Abstract
Psoriasis is a type I interferon-driven T cell–mediated disease characterized by the recruitment of plasmacytoid dendritic cells (pDC) into the skin. The molecules involved in pDC accumulation in psoriasis lesions are unknown. Chemerin is the only inflammatory chemotactic factor that is directly active on human blood pDC in vitro. The aim of this study was to evaluate the role of the chemerin/ChemR23 axis in the recruitment of pDC in psoriasis skin. Prepsoriatic skin adjacent to active lesions and early lesions were characterized by a strong expression of chemerin in the dermis and by the presence of CD15+ neutrophils and CD123+/BDCA-2+/ChemR23+ pDC. Conversely, skin from chronic plaques showed low chemerin expression, segregation of neutrophils to epidermal microabscesses, and few pDC in the dermis. Chemerin expression was localized mainly in fibroblasts, mast cells, and endothelial cells. Fibroblasts cultured from skin of psoriatic lesions expressed higher levels of chemerin messenger RNA and protein than fibroblasts from uninvolved psoriatic skin or healthy donors and promoted pDC migration in vitro in a chemerin-dependent manner. Therefore, chemerin expression specifically marks the early phases of evolving skin psoriatic lesions and is temporally strictly associated with pDC. These results support a role for the chemerin/ChemR23 axis in the early phases of psoriasis development.
Plasmacytoid DC (pDC) represent a rare DC subset characterized by plasma cell morphology and a distinctive surface phenotype (CD4+, CD45RA+, CD123+, BDCA-2+, BDCA-4+, CD62L+, CLA+, and CD11c−) (1, 2). pDC are considered key effector cells in innate antiviral immunity because of their peculiar capacity to produce massive amounts of type I IFN. Upon viral stimulation, pDC differentiate into a unique type of mature DC, which directly regulates T cell function (1, 3, 4). Circulating pDC directly migrate to secondary lymphoid organs through the expression of CD62L and some chemotactic receptors, namely CXCR4, CXCR3, CCR5, and ChemR23 (5–10). pDC also infiltrate peripheral tissues in a few pathological conditions, such as some skin immune-mediated diseases (e.g., systemic lupus erythematosus, lichen planus, and psoriasis), allergic contact dermatitis reactions, and certain tumors (7, 11–14).
Psoriasis is one of the most common T cell–mediated inflammatory diseases in humans. It is characterized by a chronic course with relapses triggered by environmental factors such as trauma, infections, stress, and drugs (15, 16). Recently, it was reported that resting pDC infiltrate lesional (LS) skin of patients affected by psoriasis and, once activated, produce high levels of IFN-α that in turn drive the local activation and expansion of pathogenetic T cells (17, 18). These are mainly represented by Th17 and Tc1 lymphocytes and are directly responsible for the development of psoriatic skin lesions (19, 20). The chemotactic factors involved in the recruitment of pDC to prepsoriatic skin are currently unknown. Accumulating evidence supports a role for chemerin and its cognate receptor ChemR23 in the recruitment of pDC into pathological peripheral tissues (7, 13, 21). Chemerin is a protein, originally isolated from inflamed biological fluids (i.e., ovarian cancer ascites and rheumatoid arthritis synovial fluids), which is synthesized as an inactive precursor protein that binds ChemR23 with low affinity (21, 22). Prochemerin can be rapidly converted into a full ChemR23 agonist by the proteolytic removal of the last six or seven amino acids by neutrophil-derived proteases (elastase and cathepsin G), mast cell products (tryptase), proteases of the coagulation cascade (23, 24), and certain bacterial proteases (25).
The aim of the present study was to evaluate the possible role of the chemerin/ChemR23 axis in the recruitment of pDC to the skin of psoriasis patients. This study shows that chemerin expression selectively marks the early phases of psoriasis lesions and strongly parallels pDC and neutrophil dermal infiltration. Collectively, these results propose chemerin as a key protein in the early phases of psoriasis lesion development.
RESULTS
Chemerin is highly expressed in psoriatic LS skin
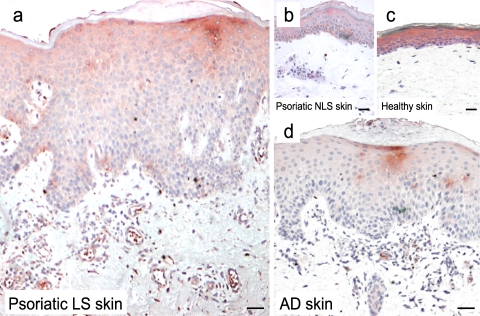
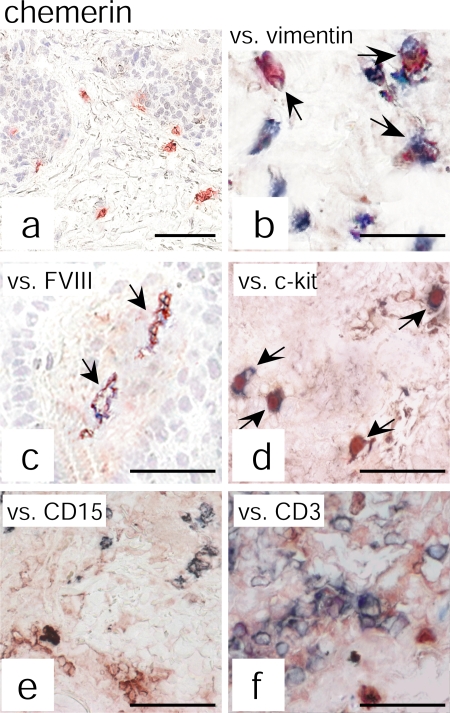
Based on previous work showing that pDC abundantly infiltrate psoriatic skin, we sought to assess chemerin presence in psoriatic plaque lesions. Chemerin expression and distribution were investigated by immunohistochemistry in both uninvolved and LS skin of psoriatic patients and, in parallel, in skin of patients affected by atopic dermatitis (AD) or healthy individuals. Skin biopsies from psoriatic lesions showed numerous chemerin+ cells localized in the dermis and scattered throughout the T cell–rich infiltrate (Fig. 1 A). In contrast, only a few chemerin immunoreactive cells were detected in the dermis of uninvolved psoriatic skin or healthy skin (Fig. 1, B and C, respectively) or in the dermis of AD lesions (Fig. 1 D). Double staining analysis revealed that the majority of chemerin+ cells were dermal cells with a fibroblast morphology coexpressing vimentin, which is a typical mesenchymal cell marker (Fig. 2, A and B). Some of the dermal chemerin+ cells also coexpressed FVIII+, which stains blood endothelial cells (∼20% of dermal vessels), and ∼5% of total chemerin+ cells expressed c-kit+, which is a mast cell marker (Fig. 2, C and D, respectively). Conversely, chemerin immunoreactivity did not colocalize with CD15+, CD3+, BDCA-2+, CD1a+, CD1b+, or CD14+ markers (Fig. 2, E and F; and not depicted). Finally, expression of chemerin immunoreactivity was also observed in the epidermal compartment of healthy or uninvolved psoriatic skin (Fig. 1, B and C). On the contrary, and in agreement with a previous study (26), LS psoriatic plaques displayed a weak chemerin expression in the epidermis that was confined to the keratinocyte suprabasal layer (Fig. 1 A).
