IL-1α and IL-10, n = 5 each. Adult: IL-1α and IL-10, n = 7 each.
It is well established that potent T cell stimulation requires high levels of costimulatory molecules on DCs (36). To test whether embryonic HLA-DR–expressing skin leukocytes up-regulate molecules relevant for T cell activation, single cell suspensions were cultured. Freshly isolated HLA-DR+ leukocytes express neither CD80 nor CD83 but low levels of CD86 (unpublished data). However, after 48 h of culture, HLA-DRhigh but not HLA-DRlow leukocytes up-regulate CD80, CD83, and CD86, thus representing a phenotype consistent with the ability of priming immune responses (Fig. 8 B). To confirm this, we enriched CD1c+ cells (purity range 40–70%) using magnetic bead separation and compared their allostimulatory capacity with those of the CD1c− fraction, containing <0.1% CD1c+ cells. Repeated experiments showed that embryonic CD1c+ cells, but not CD1c− cells, elicit vigorous proliferation of allogeneic T cells (Fig. 8 C).
To address whether the phenotype and functionality of leukocytes in developing skin may be influenced by a specific development-related milieu, we comparatively assessed the concentrations of the proinflammatory cytokine IL-1α and the immunosuppressive cytokine IL-10 in supernatants of embryonic, fetal, and adult skin cell cultures. Although IL-1α levels in developing skin are half of what is found in adult skin, IL-10 concentrations in embryonic (50-fold) and fetal (18-fold) skin cultures far exceed those secreted by skin cells from adults (Fig. 8 D), suggesting that developing skin represents an immunosuppressive environment.
DISCUSSION
We showed in this study that human embryonic skin harbors phenotypically diverse and functionally potent HLA-DR+ leukocytes. We also demonstrated that human developing skin generates an environment that promotes proliferation of skin-resident leukocytes and probably immigration of blood borne precursors, thereby actively contributing to the formation of the skin immune system. Given that only small amounts of embryonic and fetal tissue were available because of ethical limitations and that embryonic skin contains only few leukocytes, we primarily investigated the development of HLA-DR+ leukocytes.
The increase in leukocyte numbers in developing skin is dependent on their local proliferation and immigration
Adult skin contains a dense network of DCs and macrophages that control a spectrum of innate and adaptive immune responses (37, 38). In human embryonic skin, this network has not yet formed, implicating an insufficient host defense in case the epidermal barrier is breached. During skin development, this network continuously develops, probably as a consequence of proliferating resident leukocytes and of leukocyte immigration. The first assumption is based on our observations of a high rate of leukocytes expressing the proliferation marker Ki67 in embryonic and fetal skin. In support of this observation, we found small clusters of two to four leukocytes in skin sections, which is also indicative of local proliferation. Moreover, our findings that supernatants of embryonic and, to a lesser extent, fetal skin cell cultures contain higher levels of growth factors, such as GM-CSF, SCF, and IL-6, compared with adults are suggestive of the presence of a cytokine milieu in developing skin that stimulates proliferation of skin-resident leukocytes. These growth factors, either alone or in combination, have been described to stimulate the proliferation of cells such as hematopoietic stem and progenitor cells, DCs, and other cell types (39–43). M-CSF, which is present in similar amounts in supernatants of embryonic, fetal, and adult skin cells, may also stimulate proliferation of hematopoietic progenitor cells but, in concert with GM-CSF, might also play a role in the maturation of macrophages and DCs (43). Apart from the local proliferation of immune cells, the leukocyte network in developing skin is probably reinforced by the immigration of leukocyte precursors from the circulation. Indeed, we occasionally observed leukocytes sticking to the luminal surface of CD34+ vessels in developing skin (unpublished data), corroborating leukocyte recruitment into skin.
The migration of leukocytes into skin largely depends on chemokines (44, 45), a concept which can also be expected in developing skin. Various leukocyte-attracting chemokines are produced in high levels during inflammation, including MCP-1 (CCL2), RANTES (CCL5), MIP-3α (CCL20), and MDC (CCL22) (45–50). We found higher concentrations of MCP-1 and RANTES, but not of MIP-3α and MDC, in supernatants of developing skin than in adult skin. In addition, RANTES levels reach adult-like levels by 18–24 wk EGA, whereas MCP-1 levels remain high. Collectively, these findings point to a tightly regulated program of leukocyte attraction into skin during development that differs from that found during skin inflammation. Collectively, these results suggest that blood-borne precursors are recruited to the skin owing to this unique chemokine milieu, yet definitive evidence cannot be provided from our studies. However, it cannot be ruled out that chemokines that have so far not been implicated in the recruitment of leukocytes in skin may play a role during skin development. Furthermore, our data that the cellular composition of embryonic and adult single cell suspensions differs with regard to numbers of keratinocytes, leukocytes, and dermal CD45− cells complicates the elucidation of which cells these cytokines are produced by.
