Abstract
The nucleosome remodeling complex SWI/SNF is a coactivator for yeast transcriptional activator Gcn4p. We provide strong evidence that Gcn4p recruits the entire SWI/SNF complex to its target genes ARG1 and SNZ1 but that SWI/SNF is dispensable for Gcn4p binding to these promoters. It was shown previously that Snf2p/Swi2p, Snf5p, and Swi1p interact directly with Gcn4p in vitro. However, we found that Snf2p is not required for recruitment of SWI/SNF by Gcn4p nor can Snf2p be recruited independently of other SWI/SNF subunits in vivo. Snf5p was not recruited as an isolated subunit but was required with Snf6p and Swi3p for optimal recruitment of other SWI/SNF subunits. The results suggest that Snf2p, Snf5p, and Swi1p are recruited only as subunits of intact SWI/SNF, a model consistent with the idea that Gcn4p makes multiple contacts with SWI/SNF in vivo. Interestingly, Swp73p is necessary for efficient SWI/SNF recruitment at SNZ1 but not at ARG1, indicating distinct subunit requirements for SWI/SNF recruitment at different genes. Optimal recruitment of SWI/SNF by Gcn4p also requires specific subunits of SRB mediator (Gal11p, Med2p, and Rox3p) and SAGA (Ada1p and Ada5p) but is independent of the histone acetyltransferase in SAGA, Gcn5p. We suggest that SWI/SNF recruitment is enhanced by cooperative interactions with subunits of SRB mediator and SAGA recruited by Gcn4p to the same promoter but is insensitive to histone H3 acetylation by Gcn5p.
Eukaryotic activator proteins depend on coactivator complexes to eliminate repressive chromatin structures at their target promoters. One class of nucleosome remodeling complexes uses the energy of ATP hydrolysis to disrupt histone-DNA contacts and alter the accessibility of protein binding sites in the promoter. The best-studied coactivator of this type in Saccharomyces cerevisiae is the SWI/SNF complex (37, 41, 51). Deletion of Snf2p/Swi2p, the ATPase subunit of SWI/SNF, is not lethal but leads to altered transcription of a subset (1 to 2%) of genes in nutrient-rich medium (20, 52). SWI/SNF interacts directly with yeast activators (35, 58) and can be recruited to promoters for nucleosome remodeling and transcriptional activation in vitro (16, 38, 58). Recruitment of SWI/SNF by yeast activators has also been demonstrated in vivo by using chromatin immunoprecipitation (ChIP) assays (8, 13, 53, 54).
The yeast SWI/SNF complex contains 11 different subunits, and genetic studies suggest that many of the subunits are required for the chromatin remodeling function of the entire complex (52). Six subunits have homologs in human SWI/SNF, and four of the latter (related to yeast Snf2p/Swi2p, Snf5p, and Swi3p) form a core complex capable of nucleosome remodeling in vitro (42). However, Swp73p is not necessary for transcriptional stimulation by certain activators that require Snf2p (6), and deletion of Snf11p has little effect on SWI/SNF-dependent genes even though it seems to interact directly with Snf2p (55). Interestingly, Snf2p can mediate repression of SER3 without the cooperation of certain other SWI/SNF subunits (33, 52).
SWI/SNF remodeling can stimulate various steps in gene activation. It enhances binding of TATA-binding protein (TBP) and RNA polymerase II (PolII) to the yeast RNR3 promoter (46). Recruitment of SWI/SNF to the HO gene stimulates binding of the coactivator complexes SAGA and SRB mediator (4, 8, 26). SAGA is a multifunctional coactivator containing the histone H3 acetyltransferase (HAT) Gcn5p and multiple TBP-interacting proteins (49). SRB mediator is a multifunctional complex associated with PolII and certain general transcription factors (34). The requirement for SWI/SNF function in the recruitment of SAGA to HO may be a special case restricted to mitosis involving a hypercondensed state of chromatin (25). Indeed, recruitment of Gcn5p occurred independently of Snf2p at a synthetic PHO5 promoter regulated by Gcn4p (54). Recruitment of SWI/SNF by Gcn4p to a plasmid-borne copy of HIS3 leads to a highly labile chromatin domain that extends beyond the promoter region and includes the coding sequences (24). There is also evidence that SWI/SNF can stimulate transcription elongation (9).
The mechanism of SWI/SNF recruitment by activators is not well understood. Gcn4p can bind in vitro to SWI/SNF (35, 38), dependent on bulky hydrophobic residues in the Gcn4p activation domain that are required for transcriptional stimulation in vivo (10, 22). The Snf2p, Snf5p, and Swi1p subunits were photo-cross-linked to Gcn4p and to activator Hap4p in the context of native SWI/SNF, and the corresponding recombinant subunits can bind individually to both activator proteins (37). It was unknown, however, whether these interactions are important for SWI/SNF recruitment in living cells. Recruitment of SWI/SNF to SUC2 requires both Snf5p and Snf2p (13, 17). At SER3, by contrast, Snf5p binding requires Snf2p, while Snf2p binding is independent of Snf5p (33). Experiments with purified components indicate that histone acetylation by SAGA can increase the retention of SWI/SNF on promoter nucleosomes (16) dependent on the Snf2p bromodomain and that the bromodomain is required for activator-induced SWI/SNF recruitment to SUC2 in vivo (17). However, H3 acetylation by Gcn5p is not required for SWI/SNF recruitment, even though it promotes nucleosome remodeling by SWI/SNF at the synthetic PHO5 promoter mentioned above (54). Similar conclusions were reached for RNR3 (46) and PHO8 (15, 44). Interestingly, SWI/SNF binding at RNR3 requires the functions of TFIID and the SRB mediator/PolII holoenzyme (46).
Previously, we showed that multiple SWI/SNF subunits are required for wild-type (WT) transcriptional activation of a subset of genes regulated by Gcn4p. Expression of GCN4 is induced at the translational level by starvation for any amino acid (18), and the induced Gcn4p stimulates transcription of hundreds of genes, including those involved in amino acid, vitamin, and purine biosynthesis (36). Transposon insertions or deletions of SWI/SNF subunits Snf2p, Swi1p, Swp73p, Snf5p, Snf6p, and Swi3p impaired Gcn4p activation of a lacZ reporter and conferred sensitivity to amino acid analogs that inhibit biosynthetic enzymes induced by Gcn4p. Mutations in certain SWI/SNF subunits also impaired induction by Gcn4p of a HIS3-GUS reporter and the authentic target genes SNZ1 and HIS4; however, other Gcn4p-dependent genes can be induced independently of Snf2p (35, 53). The SWI/SNF-independence of a subset of Gcn4p target genes may reflect the absence of repressive chromatin in their promoter regions; alternatively, SWI/SNF may have overlapping functions at these genes with SAGA, SRB mediator (45), or RSC complexes—all employed as coactivators by Gcn4p in vivo (27, 35, 53).
We wished to determine the roles of different subunits of SWI/SNF and the coactivators SAGA and SRB mediator in recruitment of SWI/SNF by Gcn4p in vivo. Toward this end, we performed ChIP analysis on deletion mutants lacking different subunits of these coactivators and bearing epitope tags on six different SWI/SNF subunits. We show that Gcn4p recruits all of these SWI/SNF subunits to the ARG1 and SNZ1 promoters and that SWI/SNF recruitment is dependent on hydrophobic clusters in the Gcn4p activation domain. Binding of Gcn4p itself to the ARG1 promoter occurs independently of the activation domain and SWI/SNF function. In contrast to the situation described at SER3 (33) and SUC2 (13), we find that recruitment of SWI/SNF by Gcn4p is independent of Snf2p but is strongly dependent on Snf5p, Snf6p, and Swi3p. Since deletions of these latter subunits disrupt the SWI/SNF complex, efficient recruitment by Gcn4p most likely depends on interactions of the activation domain with multiple subunits of the complex. Consistent with this model, overexpressing Snf2p or Snf5p alone does not increase their recruitment by Gcn4p. SWI/SNF recruitment by Gcn4p is independent of Gcn5p but, interestingly, requires the Ada1p and Ada5p/Spt20p subunits of SAGA and multiple subunits of SRB mediator. The latter findings suggest that SWI/SNF recruitment is enhanced by cooperative interactions with SRB mediator and SAGA recruited by Gcn4p to the same promoters.
MATERIALS AND METHODS
Yeast strains and plasmids.
All strains listed in Table 1 are derived from BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) or BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) or deletion derivatives thereof generated by the Saccharomyces Genome Deletion Project and purchased from Research Genetics. The presence of all reported deletion alleles was confirmed by PCR amplification with lysed cells as the source of template chromosomal DNA and commercially available primers. In each case, the upstream primer derives from sequences 5′ of the deleted sequences and the downstream primer corresponds to sequences in the KanMX4 cassette that replaces the deleted sequences. Except for the snf11Δ strain, the presence of the deletion alleles was also verified by showing that sensitivity of the strains to sulfometuron methyl (SM) was complemented by the relevant WT plasmid-borne alleles (53). The absence of Snf2p in the snf2Δ strain was additionally confirmed by Western blot analysis using anti-Snf2p antiserum. Insertion of the coding sequences for 13 tandem myc epitopes at the C termini of SWI/SNF genes using the myc13-HIS3 cassette in pFA6a-13Myc-HIS3 (31) was conducted as described previously (53). The presence of the myc-tagged alleles was verified by colony PCR and by Western blot analysis using anti-Myc antibodies. The deletion of GCN4 (in all strains except 249) was conducted by transforming with plasmid pHQ1240, containing gcn4Δ::hisG::URA3::hisG and digested with SspI, and selecting for Ura+ colonies. The URA3 gene was subsequently evicted by selecting for growth on medium containing 5-fluoroorotic acid (2).
TABLE 1.
