Abstract
Th17 cell differentiation is dependent on interleukin (IL)-6 and transforming growth factor (TGF)-β, and it is modulated by activation of the aryl hydrocarbon receptor (AhR). In this study, we show that differentiation of Th17 cells, but not Th1 or induced regulatory T (iT reg) cells, is increased by endogenous AhR agonists present in culture medium. Th17 development from wild-type mice is suboptimal in the presence of the AhR antagonist CH-223191, similar to the situation in AhR-deficient mice, which show attenuated IL-17 production and no IL-22 production. The presence of natural AhR agonists in culture medium is also revealed by the induction of CYP1A1, a downstream target of AhR activation. However, the most commonly used medium, RPMI, supports very low levels of Th17 polarization, whereas Iscove's modified Dulbecco's medium, a medium richer in aromatic amino acids, which give rise to AhR agonists, consistently results in higher Th17 expansion in both mouse and human cells. The relative paucity of AhR agonists in RPMI medium, coupled with the presence of factors conducive to IL-2 activation and enhanced Stat5 phosphorylation, conspire against optimal Th17 differentiation. Our data emphasize that AhR activation plays an essential part in the development of Th17 cells and provide a rational explanation for the poor in vitro polarization of Th17 cells that is reported in the majority of publications for both mouse and human cells.
Differentiation of the new CD4 effector subset Th17 requires the presence of IL-6 and TGF-β and is further enhanced by IL-1β and -21 (1, 2). In addition, Th17 polarization is promoted by stimulation of the aryl hydrocarbon receptor (AhR) (3), a ligand-dependent transcription factor that responds to a wide range of ligands. Ligands include environmental toxins, such as halogenated aromatic hydrocarbons represented by tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons (e.g., 3-methylcholanthrene), as well as potential endogenous ligands, including dietary components, heme metabolites, indigoids, and tryptophan metabolites (4). The tryptophan metabolite 6-formylindolo[3,2-b] carbazole (FICZ) was shown to have very high affinity for AhR, comparable to that of TCDD (5–7). Exposure of CD4 T cells to FICZ under Th17-polarizing conditions enhances Th17 development and functions such as autoimmune pathology in EAE, which is substantially exacerbated by AhR ligation, and it induces expression of IL-22 (3). Despite the fact that AhR binds many toxins, its evolutionary conservation (8) suggests that this is not its primary function. Although invertebrate AhR shares high protein sequence homology with the vertebrate receptor, it does not recognize xenobiotic ligands such as TCDD or β-naphthoflavone, which indicates that the AhR has physiological functions that are not restricted to recognition of environmental pollutants (5).
Involvement of an endogenous AhR ligand in Th17 development in vivo is suggested by the fact that AhR-deficient mice have an attenuated Th17 program, with fewer Th17 cells activated upon immunization with MOG peptide/CFA, resulting in milder pathology in the EAE model and the absence of IL-22 production (3). The attenuated Th17 development from naive CD4 T cell precursors of AhR-deficient mice is also noticeable in vitro.
There are several studies in the toxicology field suggesting that high affinity ligands for AhR are generated in culture medium and play a role in the baseline activation of AhR in hepatocyte cell lines, resulting in activation of genes encoding xenobiotic metabolizing cytochrome P450 enzymes such as CYP1A (for review see reference [9]). These findings prompted us to test whether such endogenous AhR activity might influence the differentiation of Th17 effector cells in vitro. Indeed, our studies show that addition of an AhR antagonist during Th17 polarization decreases Th17 polarization, whereas it does not influence differentiation of other effector T cell subsets. Interestingly, we also found that there are substantial differences in regard to generation of endogenous AhR ligands in different culture media, with RPMI having far less activity than IMDM. This could explain the puzzling variability in the literature in regard to Th17 polarization, which is generally in the range of 5–20%, whereas our values usually range from 40–60%. RPMI is by far the most commonly used culture medium, but as we show here, IMDM supports better Th17 polarization from both mouse and human CD4 T cells. We also observed that in RPMI, but not IMDM, CD4 T cells cultured under Th17 conditions show increased Stat5 phosphorylation. Given that IL-2 dampens Th17 differentiation via the induction of Stat5 (10), it is not surprising that Th17 differentiation is difficult to obtain in this medium. Our results emphasize the important role AhR agonists play in the modulation of the Th17 program and illustrate that differentiation of this effector T cell subset is inhibited by factors that are conducive to generation of the other T cell subsets, a fact that is reflected in the formulation of some culture media that support Th1, Th2, and regulatory T (T reg) cell differentiation, but lead to inefficient Th17 development.