Leukocytes from embryonic skin are functionally not intrinsically inhibited
Adequate numbers and functional maturity are needed for leukocytes to exhibit a protective role in host defense. For the first time, this study sheds light on the functional state of APCs in human embryonic and fetal skin because we were able to isolate the rare DC precursors from embryonic skin in numbers sufficient to perform meaningful functional experiments. Previous studies investigating the efficiency of APCs of human fetuses have relied on the use of APCs in umbilical cord blood, given the difficulties in obtaining human embryonic and fetal tissue (34). These studies yielded controversial results, which may be partially explained by the use of different cell isolation techniques. Some studies describe diminished levels of HLA-DR on cord blood monocytes, which should explain the deficient T cell priming of these cells (51). In contrast, other investigators did not find deficient function of cord blood DCs (52, 53). In this paper, we show that APCs already in embryonic skin efficiently take up antigen, up-regulate costimulatory molecules, and stimulate allogeneic T cells in vitro, suggesting that they are not intrinsically inhibited and that the skin already in utero possesses the machinery to mount vigorous immune responses. Indeed, various studies have already shown that human fetuses are capable of generating vigorous immune responses after transplacental spread of infectious agents, including parasites and viruses (54–57). It has been therefore hypothesized that the immune system of human fetuses is actively suppressed rather than functionally deficient (58). This suppression may be related to high numbers of regulatory T cells in fetal circulation, lymph nodes (58), and decidual tissue (59) as well as to an immunosuppressive environment generated by the placenta (60). Furthermore, it is known that IL-10 is present in amniotic fluid, and its antiinflammatory function is postulated to play an important role in the prevention of an inflammatory response to the fetus and fetal “foreign” antigens (61, 62). Similar to our previous observations in mice (63), our results show that in developing human skin high levels of the immunosuppressive cytokine IL-10 together with low levels of the proinflammatory cytokine IL-1α could inhibit the activation and mobilization of skin DCs and macrophages, thus contributing to the acquisition or maintenance of immune tolerance.
LC and dermal DCs acquire their marker profile during skin development
The study of the ontogeny of skin APCs also provides a unique opportunity to evaluate the development and, thus, the phenotype of their precursor cells. In addition, despite considerable research, the relationship among LCs, dermal DCs, and skin macrophages still remains unclear, not least because of the high plasticity of precursors to differentiate into each of these cells in different microenvironments (7, 12, 15). In this study, we show that at 9 wk EGA, skin macrophages and DCs can already be phenotypically separated by the distinct expression of the DC marker CD1c on some HLA-DRhigh cells. HLA-DRhigh leukocytes are capable of phagocytosing bacteria, up-regulating costimulatory molecules, and stimulating proliferation of allogeneic T cells in vitro, thus confirming their DC nature. In contrast, HLA-DRlow skin macrophages neither express CD1c nor up-regulate costimulatory molecules during culture. Collectively, these data show that at 9 wk EGA, skin macrophages and DCs can already be identified and distinguished. Intriguingly, some HLA-DR+ epidermal leukocytes do not express CD1c at 9 wk EGA and, hence, acquire this antigen in the epidermis. It is therefore conceivable that CD45+HLA-DR+ leukocytes represent common precursors for skin macrophages, dermal DCs, and LCs that develop into each subset owing to local microenvironmental cues. Alternatively, it might also be possible that CD45+HLA-DR+ precursors committed to differentiate into skin macrophages, LCs, and dermal DCs migrate into their residential compartment where they eventually acquire their mature phenotype. Further studies investigating the expression of CD11b, CD11c, and CD14 on these CD45+HLA-DR+ cells, as well as their departmental localization, should help to clarify the relationship of skin DCs and macrophages in humans.
In contrast to the heterogeneity of dermal immune cells, the sole presence of LCs in the epidermis facilitates the investigation of their development. We found that LC precursors sequentially acquire their phenotypic repertoire in the epidermis. They colonize the epidermis as CD45+HLA-DR+ leukocytes and then acquire CD1c, Langerin, and CD1a in a stepwise manner. Flow cytometric analyses suggest that the phenotype of LC precursors changes from Langerin−CD1a− to LangerinhighCD1ahigh via LangerinhighCD1a− and LangerinhighCD1a+. Because we could not detect apoptotic leukocytes in embryonic and fetal skin, we conclude that epidermal LC precursors are not replaced by immigrating cells but acquire their phenotype in the epidermis. These findings contrast with an earlier study, showing that CD1a expression precedes Lag expression (19). As it has been shown that the anti-Lag mAb recognizes an intracellular epitope of Langerin (3), it is conceivable that the Langerin antibody recognizes a more accessible form of this antigen and, thus, allows an earlier identification of these cells. However, data from a recent study supports our view that Langerin expression on LC precursors precedes CD1a acquisition. In this study, a migratory CD1a−CD14+CD207+ population is described that acquires CD1a and E-cadherin while down-regulating CD14 during short term culture with TGF-β1 (12). It remains to be investigated whether embryonic CD45+HLA-DR+ epidermal leukocytes express CD14. However, alternatives to the serial acquisition of markers on LCs in developing skin are conceivable. As the changes of the marker profile on LCs take place during the transition of hematopoiesis from fetal liver to the bone marrow (64), it is possible that bone marrow–derived DCs that colonize developing skin display already a more mature surface marker profile. Also, it cannot be excluded that LCs loose and regain certain surface markers during development. Yet, given the ethical limitations when using human prenatal tissue, it is to date not possible to exclude any of these alternatives.