S. cerevisiae strains
| Strain | Parent strain | Relevant genotype | Reference or source |
|---|---|---|---|
| BY4741 | NA | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| BY4742 | NA | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Research Genetics |
| SY1 | BY4741 | SNF2-myc13::HIS3*a | 53 |
| SY162 | BY4741 | SNF5-myc13::HIS3* | This study |
| SY163 | BY4741 | SNF6-myc13::HIS3* | This study |
| SY160 | BY4741 | SWI1-myc13::HIS3* | This study |
| SY161 | BY4741 | SWI3-myc13::HIS3* | This study |
| SY175 | BY4741 | SWP73-myc13::HIS3* | This study |
| SY127 | BY4741 | SNF2-myc13::HIS3* gcn4Δ | This study |
| 249 | BY4741 | gcn4Δ::kanMX4 | Research Genetics |
| SY166 | 249 | SNF5-myc13::HIS3* gcn4Δ::kanMX4 | This study |
| SY167 | 249 | SNF6-myc13::HIS3* gcn4Δ::kanMX4 | This study |
| SY164 | 249 | SWI1-myc13::HIS3* gcn4Δ::kanMX4 | This study |
| SY165 | 249 | SWI3-myc13::HIS3* gcn4Δ::kanMX4 | This study |
| SY176 | 249 | SWP73-myc13::HIS3* gcn4Δ::kanMX4 | This study |
| 1586 | BY4741 | swi2Δ::kanMX4 | Research Genetics |
| SY169 | 1586 | swi2Δ::kanMX4 gcn4Δ | This study |
| SY206 | 1586 | swi2Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY207 | 1586 | swi2Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| SY210 | 1586 | swi2Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| SY228 | 1586 | swi2Δ::kanMX4 SWI3-myc13::HIS3* | This study |
| SY282 | 1586 | swi2Δ::kanMX4 SWP73-myc13::HIS3* | This study |
| 1250 | BY4741 | swi3Δ::kanMX4 | Research Genetics |
| SY294 | 1250 | swi3Δ::kanMX4 gcn4Δ | This study |
| SY2 | 1250 | swi3Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY222 | 1250 | swi3Δ::kanMX4 SWP73-myc13::HIS3* | This study |
| SY284 | 1250 | swi3Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| SY286 | 1250 | swi3Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY324 | 1250 | swi3Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 7175 | BY4741 | snf5Δ::kanMX4 | Research Genetics |
| SY327 | 7175 | snf5Δ::kanMX4 gcn4Δ | This study |
| SY5 | 7175 | snf5Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY209 | 7175 | snf5Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| SY219 | 7175 | snf5Δ::kanMX4 SWP73-myc13::HIS3* | This study |
| SY225 | 7175 | snf5Δ::kanMX4 SWI3-myc13::HIS3* | This study |
| SY229 | 7175 | snf5Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| 6409 | BY4741 | snf6Δ::kanMX4 | Research Genetics |
| SY295 | 6409 | snf6Δ::kanMX4 gcn4Δ | This study |
| SY3 | 6409 | snf6Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY221 | 6409 | snf6Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| SY223 | 6409 | snf6Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY300 | 6409 | snf6Δ::kanMX4 SWI3-myc13::HIS3* | This study |
| SY299 | 6409 | snf6Δ::kanMX4 SWP73-myc13::HIS3* | This study |
| 4008 | BY4741 | snf11Δ::kanMX4 | Research Genetics |
| SY296 | 4008 | snf11Δ::kanMX4 gcn4Δ | This study |
| SY4 | 4008 | snf11Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY214 | 4008 | snf11Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| SY215 | 4008 | snf11Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY216 | 4008 | snf11Δ::kanMX4 SWI3-myc13::HIS3* | This study |
| SY218 | 4008 | snf11Δ::kanMX4 SWP73-myc13::HIS3* | This study |
| SY278 | 4008 | snf11Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 15398 | BY4742 | swp73Δ::kanMX4 | Research Genetics |
| SY298 | 15398 | swp73Δ::kanMX4 gcn4Δ | This study |
| SY6 | 15398 | swp73Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY217 | 15398 | swp73Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY226 | 15398 | swp73Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| SY283 | 15398 | swp73Δ::kanMX4 SWI1-myc13::HIS3* | This study |
| SY290 | 15398 | swp73Δ::kanMX4 SWI3-myc13::HIS3* | This study/PICK> |
| 7285 | BY4741 | gcn5Δ::kanMX4 | Research Genetics |
| SY202 | 7285 | gcn5Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY203 | 7285 | gcn5Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| SY204 | 7285 | gcn5Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| 1038 | BY4741 | ada1Δ::kanMX4 | Research Genetics |
| SY199 | 1038 | ada1Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY211 | 1038 | ada1Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY212 | 1038 | ada1Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 7309 | BY4741 | ada5Δ::kanMX4 | Research Genetics |
| SY205 | 7309 | ada5Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY213 | 7309 | ada5Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| 3218 | BY4741 | spt7Δ::kanMX4 | Research Genetics |
| SY273 | 3218 | spt7Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY276 | 3218 | spt7Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| 2786 | BY4741 | srb10Δ::kanMX4 | Research Genetics |
| SY270 | 2786 | srb10Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY274 | 2786 | srb10Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY285 | 2786 | srb10Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 4734 | BY4741 | srb5Δ::kanMX4 | Research Genetics |
| SY287 | 4734 | srb5Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY288 | 4734 | srb5Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY293 | 4734 | srb5Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 4393 | BY4741 | pgd1Δ::kanMX4 | Research Genetics |
| SY271 | 4393 | pgd1Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY343 | 4393 | pgd1Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| SY346 | 4393 | pgd1Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| 3119 | BY4741 | rox3Δ::kanMX4 | Research Genetics |
| SY289 | 3119 | rox3Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY360 | 3119 | rox3Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY361 | 3119 | rox3Δ::kanMX4 SNF6-myc13::HIS3* | This study |
| 1742 | BY4741 | gal11Δ::kanMX4 | Research Genetics |
| SY197 | 1742 | gal11Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY198 | 1742 | gal11Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| LSO2 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 med2Δ::kanMX4 | 53 | |
| SY292 | LSO2 | med2Δ::kanMX4 SNF5-myc13::HIS3* | This study |
| SY305 | LSO2 | med2Δ::kanMX4 SNF2-myc13::HIS3* | This study |
| SY341 | LSO2 | med2Δ::kanMX4 SNF6-myc13::HIS3* | This study |
HIS3* designates the HIS3 allele from S. kluyveri (31).
Plasmids p2382, pHQ1239, and pSK1 were described previously (53). The empty vector employed throughout was the URA3 CEN4 plasmid YCp50 (39). Plasmid pHQ1240 was constructed by inserting the EcoRI-SalI fragment from p2382 between the corresponding sites in pUC19 and replacing the BamHI-BglII fragment of the resulting plasmid with a 3.8-kb BamHI fragment containing the hisG::URA3::hisG cassette from pNKY51 (2). Single-copy plasmids pSY284 and pSY285, harboring gcn4-14Ala-myc or gcn4-14Ala-HA, respectively, were constructed by ligating the larger XbaI-MluI fragment of p2240 (22), a single-copy plasmid harboring gcn4-14Ala, with the smaller XbaI-MluI fragments of pSK1 or p2382. The constructs were verified by sequencing and by Western blot analysis of whole-cell extracts (WCEs) of the appropriate yeast transformants by using anti-Myc or -HA antibodies. The high-copy-number plasmids pHQ1303 and pHQ1304, harboring GCN4 or gcn4-14Ala, respectively (see Fig. 3), were produced as follows. The EcoRI-SalI fragment of pCD35 (10) was inserted into YEplac195 (14) between the EcoRI and SalI sites to produce pHQ1303. The SalI to XbaI fragment from p2240 and the XbaI to EcoRI fragment from pCD35 were inserted into YEplac195 between the EcoRI and SalI sites to produce pHQ1304. The constructs were verified by sequencing.
FIG. 3.
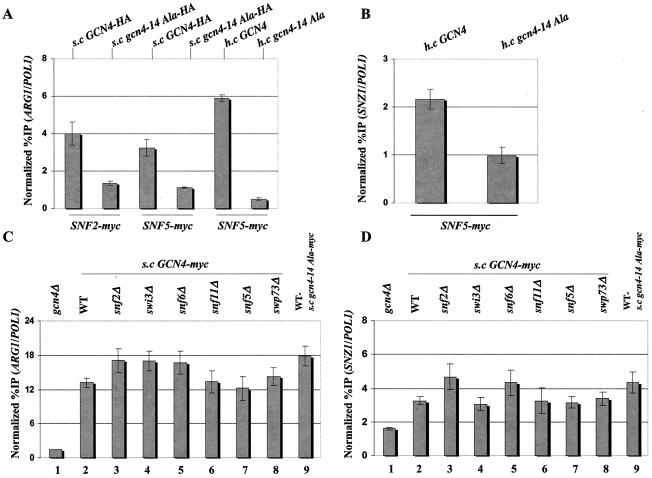
Hydrophobic clusters in the Gcn4p activation domain are required for recruitment of Snf2p and Snf5p to ARG1 and SNZ1, but Gcn4p binding at these promoters is independent of SWI/SNF. (A and B) The gcn4Δ SNF2-myc (SY127) and gcn4Δ SNF5-myc (SY166) strains described in the legend to Fig. 1 were transformed with single-copy (s.c) plasmid p2382 or pSY285, harboring GCN4-HA or gcn4-14Ala-HA, respectively, or high-copy-number (h.c.) plasmid pHQ1303 or pHQ1304, harboring GCN4 or gcn4-14Ala, respectively, and subjected to ChIP analysis as described in the legend to Fig. 1 to measure binding of myc-tagged Snf2p or Snf5p to ARG1 (A) or SNZ1 (B). (C and D) ChIP analysis was conducted as described above to measure binding of myc-Gcn4p to ARG1 (C) or SNZ1 (D) in the following strains: transformants of the gcn4Δ strains containing either all WT SWI/SNF genes (249), snf2Δ (SY169), swi3Δ (SY294), snf6Δ (SY295), snf11Δ (SY296), snf5Δ (SY327), or swp73Δ (SY298), all bearing single-copy plasmid pSK1 containing GCN4-myc, lanes 2 through 8; gcn4Δ strain (249) transformed with empty vector YCp50 or single-copy plasmid pSY284 containing gcn4-14Ala-myc, respectively, lanes 1 and 9.