Figure 1.
Chemerin expression in healthy and diseased skin. Immunohistochemistry was performed with an anti-chemerin mAb (red) on the following: a, LS (12 different subjects); b, nonlesional (NLS; 10 different subjects); c, normal skin from healthy donors (6 different subjects); and d, AD LS skin (5 different subjects). Bars, 20 μm. Chemerin was highly expressed in the dermis of psoriatic lesions but not in NLS psoriatic or healthy skin. Few chemerin+ cells were present in the dermis of AD LS skin. In addition, chemerin immunoreactivity was intense throughout the epidermis of healthy and NLS psoriatic skin, whereas in LS it was weak and confined to the suprabasal layer keratinocytes. The figure is representative of the results on skin biopsies obtained from all the different donors indicated.
Figure 2.
Chemerin expression colocalizes with vimentin+, FVIII+, and c-kit+ cells. Chemerin expression was evaluated by double immunohistochemistry in psoriatic plaque lesions. Anti-chemerin mAb (red) immunoreactivity was detected in dermal cells having fibroblast-like morphology (a). Double-staining analysis revealed that chemerin colocalizes with vimentin (b), FVIII (c), and c-kit (d) but not with CD15 (e) or CD3 (f) staining (all blue). The figure shows the staining of one biopsy that is representative of eight different patients evaluated. Arrows indicate cells that are double positive for chemerin and for vimentin (b), FVIII (c), or c-kit (d). Bars, 20 μm.
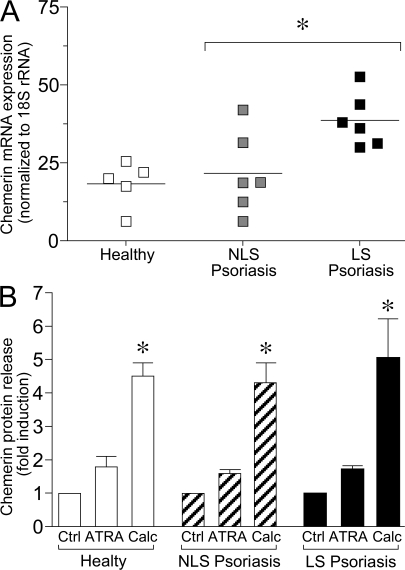
Chemerin production by fibroblasts was further evaluated in vitro using primary cell cultures. Consistent with the immunohistochemical findings, chemerin messenger RNA (mRNA) levels, evaluated by quantitative PCR, were significantly higher (>50%; P < 0.01) in fibroblasts obtained from LS psoriatic skin compared with those from NLS skin of the same patients (Fig. 3 A). Fibroblasts from healthy donors expressed levels of chemerin mRNA that were only slightly reduced compared with fibroblasts from uninvolved skin of psoriatic patients, suggesting that the different genetic background of psoriatic cells does not have an influence on the basal levels of chemerin expression. Regulation of chemerin production was investigated at the protein level in cultures of fibroblasts stimulated with some of the proinflammatory cytokines that characterize psoriatic plaque lesions (e.g., IFN-γ, IFN-α, TNF-α, and CXCL8) (27, 28) or with factors known to regulate fibroblast growth, such as retinoic acid, calcitriol, and TGF-β. Basal chemerin release from healthy donor and NLS psoriatic skin fibroblasts was similar (321 ± 31 vs. 436 ± 84 pg/ml; mean ± SD; P = 0.07), whereas LS psoriatic fibroblast cultures released higher amounts of chemerin (702 ± 110 pg/ml; P < 0.01), which is in agreement with mRNA data. Among the different experimental conditions tested, only calcitriol induced a significant up-regulation of chemerin production (P < 0.01). Retinoic acid induced only a minor increment of chemerin release (Fig. 3 B), whereas all the other agonists tested did not show any effect on chemerin basal production independent of the source of fibroblasts (not depicted). The three fibroblast types investigated, namely from healthy donor skin, and NLS and LS skin from the same patients with psoriasis showed the same degree of chemerin regulation independent of basal levels of chemerin production (Fig. 3 B).
Figure 3.
Selective expression of chemerin by fibroblasts isolated of psoriatic LS skin. (A) Chemerin expression was determined in fibroblast primary cultures prepared from skin cells isolated from healthy donors (five donors) as well as NLS and LS skin of the same psoriatic patients (six donors). Levels of mRNA expression were determined after normalization with 18S ribosomal RNA values. Horizontal lines indicate mean values for each experimental group. (B) Chemerin protein expression was evaluated by ELISA on supernatants from fibroblast cultures derived from NLS and LS psoriatic patients (10 donors) and healthy subject (10 donors) skin cells. Cells were stimulated or not with 10 μM retinoic acid (ATRA) or 1 μM calcitriol (Calc) for 24 h. Data are represented as fold of induction, with basal chemerin release being 321 ± 31, 436 ± 84, and 702 ± 110 pg/ml for healthy, NLS, and LS psoriatic cells, respectively. Error bars are SD of mean values of 10 different donors per group. *, P < 0.05.