TGF-β1 has been shown to be a prerequisite for the formation of the LC phenotype in vitro and in vivo (15, 28, 30). In concordance with these studies, we demonstrate that the production of TGF-β1 in developing epidermis after the onset of stratification correlates with the appearance of Langerin and CD1a, corroborating the possible role of TGF-β1 in humans in the acquisition of the LC phenotype in vivo. In addition, the increasing staining intensity of Langerin and CD1a in fetal skin may reflect the consistently increasing total amount of TGF-β1 in the developing epidermis. However, TGF-β1 is also known to exert a wide range of effects on keratinocytes, including potent growth inhibition. The exclusive suprabasal staining pattern found in healthy adult epidermis may represent a compartmental regulation to exclude the proliferating basal layer from the inhibitory growth effect of TGF-β1 (31). This hypothesis is supported by our finding that, after the onset of epidermal stratification around 9 wk EGA, TGF-β1 is found exclusively suprabasally. The production of TGF-β1 in suprabasal layers, and temporarily in the periderm, implies an important regulatory role of TGF-β1 in the differentiation and proliferation of keratinocytes also in developing epidermis, apart from its possible role in the generation of the LC phenotype. The secreted inactive form of TGF-β1, LAP, is found in the primitive epidermis earlier than the active form, suggesting that the release of active TGF-β1 is regulated by the incipient LAP activation that develops around 9 wk EGA. The mechanism by which this is regulated has to be determined in further studies.
It has recently been shown that CD1a+Langerin− dermal DCs represent a subset of dermal DCs distinct from LCs that is capable of carrying antigen to lymph nodes and stimulating naive T cells (8). We show that CD1a+Langerin− dermal DCs develop at 18–24 wk EGA, thereby confirming that these cells are distinct from CD1a+Langerin+ epidermal LCs. To date, the origin of CD1a+Langerin− dermal DCs is speculative. It is possible that skin-resident dermal DC precursors, such as dermal CD1c+ cells which are already present in the skin at 9 wk EGA, acquire CD1a as a result of an intrinsic program or changing environmental factors.
HLA-DR expression on endothelial cells is not found before 18–24 wk EGA
It is well established that a fraction of endothelial cells in healthy adult human skin express HLA-DR (24, 25). HLA-DR+ endothelial cells are believed to play an important role in dampening T cell responses and in the maintenance of tolerance (24). Alternatively, it has been speculated that these cells are involved in the activation of skin-homing T cells (65). We show that HLA-DR expression on endothelial cells is not acquired before 18–24 wk EGA. IFN-γ has been shown to induce HLA-DR expression in a variety of cells, including endothelial cells and keratinocytes (66). Whether this proinflammatory cytokine controls the acquisition of HLA-DR on endothelial cells remains to be investigated. Even though the function of HLA-DR–expressing endothelial cells during midgestation is yet unknown, one may speculate that they have the potential to present peptide antigen and subsequently influence the cutaneous immune environment.
In summary, we have shown that the study of the development of the human skin immune system yields valuable information about the origin and relationship of leukocytes. Moreover, this study adds further data to the growing body of evidence that the human embryo and fetus possess the principal machinery to mount vigorous immune responses once the immunosuppressive environment is overcome.
MATERIALS AND METHODS
Skin samples.
After legal termination of pregnancy, 40 specimens of human embryonic and fetal trunk skin ranging from 8–24 wk EGA were studied. The age was estimated by crown-rump length and maternal history. The study was approved by the local ethics committee (Medical University of Vienna), and informed written consent was obtained from the parents. The ethics committee permitted the removal of 1.4 cm2/individual. Healthy adult skin was obtained after abdominal and breast surgery in accordance with the regulations of the Medical University of Vienna.
Preparation of skin cell suspensions.
After removal of subcutaneous tissue, embryonic, fetal, and adult skin was incubated on 1.2 U/ml Dispase II (Roche) in PBS overnight at 4°C. It was not possible to efficiently separate dermis and epidermis in embryonic and fetal skin. Thus, unseparated skin, regardless of age, was vigorously agitated in a shaking bath in 0.53 U/ml Liberase3 (Roche) in PBS for 60–90 min. The resulting single cell suspensions were analyzed by flow cytometry, cultured for 48 h, or used for functional studies. Cells were washed and cultured in normal cell medium (106 cells/ml), composed of RPMI 1640 cell medium (Invitrogen) supplemented with 10% heat-inactivated FCS (PromoCell), 25 mM Hepes, 10 μg/ml gentamycin, 2 mM l-glutamine, 0.1 mM of nonessential amino acids, 1 mM sodium pyruvate, 50 μM 2-mercapto-ethanol, and 0.002% of antibiotic antimycotic solution (all Invitrogen).