The high-copy-number plasmids pSY286 and pSY287, harboring SNF2-myc or SNF5-myc, respectively, were generated by introducing the coding sequences for 13 myc epitopes at the C termini of the corresponding genes in plasmids pLN138-4 and pAC153 (1). Briefly, yeast strains 1586 and 7175 were transformed with plasmids pLN138-4 and pAC153, respectively, along with the relevant PCR product used for tagging the C termini of chromosomal SNF2 or SNF5 with 13 tandem myc epitopes (see above). The transformants were screened by Western blot analysis with anti-Myc antibodies for the presence of the relevant myc-tagged protein. The plasmids were recovered from cells (19) and reintroduced into the S. cerevisiae strains described above to verify that they encode the relevant myc-tagged protein, as indicated by Western blot analysis, and to demonstrate their ability to complement the SM sensitivity of the resident snf2Δ or snf5Δ mutations (53) indistinguishably from that of the parental plasmids.
ChIPs.
The experiments were conducted as previously described (53), with the following modifications. Cells cross-linked with formaldehyde were sonicated for 12 cycles of 30 s at 4°C with at least 30-s cooling on ice per cycle. The primer set 5′ TGAAATGCCTGGTGTCAACT 3′ (sense) and 5′ TCTATGCAATCTTGCCAAAG 3′) (antisense) was used to amplify sequences −4724 to −4557 relative to the SER3 ATG codon (SER3−4.5kb). The primers used to amplify nucleotides −198 to −43 at the SNZ1 promoter were 5′ TAGCGCCGCCATTTCTTCAT 3′ (sense) and 5′ TCGTTCCTAAAGGTTTCTCC 3′ (antisense). The primers used to amplify the ARG1 upstream activation sequence (UAS) and POL1 open reading frame (ORF) were described previously (53). The PCR conditions were as follows: initial denaturation at 94°C for 4 min, followed by 27 cycles of 94°C for 30 s, 52°C for 30 s, 65°C for 1 min, and a final extension for 5 min at 65°C.
Western blot analysis and coimmunoprecipitation assays.
For Western blot analysis, yeast cultures were grown to an optical density at 600 nm (OD600) of 1.0 to 2.0 in synthetic complete medium lacking isoleucine and valine (SC-ILV) (47) at 30°C and treated with 0.6 μg of SM/ml for 2 h. Total proteins were extracted by the trichloroacetic acid method described previously (43) and analyzed using rabbit polyclonal anti-Snf5p antibodies (5), anti-Swp73p antibodies (6), and anti-Gcd6p antibodies (7). The WCEs for coimmunoprecipitation analysis were prepared by a method described previously for glutathione S-transferase pull-down assays (11), with the following modifications. The cells were grown in 100 ml of YPD medium (47) to an OD600 of 1.0 to 2.0 and resuspended in 200 μl of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 20% glycerol, 50 mM NaCl, 0.1% Triton X-100, 10 μg of leupeptin/ml, 10 μg of pepstatin/ml, 10 μg of aprotinin/ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and protease inhibitor cocktail tablet [Boehringer]). The cell suspension was lysed by vortexing with glass beads for five cycles of 30 s, and the supernatant was recovered after centrifugation at 12,000 rpm in an Eppendorf Microfuge for 15 min at 4°C. Coimmunoprecipitation analysis was performed using mouse monoclonal anti-Myc antibodies (Roche). Briefly, the WCEs were incubated for 1 h at 4°C with 1 μg of anti-Myc antibody, 100 μg of bovine serum albumin dissolved in phosphate-buffered saline, and MT buffer (11). The immune complexes were washed three times with 1 ml of MT buffer, dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (Invitrogen), and subjected to Western blot analysis with the anti-Myc and anti-Snf5p antibodies described above.
RESULTS
Gcn4p recruits multiple subunits of the SWI/SNF complex to the ARG1 and SNZ1 promoters in vivo.
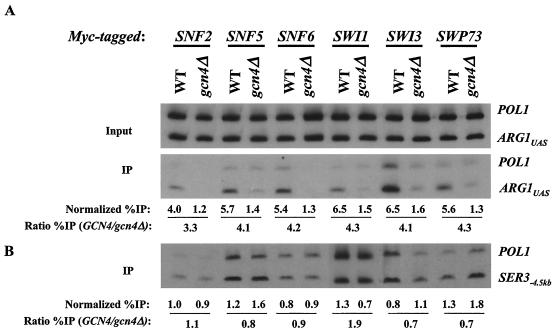
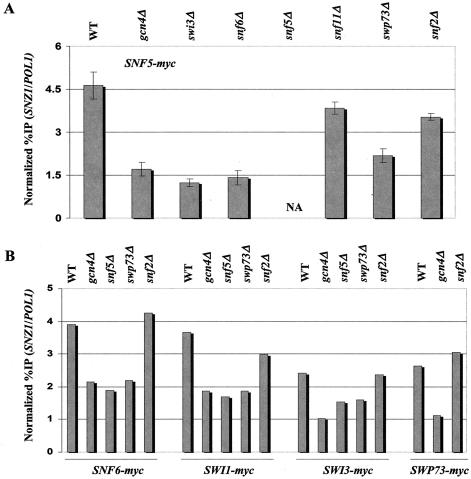
Previously, we showed that Gcn4p recruits myc-tagged Snf2p to the UAS of the ARG1 promoter when Gcn4p expression is induced from a high-copy-number plasmid by starvation for isoleucine and valine with the drug SM (53). We wished to determine whether Snf2p and other subunits of SWI/SNF would be recruited to ARG1 when Gcn4p is being expressed from a single-copy gene. Accordingly, we constructed six pairs of yeast strains, each harboring a different subunit of SWI/SNF (Snf2p, Snf5p, Snf6p, Swi1p, Swi3p, or Swp73p) tagged at the C terminus with 13 myc epitopes. One member of each pair bears the WT chromosomal GCN4 allele, and the other has a deletion of GCN4. All strains were grown in the presence of SM for 2 h to induce Gcn4p synthesis in the GCN4 strains (Fig. 1A). The results showed that the ARG1 UAS was immunoprecipitated with each myc-tagged SWI/SNF subunit at a level 3.3- to 4.3-fold higher in the WT strain than in the gcn4Δ strain (Fig. 1A). As a negative control, we monitored immunoprecipitation of a sequence located 4.5-kb upstream of the SER3 gene. As shown in Fig. 1B, we observed low-level immunoprecipitation of this SER3−4.5kb sequence relative to POL1 in WT and gcn4Δ strains alike. The comparable levels of GCN4-dependent immunoprecipitation of the ARG1 UAS observed for all six myc-tagged strains suggest that Gcn4p recruits the entire SWI/SNF complex to the ARG1 UAS. We consistently observed a lower level of ARG1 immunoprecipitation in the strain with myc-tagged Snf2p than in strains with other myc-tagged SWI/SNF subunits. We assume that this finding reflects a relatively low efficiency of cross-linking by myc-tagged Snf2p to the promoter.
FIG. 1.
Gcn4p at native levels recruits the SWI/SNF complex to the ARG1 promoter. (A) WT (BY4741) and gcn4Δ (249) strains containing 13-myc tagged alleles of SNF2 (SY1 and SY127), SNF5 (SY162 and SY166), SNF6 (SY163 and SY167), SWI1 (SY160 and SY164), SWI3 (SY161 and SY165), and SWP73 (SY175 and SY176) were grown to an OD600 of 0.8 to 1.0 in SC-ILV medium at 30°C and treated with 0.6 μg of SM/ml for 2 h. Cells were harvested, treated with formaldehyde, and broken by vortexing with glass beads, and the extracts were sonicated to produce chromatin fragments with an average length of ∼500 bp. Aliquots (5% of the total) were immunoprecipitated with anti-myc antibodies, and DNA was extracted from the immunoprecipitate (IP) after reversing the cross-links. DNA was extracted directly from another aliquot of the chromatin preparation (5% of the total) to serve as the input control. (A) A 600-fold dilution of the input control and the undiluted IP DNA were amplified by PCR by using primers specific for the ARG1 UAS or POL1 ORF in the presence of [33P]dATP. The PCR products were resolved by PAGE and quantified by phosphorimaging analysis, and the ratio of the ARG1 UAS signals in the IP-to-input samples was calculated and normalized for the corresponding ratio calculated for the POL1 signals to yield the normalized percentage of IP (Normalized %IP). The ratio of the normalized percentage of IP values for the GCN4 to gcn4Δ strains was calculated to yield the ratio percentage of IP (Ratio %IP) (GCN4/gcn4Δ). (B) As a negative control, the same IP and input samples described in panel A were analyzed using primers to amplify a sequence located 4.5-kb upstream of the SER3 gene.
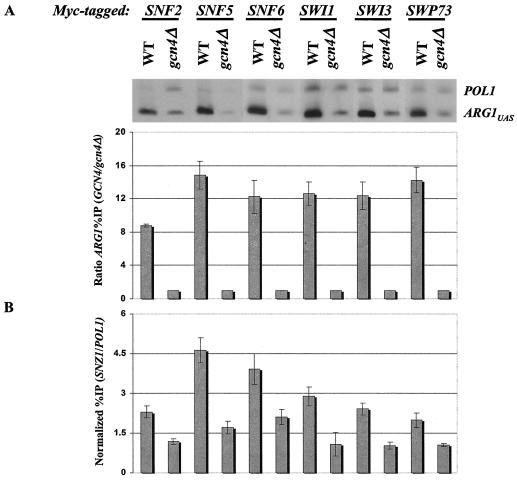
A similar analysis was carried out after transforming each of the myc-tagged GCN4 strains with a high-copy-number plasmid bearing GCN4-HA, a fully functional allele tagged at the C terminus with the triple-HA tag (53). As shown in Fig. 2A, the ARG1 UAS was immunoprecipitated with each myc-tagged subunit at a level 8- to 15-fold higher in the WT than in the gcn4Δ strains. The relatively higher level of ARG1 UAS immunoprecipitation shown in Fig. 2A than in Fig. 1A presumably reflects increased Gcn4p binding at ARG1 when Gcn4p is overexpressed. Indeed, other ChIP experiments show that overexpression of myc-tagged Gcn4p from a high-copy-number plasmid produces an approximately twofold increase in binding to ARG1 than that seen with single-copy GCN4-myc (H. Qiu and A. G. Hinnebusch, unpublished data). We also analyzed the GCN4-dependent recruitment of all six SWI/SNF subunits to the 5′ noncoding region of SNZ1, another Gcn4p target gene (36), in the same strains that contain high-copy-number GCN4-HA. The percentages of the SNZ1 promoter that immunoprecipitated with myc-tagged SWI/SNF subunits from the WT strains (Fig. 2B) were uniformly lower than those observed for the ARG1 UAS (Fig. 2A); nevertheless, in all cases they were significantly greater than those seen in the gcn4Δ strains (Fig. 2B). Hence, it is likely that the entire SWI/SNF complex is recruited by Gcn4p to both SNZ1 and ARG1.