RESULTS AND DISCUSSION
AhR antagonist CH-223191 impairs Th17 differentiation
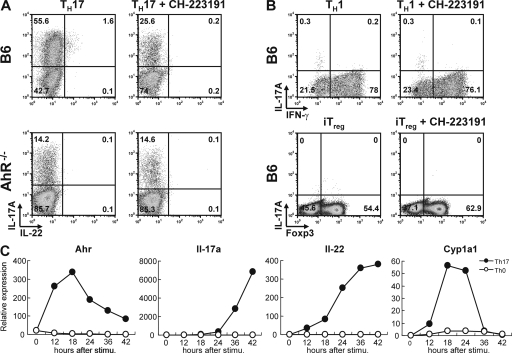
Because tryptophan metabolites generated by light exposure, such as FICZ, are potent enhancers of Th17 differentiation and there are reports suggesting that light exposure of culture medium generates AhR ligands that contribute to background AhR activation (11), we set out to assess Th17 development from naive precursors in the absence or presence of an AhR antagonist. Several AhR antagonists found in food components, such as flavones and resveratrol have been previously described (12), but all of them seem to exert AhR agonist activity at high concentrations (13). In contrast, screening of a chemical library identified CH-223191 as an antagonist that lacked agonist activity even at high doses (14). Th17 differentiation of naive CD4 T cells from B6 mice was markedly inhibited in the presence of the AhR antagonist (Fig. 1 A, left). CD4 T cells from AhR-deficient mice showed attenuated Th17 development, as previously described (3), and this was not influenced by the presence of AhR antagonist (Fig. 1 A, right). IL-22 expression is low, but detectable, in Th17 cells from wild-type B6 mice, but abrogated in the presence of AhR antagonist and absent in Th17 cells from AhR-deficient mice.
Figure 1.
Increased Th17 polarization caused by endogenous AhR ligands. (A) Intracellular staining for IL-17 versus IL-22 of CD4 T cells from control B6 (top) or AhR-deficient (bottom) cells polarized under Th17 conditions in IMDM for 5 d in the absence (left) or presence (right) of AhR antagonist CH-223191. Representative dot plots of four independent experiments are shown. (B) Intracellular staining for IL-17A versus IFN-γ or IL-17A versus Foxp3 of CD4 T cells from B6 mice cultured under Th1 conditions (top) or iT reg cell conditions (bottom) in the absence (left) or presence (right) of AhR antagonist CH-223191. Dot plots are representative of three independent experiments. (C) qPCR kinetic analysis of AhR, IL-17, IL-22, and CYP1A1 expression after activation of naive CD4 T cells under Th17 conditions. Expression in CD4 T cells activated under neutral conditions is shown for comparison. The figure shows mRNA levels normalized to Hprt expression and is representative of three independent experiments.
In contrast to Th17 cells, the generation of Th1 or induced T reg (iT reg) effector cells was not inhibited in the presence of AhR antagonist (Fig. 1 B), which is in line with our finding that they do not express AhR (3). It has been suggested that AhR ligation by TCDD, a high affinity, nonmetabolized AhR ligand, induces regulatory T cells (15, 16). However, it remains to be investigated whether the AhR ligand directly affected regulatory T cells, as so far only changes in percentage of T reg cells were measured under conditions in which other T cell subsets, such as Th17, underwent apoptosis; this would result in a proportional shift toward T reg cells, especially if these cells are not affected by AhR ligands. The presence of endogenous AhR agonists in culture medium was additionally supported by kinetic analysis of the expression of AhR and CYP1A1, which is an AhR-dependent enzyme whose expression is induced upon AhR activation. Under Th17 conditions, AhR expression was induced ∼12 h after the onset of culture and, concordantly, induction of CYP1A1 expression was observed, followed by expression of IL-17 and -22 (Fig. 1 C). It should be emphasized that because we culture highly FACS-purified naive CD4 T cells without the presence of APCs, there is no source of IL-23 in these cultures, so the induction of IL-22 is independent of this cytokine.