Flow cytometry.
Single cell suspensions were stained with the following mAbs: PE anti-Langerin (DCGM4), PE-Cy7 anti-CD45 (J.33; both Beckman Coulter), FITC anti-CD1a (HI-149), FITC anti-CD80 (L307.4), FITC anti-CD83 (HB15e), FITC anti-CD86 (2331), PE anti-CD318 (CUB1), allophycocyanin and PE-anti-HLA-DR (L243; all BD), PE-anti-CD19 (SJ25-C1; Invitrogen), and FITC anti-CD1c (M241; Ancell). Appropriate isotype controls were included. Dead cells were excluded with 7-AAD (EMD). Five-color flow cytometry analyses were performed on an LSR-II (BD) and data were analyzed using FlowJo software (Tree Star, Inc.).
Immunohistochemistry.
Embryonic, fetal, and adult skin specimens were embedded in optimum cutting tissue compound (Tissue-Tek; Sakura), snap frozen in liquid nitrogen, and stored at −80°C until further processing. 6-μm sections were cut, air dried, fixed in ice-cold acetone for 10 min, and washed in PBS. Sections were then incubated with anti-collagen type IV (24.12.8; Millipore) or anti-Laminin5 (D4B5; Millipore) overnight at 4°C, followed by blocking of endogenous peroxidase activity by incubating sections for 10 min in methanol containing 0.03% hydrogen peroxide. Subsequently, sections were incubated for 2 h at room temperature with biotin-conjugated goat anti–mouse IgG using the Elite mouse IgG Vectastain kit (Vector Laboratories). Biotinylated antibodies were detected with HRP streptavidin and staining was visualized with DAB (Vector Laboratories) supplemented with Ni2+. Remaining peroxidase activity was blocked for 10 min with 0.03% hydrogen peroxide/methanol. Tissue sections were then incubated with anti-LAP (27232; R&D Systems) and anti–TGF-β1 serum (Santa Cruz Biotechnology, Inc.) overnight at 4°C, followed by biotin-conjugated goat anti–mouse or goat anti–rabbit IgG for 2 h at room temperature using the Elite mouse IgG and Elite rabbit IgG Vectastain kits. Biotinylated antibodies were detected with HRP streptavidin and staining was visualized with amino-ethyl-carbazole (AEC; Dako). Finally, sections were mounted with Aquatex (Merck) and examined using a microscope (Eclipse 80; Nikon). Appropriate rabbit control serum (Dako) and isotype controls (BD) were included.
Immunofluorescence.
Fixed sections were stained with the following unconjugated primary antibodies: anti-CD1a (WM35; Sanquin,), anti-CD1c (L161; AbD Serotec), anti-Langerin (DCGM4; Beckman Coulter), anti–collagen type IV (24.12.8; Millipore), and anti-Ki67 (Leica) overnight at 4°C. Primary antibodies were detected with the corresponding species-specific donkey anti–mouse NorthernLight637 (R&D Systems) or goat anti–rabbit Alexa Fluor 647 (Invitrogen) antibodies. Subsequently, sections were blocked with 10% mouse serum and 2% mouse IgG (BD) for 1 h at room temperature and stained with the following conjugated antibodies overnight at 4°C: Alexa Fluor 488 anti-Langerin (DCGM4; Dendritics), FITC anti–HLA-DR (L243; BD), and Alexa Fluor 546 anti-CD45 (MEM28; provided by A. Muhammad, Medical University of Vienna, Vienna, Austria). Alexa Fluor 488 anti-Laminin5 (D4B5; Millipore) was used to visualize the dermo-epidermal junction. Slides were mounted using Vectashield (Vector Laboratories) and images were recorded using a confocal laser scanning microscope (LSM 410; Carl Zeiss, Inc.) equipped with three lasers emitting lights at 488, 543, and 633 nm.
Identification of apoptotic cells.
To detect DNA breaks in leukocytes in situ, cryostat sections were fixed with 4% paraformaldehyde for 20 min at room temperature and then washed three times in PBS. Sections were subsequently subjected to TUNEL technique according to the manufacturer's instructions (Roche). After rinsing in PBS, sections were counterstained with Alexa Fluor 546 anti-CD45. As a positive control, sections were first incubated with 1,500 U/ml DNase I for 45 min at room temperature to induce DNA breaks in the nucleus and were then subjected to CD45/TUNEL staining. Images from four individuals from embryonic and three individuals from fetal skin were recorded using a confocal laser scanning microscope.
Quantification of cells in skin sections.
Ki67+ and CD45+ cells were enumerated by two independent investigators in at least 20 randomly selected microscopic fields per sample (four to eight sections from seven to nine individuals were tested as specified in the figures; ×20 objective lens; Nikon).
Phagocytosis assay.