FIG. 2.
Overexpression of Gcn4p increases the recruitment of SWI/SNF at ARG1 and allows detection of SWI/SNF recruitment at SNZ1. (A) High-copy-number 2μm plasmid pHQ1239 harboring the GCN4-HA allele was introduced into the GCN4 strains containing myc-tagged SWI/SNF subunits, and empty vector YCp50 was introduced into the gcn4Δ myc-tagged strains that were described in the legend to Fig. 1. The strains were cultured in SC-ILV-Ura (SC-ILV also lacking uracil) and induced with SM and then subjected to ChIP analysis as described in the legend to Fig. 1. The results of a typical experiment are depicted at the top, while the histograms below summarize the averages and standard errors of the ratio percent IP (GCN4/gcn4Δ) (as defined in the legend to Fig. 1) that were derived from multiple determinations for each strain. Two independent cultures and two to four independent immunoprecipitations were analyzed for each strain to provide the data used to calculate the mean values and standard errors shown in the histogram. (B) The same process was used as for panel A except that the SWI/SNF subunit binding to the SNZ1 promoter was analyzed.
In most of the ChIP experiments described below, we measured SWI/SNF recruitment to SNZ1 and ARG1 in strains overexpressing Gcn4p from a high-copy-number plasmid. This was done because the level of SWI/SNF recruitment in strains expressing the native amount of Gcn4p is too low to distinguish statistically significant differences in SWI/SNF binding between mutant and WT strains at SNZ1. As indicated above, promoter occupancy by Gcn4p increases approximately twofold when it is overexpressed from a high-copy-number plasmid. This level of promoter binding by Gcn4p may exist under conditions of severe amino acid limitation in which cell division is blocked, whereas cells continue to grow and divide at the SM concentration employed in our experiments (data not shown). In addition, cells overexpressing Gcn4p exhibit approximately twofold-greater transcriptional induction of SNZ1 than occurs in WT cells (S. Yoon, S. Kim, and A. G. Hinnebusch, unpublished data), showing that the increased promoter occupancy by Gcn4p is functionally significant. In any event, the experiments described below allowed us to define the requirements for efficient SWI/SNF recruitment at maximal promoter occupancy by the activator Gcn4p.
Recruitment of SWI/SNF is dependent on the Gcn4p activation domain, but promoter binding by Gcn4p is independent of SWI/SNF.
In vitro interaction between Gcn4p and the SWI/SNF complex is strongly dependent on bulky hydrophobic clusters in the Gcn4p activation domain, and multiple clusters must be inactivated to observe a strong defect in SWI/SNF binding (35). To determine whether the hydrophobic clusters are required for recruitment of SWI/SNF by Gcn4p in vivo, we conducted ChIP experiments using the SNF2-myc gcn4Δ and SNF5-myc gcn4Δ strains described above harboring plasmids containing GCN4 or gcn4-14Ala (or the corresponding HA-tagged versions of these alleles). The gcn4-14Ala allele contains 14 alanine substitutions in the 7 hydrophobic clusters in the Gcn4p activation domain that contribute to transcriptional activation in vivo (22). As shown in Fig. 3A and B, we consistently observed greater binding of myc-Snf2p and myc-Snf5p to the ARG1 and SNZ1 promoters and greater binding of myc-Snf2p at ARG1 in the strains harboring GCN4 than in those with gcn4-14Ala.
It was conceivable that the 14 Ala substitutions in the activation domain reduce SWI/SNF recruitment by impairing the binding of Gcn4p to the ARG1 and SNZ1 promoters. As shown in Fig. 3C and D, similar or even greater amounts of ARG1 and SNZ1 promoter sequences were immunoprecipitated with myc-gcn4p-14Ala than with myc-Gcn4p when these proteins were expressed from single-copy plasmids (Fig. 3C, compare lanes 2 and 9; Fig. 3D, compare lanes 2 and 9). The somewhat higher level of promoter binding observed for the 14Ala mutant protein can be attributed to its higher steady-state level, as determined by Western blot analysis (data not shown). Higher steady-state levels are frequently observed for Gcn4 proteins with mutations in the activation domain (22).
The results shown in Figs. 3C to D also reveal that deletions of SWI/SNF subunits have little or no effect on binding of myc-tagged Gcn4p to the ARG1 and SNZ1 promoters. This finding is especially important for the snf2Δ mutant, which lacks the ATPase subunit of the complex. Hence, WT binding of Gcn4p at these promoters does not depend on prior nucleosome remodeling by SWI/SNF, nor does Gcn4p binding require prior interaction of any cofactors with the activation domain in a manner that depends on the hydrophobic clusters.
Recruitment of SWI/SNF by Gcn4p is independent of Snf2p but requires Snf5p, Snf6p, and Swi3p.
It was shown previously that Swi2p/Snf2p, Snf5p, and Swi1p were photo-cross-linked to Gcn4p in the context of intact SWI/SNF and that each recombinant subunit can interact directly with recombinant Gcn4p in vitro (37). We wished to determine whether these interactions are necessary or sufficient for recruitment of the SWI/SNF complex to the ARG1 and SNZ1 promoters in vivo. Towards this end, we constructed a panel of six strains harboring deletions of SNF6, SNF5, SWI3, SNF11, SWP73 or SNF2 for each of the six strains containing a different myc-tagged subunit (myc-Snf2p, myc-Snf5p, myc-Swp73, myc-Swi3p, myc-Snf6p, and myc-Swi1p). The resulting 31 strains, along with the 12 tagged strains containing all WT SWI/SNF subunits (WT and gcn4), were subjected to ChIP analysis as described above.
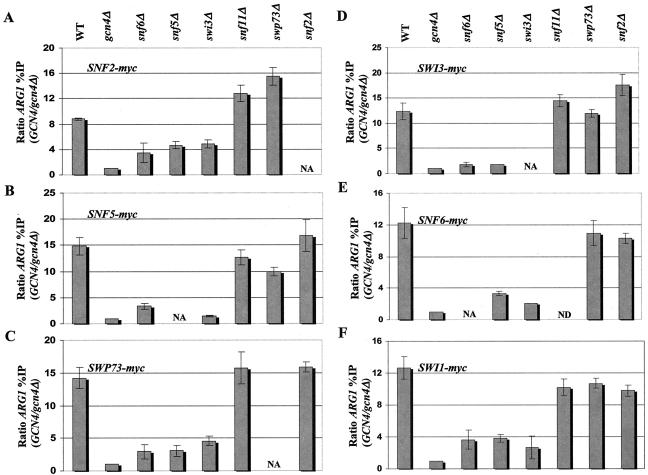
The results shown in Fig. 4A through F indicate that the snf6Δ, snf5Δ, and swi3Δ strains consistently showed marked reductions in the Gcn4p-dependent recruitment of all tagged SWI/SNF subunits to the ARG1 UAS. Recruitment was not completely eliminated by any of these mutations, as the residual binding was consistently higher than that seen in the gcn4Δ strain lacking the activator. By contrast, the snf11Δ, swp73Δ, and snf2Δ strains showed little or no reduction in binding of all SWI/SNF subunits to ARG1. Similar results were obtained when we analyzed binding to SNZ1 except that the snf6Δ and swi3Δ mutations greatly reduced myc-Snf5p binding to the same low level observed in the gcn4Δ strain. Moreover, the binding of all tagged SWI/SNF subunits to this promoter was impaired by swp73Δ (Fig. 5A and B), even though this mutation had little or no effect on SWI/SNF recruitment to ARG1 (Fig. 4). Even though Snf2p, Snf5p, and Swi1p are capable of direct binding to Gcn4p in vitro (37), the data in Fig. 4 and 5 suggest that high-level recruitment of these proteins is dependent on SWI/SNF subunits Snf6p, Snf5p, and Swi3p. Interestingly, it appears that interactions between Gcn4p and Snf2p are dispensable for recruitment of other SWI/SNF subunits in vivo.
FIG. 4.
Recruitment of SWI/SNF subunits by Gcn4p to ARG1 is impaired in snf6Δ, snf5Δ, and swi3Δ mutants. (A through F) We generated a panel of strains harboring the chromosomal myc-tagged SWI/SNF alleles designated in the upper left of each histogram from GCN4 strains containing all WT SWI/SNF subunits or the indicated swi/snf deletion alleles (shown at the top) and from the gcn4Δ strain containing all WT SWI/SNF subunits (gcn4Δ). (See Table 1 for a list of all strains employed.) High-copy-number plasmid pHQ1239 was introduced into the GCN4 strains, and empty vector YCp50 was introduced into the gcn4Δ strains. The resulting transformants were subjected to ChIP analysis as described in the legend to Fig. 1. NA, not applicable; ND, not determined.
FIG. 5.
Recruitment of SWI/SNF subunits by Gcn4p to SNZ1 is impaired in snf6Δ, snf5Δ, swi3Δ, and swp73Δ mutants. The selected transformants described in the legend to Fig. 4 were subjected to ChIP analysis to measure binding of the indicated myc-tagged SWI/SNF subunits to SNZ1 in GCN4 or gcn4Δ strains containing all WT SWI/SNF subunits (WT and gcn4Δ, respectively) and in GCN4 strains containing the indicated swi/snf deletion alleles.
Impaired recruitment of SWI/SNF in snf6Δ, snf5Δ, swi3Δ, and swp73Δ mutants likely results from complex disruption.