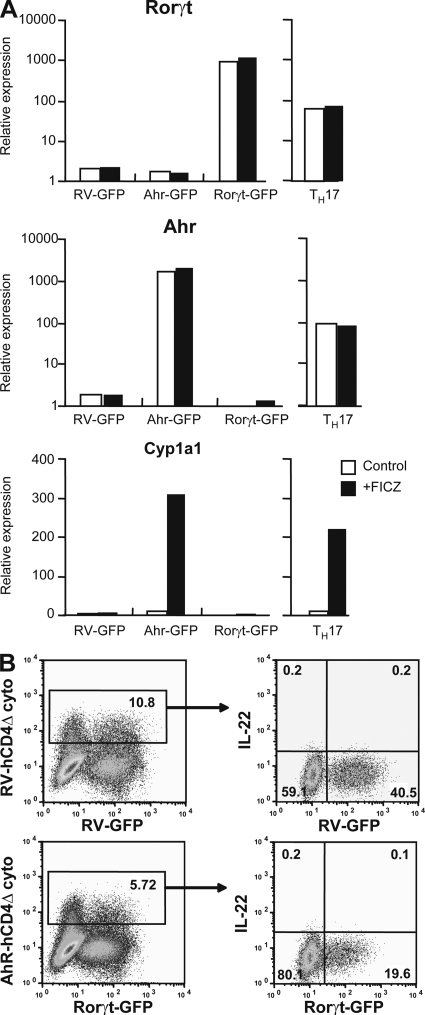
Thus, our results indicate that there are endogenous AhR agonists in culture medium, resulting in AhR activation, which plays an important role in shaping Th17 polarization, whereas it does not play a role for Th1 or iT reg cells. The molecular mechanisms involved in AhR modulation of the Th17 program are currently not well defined. Our recent data indicated that AhR expression on its own does not drive expression of IL-17 or -22 (3). Furthermore, forced expression of either RORγt or AhR does not impact on the expression of the other transcription factor (Fig. 2 A). Even cotransduction of both transcription factors is insufficient to affect the characteristic Th17 modulation with IL-22 induction (Fig. 2 B), suggesting that other pathways are involved. Thus, it was recently suggested that AhR interacts with Stat1 and Stat5 and that it may regulate Th17 development by modifying the activation of these two negative regulators of Th17 generation (17).
Figure 2.
Independent RORγt and AhR regulation in Th17 cells. (A) FACS-sorted naive CD4 T cells retrovirally transduced with RV-GFP control, AhR-GFP, or RORγt-GFP vector as indicated on abscissa were cultured in the presence (shaded bars) or absence (open bars) of FICZ. As a comparison, FACS-sorted naive CD4 T cells cultured under Th17 condition in the presence (shaded bars) or absence (open bars) of FICZ are shown. The figure shows mRNA expression for the indicated genes normalized for HPRT mRNA expression. The figure is representative of three independent experiments. (B) FACS-sorted naive CD4 T cells were retrovirally transduced with either control vector containing truncated human CD4 (RV-hCD4Δcyto) together with RV-GFP control vector (top) or with Ahr-hCD4Δcyto together with RORγt-GFP (bottom) and cultured in the presence of FICZ. Expression of hCD4 versus GFP is shown on the left. Intracellular IL-22 expression in gated populations is shown on the right. Results represent three independent experiments.