The phagocytic capacity of leukocytes from embryonic skin (9–12 wk EGA) was assessed using a modified protocol for the Phagotest (ORPEGEN Pharma). In brief, 2 × 106 skin cells were incubated for 90 min at 4 and 37°C with 107 inactivated opsonized FITC-conjugated E. coli cells in RPMI 1640 medium containing 10% FCS. To quench the fluorescence signal from attached but not internalized E. coli, cells were incubated with the provided quenching solution. Cells were then washed with PBS and stained with the respective antibodies and isotype controls before flow cytometric analysis.
DC isolation from embryonic skin.
CD1c+ skin cells (11–14 wk EGA) were positively selected using PE anti-CD1c (AD5-8E7; Miltenyi Biotec), followed by anti-PE magnetic beads (Miltenyi Biotec). The purity of isolated CD1c+ cells was 40–70%, as determined by flow cytometric analysis. CD1c+ and CD1c− (containing <0.1% CD1c+ cells) cells were then cultured for 24 h in medium containing 10% FCS and used for functional analysis.
Mixed leukocyte reaction.
Allogeneic CD3+ responder T cells were purified from PBMCs (>95% purity) by negative selection using a CD3 T cell isolation kit (Miltenyi Biotec). Graded numbers of stimulator cells (CD1c+ and CD1c−) were cocultured with allogeneic CD3+ T cells (5 × 104/well) in 96-well round-bottom microtiter plates (Costar; Corning) for 5 d. For the final 16 h of culture, cells were pulsed with [3H]thymidine (37 kBq/well; Hartmann Analytic), and the amount of incorporation of the radioisotope was determined with a 1450 Microbeta liquid scintillation counter (PerkinElmer). CD1c+, CD1c−, and purified T cells alone did not proliferate when cultured with medium (<600 cpm). Data are expressed as the mean cpm ± SD of triplicate cultures.
Cytokine determination in skin cell supernatants.
Skin single cell suspensions (106/ml) were cultured in 24-well plates (Costar; Corning). After 48 h, supernatants were harvested and stored at −80°C until use. IL-3, M-CSF, CCL22, CCL20, and SCF (all R&D Systems) were determined by ELISA according to the manufacturer's instructions. IL-6, GM-CSF, MCP-1, RANTES, IL-10, and IL-1α (all R&D Systems) were determined using the Luminex Laboratory Multi-Analyte Profiling technology (Invitrogen) as previously described (67). Experiments were performed in duplicates.
Statistical analysis.
Differences between groups were assessed with the Mann-Whitney U Test (GraphPad Software, Inc.). The reported p-value is a result of a two-sided test. A p-value <5% was considered statistically significant.
Online supplemental material.
Fig. S1 depicts the percentage of CDCP1 (CD318)-expressing keratinocytes and CD45-expressing leukocytes of whole embryonic and adult skin single cell suspensions. Fig. S2. displays HLA-DR expression kinetics on endothelial cells in fetal skin. Fig. S3. shows that leukocytes do not undergo apoptosis during skin development using cryostat sections of embryonic and fetal skin. Fig. S4. illustrates that epidermal LAP production precedes TGF-β1 production. Table S1 shows the percentage of CDCP1+ (CD318) keratinocytes, CD45+ leukocytes, and CD45− dermal cells of whole embryonic and adult skin single cell suspensions. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081747/DC1.
Supplementary Material
Acknowledgments
We are grateful for the invaluable help of all midwifes, nurses and clinical psychologists. We also thank Dr. A. Stift (Department of Surgery, Medical University of Vienna, Austria) for the organization of adult human skin samples, Dr. C. Kopetzki (Department of Constitutional and Administrative Law, University of Vienna) for judicial support, Drs. G. Cohen (Department of Nephrology, Medical University of Vienna), W. Bauer, and D. Spazierer (both Department of Dermatology, Medical University of Vienna) for technical support, and Drs. F. Bach (Immunobiology Research Center, Beth Israel Deaconess Medical Center, Boston) and H. Strobl (Institute of Immunology, Medical University Vienna) for fruitful discussions.
This study was supported by a research grant of the Austrian Science Fund (FWF, P19474-B13).
The authors have no conflicting financial interests.
Abbreviations used: DAB, diamino-benzidine-tetrahydrochloride; EGA, estimated gestational age; LC, Langerhans cell; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling.
References
- 1.Modlin, R.L., J. Kim, D. Maurer, C. Bangert, and G. Stingl. 2007. Innate and adaptive immunity in the skin. In Fitzpatrick's Dermatology in General Medicine. K. Wolff, L.A. Goldsmith, S.I. Katz, B.A. Gilchrest, A.S. Paller, and D. J. Leffell, eds., McGraw Hill, New York. 95–114.