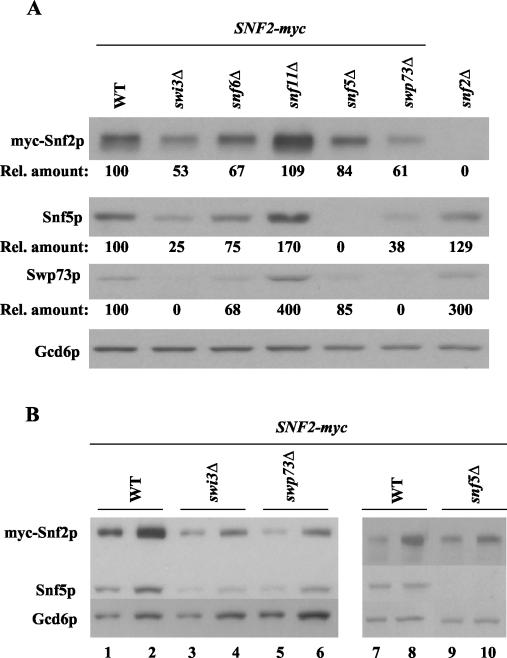
It was shown previously that deletions of Snf6p, Snf5p, and Swi3p affect the integrity of the SWI/SNF complex (40). Thus, the reduced recruitment of SWI/SNF subunits to ARG1 and SNZ1 we observed in snf6Δ, snf5Δ, and swi3Δ mutants and to SNZ1 in swp73Δ cells might result simply from reduced steady-state levels of these subunits in the mutant strains. To address this possibility, we conducted Western blot analysis of WCEs from swi/snf mutant and WT strains harboring SNF2-myc by using rapid extraction with trichloroacetic acid to minimize protein degradation during preparation of the extracts. The blots were probed with Myc antibodies to visualize myc-Snf2p, with antibodies against SWI/SNF subunits Snf5p and Swp73p, and with antibodies against Gcd6p to control for protein loading. Quantification of the results shown in Fig. 6A and B indicated that the steady-state levels of myc-Snf2p, Snf5p, and Swp73p (normalized for Gcd6p) were reduced by one-third or less in snf6Δ and snf5Δ cells. Greater reductions, of between 40 to 75%, were observed in swi3Δ and swp73Δ cells. (Owing to the relatively weak Western blotting signals for Swp73p, we could not estimate its reduction in swi3Δ cells.)
FIG. 6.
Western blot analysis of SWI/SNF subunits in swi/snf mutants. (A) The GCN4 strains containing SNF2-myc and either WT SWI/SNF subunits or the indicated swi/snf deletions were grown in SC-ILV, and total proteins were extracted as described in Materials and Methods. Aliqouts with equal amounts of total proteins were separated by SDS-PAGE by using 10% gels, transferred to a nitrocellulose membrane, and probed with antibodies against myc, Snf5p, or Swp73p, as indicated on the left of the blot. Probing with Gcd6p antibodies provided a loading control. The Western blotting signals were quantified by video densitometry by using NIH Image software, normalized for the Gcd6p signals, expressed relative to the normalized value measured in WT cells, and listed below the corresponding blots for myc-Snf2p, Snf5p, and Swp73p (Rel. amount). (B) The swp73Δ and swi3Δ strains exhibit similar reductions in SWI/SNF subunit levels. The analysis described for panel A was repeated for the WT, swi3Δ, swp73Δ, and snf5Δ strains harboring SNF2-myc, loading two different amounts of extract in adjacent lanes; thus, the sizes of samples in lanes 1, 3, 5, 7, and 9 were 50% of those loaded in lanes 2, 4, 6, 8, and 10.
As shown above, the Gcn4p-dependent recruitment of myc-Snf5p and myc-Swp73p to ARG1 was reduced by ∼80% in snf6Δ and snf5Δ mutants (Fig. 4B and C) and recruitment of myc-Snf5p to SNZ1 was completely eliminated in the snf6Δ mutant (Fig. 5A). These reductions in recruitment are considerably larger than the <33% decreases in expression of SWI/SNF subunits observed in snf6Δ and snf5Δ mutants (Fig. 6A). A similar but less pronounced disparity holds for myc-Snf2p, whose recruitment to ARG1 was decreased by 50 to 65%, versus the <33% reduction in myc-Snf2p expression seen in snf6Δ and snf5Δ cells. Hence, it seemed unlikely that reduced expression alone could account for the defective recruitment of SWI/SNF subunits in snf6Δ and snf5Δ cells. The same conclusion cannot be drawn for the swi3Δ mutant, because the reductions in recruitment to ARG1 were comparable to the reductions in subunit expression for myc-Snf2p, Snf5p, and Swp73p in swi3Δ cells (compare Fig. 4A through C and 6A and B). It is noteworthy, however, that we observed comparable, strong reductions in SWI/SNF subunit expression in swi3Δ and swp73Δ cells (Fig. 6B), yet recruitment of myc-Snf2p and myc-Snf5p to ARG1 occurred at nearly WT levels in swp73Δ cells (Fig. 4). Thus, the extensive decreases in myc-Snf2p, Snf5p, and Swp73p expression in swi3Δ cells probably cannot fully explain the dramatic reduction in recruitment of these proteins to ARG1 in the swi3Δ mutant. It appears that high-level recruitment of Snf2p and Snf5p by Gcn4p requires contributions from other SWI/SNF subunits that go beyond their roles in maintaining WT levels of Snf2p and Snf5p.
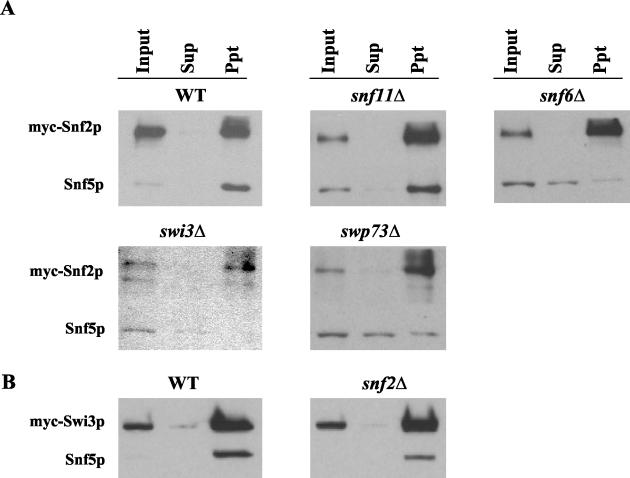
As mentioned above, previous gel filtration analysis suggested that the SWI/SNF complex is partially disrupted in snf2Δ, swi3Δ, snf5Δ, and snf6Δ strains, with a particularly strong defect in swi3Δ cells (40). We confirmed by coimmunoprecipitation analysis that the association between myc-Snf2p and Snf5p was impaired in our snf6Δ and swi3Δ strains, and we obtained the same result for the swp73Δ mutant (Fig. 7A). By contrast, myc-Snf2p/Snf5p association was intact in snf11Δ cells, as was the myc-Swi3p/Snf5p association in snf2Δ cells (Fig. 7B). The latter finding is in accordance with our Western blot analysis of snf2Δ cells showing high-level expression of SWI/SNF subunits (Fig. 6A) and our ChIP data showing little or no defect in SWI/SNF recruitment in the snf2Δ mutant (Fig. 4B through F and 5A and B). To account for the previous data indicating disruption of SWI/SNF in snf2Δ cells (40), we propose that SWI/SNF is largely intact in snf2Δ cells and dissociated in vitro under the conditions of gel filtration analysis. The fact that the SWI/SNF complex is disrupted (Fig. 7A) and subject to proteolysis (Fig. 6B) in swp73Δ cells yet shows no defect in recruitment by Gcn4p to ARG1 could indicate that a low level of intact SWI/SNF complex remains in this strain that is sufficient for nearly WT recruitment by Gcn4p to ARG1 but inadequate for SWI/SNF recruitment to SNZ1 (Fig. 5A and B). It is also possible that SWI/SNF subcomplexes which presumably occur in swp73Δ cells can be recruited independently of one another by Gcn4p, at least to ARG1 (see Discussion).
FIG. 7.
Examination of SWI/SNF complex integrity in swi/snf mutants by coimmunoprecipitation analysis. (A) SNF2-myc strains containing WT SWI/SNF subunits or the indicated swi/snf deletions were grown in YPD, and WCEs were prepared as described in Materials and Methods. Aliqouts containing 0.3 to 0.5 mg of protein were immunoprecipitated with mouse monoclonal myc antibodies. Ten percent of the input samples (Input), 100% of the immunoprecipitates (Ppt), and 10% of the supernatant (Sup) fractions were resolved by 4 to 20% SDS-PAGE and subjected to Western blot analysis using monoclonal mouse antibodies against myc or rabbit antibodies against Snf5p. The upper and lower portions of each membrane were probed separately with different antibodies. (B) The same kind of analysis as that described for panel A was carried out by using the WT or snf2Δ strains harboring SWI3-myc.
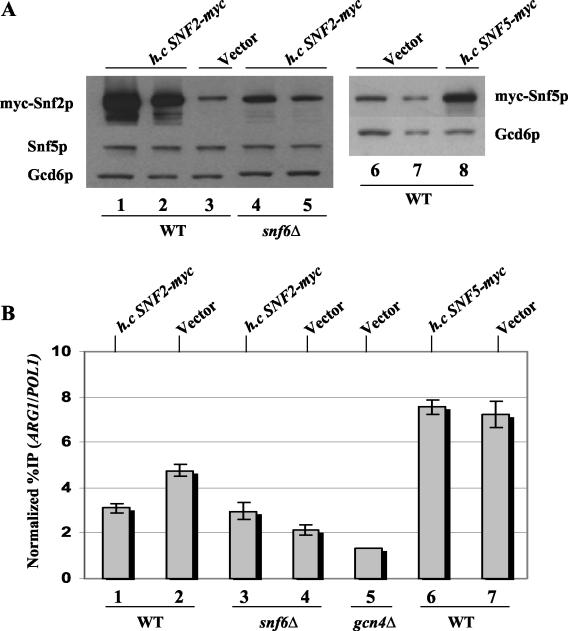
The results presented above suggest that efficient recruitment of Snf2p and Snf5p by Gcn4p in vivo is strongly dependent on their association with other subunits in the SWI/SNF complex. If so, then overexpressing these proteins individually from high-copy-number plasmids should not increase their level of recruitment by Gcn4p. To test this prediction, we introduced a multicopy SNF2-myc plasmid, or empty vector, into the otherwise WT strain harboring SNF2-myc. Likewise, we transformed the WT SNF5-myc strain with a multicopy SNF5-myc plasmid or with vector alone. In both instances, increasing the gene dosage of SNF2-myc or SNF5-myc led to overexpression of the cognate-tagged protein (Fig. 8A). Importantly, overexpression of myc-Snf2p led to a decrease, rather than an increase, in its recruitment to ARG1 (Fig. 8B, compare lanes 1 and 2). Overexpression of myc-Snf5p had no impact on the amount of this protein that was recruited by Gcn4p to ARG1 (Fig. 8B, compare lanes 6 and 7). To explain the negative effect of myc-Snf2p overexpression on its recruitment, we suggest that increasing the amount of Snf2p leads to the formation of Snf2p-containing subcomplexes that lack one or more other subunits required for efficient recruitment of SWI/SNF by Gcn4p.