Reduced endogenous AhR ligands in RPMI medium
We noticed in the course of experiments on Th17 polarization that different individuals consistently obtained different levels of Th17 polarization, despite our using the same pool of FACS-sorted naive CD4 T cells as a starting population. Eventually, it became clear that some researchers used RPMI as culture medium, like most immunological laboratories, whereas IMDM, a richer culture medium developed by Norman Iscove at the Basel Institute for Immunology (18), was typically used by former Basel Institute researchers and was the medium used in the original studies showing that TGF-β and IL-6 drive Th17 differentiation (19). It is noteworthy that IMDM contains three- to fivefold higher amounts of aromatic amino acids, such as tryptophan, tyrosine, and phenylalanine (Table S1, available at http://www.jem.org/cgi/content/full/jem.20081438/DC1), than RPMI medium, which could give rise to different levels of endogenous AhR ligands in the two types of media.
Th17 differentiation in RPMI medium was consistently lower and similar to that of AhR-deficient cells that show attenuated Th17 polarization irrespective of the source of culture medium (Fig. 3 A). The AhR antagonist CH-223191 strongly reduced Th17 development from B6 CD4 T cells in IMDM, but even in RPMI medium, antagonizing AhR resulted in a further reduction of Th17 differentiation. In contrast to its effect on development of Th17 cells, Th1 polarization was not affected by the choice of culture medium or the presence of AhR antagonist. The development of iT reg cells was even increased in RPMI medium. Human Th17 cells also express AhR (3), and, similar to mouse T cells, the expansion of human Th17 cells from total CD4 T cells in peripheral blood was markedly better in IMDM compared with RPMI medium and strongly reduced in the presence of the AhR antagonist (Fig. 3 B).
Figure 3.
Suboptimal Th17 differentiation in RPMI medium. (A) Scatter plots showing the percentage of IL-17 polarization from individual experiments with CD4 T cells from B6 (filled triangles) or AhR-deficient mice (open circles) cultured under Th17-polarizing conditions in IMDM or RPMI in the presence or absence of AhR antagonist (left). P value control versus AhR antagonist for B6 are <0.001; between IMDM and RPMI B6 control values P < 0.001. Data from four independent experiments are shown. (right) Percentage of IFN-γ producers (filled diamonds) or Foxp3 expression (open squares) in B6 CD4 T cells cultured under Th1 or iT reg cell conditions, respectively in IMDM or RPMI. P value for iT reg cell generation in IMDM versus RPMI is P < 0.01. (B) Total CD4 T cells purified from PBMCs of human blood and stimulated under Th17 conditions in IMDM (left) or RPMI (right) medium in the absence (top) or presence (bottom) of AhR antagonist. IL-17 versus IFN-γ intracellular staining is shown in the dot plots. The scatter plot shows the percentage of IL-17 producers from three different individuals (representing three independent experiments) obtained in IMDM versus RPMI medium.
Supplementation of tryptophan partially restores Th17 polarization
To test whether the increased amount of tryptophan in IMDM is involved in the enhanced Th17 polarization in IMDM versus RPMI medium, we supplemented RPMI medium with l-tryptophan to adjust levels to those in IMDM. Tryptophan supplementation of RPMI significantly improved Th17 polarization, but levels were still much lower than those in IMDM. On the other hand, Th17 polarization of AhR-deficient cells was not influenced by tryptophan (Fig. 4 A). There could be several AhR ligands generated in IMDM, which is considerably richer in aromatic amino acids than RPMI. One of them may be derived from tryptophan, an essential amino acid that functions as the precursor to many substances, including melatonin, serotonin, or kynurenic acid. The light and culture medium–related activation of AhR has been observed as early as 1976 (20). More recently, it was shown that the tryptophan metabolite FICZ is produced in culture medium not only under UV irradiation but also under normal laboratory light conditions, and is stable during storage in the refrigerator for 3 d (11). In this respect, it is notable that the other aromatic amino acids that could be metabolic precursors for AhR ligands, l-tyrosine and l-phenylalanine, are also represented in substantially lower amounts in RPMI medium (Table S1). Furthermore, it has been suggested that oxidization of histidine could also generate an AhR ligand, and this amino acid is likewise underrepresented in RPMI compared with IMDM (21). However, we found that supplementation with these other components did not improve Th17 differentiation beyond that seen with tryptophan supplementation (unpublished data). Thus, it appears that the relative paucity of endogenous AhR ligands only partially explains the defective Th17 development in RPMI medium.