In vitro migration of pDC in response to supernatants from psoriatic fibroblast cultures
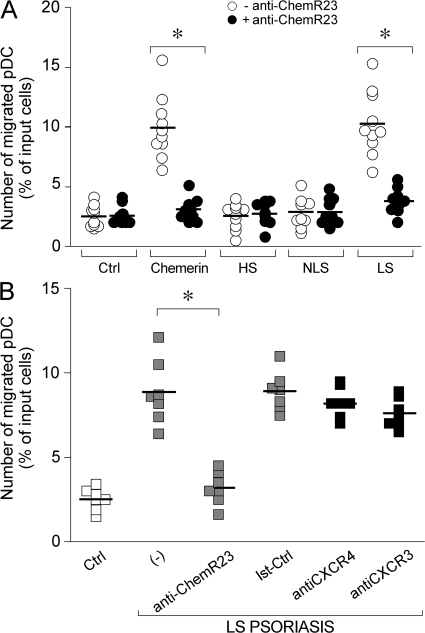
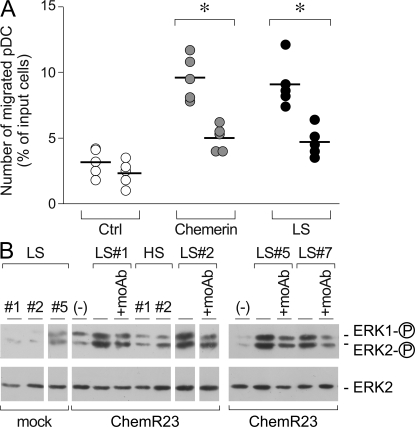
The ability of fibroblast-derived chemerin to induce pDC migration was evaluated in vitro in transmigration experiments. Fig. 4 A shows that blood-purified pDC were induced to transmigrate across an endothelial cell monolayer by conditioned medium obtained from LS skin fibroblast cultures but not from NLS skin or healthy donor-derived fibroblast supernatants. This migration was dependent on chemerin because it was completely blocked in the presence of an anti-ChemR23–blocking mAb (Fig. 4, A and B). The specificity of the antibody was confirmed by the lack of effect on CXCL12-induced pDC migration (unpublished data). pDC migration was not affected by the presence of blocking mAbs to CXCR4 or CXCR3, two potentially relevant chemotactic receptors for pDC (Fig. 4 B). Furthermore, migration to LS skin fibroblast conditioned medium was inhibited by pretreatment of cells with recombinant chemerin (Fig. 5 A). Finally, conditioned medium obtained from LS skin fibroblast cultures, but not from healthy donors, induced the phosphorylation of ERK1/2 in H460 epithelial cell line stably transfected with ChemR23 (Fig. 5 B). Altogether, these data strongly suggest that fibroblasts from LS skin constitutively release biological active chemerin that promotes pDC migration.
Figure 4.
Psoriatic fibroblasts induced pDC migration in vitro. Supernatants from cultured fibroblasts were tested for their ability to induce blood-purified pDC migration in Transwell experiments. (A) pDC transmigration was assessed using supernatants collected from healthy (HS; 1:9 dilution), NLS (1:9 dilution), and LS (1:81 dilution) psoriasis fibroblast cultures or 100 pM of recombinant chemerin. Anti-ChemR23 antibody was used at 10 μg/ml. (B) Migration of pDC to LS psoriasis supernatants was completely inhibited in the presence of a blocking anti-ChemR23 mAb (IgG2b) but not by an isotype-matched mAb (Ist-Ctrl; anti-CCR6 mAb) or blocking anti CXCR4 and CXCR3 mAbs. Squares represents the results obtained from normal subjects (white), psoriasis patients (gray), and psoriasis patients treated with control mAbs (black). *, P < 0.01 versus respective control group (i.e., without blocking mAb). Results are expressed as single values obtained using supernatants generated from individual cultures obtained from 10 and 7 different donors investigated (A and B, respectively). Horizontal lines indicate mean values for each experimental group.
Figure 5.
LS skin fibroblasts from psoriatic lesions release bioactive chemerin. (A) Blood-purified pDC were preincubated with 300 pM of recombinant human chemerin at 37°C. Cells were then washed and tested in Transwell assay for their ability to transmigrate an endothelial cell monolayer in response to medium (Ctrl), 100 pM chemerin, or LS supernatant (1:81 dilution). Data were obtained with supernatants obtained from five different donors. *, P < 0.05. Horizontal lines indicate mean values for each experimental group. (B) H460 cells transfected with the empty vector (mock) or ChemR23 were stimulated with fibroblast-derived supernatant from LS skin (1:81 dilution) or healthy donors (HS; 1:9 dilution). Anti-ChemR23 mAb was used at 10 μg/ml. Western blots were performed as detailed in Materials and methods.
pDC infiltration correlates with chemerin expression and marks early psoriatic lesions
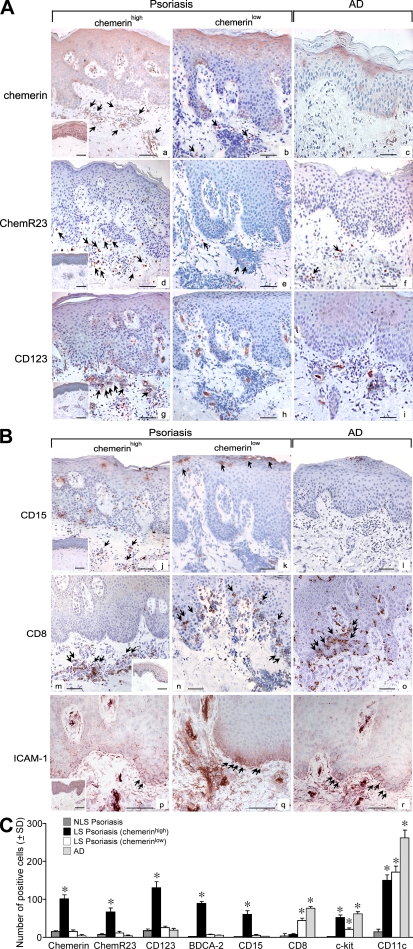
Only a subset of psoriatic LS skin samples showed a chemerinhigh phenotype (Fig. 6 A, a), whereas other skin samples could be defined as chemerinlow based on the modest number of chemerin+ cells (6.3-fold lower; Fig. 6, A [a vs. b] and C). Chemerinhigh, but not chemerinlow, psoriatic lesions exhibited a prominent infiltrate of ChemR23+ cells (Fig. 6, A [d and e, respectively] and C) and CD123+/BDCA-2+ pDC in the dermis (Fig. 6 A, g and h; and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080129/DC1). Double staining showed that ChemR23+ cells were mostly CD123+ or BDCA-2+ (∼60 and 85%, respectively) with some CD11c+ cells (∼15%). Conversely, ChemR23 was not coexpressed by c-kit+, CD3+, CD15+, or CD14+ cells (Fig. S1 and not depicted). In chemerinlow biopsies, the anti-CD123 mAb only stained blood vessels (Fig. 6 A, panel h). The lack of chemerin expression in uninvolved psoriatic skin correlated with the absence of ChemR23+ cells in the dermis (Fig. 6 A, a and d, insets).