FIG. 8.
Overexpression of myc-Snf2p or myc-Snf5p does not increase their recruitment to ARG1. (A) The SNF2-myc (SY1), SNF5-myc (SY162), and snf6Δ SNF2-myc (SY3) strains described in the legends to Fig. 1 and 4 were transformed with high-copy-number plasmid pSY286 or pSY287, harboring SNF2-myc or SNF5-myc, respectively, or with empty vector YCp50 and subjected to Western blot analysis as described in the legend to Fig. 6. Lanes 1 through 3, SNF2-myc strain transformed with the plasmids indicated at the top; lanes 4 and 5, snf6Δ SNF2-myc strain transformed with pSY286; lanes 6 through 8, SNF5-myc strain transformed with the plasmids indicated at the top. The samples in lanes 2, 5, and 7 contained 50% of those loaded in lanes 1, 4, and 6, respectively. (B) ChIP analysis was performed on the transformants described for panel A. Lanes 1 through 4, transformants of the SNF2-myc and snf6Δ SNF2-myc strains harboring the plasmids indicated at the top; lanes 6 and 7, transformants of the SNF5-myc strain harboring the plasmids indicated at the top; lane 5, transformant of the gcn4Δ SNF2-myc strain containing YCp50.
The multicopy SNF2-myc plasmid also led to greater-than-WT levels of myc-Snf2p in the snf6Δ mutant, compensating for the slight effect of this mutation on myc-Snf2p expression (Fig. 8A, compare lanes 3 through 5). Nonetheless, the recruitment of myc-Snf2p was impaired in this transformant relative to that seen in the WT strain with native levels of myc-Snf2p (Fig. 8B, compare lanes 2 and 3). We found by coimmunoprecipitation analysis that overexpressing myc-Snf2p in the snf6Δ mutant slightly increased SWI/SNF complex formation (data not shown), most likely accounting for the small increase in myc-Snf2p recruitment observed in this situation (Fig. 8B, compare lanes 3 and 4). The results shown in Fig. 8 support our conclusion that Snf6p contributes to SWI/SNF recruitment in a manner that exceeds its contribution to SWI/SNF subunit expression, presumably by promoting SWI/SNF complex integrity (see Discussion).
Recruitment of SWI/SNF by Gcn4p requires SRB mediator subunits and a non-Gcn5p function of SAGA.
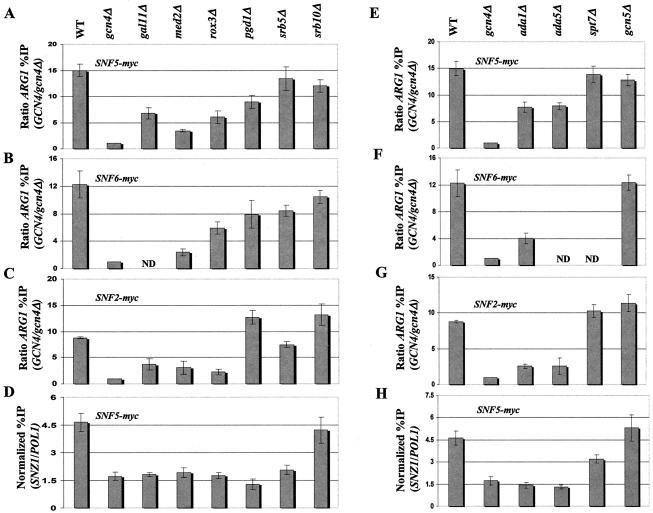
We next turned to the question of whether recruitment of the SWI/SNF complex is dependent on SRB mediator. To address this possibility, we myc13-tagged Snf2p, Snf5p, or Snf6p in deletion mutants lacking single subunits of SRB mediator (gal11Δ, med2Δ, rox3Δ, pgd1Δ, srb5Δ, or srb10Δ). All of these strains showed defects in transcriptional activation of Gcn4p-dependent reporters and selected Gcn4p target genes in vivo (53). The resulting set of tagged strains was analyzed by ChIP assays as described above. Deletion of GAL11, MED2, and ROX3 had relatively strong effects on recruitment of SWI/SNF subunits to ARG1 (Fig. 9A through C). Deletion of SRB5 or SRB10 produced either a modest reduction or had no effect on recruitment of SWI/SNF, while deletion of PGD1 gave various results (Fig. 9A through C). Interestingly, Gal11p, Med2p, Rox3p, Pgd1p, and Srb5p were all strongly required for efficient recruitment of myc-Snf5p to SNZ1, as deletion of each protein lowered recruitment to nearly the same low level seen in the gcn4Δ strain (Fig. 9D).
FIG. 9.
Subunits of mediator and SAGA are required for optimal recruitment of SWI/SNF by Gcn4p. (A through C and E through G) We generated a panel of strains harboring the chromosomal myc-tagged SWI/SNF alleles designated in the upper left of each histogram from GCN4 strains containing all WT coactivator subunits (WT) or the indicated deletion alleles (shown at the top) that remove subunits of mediator (A through C) or SAGA (E through G) and from the gcn4Δ strain containing all WT coactivator subunits (gcn4Δ) (see Table 1 for a list of strains). High-copy-number plasmid pHQ1239 was introduced into the GCN4 strains, and empty vector YCp50 was introduced into the gcn4Δ strains. The resulting transformants were subjected to ChIP analysis to measure binding of the myc-tagged SWI/SNF subunit to ARG1, as described in the legend to Fig. 1. (D and H) The strains described for panels A and E that harbor the SNF5-myc allele were subjected to ChIP analysis to measure binding of myc-Snf5p to SNZ1. ND, not determined.
We carried out similar experiments to address the importance of SAGA subunits Ada1p, Ada5p, Spt7p, and Gcn5p in recruitment of SWI/SNF to ARG1 and SNZ1. Deletions of these proteins also impair transcriptional activation of Gcn4p-dependent reporters and certain Gcn4p target genes (53). The results revealed a moderate to strong requirement for Ada1p and Ada5p in recruitment of SWI/SNF subunits to ARG1 (Fig. 9E through G) and a complete dependence on both proteins for SWI/SNF recruitment to SNZ1 (Fig. 9H). By contrast, Spt7p was dispensable for recruitment of SWI/SNF subunits to ARG1 and made only a small contribution to SWI/SNF recruitment at SNZ1. Gcn5p was dispensable for a WT level of SWI/SNF recruitment at both genes (Figs. 9E through H).
It is important to note that ChIP experiments using GCN4-myc13 derivatives of the mediator and the SAGA mutants analyzed in Fig. 9 revealed no reductions in myc-Gcn4p binding at ARG1 or SNZ1 in any of these strains (Qiu and Hinnebusch, unpublished). Thus, the diminished recruitment of SWI/SNF in mediator and SAGA mutants cannot be attributed to reduced binding of the activator Gcn4p at the target promoters. It was also important to consider the possibility that expression of the tagged SWI/SNF subunit or the integrity of the SWI/SNF complex was compromised in the mediator and SAGA mutants. To eliminate this possibility, we conducted Western blot analysis of myc-Snf2p and Snf5p levels in WCEs prepared from the relevant mutants. In addition, we compared the amounts of Snf5p that coimmunoprecipitated with Snf2-myc in the mediator and SAGA mutants with those of WT. The results showed little or no effect of the mediator or SAGA mutations on the steady-state levels of myc-Snf2p or Snf5p or the integrity of the SWI/SNF complex (data not shown). We conclude that recruitment of the intact SWI/SNF complex by Gcn4p is strongly enhanced by SRB mediator and SAGA.
DISCUSSION
The results presented in this report increase our understanding of the recruitment of SWI/SNF by the activator Gcn4p in several important respects. First, we provide the strongest evidence to date that Gcn4p recruits the entire SWI/SNF complex, not just the catalytic subunit Snf2p, to authentic target genes in vivo. Second, we show that recruitment of SWI/SNF is dependent on the hydrophobic clusters in the activation domain that are required for activation by Gcn4p in vivo (22) and for the interaction between Gcn4p and purified SWI/SNF in vitro (35). It was possible that low constitutive binding of SWI/SNF to the target promoters, with attendant nucleosome remodeling, would be required for high-level binding of Gcn4p to its recognition sites. Our results eliminate this possibility at ARG1 and SNZ1 by showing that a WT level of Gcn4p binding occurs at these promoters in the snf2Δ mutant. It could also be proposed that another subunit of SWI/SNF, e.g., Snf5p or Swi3p, would enhance Gcn4p binding through direct physical contact with the activation domain in the snf2Δ mutant, given that the SWI/SNF complex remains intact and binds to ARG1 and SNZ1 in snf2Δ cells. At odds with this possibility, we observed nearly WT levels of Gcn4p binding to both promoters in mutants lacking Swi3p, Snf6p, Snf11p, Snf5p, or Swp73p. Thus, the simplest explanation for our results is that Gcn4p binds to ARG1 and SNZ1 independently of nucleosome remodeling by SWI/SNF and then recruits SWI/SNF via interactions with the hydrophobic clusters in the Gcn4p activation domain. Because the gcn4-14Ala protein showed a WT level of binding to ARG1 and SNZ1, it appears that Gcn4p binding is not enhanced by any coactivators that interact with the Gcn4p activation domain, including SAGA, SRB mediator, RSC, and CCR4-NOT (11, 53).
Snf2p is dispensable, but Snf5p, Snf6p, and Swi3p are required for optimal recruitment of SWI/SNF by Gcn4p.