Figure 4.
Substitution of RPMI with tryptophan improves Th17 differentiation. (A) Scatter plot showing the percentage of IL-17 producers in CD4 T cells from wild-type B6 (filled triangles) or AhR-deficient mice (open circles) cultured under Th17-polarizing conditions in IMDM (left) or RPMI (right) with or without addition of 11 mg/liter of l-tryptophan. P values are shown in the graph. Data from four independent experiments are shown. (B) IL-17/IL-22 expression in CD4 T cells cultured under Th17 conditions in either IMDM (left) or RPMI (right) in the absence or presence of AhR agonist FICZ after 4 d of culture or (C) in the presence of neutralizing antibodies to IFN-γ and IL-4. Dot plots are representative of three independent experiments.
We previously reported that addition of FICZ, a tryptophan-derived AhR agonist with high affinity, substantially increases Th17 polarization and induces IL-22 production (3). To test whether FICZ can overcome the defect in Th17 polarization in RPMI medium, we differentiated naive CD4 T cells to Th17 in the presence of FICZ. This improved Th17 polarization and, importantly, resulted in some IL-22 expression. However, similar to tryptophan, supplementation of FICZ did not fully restore the defect (Fig. 4 B).
IL-2 depletion restores Th17 differentiation, but not IL-22 production, in RPMI medium
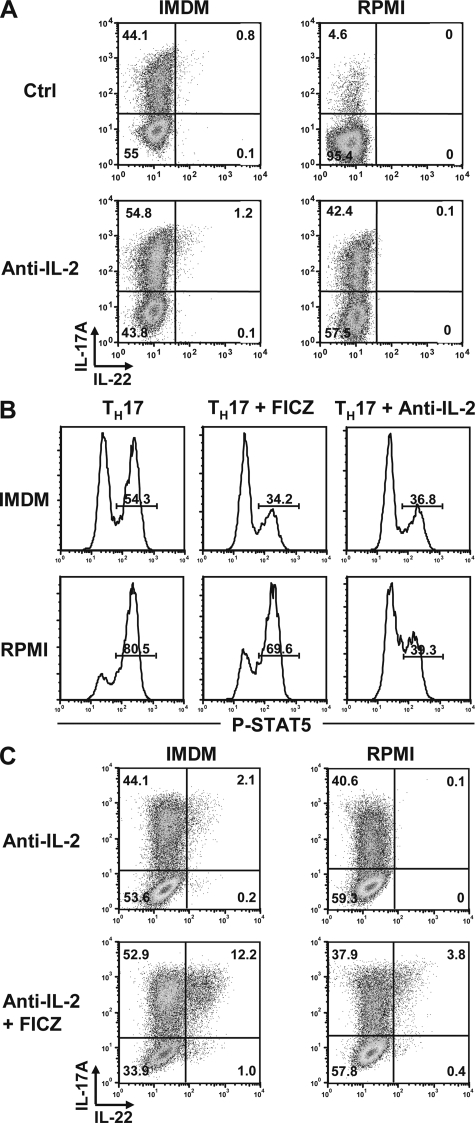
Because the differentiation of Th17 cells is strongly inhibited by cytokines that support development of the other CD4 T cell subsets, we tested whether addition of anti–IFN-γ and –IL-4, which are frequently used in Th17 polarization to inhibit Th1 and Th2 polarization (22), would have a positive influence on generation of this effector T cell subset. However, blockade of IFN-γ and IL-4 had little effect on Th17 differentiation under our culture conditions (Fig. 4 C). Given that our cultures contain highly purified T cells without APCs, this is perhaps not surprising, as blocking IFN-γ and IL-4 is effective mainly in mixed cultures with insufficiently purified T cells that may contain Th1 or Th2 effector cells and residual APCs that may promote Th1 development via secretion of IL-12. In contrast, neutralization of IL-2 markedly increased Th17 polarization in RPMI medium to levels similar to those obtained in IMDM (Fig. 5 A). In contrast, blockade of IL-2 had only a marginal effect on Th17 differentiation in IMDM. We checked IL-2 production in the two media and could not find a notable difference. Furthermore, careful analysis of IL-2 mRNA induction and expression of CD25 did not establish any differences in the two media (unpublished data).
