Abstract
Objective
To evaluate the clinical efficacy and safety of transcatheter arterial embolization (TAE) with N-Butyl Cyanoacrylate (NBCA) for nonvariceal upper gastrointestinal bleeding.
Materials and Methods
Between March 1999 and December 2002, TAE for nonvariceal upper gastrointestinal bleeding was performed in 93 patients. The endoscopic approach had failed or was discarded as an approach for control of bleeding in all study patients. Among the 93 patients NBCA was used as the primary embolic material for TAE in 32 patients (28 men, four women; mean age, 59.1 years). The indications for choosing NBCA as the embolic material were: inability to advance the microcatheter to the bleeding site and effective wedging of the microcatheter into the bleeding artery. TAE was performed using 1:1-1:3 mixtures of NBCA and iodized oil. The angiographic and clinical success rate, recurrent bleeding rate, procedure related complications and clinical outcomes were evaluated.
Results
The angiographic and clinical success rates were 100% and 91% (29/32), respectively. There were no serious ischemic complications. Recurrent bleeding occurred in three patients (9%) and they were managed with emergency surgery (n = 1) and with a successful second TAE (n = 2). Eighteen patients (56%) had a coagulopathy at the time of TAE and the clinical success rate in this group of patients was 83% (15/18).
Conclusion
TAE with NBCA is a highly effective and safe treatment modality for nonvariceal upper gastrointestinal bleeding, especially when it is not possible to advance the microcatheter to the bleeding site and when the patient has a coagulopathy.
Keywords: Gastrointestinal tract, hemorrhage; Angiography, therapeutic embolization
Transcatheter arterial embolization (TAE) has been widely used to control nonvariceal upper gastrointestinal bleeding (1-4). A variety of embolic materials have been used for embolization; the more commonly used agents are gelatin sponge pieces, coils and polyvinyl alcohol particles. However, liquid embolic materials such as N-Butyl Cyanoacylate (NBCA) have been rarely used, because of concerns about complicated ischemic injury and difficulty with handling the materials.
N-Butyl Cyanoacylate has several advantages compared with other embolic materials. NBCA allows rapid and permanent embolization with fast polymerization when contacted with blood. Complete hemostasis can be achieved by a single injection with simultaneous embolization of collateral vessels connected to the bleeding focus, which can cause backbleeding or rebleeding if not treated. NBCA is also useful in patients with coagulopathy because it does not depend on the coagulation process for its therapeutic effect. Serious complications, such as bowel ischemia or innocent vessel embolization, can be minimized by treating patients with appropriate indications and careful procedures by adequately trained interventionists.
Thus, NBCA can be an alternative embolic material which is effective, rapid and safe in transcatheter embolization of gastrointestinal bleeding and it may possibly be lifesaving in very emergent situations, especially when the patient is critical. The purpose of this study was to evaluate the clinical efficacy and safety of TAE with NBCA for nonvariceal upper gastrointestinal bleeding that could not be controlled by conventional endoscopic procedures.
MATERIALS AND METHODS
Study Subjects
Between March 1999 and December 2002, TAE for nonvariceal upper gastrointestinal bleeding was performed in 93 patients at our institution. The endoscopic approach had failed or was discarded because of its inability to control the bleeding in participating patients. Prospective collection of data about clinical records and medical imagings was carried out for all patients. Among the 93 patients, NBCA was used as the primary embolic material for TAE in 32 patients and they were included in this study. There were 28 men and four women, with an age range of 33-84 years (mean age, 59.1 years). Pertinent patient information including comorbid conditions are summarized in the Table 1. The causes of bleeding included: benign ulcers (n = 20), gastric cancers (n = 7), pancreatitis (n = 2), duodenal cancer (n = 1), duodenal tuberculosis (n = 1) and esophageal candidiasis (n = 1). The embolized arteries were: duodenal branch of gastroduodenal arteries (n = 11), left gastric arteries (n = 9), right gastric arteries (n = 5), right gastroepiploic arteries (n = 2), esophageal arteries (n = 2), antral branch of gastroduodenal artery (n = 1), dorsal pancreatic artery (n = 1) and the transverse pancreatic artery (n = 1). The angiographic findings were extravasation of contrast media in 27 patients and pseudoaneurysms in five patients. Written informed consent for transcatheter embolization was obtained from each patient or a family member.
Table 1.