- 2.Elder, J.T., N.J. Reynolds, K.D. Cooper, C.E. Griffiths, B.D. Hardas, and P.A. Bleicher. 1993. CD1 gene expression in human skin. J. Dermatol. Sci. 6:206–213. [DOI] [PubMed] [Google Scholar]
- 3.Valladeau, J., O. Ravel, C. Dezutter-Dambuyant, K. Moore, M. Kleijmeer, Y. Liu, V. Duvert-Frances, C. Vincent, D. Schmitt, J. Davoust, et al. 2000. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 12:71–81. [DOI] [PubMed] [Google Scholar]
- 4.Turville, S.G., P.U. Cameron, A. Handley, G. Lin, S. Pohlmann, R.W. Doms, and A.L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. [DOI] [PubMed] [Google Scholar]
- 5.Ebner, S., Z. Ehammer, S. Holzmann, P. Schwingshackl, M. Forstner, P. Stoitzner, G.M. Huemer, P. Fritsch, and N. Romani. 2004. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int. Immunol. 16:877–887. [DOI] [PubMed] [Google Scholar]
- 6.Zaba, L.C., J. Fuentes-Duculan, R.M. Steinman, J.G. Krueger, and M.A. Lowes. 2007. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J. Clin. Invest. 117:2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestle, F.O., X.G. Zheng, C.B. Thompson, L.A. Turka, and B.J. Nickoloff. 1993. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 151:6535–6545. [PubMed] [Google Scholar]
- 8.Angel, C.E., E. George, A.E.S. Brooks, L.L. Ostrovsky, T.L.A. Brown, and P.R. Dunbar. 2006. CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J. Immunol. 176:5730–5734. [DOI] [PubMed] [Google Scholar]
- 9.Ochoa, M.T., A. Loncaric, S.R. Krutzik, T.C. Becker, and R.L. Modlin. 2008. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J. Invest. Dermatol. 128:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strunk, D., K. Rappersberger, C. Egger, H. Strobl, E. Krömer, A. Elbe, D. Maurer, and G. Stingl. 1996. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 87:1292–1302. [PubMed] [Google Scholar]
- 11.Ito, T., M. Inaba, K. Inaba, J. Toki, S. Sogo, T. Iguchi, Y. Adachi, K. Yamaguchi, R. Amakawa, J. Valladeau, et al. 1999. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J. Immunol. 163:1409–1419. [PubMed] [Google Scholar]
- 12.Larregina, A.T., A.E. Morelli, L.A. Spencer, A.J. Logar, S.C. Watkins, A.W. Thomson, and L.D. Falo Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158. [DOI] [PubMed] [Google Scholar]
- 13.Strunk, D., C. Egger, G. Leitner, D. Hanau, and G. Stingl. 1997. A skin homing molecule defines the Langerhans cell progenitor in human peripheral blood. J. Exp. Med. 185:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaksits, S., E. Kriehuber, A.S. Charbonnier, K. Rappersberger, G. Stingl, and D. Maurer. 1999. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J. Immunol. 163:4869–4877. [PubMed] [Google Scholar]
- 15.Strobl, H., E. Riedl, C. Scheinecker, C. Bello-Fernandez, W.F. Pickl, K. Rappersberger, O. Majdic, and W. Knapp. 1996. TGF-β1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J. Immunol. 157:1499–1507. [PubMed] [Google Scholar]
- 16.Foster, C.A., K.A. Holbrook, and A.G. Farr. 1986. Ontogeny of Langerhans cells in human embryonic and fetal skin: expression of HLA-DR and OKT-6 determinants. J. Invest. Dermatol. 86:240–243. [DOI] [PubMed] [Google Scholar]
- 17.Breathnach, A.S. 1978. Development and differentiation of dermal cells in man. J. Invest. Dermatol. 71:2–8. [DOI] [PubMed] [Google Scholar]
- 18.Janossy, G., M. Bofill, L.W. Poulter, E. Rawlings, G.D. Burford, C. Navarrete, A. Ziegler, and E. Kelemen. 1986. Separate ontogeny of two macrophage-like accessory cell populations in the human fetus. J. Immunol. 136:4354–4361. [PubMed] [Google Scholar]
- 19.Fujita, M., F. Furukawa, Y. Horiguchi, M. Ueda, M. Kashihara-Sawami, and S. Imamura. 1991. Regional development of Langerhans cells and formation of Birbeck granules in human embryonic and fetal skin. J. Invest. Dermatol. 97:65–72. [DOI] [PubMed] [Google Scholar]
- 20.Breathnach, A.S., and L.M. Wyllie. 1965. Electron microscopy of melanocytes and Langerhans cells in human fetal epidermis at fourteen weeks. J. Invest. Dermatol. 44:51–60. [DOI] [PubMed] [Google Scholar]
- 21.Gibran, N.S., B.J. Nickoloff, and K.A. Holbrook. 1996. Ontogeny and characterization of factor XIIIa+ cells in developing human skin. Anat. Embryol. (Berl.). 193:35–41. [DOI] [PubMed] [Google Scholar]
- 22.Brown, T.A., T.M. Yang, T. Zaitsevskaia, Y. Xia, C.A. Dunn, R.O. Sigle, B. Knudsen, and W.G. Carter. 2004. Adhesion or plasmin regulates tyrosine phosphorylation of a novel membrane glycoprotein p80/gp140/CUB domain-containing protein 1 in epithelia. J. Biol. Chem. 279:14772–14783. [DOI] [PubMed] [Google Scholar]