Figure 6.
Infiltration of pDC in psoriatic LS skin correlates with the expression of chemerin and the presence of neutrophils in the dermis. (A and B) Immunohistochemistry was performed using sections from AD lesions (n = 5) and from psoriatic skin showing a chemerinhigh (n = 8) and chemerinlow (n = 12) phenotype. Bars, 20 μm. The insets represent stainings of uninvolved psoriatic skin. Arrows point to positive cells. (C) Positive cells were enumerated in the dermis of psoriatic uninvolved skin (NLS) in chemerinhigh, chemerinlow, and AD skin sections (n = 3). Slides were analyzed blind by two observers and positive cells were counted in 10 adjacent fields at a magnification of 200×. Results are expressed as the mean number of positive cells ±SD. *, P < 0.05 versus NLS skin.
Previous work described infiltration of dermis and epidermis by neutrophils as discriminating the early phase of psoriasis development from the chronic phase, which is characterized by the accumulation of neutrophils in the epidermis to form focal subcorneal aggregates (29). Other immunological aspects of chronic lesions include a prominent CD8+ T cell infiltrate in the epidermis and a strong ICAM-1 positivity of basal layer keratinocytes (30). To better understand the regulation of chemerin in psoriatic skin, the expression of chemerin was correlated with the degree and distribution of CD15+ neutrophils as well as CD8+ and ICAM-1+ cells. Fig. 6 B (j and k) shows that the presence of CD15+ cells in the mid and papillary dermis and in the epidermis was exclusively associated with the chemerinhigh phenotype and with the presence of a prominent infiltrate of CD123+ pDC (Fig. 6 A, g) as well as c-kit+ mast cells (Fig. S2 a, available at http://www.jem.org/cgi/content/full/jem.20080129/DC1). Conversely, in chemerinlow biopsies, CD15+ cells were localized only in epidermal microabscesses (Fig. 6 B, k). Chemerinlow skin lesions also showed the markers of chronic inflammation, such as CD8+ T cell infiltration in the epidermis and the intense ICAM-1 immunoreactivity of basal keratinocytes (Fig. 6 B, n and q). CD11c+ cells were present in a similar manner in both chemerinhigh and chemerinlow biopsies (Fig. S3, a and b, available at http://www.jem.org/cgi/content/full/jem.20080129/DC1). Similarly, CXCL10 and CXCL12 expression was equally present in early (chemerinhigh) and chronic (chemerinlow) LS skin (Fig. S3, d, e, g, and h). AD skin lesions presented only a negligible infiltration of pDC (Fig. 6 A, i), as previously described (14), which was not associated with dermal chemerin+ cells (Fig. 6 A, c) or CD15+ neutrophils (Fig. 6 B, l). The presence of CD8+ T lymphocytes (Fig. 6 B, o), CD11c+ DC (Fig. S3, c), and focal ICAM-1 positivity (Fig. 6 B, r) in the epidermis of AD skin is consistent with previous reports (31). The quantification of chemerin+, ChemR23+, CD123+, BDCA-2+, CD15+, CD8+, c-kit+, and CD11c+ cells in the different experimental conditions is reported in Fig. 6 C.
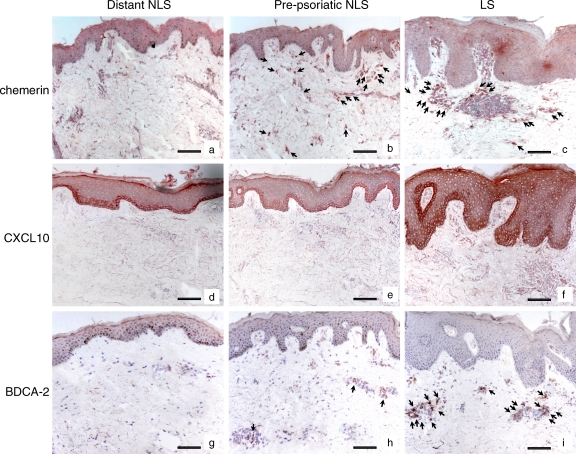
Finally, to definitively establish whether chemerin expression might be an early event leading to the initial infiltration of pDC in psoriatic skin, chemerin expression and pDC infiltration were investigated in NLS prepsoriatic skin adjacent to newly developed plaque lesions. Fig. 7 (b and h) shows that chemerin was expressed in the dermis of prepsoriatic skin and was associated with the initial, still limited infiltration of BDCA-2+ (Fig. 7) and CD123+ (not depicted) pDC. Dermal chemerin expression was further increased in the proximal plaque lesions and was associated with extensive infiltration of BDCA-2+/CD123+ pDC (Fig. 7, c and i; and not depicted). Both chemerin expression and pDC recruitment was not observed in the dermis of distant NLS psoriatic skin (Fig. 7, a and g). In contrast, CXCL10 expression was detectable only within the plaque lesions and was confined to basal layer epidermis in both adjacent and 3-cm distant NLS psoriatic skin (Fig. 7, d-f), showing a pattern of expression different from that of chemerin and pDC infiltration. Collectively, these results indicate that chemerin expression is an early event in psoriasis plaque development, and it precedes and parallels the recruitment of pDC into developing skin lesions.
Figure 7.
Chemerin is highly expressed in prepsoriatic skin where BDCA-2+ pDC start to be accumulated. Immunohistochemistry was performed using sections from early psoriatic plaques at sites of peripheral lesions (LS) together with the adjacent prepsoriatic NLS skin and biopsies from normal skin appearing 3 cm distant from the evolving plaques of the same patients (five total patients examined). Arrows indicate cells that are positive for chemerin (b and c) or BDCA-2 (h and i). Bars, 20 μm.