Clinical Data on 32 Patients Who Underwent Embolization with the NBCA Mixture
Note.-GU = gastric ulcer, DU = duodenal ulcer, DM = diabetes mellites, ESRD = end stage renal disease, LC = liver cirrhosis, HCC = hepatocellular carcinoma, CVA = cerebrovascular accident, RGA = right gastric artery, LIPA = left inferior phrenic artery, GDA = gastroduodenal artery, LGA = left gastric artery, GEA = gastroepiploic artery, SMA = superior mesenteric artery, NBCA = N-Butyl Cyanoacrylate, TAE = transcatheter arterial embolization, MOF = multi-organ failure
Embolization Techniques
N-Butyl Cyanoacrylate (Histoacryl, Braun, Melsungen, Germany) was used as an embolic agent in all participating patients. After a common femoral artery puncture, a standard 5-F angiographic catheter was used to access the sites of suspected bleeding and it was also used as an introducing catheter. Diagnostic angiography was performed to identify the bleeding site; next a 3-F microcatheter (Microferret; Cook, Bloomington, IN) was advanced, to as close as possible, to the bleeding point. The indications for using NBCA as an embolic material were inability to adequately reach the bleeding site and effective wedging of the microcatheter into the bleeding artery to restrict pericatheter flow (Figs. 1, 2).
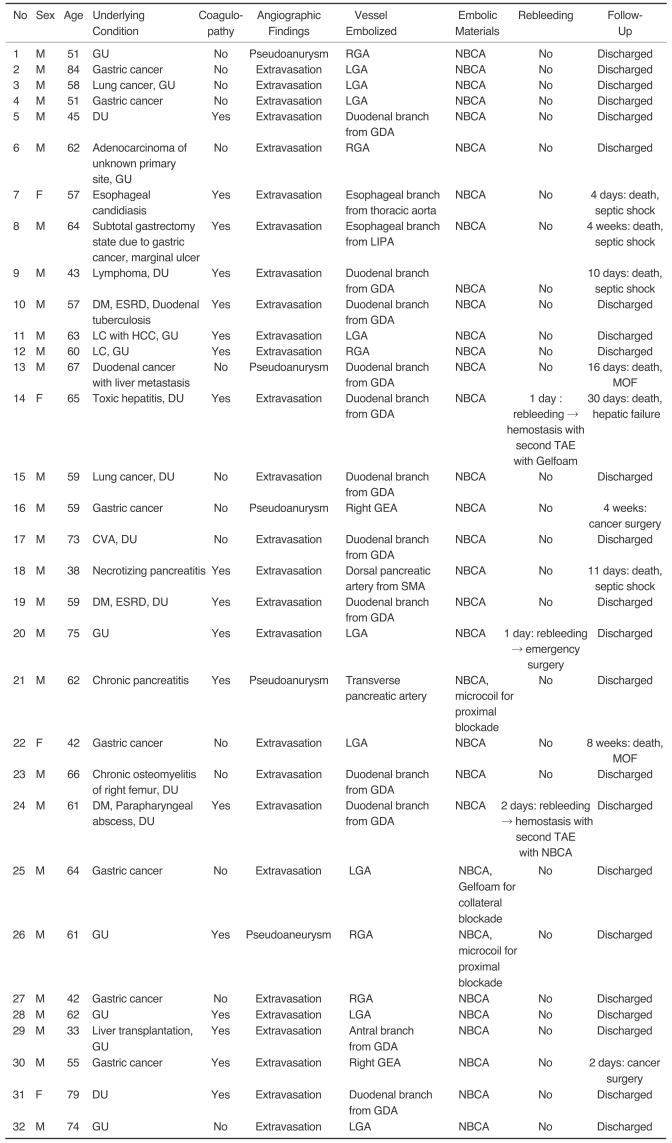
Fig. 1.
Thirty-three-year-old male presented with gastric ulcer bleeding who underwent liver transplantation 11 days previously.
A. Celiac arteriography shows suspected extravasation of contrast media in the antrum of the stomach (arrow).
B. Superselective gastroduodenal arteriography using a microcatheter reveals extravasation of contrast media from the antral branch (arrow).
C. Radiography obtained after test injection of contrast media. The tip of the microcatheter (white arrow) could not advance to the bleeding point, but effective wedging of the microcatheter, into the bleeding artery, was achieved to restrict pericatheter flow.
D. Post-embolic celiac arteriography shows the successful embolization of the bleeding focus after embolization with NBCA mixture.