Figure 5.
IL-2 blockade together with AhR activation restored Th17 differentiation. (A) Intracellular IL-17/IL-22 in Th17 cells developing in IMDM (left) or RPMI medium (right) without (top) or with addition of neutralizing antibodies to IL-2 (bottom). Dot plots are representative of three independent experiments. (B) Expression of phospho-Stat5 in CD4 T cells cultured under Th17 conditions in IMDM (top) or RPMI (bottom) in the absence of exogenous AhR agonist (left), with FICZ (middle), or in the presence of neutralizing antibodies to IL-2 (right). Histograms (representative of three independent experiments) of gated CD4 T cells showing phospho-Stat5 expression at 48 h after onset of culture are shown. (C) IL-17/IL-22 expression in Th17 cells cultured in IMDM (left) or RPMI (right) in the presence of neutralizing antibodies to IL-2 and FICZ. Dot plots are representative of three independent experiments.
Despite recovery of Th17 differentiation in RPMI by blockade of IL-2, this was not sufficient to rescue IL-22 expression. However, IL-22 production in RPMI medium was obtained by a combination of IL-2 neutralization and AhR ligation, emphasizing the important role for AhR activation in induction of IL-22 production (Fig. 5 C).
It has been shown that Th17 differentiation is inhibited by IL-2 signaling via induction of Stat5 (10). In line with these findings, we observed considerably more Stat5 phosphorylation during Th17 culture in RPMI medium (80.5%) compared with IMDM (54.3%), whereas blockade of IL-2 reduced Stat5 phosphorylation and the addition of FICZ caused a reduction in Stat5 phosphorylation, albeit less dramatic than that seen with anti–IL-2 (Fig. 5 B). It is currently unknown how AhR interacts with the Th17 program. We have shown that there is no direct interaction between AhR and RORγt (Fig. 2), but it is conceivable that AhR interacts with other transcription factors that positively or negatively influence Th17 differentiation. The latter is in agreement with data by Kimura et al. (17) showing direct interaction of AhR with Stat1 and Stat5 protein. Nevertheless, the interactions between IL-2, AhR, and Stat5 are yet to be defined on a molecular level.
Collectively, Th17 development is clearly controlled not only by multiple cytokines, but also by modulation via activation of the AhR, whose involvement in shaping Th17 differentiation in vivo is highlighted by the phenotype of AhR-deficient mice that show decreased IL-17 development and absence of IL-22 production (3). The tryptophan metabolite FICZ has been suggested to be an endogenous AhR agonist that is likely to play a role in vivo because exposure of human skin to UV-B induces CYP1A1 expression (23), but the number and nature of endogenous AhR agonists are still a matter of debate (5). Here, we describe that endogenous AhR activity present in culture medium has a strong influence on Th17 polarization. Thus, Th17 differentiation represents an alternative biological system in which the effects of potential AhR agonists or antagonists can be directly tested. Whereas the assessment of CYP1A1 reporter activity in hepatocyte or other cell lines, as currently used in the toxicology field, is a reliable and convenient readout for AhR activity, the connection of AhR activation to the Th17 program opens a wide range of possibilities in regard to testing the influence of AhR agonists and antagonists on biological processes dependent on these cells.