- 23.Alvares, S.M., C.A. Dunn, T.A. Brown, E.E. Wayner, and W.G. Carter. 2008. The role of membrane microdomains in transmembrane signaling through the epithelial glycoprotein Gp140/CDCP1. Biochim. Biophys. Acta. 1780:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amatschek, S., E. Kriehuber, W. Bauer, B. Reininger, P. Meraner, A. Wolpl, N. Schweifer, C. Haslinger, G. Stingl, and D. Maurer. 2007. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 109:4777–4785. [DOI] [PubMed] [Google Scholar]
- 25.Angel, C.E., E. George, L.L. Ostrovsky, and P.R. Dunbar. 2007. Comprehensive analysis of MHC-II expression in healthy human skin. Immunol. Cell Biol. 85:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meunier, L., A. Gonzalez-Ramos, and K.D. Cooper. 1993. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J. Immunol. 151:4067–4080. [PubMed] [Google Scholar]
- 27.Delia, D., G. Cattoretti, N. Polli, E. Fontanella, A. Aiello, R. Giardini, F. Rilke, and P.G. Della. 1988. CD1c but neither CD1a nor CD1b molecules are expressed on normal, activated, and malignant human B cells: identification of a new B-cell subset. Blood. 72:241–247. [PubMed] [Google Scholar]
- 28.Borkowski, T.A., J.J. Letterio, A.G. Farr, and M.C. Udey. 1996. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184:2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annes, J.P., J.S. Munger, and D.B. Rifkin. 2003. Making sense of latent TGFbeta activation. J. Cell Sci. 116:217–224. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan, D.H., M.O. Li, M.C. Jenison, W.D. Shlomchik, R.A. Flavell, and M.J. Shlomchik. 2007. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 204:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane, C.J., A.M. Knapp, J.N. Mansbridge, and P.C. Hanawalt. 1990. Transforming growth factor-beta 1 localization in normal and psoriatic epidermal keratinocytes in situ. J. Cell. Physiol. 144:144–150. [DOI] [PubMed] [Google Scholar]
- 32.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553–564. [DOI] [PubMed] [Google Scholar]
- 33.Levy, O. 2005. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J. Endotoxin Res. 11:113–116. [DOI] [PubMed] [Google Scholar]
- 34.Fadel, S., and M. Sarzotti. 2000. Cellular immune responses in neonates. Int. Rev. Immunol. 19:173–193. [DOI] [PubMed] [Google Scholar]
- 35.Holt, P.G., and C.A. Jones. 2000. The development of the immune system during pregnancy and early life. Allergy. 55:688–697. [DOI] [PubMed] [Google Scholar]
- 36.Stingl, G., D. Maurer, C. Hauser, and K. Wolff. 2003. The skin: an immunologic barrier. In Fitzpatrick's Dermatology in General Medicine. I.M. Freedberg, A.Z. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, and S.I. Katz, eds. McGraw-Hill, New York. 253–272.
- 37.Larregina, A.T., and L.D. Falo Jr. 2005. Changing paradigms in cutaneous immunology: Adapting with dendritic cells. J. Invest. Dermatol. 124:1–12. [DOI] [PubMed] [Google Scholar]
- 38.Rossi, M., and J.W. Young. 2005. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 175:1373–1381. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber, S., O. Kilgus, E. Payer, R. Kutil, A. Elbe, C. Mueller, and G. Stingl. 1992. Cytokine pattern of Langerhans cells isolated from murine epidermal cell cultures. J. Immunol. 149:3524–3534. [PubMed] [Google Scholar]
- 40.Dexter, T.M., and C.M. Heyworth. 1994. Growth factors and the molecular control of haematopoiesis. Eur. J. Clin. Microbiol. Infect. Dis. 13:S3–S8. [DOI] [PubMed] [Google Scholar]
- 41.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metcalf, D. 2008. Hematopoietic cytokines. Blood. 111:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamilton, J.A. 2008. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8:533–544. [DOI] [PubMed] [Google Scholar]
- 44.Ono, S.J., T. Nakamura, D. Miyazaki, M. Ohbayashi, M. Dawson, and M. Toda. 2003. Chemokines: roles in leukocyte development, trafficking, and effector function. J. Allergy Clin. Immunol. 111:1185–1199. [DOI] [PubMed] [Google Scholar]
- 45.Koch, S., K. Kohl, E. Klein, D. von Bubnoff, and T. Bieber. 2006. Skin homing of Langerhans cell precursors: adhesion, chemotaxis, and migration. J. Allergy Clin. Immunol. 117:163–168. [DOI] [PubMed] [Google Scholar]