DISCUSSION
Chemerin is the only inflammatory chemotactic factor known to induce pDC migration in vitro (10). In vivo, chemerin expression was shown to correlate with pDC infiltration into peripheral tissues during autoimmune diseases such as systemic lupus erythematosus and lichen planus (7, 13). In this paper, we report that expression of chemerin is temporally strictly associated with pDC recruitment in psoriatic skin biopsies. Psoriasis is an immune-mediated disease characterized by a profound T cell dysregulation (15, 16). Growing evidences propose a pivotal role for pDC in the triggering of the inflammatory reactions associated with psoriasis, and the current model depicts pDC as key effector cells that drive the activation of pathogenetic T cells through their ability to release high levels of IFN-α (17, 18). Previous studies have focused on the role of innate immunity in psoriasis. Neutrophils and mast cells are known to infiltrate psoriasis skin plaque lesions during the early phases of the disease, whereas both cell types are more rarely found in the late chronic phase (32). Immunohistological features of chronic lesions are also characterized by a prominent CD8+ T cell infiltrate in the epidermis and by ICAM-1 expression by basal layer keratinocytes (30). In addition, CD11c+ iNOS+ myeloid DC also infiltrate psoriatic lesions (32, 33). These data indicate that psoriatic lesions markedly change their inflammatory cell profile according to the development stage, with different cell types playing distinct roles in earlier versus later stages.
In this paper, we report that in psoriatic skin lesions, pDC accumulation is almost exclusively associated with the concomitant presence of neutrophils and mast cells in the dermis. In contrast, psoriatic skin specimens characterized by the presence of intraepidermal neutrophils and CD8+ T cells, ICAM+ keratinocytes, and a few c-kit+ dermal mast cells were associated to a scanty presence of pDC. The majority of CD123+ and BDCA-2+ pDC expressed ChemR23, the chemerin receptor, and chemerin expression in the dermis was only observed in association with pDC infiltration. Analysis of uninvolved prepsoriatic skin that was adjacent to active plaque lesions demonstrated that chemerin is already expressed in the dermis in pre-LS sites, where pDC start to become recruited. Therefore, chemerin expression represents an early event that precedes and strictly parallels accumulation of pDC and clinical expression of psoriasis. Moreover, recruitment of pDC in psoriatic skin lesions is associated with infiltration of neutrophils and numerous mast cells in the dermis. In this context, it is interesting to note that activated neutrophils and mast cells can convert prochemerin into active chemerin through the release of cathepsin G, elastase, and tryptase (23, 24). These proteolytic enzymes can also regulate keratinocyte proliferation, although their precise role in psoriasis pathogenesis is still elusive (34, 35). Of note, immunohistochemical stainings of CXCL12 and CXCL10 showed that these two chemokines were equally expressed in chronic versus early psoriatic skin lesions (Fig. S3), arguing against a direct role in pDC recruitment into psoriatic lesions. To confirm the specificity of these findings, we studied LS AD skin, which did not express chemerin and was scantly infiltrated by pDC.
In psoriatic dermis, chemerin immunoreactivity was observed in endothelial cells, mast cells, and fibroblasts. Chemerin+ blood vessel endothelial cells have been also observed in the skin of lupus erythematosus and lichen planus patients. In addition, chemerin is expressed by high endothelial cells in the lymph node (7, 13). The expression of chemerin by mast cells represents a new finding, and it is interesting in light of the ability of these cells to generate active chemerin through the release of tryptase. However, fibroblasts apparently represent the major source of chemerin in psoriatic dermis. This in vivo finding was further substantiated in vitro by the use of primary cell cultures. Fibroblasts isolated from LS skin expressed higher levels of chemerin mRNA than cells obtained from uninvolved skin of the same patients or skin obtained from healthy donors. Moreover, only supernatants of fibroblasts obtained from LS skin of psoriatic patients, and not from healthy donor or NLS psoriatic skin, induced the migration of pDC in vitro and activated ERK1/2 phosphorylation, which is an early step of chemotactic receptor signaling cascade, in ChemR23-transfected cells. These activities were completely blocked in the presence of an anti-ChemR23 blocking antibody with no effect of anti-CXCR3 and -CXCR4 antibodies. Furthermore, pDC migration to psoriatic skin fibroblast supernatants was completely inhibited (desensitized) by a precedent exposure of the cells to recombinant chemerin. Altogether, these results strongly indicate that chemerin is the main, if not the only, protein responsible for the pDC chemotactic activity that is present in the conditioned medium of psoriatic skin fibroblasts. Taking into consideration that fibroblasts from healthy and NLS psoriatic skin also release chemerin/prochemerin, the selective ability of psoriatic fibroblasts to promote pDC migration is likely to be crucially regulated by the concomitant higher expression of proteases by these cells and, thus, by their higher efficiency in generating bioactive chemerin (36).
To better understand the nature of the signals present within the psoriatic inflammatory milieu that is responsible for the induction of chemerin production, fibroblasts at early culture passages were stimulated with proinflammatory cytokines and growth factors that characterize psoriatic lesions such as IFN-γ, IFN-α, TNF-α, CXCL8, retinoic acid, calcitriol, and TGF-β. Among the different experimental conditions investigated, only calcitriol and, to a lesser extent, retinoic acid were able to induce the up-regulation of chemerin protein release. Therefore, as previously described for endothelial cells (7), the mechanisms that govern chemerin expression in pathological conditions still need to be fully elucidated.