Our results also illuminate several aspects of the mechanism of SWI/SNF recruitment by Gcn4p. Because Snf2p, Snf5p, and Swi1p interact with the Gcn4p activation domain in vitro (37), we sought to determine whether these subunits are necessary or sufficient for recruitment of native SWI/SNF by Gcn4p in vivo. We found that deletion of SNF2 did not significantly reduce the recruitment of other SWI/SNF subunits by Gcn4p to ARG1 and SNZ1. (We verified the absence of both SNF2 coding sequences and immunologically detectable Snf2p in the snf2Δ strain.) Additionally, the excess Snf2p present in a strain overexpressing this subunit alone was not recruited to ARG1, suggesting that Snf2p cannot be recruited directly by Gcn4p in vivo. In contrast to these results, deletion of SNF5 decreased Gcn4p-dependent recruitment of other SWI/SNF subunits to ARG1 by 50 to 80%, with an average reduction of 70%. Moreover, deletion of SNF5 severely impaired SWI/SNF recruitment to SNZ1, lowering the binding of myc-Snf6p and myc-Swi1p to the same low level observed in gcn4Δ cells. These recruitment defects are substantially greater than the ∼15% reductions in subunit expression we observed in snf5Δ cells, suggesting that Snf5p makes a contribution to SWI/SNF recruitment beyond its role in maintaining WT levels of SWI/SNF subunits.
One way to account for the role of Snf5p in SWI/SNF recruitment is to propose that Snf5p is a direct target of Gcn4p (37). However, we found that Snf5p cannot be efficiently recruited by Gcn4p in a snf6Δ mutant, in which Snf5p expression is only slightly reduced, and that excess Snf5p produced in WT cells was not recruited by Gcn4p. To explain these findings, we propose that the Gcn4p-Snf5p interaction is only one of several contacts between Gcn4p and the SWI/SNF complex and that this interaction is not strong enough to support recruitment of Snf5p outside of intact SWI/SNF. Because SWI/SNF is disrupted in the snf6Δ mutant, Gcn4p cannot interact simultaneously with Snf5p and other targets in the complex, such as Snf2p or Swi1p, leading to defective recruitment of Snf5p in the snf6Δ strain. Similarly, deletion of SNF5 disrupts the complex and prevents concerted interaction of Gcn4p with multiple other subunits in addition to abolishing the contact with Snf5p.
Ostensibly at odds with the idea that complex integrity is required for SWI/SNF recruitment by Gcn4p, the swp73Δ mutation did not impair the recruitment of SWI/SNF to ARG1, even though it disrupted interaction between myc-Snf2p and Snf5p, as indicated by coimmunoprecipitation experiments. However, swp73Δ did reduce the recruitment of SWI/SNF to SNZ1. One way to explain these findings is to propose that a low level of the SWI/SNF complex lacking only Swp73p persists in swp73Δ cells and is recruited efficiently by Gcn4p to ARG1 because it contains multiple contact sites for Gcn4p. Indeed, a low level of myc-Snf2p/Snf5p association was detected in the swp73Δ extracts in our coimmunoprecipitation assays. By contrast, low levels of otherwise intact SWI/SNF complexes lacking only Snf5p, Snf6p, or Swi3p may not exist in snf5Δ, snf6Δ, or swi3Δ mutants. The inability of Gcn4p to recruit the putative SWI/SNF complex lacking only Swp73p to SNZ1 in swp73Δ cells may be related to the fact that binding of Myc-Gcn4p and recruitment of WT SWI/SNF to SNZ1 occurs at only one-fourth or one-third, respectively, of the levels seen at ARG1. SAGA and SRB mediator are also recruited at lower levels to SNZ1 than to ARG1 (data not shown). Thus, the low concentration of intact SWI/SNF complexes in swp73Δ cells may be insufficient for their recruitment to SNZ1 because of reduced levels of Gcn4p and other coactivators bound at the promoter.
Another way to account for the recruitment of SWI/SNF subunits to ARG1 in swp73Δ cells would be to propose that particular subcomplexes remaining in swp73Δ cells retain sufficient contacts with Gcn4p to support their recruitment to ARG1 but not to SNZ1. By contrast, the snf5Δ, snf6Δ, and swi3Δ mutations would produce different, more defective subcomplexes containing too few contacts for Gcn4p. The latter suggestion is consistent with the fact that Snf5p and Swi3p belong to the evolutionarily conserved core of the SWI/SNF complex (42) and that Swi3p and Snf6p are the only two subunits present in two copies per complex (48). Hence, Snf5p, Snf6p, and Swi3p may play greater roles than Swp73p in linking together multiple subunits of the SWI/SNF complex.
Our finding that Snf2p is dispensable for SWI/SNF recruitment by Gcn4p differs from results described recently for SWI/SNF binding at the SUC2 and SER3 promoters. At SER3, Snf2p functions in repression in a manner largely independent of other SWI/SNF subunits. In this instance, Snf2p was crucial for Snf5p binding at SER3, whereas Snf5p was dispensable for Snf2p binding to the promoter (33). Thus, the relative importance of Snf2p and Snf5p for recruitment seems to be reversed between SER3 and ARG1/SNZ1. It is unknown whether Snf2p is actively recruited to SER3; perhaps it binds directly to acetylated histone tails through its bromodomain (17). At SUC2, it was found that Snf2p and Snf5p are mutually interdependent for promoter binding, and it was concluded there, as in our study, that an intact complex is crucial for recruitment of SWI/SNF to this promoter (13). It remains possible that the optimal rate of SWI/SNF recruitment by Gcn4p is dependent on Snf2p, even if the final extent of binding is not significantly impaired in snf2Δ cells. It was conceivable that Snf2p would be required for efficient SWI/SNF recruitment at lower levels of promoter occupancy by Gcn4p than were examined in our experiments; however, we found recently that deleting SNF2 does not decrease SWI/SNF recruitment to ARG1 in cells expressing native levels of Gcn4p (data not shown).
Recruitment of SWI/SNF is dependent on particular mediator and SAGA subunits but independent of Gcn5p.
We found that deletions of the Gal11p, Med2p, and Rox3p subunits of SRB mediator led to substantial reductions in recruitment of SWI/SNF subunits Snf5p, Snf6p, and Snf2p to ARG1 and SNZ1. None of these mutations reduced binding of Gcn4p itself to ARG1, nor did they produce any obvious reductions in the integrity or abundance of the SWI/SNF complex. Previously, we reported ChIP data showing that SRB mediator is recruited by Gcn4p to ARG1 in vivo (53). Thus, our findings are consistent with the idea that SWI/SNF binding is enhanced by prior recruitment of mediator to the same promoter. Given that Gcn4p can interact independently with purified SWI/SNF and SRB mediator complexes in vitro (29, 35) and considering that SWI/SNF copurifies with holoenzyme under certain conditions (56), it is possible that these coactivators cooperate in binding simultaneously to Gcn4p at the promoter. This model would be analogous to the activator-dependent cooperative assembly of human mediator and TFIID on promoters observed in vitro (23). Consistent with this idea, Snf2p binding and Snf2p-dependent remodeling at RNR3 were shown to be impaired by Ts− mutations in the PolII subunit Rpb1p and the mediator subunit Srb4p (46). Our finding that Pgd1p and Srb5p are required for SWI/SNF recruitment to SNZ1 but not to ARG1 might indicate that more extensive contacts between SWI/SNF and mediator, or a higher level of mediator recruitment, are required to stabilize SWI/SNF binding when there is a lower level of activator associated with the promoter, as occurs at SNZ1 versus at ARG1.
We found that SAGA subunits Ada1p and Ada5p are additionally required for WT recruitment of SWI/SNF by Gcn4p at ARG1 and SNZ1, whereas Gcn5p is dispensable for SWI/SNF binding at both genes. Hassan et al. reported that the Snf2p bromodomain is required for recruitment of Snf6p to the SUC2 promoter in vivo and for cell growth on a carbon source (raffinose) requiring SUC2 induction (17). These results are consistent with the idea that histone acetylation by Gcn5p can enhance SWI/SNF recruitment through interaction of the Snf2p bromodomain with acetylated histones. In contrast, deletion of the Snf2p bromodomain had no apparent effect on transcriptional activation by Gcn4p unless it was combined with mutations in SAGA subunits Gcn5p or Tra1p (17). This observation fits with our findings that Snf2p and Gcn5p are dispensable for recruitment of SWI/SNF by Gcn4p in otherwise WT cells. It is possible that H3 acetylation by Gcn5p would enhance the rate of SWI/SNF recruitment or that it becomes critical for recruitment in mutants lacking other coactivator functions. Furthermore, it was shown that H3 acetylation by Gcn5p promotes nucleosome remodeling by SWI/SNF independently of SWI/SNF recruitment (54).
Although the HAT activity of Gcn5p is dispensable, other functions of SAGA dependent on Ada1p and Ada5p are needed for optimal recruitment of SWI/SNF by Gcn4p. Ada1p and Ada5p are required with Spt7p for integrity of SAGA during purification, and mutations in these three subunits have more severe phenotypes than do mutations in other nonessential SAGA subunits, including Gcn5p. These findings indicate that SAGA has critical functions beyond HAT activity that are disrupted when Ada1p, Ada5p, or Spt7p is deleted (21, 32, 50). Consistently, it was shown that Ada5p and Spt3p, but not Gcn5p, are required for TBP recruitment by Gal4p to the promoter (3, 12). Our results indicate that enhancing SWI/SNF recruitment can be viewed as another important non-HAT function of SAGA.
The dependence of SWI/SNF recruitment on SAGA could be a manifestation of the cooperative assembly of coactivator complexes proposed above to explain the stimulatory effect of mediator subunits on SWI/SNF recruitment. While it might seem difficult to accommodate several large coactivator complexes simultaneously at the same promoter, Gcn4p binds as a homodimer and frequently has multiple binding sites in the promoter. Hence, several Gcn4p activation domains may function simultaneously, each tethering a different coactivator to the promoter. The fact that mutations in TFIID subunits impaired SWI/SNF binding at RNR3 could indicate a role for TBP in SWI/SNF recruitment (46). Thus, the ada1Δ and ada5Δ mutations might reduce SWI/SNF recruitment by Gcn4p indirectly by impairing TBP recruitment. Given the stimulatory role of SRB mediator in TBP recruitment (28, 30), the same indirect mechanism could explain the requirement for mediator subunits in SWI/SNF recruitment by Gcn4p. Additional ChIP experiments are needed to distinguish between these hypotheses.