- 46.Charbonnier, A.S., N. Kohrgruber, E. Kriehuber, G. Stingl, A. Rot, and D. Maurer. 1999. Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal Langerhans cells. J. Exp. Med. 190:1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godiska, R., D. Chantry, C.J. Raport, S. Sozzani, P. Allavena, D. Leviten, A. Mantovani, and P.W. Gray. 1997. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 185:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barker, J.N., M.L. Jones, C.L. Swenson, V. Sarma, R.S. Mitra, P.A. Ward, K.J. Johnson, J.C. Fantone, V.M. Dixit, and B.J. Nickoloff. 1991. Monocyte chemotaxis and activating factor production by keratinocytes in response to IFN-gamma. J. Immunol. 146:1192–1197. [PubMed] [Google Scholar]
- 50.Sebastiani, S., C. Albanesi, P.O. De, P. Puddu, A. Cavani, and G. Girolomoni. 2002. The role of chemokines in allergic contact dermatitis. Arch. Dermatol. Res. 293:552–559. [DOI] [PubMed] [Google Scholar]
- 51.Hunt, D.W., H.I. Huppertz, H.J. Jiang, and R.E. Petty. 1994. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 84:4333–4343. [PubMed] [Google Scholar]
- 52.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salio, M., N. Dulphy, J. Renneson, M. Herbert, A. Mcmichael, A. Marchant, and V. Cerundolo. 2003. Efficient priming of antigen-specific cytotoxic T lymphocytes by human cord blood dendritic cells. Int. Immunol. 15:1265–1273. [DOI] [PubMed] [Google Scholar]
- 54.Marchant, A., V. Appay, S.M. Van Der, N. Dulphy, C. Liesnard, M. Kidd, S. Kaye, O. Ojuola, G.M. Gillespie, A.L. Vargas Cuero, et al. 2003. Mature CD8+ T lymphocyte response to viral infection during fetal life. J. Clin. Invest. 111:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malhotra, I., J. Ouma, A. Wamachi, J. Kioko, P. Mungai, A. Omollo, L. Elson, D. Koech, J.W. Kazura, and C.L. King. 1997. In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J. Clin. Invest. 99:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim, Y.M., R. Romero, T. Chaiworapongsa, J. Espinoza, G. Mor, and C.J. Kim. 2006. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 49:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rastogi, D., C. Wang, X. Mao, C. Lendor, P.B. Rothman, and R.L. Miller. 2007. Antigen-specific immune responses to influenza vaccine in utero. J. Clin. Invest. 117:1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michaelsson, J., J.E. Mold, J.M. McCune, and D.F. Nixon. 2006. Regulation of T cell responses in the developing human fetus. J. Immunol. 176:5741–5748. [DOI] [PubMed] [Google Scholar]
- 59.Tilburgs, T., D.L. Roelen, B.J. van der Mast, G.M. de Groot-Swings, C. Kleijburg, S.A. Scherjon, and F.H. Claas. 2008. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J. Immunol. 180:5737–5745. [DOI] [PubMed] [Google Scholar]
- 60.Roth, I., D.B. Corry, R.M. Locksley, J.S. Abrams, M.J. Litton, and S.J. Fisher. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heyborne, K.D., J.A. McGregor, G. Henry, S.S. Witkin, and J.S. Abrams. 1994. Interleukin-10 in amniotic fluid at midtrimester: immune activation and suppression in relation to fetal growth. Am. J. Obstet. Gynecol. 171:55–59. [DOI] [PubMed] [Google Scholar]
- 62.Cadet, P., P.L. Rady, S.K. Tyring, R.B. Yandell, and T.K. Hughes. 1995. Interleukin-10 messenger ribonucleic acid in human placenta: implications of a role for interleukin-10 in fetal allograft protection. Am. J. Obstet. Gynecol. 173:25–29. [DOI] [PubMed] [Google Scholar]
- 63.Chang-Rodriguez, S., R. Ecker, G. Stingl, and A. Elbe-Bürger. 2004. Autocrine IL-10 partially prevents differentiation of neonatal dendritic epidermal leukocytes into Langerhans cells. J. Leukoc. Biol. 76:657–666. [DOI] [PubMed] [Google Scholar]
- 64.Tavian, M., and B. Peault. 2005. Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. 49:243–250. [DOI] [PubMed] [Google Scholar]
- 65.Pober, J.S., M.S. Kluger, and J.S. Schechner. 2001. Human endothelial cell presentation of antigen and the homing of memory/effector T cells to skin. Ann. N. Y. Acad. Sci. 941:12–25. [DOI] [PubMed] [Google Scholar]
- 66.Wedgwood, J.F., L. Hatam, and V.R. Bonagura. 1988. Effect of interferon-gamma and tumor necrosis factor on the expression of class I and class II major histocompatibility molecules by cultured human umbilical vein endothelial cells. Cell. Immunol. 111:1–9. [DOI] [PubMed] [Google Scholar]
- 67.Kalthoff, F.S., A. Winiski, P. Fichtinger, B. Schwendinger, S. Wang, C. Weishaeupl, and A. Stuetz. 2007. Differential inhibition of primary versus preactivated T cells by pimecrolimus but not by tacrolimus in vitro. Int. Arch. Allergy Immunol. 142:255–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.