Immunohistochemistry analyses also showed a diffuse chemerin expression in the epidermis. Chemerin epidermal expression was higher in healthy and uninvolved psoriatic skin than in plaque lesions. The biological role of chemerin in the epidermis is unknown, but it can be speculated that under homeostatic conditions chemerin may represent a ready-to-use mechanism to rapidly recruit effector cells upon the generation of danger signals. Recently, chemerin was shown to play a role in the resolution of the inflammatory process at the mucosal sites by promoting the clearance of epithelial bound neutrophils through a CD55/ICAM-1–dependent mechanism (37). This function might sustain the transmigration of neutrophils in the parakeratotic areas as observed in chronic psoriatic skin, and it is consistent with the expression of chemerin found both in vitro and in vivo in differentiated keratinocytes (Fig. 1 A and not depicted). The possible antiinflammatory action of chemerin in the epidermis was also suggested in a previous study showing the up-regulation of chemerin (Tig-2) in psoriatic epidermis after topical application of tazarotene, an antipsoriatic synthetic retinoid (26). In contrast, calcitriol, an effective antipsoriatic agent (38), was the most potent inducer of chemerin in cultured fibroblasts. Finally, a recent study reported that chemerin exhibits antiinflammatory activity on macrophages in vitro and in vivo in a proteolysis-dependent fashion (39). Therefore, chemerin might have a dual opposite role during the evolution of psoriatic skin lesions: first, a crucial role in the recruitment of pathogenetic pDC during the early phases of the disease when expressed in the dermis; and subsequently, during the chronic phase, the expression of chemerin in the epidermis may favor leukocyte clearance from inflamed tissue and restoration of skin homeostasis.
In conclusion, we identified the chemerin/ChemR23 axis as a new important player in the pathogenesis of psoriasis. The concomitant presence of chemerin, pDC, neutrophils, and mast cells during the early phases of the disease proposes chemerin as a target for prevention and early therapeutic intervention in psoriasis.
MATERIALS AND METHODS
Tissue samples.
For immunohistochemistry, 4-mm punch biopsies were taken from LS (involved) skin of adult patients with early lesions (<10 d; n = 8) and late plaque psoriasis (>2 mo; n = 12). Biopsies were also obtained from patients with chronic AD (n = 5) and normal skin of healthy subjects undergoing plastic surgery (n = 10). For six psoriatic patients, a skin biopsy from NLS normal-appearing (uninvolved) skin distant from the plaques was also taken. Biopsies were also taken from early plaques at sites of peripheral lesions together with the adjacent normal-appearing skin (NLS prepsoriatic skin) and from normal-appearing skin 3 cm distant from the evolving plaques of the same patients (n = 5). Biopsies were taken after informed consent and the study was approved by the Ethical Committee of the Istituto Dermopatico dell'Immacolata (IDI)–IRCCS (Rome, Italy). Patients did not receive topical or systemic therapy for at least 2 wk before the study. Fibroblast cultures were established from 6-mm punch biopsies obtained from both involved and uninvolved skin of psoriatic patients (n = 10), from uninvolved skin of AD patients (n = 5), and from healthy subjects (n = 6).
Immunohistochemistry.
Skin samples were fixed in 10% formalin and embedded in paraffin. 5-μm sections were dewaxed and rehydrated. After quenching endogenous peroxidase, achieving antigen retrieval, and blocking nonspecific binding sites, sections were incubated with mAbs against Chemerin (IgG1 clone 14G10; 1:40 dilution), ChemR23 (IgG2b clone 4C7; 1:20 dilution), CD117/c-kit (Leica; 1:50 dilution), CXCL10 (Santa Cruz Biotechnology, Inc.; 1:100 dilution), CXCL12 (R&D Systems; 1:50 dilution), and BDCA-2 (Miltenyi Biotec). mAbs against CD15 (1:100), CD3 (1:20 dilution), CD11c (1:20), and CD123 (1:50) were obtained from BD, whereas anti-vimentin (1:100) and anti-FVIII/vWF (1:10) were purchased from Dako. Secondary biotinylated mAbs and staining kits were obtained from Vector Laboratories. Single stainings were developed using 3-amino-9-ethylcarbazole (Vector Laboratories). Double immunostaining was developed using the avidin-biotin peroxidase or avidin-biotin-alkaline phosphatase system and the chromogens 3-amino-9-ethylcarbazole and Blue Vector (Vector Laboratories). Occasionally, sequential immunohistochemistry was performed. As a negative control, primary Abs were omitted or replaced with an irrelevant isotype-matched mAb. Figures depict one experiment that is representative of all the patients investigated. The complete tissue bank collected for this study (Tissue samples) was probed with the full set of antibodies described in the Results section, and the number of subjects investigated in each experiment is detailed in the figure legends.
Fibroblast cultures.
Dermal samples obtained from skin biopsies were placed in a dry cell culture plate for 10 min to allow adherence to the polystyrene surface of the culture plate. DME (Biochrom KG) supplemented with 10% FCS, 4 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin (all from Biochrom KG) was added. Fibroblast outgrowth was monitored by light microscopy. At 70–80% confluence, cells were detached with 0.05% trypsin/0.02% EDTA (Biochrom KG) and then frozen or subcultured in the same medium. Second- or third-passage fibroblasts were used in all experiments, with cells cultured in 6-well plates. The following individual cell cultures obtained from different patients were tested: LSl skin psoriasis (n = 12); NLS skin psoriasis (n = 10); healthy skin (HS; n = 6); and AD (n = 5). Treatments of fibroblast cultures with 200 U/ml of recombinant human IFN-γ, 500 U/ml IFN-α, 50 ng/ml TNF-α, 10 ng/ml TGF-β, 10 mM CXCL8 (all from R&D Systems), 10 μM retinoic acid, or 1 μM calcitriol (both Sigma-Aldrich) were also performed. Chemerin protein levels were investigated, according to the manufacturer's instructions, using an ELISA kit (R&D Systems) which does not discriminate between prochemerin and chemerin.
RNA isolation and real-time RT-PCR.
Total RNA was extracted from fibroblasts using TRIzol reagent (Invitrogen), treated with DNase (Promega), and purified using an RNA isolation kit (QIAGEN). mRNA was reverse transcribed into complementary DNA using the Moloney murine leukemia virus RT and random hexamers (Applied Biosystems). RT-PCR products were analyzed by quantitative real-time RT-PCR in the following TaqMan Gene Expression Assays of target genes: chemerin, HS001611209_g1; and 18S, HS99999901 as an endogenous control (Applied Biosystems). Fluorescence intensity was analyzed by the ABI PRISM SDS 7000 PCR Instrument (Applied Biosystems). The levels of chemerin expression were determined by normalizing to 18S ribosomal RNA expression. The fold induction value for triplicate wells was averaged, and data were presented as the mean ± SD of five (HS) and six (NLS and LS) fibroblast cultures obtained from different donors.