In the immunology field, obtaining improved Th17 polarization in vitro will facilitate their further molecular characterization that depends on methods such as gene array analysis or qPCR, which are not reliable unless the populations analyzed are relatively pure. Furthermore, the analysis of human Th17 development, which can only be assessed in vitro, will benefit from improved culture conditions. Our data show that not all media formulations that had been used successfully over decades to support the development of CD4 effector T cell subsets are conducive to Th17 differentiation through a combination of lacking AhR agonists and containing components that interfere with Th17 differentiation. All in all, our data emphasize that activation of AhR by a potentially diverse range of endogenous agonists has to be considered an essential co-factor in the optimal differentiation of Th17 effector T cell subset.
MATERIALS AND METHODS
Mice.
C57BL/6 and B6 BRA AhR KO (AhR-deficient B6) mice were bred and maintained under specific pathogen–free conditions, and all animal experiments were carried out according to institutional guidelines approved by the local ethical panel and by a project licence from the UK Home Office.
In vitro T cell differentiation and intracellular staining.
Naive CD4 T cells were isolated by FACS sorting using a MoFlo sorter of lymph nodes cell suspensions for CD44lo CD25− CD4+ cells. The culture mediums used were IMDM (Sigma-Aldrich) or RPMI 1640 (Sigma-Aldrich), both supplemented with 2 × 10−3 M l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 5 × 10−5 M β-mercaptoethanol, and 5% FCS. In some cases, RPMI medium was supplemented with 11 mg/liter l-tryptophan (Invitrogen) to adjust it to the concentrations found in IMDM.
Th17 cells were differentiated on plates coated with 2 μg/ml anti-CD3 + 5 μg/ml anti-CD28 with a cytokine cocktail of 50 ng/ml IL-6, 1 ng/ml TGF-β, and 10 ng/ml IL-1. Neutralizing antibodies to IFN-γ, IL-4, or IL-2 were added at 10 μg/ml in some experiments. Th1 cells were stimulated in the presence of 3 ng/ml IL-12, and iT reg cells were generated by adding 10 ng/ml TGF-β. The AhR antagonist CH-223191 (Calbiochem) was added at 3 μM at the start of culture. FICZ was added at 300 nM at the start of some cultures. For measurements of intracellular cytokines, T cells were restimulated with 500 ng/ml phorbol dibutyrate and 500 ng/ml ionomycin in the presence of brefeldin A for 4 h on day 5 after initiation of cultures. Measurement of Stat5 phosphorylation was done with antibodies to pStat5 (BD) according to the manufacturer's instructions.
qPCR analysis.
RNA was extracted using 1-bromo-3-chloro-propane (Sigma-Aldrich) and reverse transcribed with oligo d(T)16 (Applied Biosystems) according to the manufacturer's protocol. The cDNA served as template for the amplification of genes of interest and the housekeeping gene (Hprt) by real-time PCR, using TaqMan Gene Expression Assays and Applied Biosystems 7900HT Fast Real-Time PCR System. The primers obtained from Applied Biosystems are as follows: Hprt, Mm00446968_m1; AhR, Mm00478930_ml; IL-17A, Mm00439619_m1; IL-22, Mm00444241_m1; CYP1A1, Mm00487217_m1.
Human Th17 polarization.
CD4 T cells from PBMCs were purified by magnetic cell sorting and cultured on plates coated with 1 μg/ml anti-CD3 and 2.5 μg/ml anti-CD28 in either IMDM or RPMI supplemented with 10% serum replacement factor (Invitrogen) in the presence of 0.5 ng/ml TGF-β, 30 ng/ml IL-6, 10 ng/ml IL-1β, and 10 ng/ml IL-23.
Statistics.
P values were determined using two-tailed Student's t test.
Online supplemental material.
Table S1 is a comparison of the amino acid content in IMDM and RPMI. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081438/DC1.
Supplementary Material
Acknowledgments
We are grateful to George Kassiotis for comments on the manuscript.
This work was funded by the Medical Research Council UK.
The authors have no conflicting financial interests.