The fact that spt7Δ had no effect on SWI/SNF recruitment at ARG1 and a significantly smaller effect at SNZ1 than ada1Δ and ada5Δ seems inconsistent with the idea that SAGA is disrupted by all three of these SAGA subunit deletions. To explain our findings, we propose that a subcomplex containing Ada1p and Ada5p persists in spt7Δ cells and retains the function of SAGA involved in promoting SWI/SNF recruitment by Gcn4p. It is noteworthy that partial SAGA complexes were identified recently in ada1Δ and ada5Δ mutants (57). The requirement for Spt7p to achieve WT recruitment of SWI/SNF at SNZ1 suggests that the putative SAGA subcomplex in spt7Δ cells is inferior to WT SAGA in supporting SWI/SNF recruitment by Gcn4p.
The requirement for particular subunits of the mediator or SAGA for SWI/SNF recruitment by Gcn4p could have several underlying causes. One simple possibility is that these subunits are specifically required for recruitment of the mediator or SAGA itself to the promoter by Gcn4p. Another possibility is that the required subunits provide points of contact between the mediator and SWI/SNF or between SAGA and SWI/SNF. Finally, the required subunits of the mediator or SAGA may be needed for chromatin modification or recruitment of another factor, e.g., TBP, which in turn promotes SWI/SNF binding to the promoter (46). These various possibilities are presently under investigation.
Acknowledgments
We thank Fan Zhang for advice on experimental protocols. We are grateful to Brehon Laurent, Brad Cairns, and Joe Reese for generous gifts of antibodies. We thank Hans Hwang, Soon-ja Kim, and members of the Hinnebusch and Dever Laboratories for helpful suggestions during the course of this work.
REFERENCES
- 1.Abrams, E., L. Neigeborn, and M. Carlson. 1986. Molecular analysis of SNF2 and SNF5, genes required for expression of glucose-repressible genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 6:3643-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani, E., L. Cao, and N. Kleckner. 1987. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics 116:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaumik, S. R., and M. R. Green. 2001. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhoite, L. T., Y. Yu, and D. J. Stillman. 2001. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15:2457-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, B. R., Y. J. Kim, M. H. Sayre, B. C. Laurent, and R. D. Kornberg. 1994. A multisubunit complex containing the SWI1/ADR6, SWI2/SNF2, SWI3, SNF5, and SNF6 gene products isolated from yeast. Proc. Natl. Acad. Sci. USA 91:1950-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, B. R., R. S. Levinson, K. R. Yamamoto, and R. D. Kornberg. 1996. Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes Dev. 10:2131-2144. [DOI] [PubMed] [Google Scholar]
- 7.Cigan, A. M., M. Foiani, E. M. Hannig, and A. G. Hinnebusch. 1991. Complex formation by positive and negative translational regulators of GCN4. Mol. Cell. Biol. 11:3217-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 9.Davie, J. K., and C. M. Kane. 2000. Genetic interactions between TFIIS and the Swi-Snf chromatin-remodeling complex. Mol. Cell. Biol. 20:5960-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drysdale, C. M., E. Dueñas, B. M. Jackson, U. Reusser, G. H. Braus, and A. G. Hinnebusch. 1995. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 15:1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drysdale, C. M., B. M. Jackson, R. McVeigh, E. R. Klebanow, Y. Bai, T. Kokubo, M. Swanson, Y. Nakatani, P. A. Weil, and A. G. Hinnebusch. 1998. The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex. Mol. Cell. Biol. 18:1711-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley, A. M., C. Rougeulle, and F. Winston. 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13:2940-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng, F., Y. Cao, and B. C. Laurent. 2001. Essential roles of Snf5p in Snf-Swi chromatin remodeling in vivo. Mol. Cell. Biol. 21:4311-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 15.Gregory, P. D., A. Schmid, M. Zavari, M. Munsterkotter, and W. Horz. 1999. Chromatin remodelling at the PHO8 promoter requires SWI-SNF and SAGA at a step subsequent to activator binding. EMBO J. 18:6407-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan, A. H., K. E. Neely, and J. L. Workman. 2001. Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104:817-827. [DOI] [PubMed] [Google Scholar]
- 17.Hassan, A. H., P. Prochasson, K. E. Neely, S. C. Galasinski, M. Chandy, M. J. Carrozza, and J. L. Workman. 2002. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111:369-379. [DOI] [PubMed] [Google Scholar]
- 18.Hinnebusch, A. G. 1996. Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2, p. 199-244. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y.
- 19.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 20.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 21.Horiuchi, J., N. Silverman, B. Pina, G. A. Marcus, and L. Guarente. 1997. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol. Cell. Biol. 17:3220-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson, B. M., C. M. Drysdale, K. Natarajan, and A. G. Hinnebusch. 1996. Identification of seven hydrophobic clusters in GCN4 making redundant contributions to transcriptional activation. Mol. Cell. Biol. 16:5557-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, K. M., J. Wang, A. Smallwood, C. Arayata, and M. Carey. 2002. TFIID and human mediator coactivator complexes assemble cooperatively on promoter DNA. Genes Dev. 16:1852-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, Y., and D. J. Clark. 2002. SWI/SNF-dependent long-range remodeling of yeast HIS3 chromatin. Proc. Natl. Acad. Sci. USA 99:15381-15386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs, J. E., C. J. Fry, M. L. Samuels, and C. L. Peterson. 2000. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102:587-598. [DOI] [PubMed] [Google Scholar]
- 26.Krebs, J. E., M. H. Kuo, C. D. Allis, and C. L. Peterson. 1999. Cell cycle-regulated histone acetylation required for expression of the yeast HO gene. Genes Dev. 13:1412-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo, M. H., E. vom Bauer, K. Struhl, and C. D. Allis. 2000. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6:1309-1320. [DOI] [PubMed] [Google Scholar]
- 28.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609-613. [DOI] [PubMed] [Google Scholar]
- 29.Lee, Y. C., J. M. Park, S. Min, S. J. Han, and Y. J. Kim. 1999. An activator binding module of yeast RNA polymerase II holoenzyme. Mol. Cell. Biol. 19:2967-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, X., A. Virbasius, X. Zhu, and M. R. Green. 1999. Enhancement of TBP binding by activators and general transcription factors. Nature 399:605-609. [DOI] [PubMed] [Google Scholar]
- 31.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 32.Marcus, G. A., J. Horiuchi, N. Silverman, and L. Guarente. 1996. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol. Cell. Biol. 16:3197-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens, J. A., and F. Winston. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 16:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers, L. C., and R. D. Kornberg. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69:729-749. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan, K., B. M. Jackson, H. Zhou, F. Winston, and A. G. Hinnebusch. 1999. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and SRB/mediator. Mol. Cell 4:657-664. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan, K., M. R. Meyer, B. M. Jackson, D. Slade, C. Roberts, A. G. Hinnebusch, and M. J. Marton. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21:4347-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neely, K. E., A. H. Hassan, C. E. Brown, L. Howe, and J. L. Workman. 2002. Transcription activator interactions with multiple SWI/SNF subunits. Mol. Cell. Biol. 22:1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4:649-655. [DOI] [PubMed] [Google Scholar]
- 39.Parent, S. A., C. M. Fenimore, and K. A. Bostian. 1985. Vector systems for the expression, analysis and cloning of DNA sequences in S. cerevisiae. Yeast 1:83-138. [DOI] [PubMed] [Google Scholar]
- 40.Peterson, C. L., A. Dingwall, and M. P. Scott. 1994. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc. Natl. Acad. Sci. USA 91:2905-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson, C. L., and C. Logie. 2000. Recruitment of chromatin remodeling machines. J. Cell. Biochem. 78:179-185. [DOI] [PubMed] [Google Scholar]
- 42.Phelan, M. L., S. Sif, G. J. Narlikar, and R. E. Kingston. 1999. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell 3:247-253. [DOI] [PubMed] [Google Scholar]
- 43.Reid, G. A., and G. Schatz. 1982. Import of proteins into mitochondria. Yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J. Biol. Chem. 257:13062-13067. [PubMed] [Google Scholar]
- 44.Reinke, H., P. D. Gregory, and W. Horz. 2001. A transient histone hyperacetylation signal marks nucleosomes for remodeling at the PHO8 promoter in vivo. Mol. Cell 7:529-538. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, S. M., and F. Winston. 1997. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147:451-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma, V. M., B. Li, and J. C. Reese. 2003. SWI/SNF-dependent chromatin remodeling of RNR3 requires TAF(II)s and the general transcription machinery. Genes Dev. 17:502-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman, F., G. R. Fink, and C. W. Lawrence. 1974. Methods of yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 48.Smith, C. L., R. Horowitz-Scherer, J. F. Flanagan, C. L. Woodcock, and C. L. Peterson. 2003. Structural analysis of the yeast SWI/SNF chromatin remodeling complex. Nat. Struct. Biol. 10:141-145. [DOI] [PubMed] [Google Scholar]
- 49.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya, L. A. Pacella, F. Winston, J. L. Workman, and S. L. Berger. 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19:86-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sudarsanam, P., Y. Cao, L. Wu, B. C. Laurent, and F. Winston. 1999. The nucleosome remodeling complex, Snf/Swi, is required for the maintenance of transcription in vivo and is partially redundant with the histone acetyltransferase, Gcn5. EMBO J. 18:3101-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanson, M. J., H. Qiu, L. Sumibcay, A. Krueger, S.-J. Kim, K. Natarajan, S. Yoon, and A. G. Hinnebusch. 2003. A mulitplicity of coactivators is required by Gcn4p at individual promoters in vivo. Mol. Cell. Biol. 23:2800-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Syntichaki, P., I. Topalidou, and G. Thireos. 2000. The Gcn5 bromodomain co-ordinates nucleosome remodeling. Nature 404:414-417. [DOI] [PubMed] [Google Scholar]
- 55.Treich, I., B. R. Cairns, T. de los Santos, E. Brewster, and M. Carlson. 1995. SNF11, a new component of the yeast SNF-SWI complex that interacts with a conserved region of SNF2. Mol. Cell. Biol. 15:4240-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson, C. J., D. M. Chao, A. N. Imbalzano, G. R. Schnitzler, R. E. Kingston, and R. A. Young. 1996. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell 84:235-244. [DOI] [PubMed] [Google Scholar]
- 57.Wu, P. Y., and F. Winston. 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22:5367-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]