Transendothelial migration assay.
Transendothelial migration assay was evaluated using 24-well Costar Transwell chambers (5-μm pore size; Corning). HUVEC (passage <5) were subcultured to confluent monolayers on Transwell inserts precoated with gelatin. Monolayers were rinsed with chemotaxis medium (RPMI containing 10% FCS) before use. 100 μl DC suspension (106 cells/ml) was seeded in the upper chamber and 600 μl of chemoattractant or fibroblast conditioned medium were added to the lower well. The chamber was incubated at 37°C in humidified atmosphere in the presence of 5% CO2 for 4 h. Migrated cells were recovered from the lower well, counted, and the results were expressed as the percentage of input cells. In some experiments, a ChemR23 (clone 4C7, IgG2b), CCR6 (IgG2b; R&D Systems), CXCR4 (R&D Systems), and CXCR3 blocking mAbs were used during the assay (7). For desensitization experiments, pDC were preincubated with 100 pM of recombinant chemerin at 37°C for 30 min, washed, and subsequently tested in chemotaxis experiments. Each experiment was performed in duplicate and results represent mean values of single experiments performed using conditioned media generated using fibroblasts from 10 different donors for each experimental group.
Cell stimulation and Western blotting.
H460 epithelial cells were transfected by electroporation with pcDNA3.1 containing the full length ChemR23 ORF (provided by M. Ruiz Rosquete, University of Brescia, Brescia, Italy). Transfected cells were selected in the presence of neomycin and cloned. Membrane expression of ChemR23 was evaluated by FACS analysis. H460 cells transfected with the empty vector (mock) were used as negative control. Cells were incubated with supernatants obtained from healthy skin (HS; 1:9 dilution) or LS psoriasis skin (1:81 dilution) for 5 min. Phosphorylated ERK1/2 and total ERK2 were detected after western blotting using specific mAbs (clone #9101 [Cell Signaling Technology] and clone D-2 [Santa Cruz Biotechnology]) and ECL (GE Healthcare).
Statistical analysis.
Wilcoxon's signed rank test was used (SigmaStat; Jandel) to compare differences in mRNA chemerin content in healthy and diseased fibroblasts. P-values ≤0.05 were considered significant.
Online supplemental material.
Fig. S1 shows that in the dermis of psoriatic skin, ChemR23 expression colocalizes with myeloid DC (CD11c+) and pDC (CD123+ and BDCA2+) markers. Fig. S2 shows that c-kit+ mast cells highly infiltrate the dermis of chemerinhigh psoriatic plaques but not chemerinlow and AD skin lesions. Fig. S3 shows that CXCL10 and CXCL12 are expressed in both chemerinhigh and chemerinlow biopsies and that the expression of these two chemokines does not correlate with pDC infiltration. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080129/DC1.
Supplementary Material
Acknowledgments
We thank Michel Ruiz Rosquete for providing H460-ChemR23 cells and Fabio Facchetti and William Vermi for helpful discussion.
This work was supported by grants from Istituto Superiore di Sanità, Ministero dell'Istruzione Università e Ricerca (PRIN projects), Ministero Italiano della Sanità and Associazione Italiana per la Ricerca sul Cancro (AIRC), NOBEL Project Cariplo Genetic and functional genomics of myelomonocytic cells, and by a research grant of the European Community (Brussels, Belgium; INNOCHEM project 518167). This work was also supported by the Actions de Recherche Concertées of the Communauté Française de Belgique, the Interuniversity Attraction Poles Program (P6-14), Belgian State, Belgian Science Policy, the European Union (grant LSHB-CT-2005-518167; INNOCHEM), the Walloon Region (Progamme CIBLES), and the Fondation Médicale Reine Elisabeth to M. Parmentier.
The authors have no conflicting financial interests.
Abbreviations used: AD, atopic dermatitis; LS, lesional; mRNA, messenger RNA; NLS, nonlesional; pDC, plasmacytoid DC.
G. Girolomoni and S. Sozzani contributed equally to this paper.
References
- 1.Colonna, M., G. Trinchieri, and Y.J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- 2.Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. [DOI] [PubMed] [Google Scholar]
- 3.Colonna, M., and M. Cella. 2007. Crosspresentation: plasmacytoid dendritic cells are in the business. Immunity. 27:419–421. [DOI] [PubMed] [Google Scholar]
- 4.Sapoznikov, A., J.A. Fischer, T. Zaft, R. Krauthgamer, A. Dzionek, and S. Jung. 2007. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 204:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 6.Diacovo, T.G., A.L. Blasius, T.W. Mak, M. Cella, and M. Colonna. 2005. Adhesive mechanisms governing interferon-producing cell recruitment into lymph nodes. J. Exp. Med. 202:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermi, W., E. Riboldi, V. Wittamer, F. Gentili, W. Luini, S. Marrelli, A. Vecchi, J.D. Franssen, D. Communi, L. Massardi, et al. 2005. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 201:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoneyama, H., K. Matsuno, Y. Zhang, T. Nishiwaki, M. Kitabatake, S. Ueha, S. Narumi, S. Morikawa, T. Ezaki, B. Lu, et al. 2004. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16:915–928. [DOI] [PubMed] [Google Scholar]
- 9.Randolph, G.J., J. Ochando, and S. Partida-Sánchez. 2008. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26:293–316. [DOI] [PubMed] [Google Scholar]
- 10.Sozzani, S. 2005. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 16:581–592. [DOI] [PubMed] [Google Scholar]
- 11.Bangert, C., J. Friedl, G. Stary, G. Stingl, and T. Kopp. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Invest. Dermatol. 121:1409–1418. [DOI] [PubMed] [Google Scholar]