References
- 1.Bettelli, E., T. Korn, and V.K. Kuchroo. 2007. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol. 19:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockinger, B., and M. Veldhoen. 2007. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 19:281–286. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen, M., K. Hirota, A.M. Westendorf, J. Buer, L. Dumoutier, J.C. Renauld, and B. Stockinger. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453:106–109. [DOI] [PubMed] [Google Scholar]
- 4.Denison, M.S., and S.R. Nagy. 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43:309–334. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen, L.P., and C.A. Bradfield. 2008. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rannug, A., U. Rannug, H.S. Rosenkranz, L. Winqvist, R. Westerholm, E. Agurell, and A.K. Grafstrom. 1987. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J. Biol. Chem. 262:15422–15427. [PubMed] [Google Scholar]
- 7.Wei, Y.D., H. Helleberg, U. Rannug, and A. Rannug. 1998. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem. Biol. Interact. 110:39–55. [DOI] [PubMed] [Google Scholar]
- 8.Walker, M.K., S.E. Heid, S.M. Smith, and H.I. Swanson. 2000. Molecular characterization and developmental expression of the aryl hydrocarbon receptor from the chick embryo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 126:305–319. [DOI] [PubMed] [Google Scholar]
- 9.Rannug, A., and E. Fritsche. 2006. The aryl hydrocarbon receptor and light. Biol. Chem. 387:1149–1157. [DOI] [PubMed] [Google Scholar]
- 10.Laurence, A., C.M. Tato, T.S. Davidson, Y. Kanno, Z. Chen, Z. Yao, R.B. Blank, F. Meylan, R. Siegel, L. Hennighausen, E.M. Shevach, and J. O’Shea. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26:371–381. [DOI] [PubMed] [Google Scholar]
- 11.Oberg, M., L. Bergander, H. Hakansson, U. Rannug, and A. Rannug. 2005. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol. Sci. 85:935–943. [DOI] [PubMed] [Google Scholar]
- 12.Amakura, Y., T. Tsutsumi, K. Sasaki, T. Yoshida, and T. Maitani. 2003. Screening of the inhibitory effect of vegetable constituents on the aryl hydrocarbon receptor-mediated activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol. Pharm. Bull. 26:1754–1760. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, S., C. Qin, and S.H. Safe. 2003. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ. Health Perspect. 111:1877–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S.H., E.C. Henry, D.K. Kim, Y.H. Kim, K.J. Shin, M.S. Han, T.G. Lee, J.K. Kang, T.A. Gasiewicz, S.H. Ryu, and P.G. Suh. 2006. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol. Pharmacol. 69:1871–1878. [DOI] [PubMed] [Google Scholar]
- 15.Quintana, F.J., A.S. Basso, A.H. Iglesias, T. Korn, M.F. Farez, E. Bettelli, M. Caccamo, M. Oukka, and H.L. Weiner. 2008. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453:65–71. [DOI] [PubMed] [Google Scholar]
- 16.Funatake, C.J., N.B. Marshall, L.B. Steppan, D.V. Mourich, and N.I. Kerkvliet. 2005. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 175:4184–4188. [DOI] [PubMed] [Google Scholar]
- 17.Kimura, A., T. Naka, K. Nohara, Y. Fujii-Kuriyama, and T. Kishimoto. 2008. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. USA. 105:9721–9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iscove, N.N., and F. Melchers. 1978. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J. Exp. Med. 147:923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhoen, M., R.J. Hocking, C.J. Atkins, R.M. Locksley, and B. Stockinger. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189. [DOI] [PubMed] [Google Scholar]
- 20.Paine, A.J. 1976. Induction of benzo[a]pyrene mono-oxygenase in liver cell culture by the photochemical generation of active oxygen species. Evidence for the involvement of singlet oxygen and the formation of a stable inducing intermediate. Biochem. J. 158:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paine, A.J., and J.E. Francis. 1980. The induction of benzo[a]pyrene-3-mono-oxygenase by singlet oxygen in liver cell culture is mediated by oxidation products of histidine. Chem. Biol. Interact. 30:343–353. [DOI] [PubMed] [Google Scholar]
- 22.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 23.Katiyar, S.K., M.S. Matsui, and H. Mukhtar. 2000. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J. Invest. Dermatol. 114:328–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.