- 12.Jahnsen, F.L., F. Lund-Johansen, J.F. Dunne, L. Farkas, R. Haye, and P. Brandtzaeg. 2000. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165:4062–4068. [DOI] [PubMed] [Google Scholar]
- 13.Parolini, S., A. Santoro, E. Marcenaro, W. Luini, L. Massardi, F. Facchetti, D. Communi, M. Parmentier, A. Majorana, M. Sironi, et al. 2007. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 109:3625–3632. [DOI] [PubMed] [Google Scholar]
- 14.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Invest. Dermatol. 119:1096–1102. [DOI] [PubMed] [Google Scholar]
- 15.Boyman, O., C. Conrad, G. Tonel, M. Gilliet, and F.O. Nestle. 2007. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 28:51–57. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths, C.E., and J.N. Barker. 2007. Pathogenesis and clinical features of psoriasis. Lancet. 370:263–271. [DOI] [PubMed] [Google Scholar]
- 17.Lande, R., J. Gregorio, V. Facchinetti, B. Chatterjee, Y.H. Wang, B. Homey, W. Cao, Y.H. Wang, B. Su, F.O. Nestle, et al. 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 449:564–569. [DOI] [PubMed] [Google Scholar]
- 18.Nestle, F.O., C. Conrad, A. Tun-Kyi, B. Homey, M. Gombert, O. Boyman, G. Burg, Y.J. Liu, and M. Gilliet. 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrad, C., O. Boyman, G. Tonel, A. Tun-Kyi, U. Laggner, A. de Fougerolles, V. Kotelianski, H. Gardner, and F.O. Nestle. 2007. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med. 13:836–842. [DOI] [PubMed] [Google Scholar]
- 20.Zheng, Y., D.M. Danilenko, P. Valdez, I. Kasman, J. Eastham-Anderson, J. Wu, and W. Ouyang. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 445:648–651. [DOI] [PubMed] [Google Scholar]
- 21.Wittamer, V., J.D. Franssen, M. Vulcano, J.F. Mirjolet, E. Le Poul, I. Migeotte, S. Brezillon, R. Tyldesley, C. Blanpain, M. Detheux, et al. 2003. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198:977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wittamer, V., F. Gregoire, P. Robberecht, G. Vassart, D. Communi, and M. Parmentier. 2004. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 279:9956–9962. [DOI] [PubMed] [Google Scholar]
- 23.Wittamer, V., B. Bondue, A. Guillabert, G. Vassart, M. Parmentier, and D. Communi. 2005. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J. Immunol. 175:487–493. [DOI] [PubMed] [Google Scholar]
- 24.Zabel, B.A., S.J. Allen, P. Kulig, J.A. Allen, J. Cichy, T.M. Handel, and E.C. Butcher. 2005. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 280:34661–34666. [DOI] [PubMed] [Google Scholar]
- 25.Kulig, P., B.A. Zabel, G. Dubin, S.J. Allen, T. Ohyama, J. Potempa, T.M. Handel, E.C. Butcher, and J. Cichy. 2007. Staphylococcus aureus-derived staphopain B, a potent cysteine protease activator of plasma chemerin. J. Immunol. 178:3713–3720. [DOI] [PubMed] [Google Scholar]
- 26.Nagpal, S., S. Patel, H. Jacobe, D. DiSepio, C. Ghosn, M. Malhotra, M. Teng, M. Duvic, and R.A. Chandraratna. 1997. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 109:91–95. [DOI] [PubMed] [Google Scholar]
- 27.Albanesi, C., O. De Pita, and G. Girolomoni. 2007. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin. Dermatol. 25:581–588. [DOI] [PubMed] [Google Scholar]
- 28.Nickoloff, B.J., H. Xin, F.O. Nestle, and J.Z. Qin. 2007. The cytokine and chemokine network in psoriasis. Clin. Dermatol. 25:568–573. [DOI] [PubMed] [Google Scholar]
- 29.Christophers, E. S.W. 1993. Psoriasis. In Dermatology in General Medicine. T.B. Fitzpatrick, K. Wolff, I.M. Freedberg, K.F. Austen, editors. McGraw-Hill, New York. 489–514.
- 30.Onuma, S. 1994. Immunohistochemical studies of infiltrating cells in early and chronic lesions of psoriasis. J. Dermatol. 21:223–232. [DOI] [PubMed] [Google Scholar]
- 31.Leung, D.Y., A.K. Bhan, E.E. Schneeberger, and R.S. Geha. 1983. Characterization of the mononuclear cell infiltrate in atopic dermatitis using monoclonal antibodies. J. Allergy Clin. Immunol. 71:47–56. [DOI] [PubMed] [Google Scholar]
- 32.Lowes, M.A., F. Chamian, M.V. Abello, J. Fuentes-Duculan, S.L. Lin, R. Nussbaum, I. Novitskaya, H. Carbonaro, I. Cardinale, T. Kikuchi, et al. 2005. Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl. Acad. Sci. USA. 102:19057–19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowes, M.A., A.M. Bowcock, and J.G. Krueger. 2007. Pathogenesis and therapy of psoriasis. Nature. 445:866–873. [DOI] [PubMed] [Google Scholar]
- 34.Ashenagar, M.S., K. Sugihara, A. Maeda, R. Isogai, M. Takahashi, K. Aisu, A. Horiuchi, Y. Aragane, A. Kawada, and T. Tezuka. 2007. The presence of tryptase-positive and bikunin-negative mast cells in psoriatic skin lesions. Arch. Dermatol. Res. 298:421–426. [DOI] [PubMed] [Google Scholar]
- 35.Rogalski, C., U. Meyer-Hoffert, E. Proksch, and O. Wiedow. 2002. Human leukocyte elastase induces keratinocyte proliferation in vitro and in vivo. J. Invest. Dermatol. 118:49–54. [DOI] [PubMed] [Google Scholar]
- 36.Raynaud, F., B. Bauvois, P. Gerbaud, and D. Evain-Brion. 1992. Characterization of specific proteases associated with the surface of human skin fibroblasts, and their modulation in pathology. J. Cell. Physiol. 151:378–385. [DOI] [PubMed] [Google Scholar]
- 37.Campbell, E.L., N.A. Louis, S.E. Tomassetti, G.O. Canny, M. Arita, C.N. Serhan, and S.P. Colgan. 2007. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 21:3162–3170. [DOI] [PubMed] [Google Scholar]
- 38.Mason, J., A.R. Mason, and M.J. Cork. 2002. Topical preparations for the treatment of psoriasis: a systematic review. Br. J. Dermatol. 146:351–364. [DOI] [PubMed] [Google Scholar]
- 39.Cash, J.L., R. Hart, A. Russ, J.P. Dixon, W.H. Colledge, J. Doran, A.G. Hendrick, M.B. Carlton, and D.R. Greaves. 2008. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 205:767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.