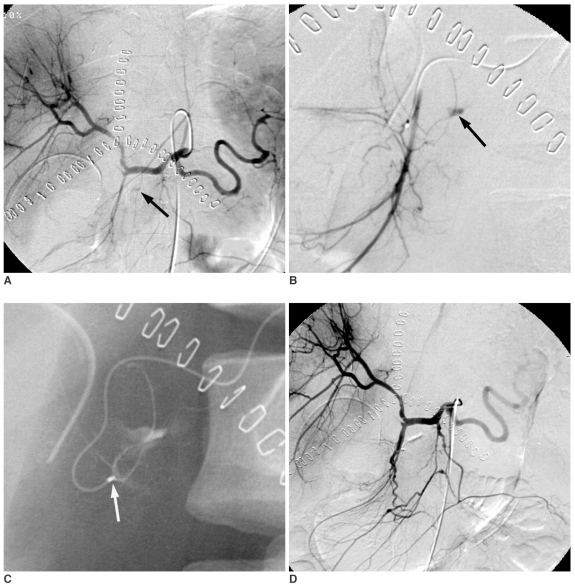
Fig. 2.
Sixty-seven-year-old male with duodenal stent placement for duodenal adenocarcinoma 20 days previously.
A. Celiac arteriography shows suspected extravasation of contrast media into the duodenum (arrow).
B. Gastroduodenal arteriography reveals a pseudoaneurysm of a duodenal branch supplied by the gastroduodenal artery (arrow).
C. The mixture of N-Butyl Cyanoacrylate and lipiodol was injected into the bleeding artery. The radiopaque N-Butyl Cyanoacrylate mixture accumulated beside the duodenal stent.
D. Post-embolic celiac arteriography shows the successful embolization of the bleeding artery and the preserved blood flow through the gastroduodenal artery.
For the embolotherapy, NBCA was mixed with iodized oil (Lipiodol, Andre Guerbet, Aulnay-Sous-Bois, France) in ratios varying from 1:1 to 1:3; the iodized oil supplied radiopacity to the mixture and delayed the polymerization time (5). Prior to injection of the NBCA mixture, the microcatheter was flushed with 5% dextrose solution to prevent premature polymerization of the mixture in contact with residual blood or saline. The NBCA mixture was injected using a 1 mL syringe under careful fluoroscopic monitoring. The end point for the injection was extravasation of the mixture from the bleeding site or filling of the pseudoaneurysm with or without appearance of anastomotic channels (Fig. 3). Only a small amount of NBCA mixture (0.2-0.6 mL) was necessary to achieve target embolization. Immediately after the injection, the microcatheter was removed to prevent adherence of the catheter tip to the vessel wall and discarded without flushing. Then the inner lumen of the guiding catheter was aspirated and post-embolic angiography was performed.
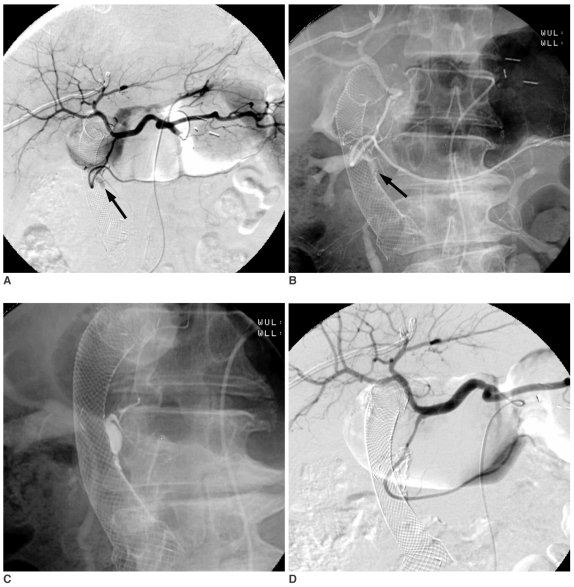
Fig. 3.
Fifty-nine-year-old male presented with gastric cancer bleeding.
A. Celiac arteriography shows a area of contrast collection supplied by the right gastroepiploic artery (arrow).
B. Selective right gastroepiploic arteriography reveals pseudoaneurysm formation (arrow).
C. After embolization with the NBCA mixture (arrow), post-embolic celiac arteriography shows the successful embolization of the bleeding artery.
D. The radiopaque N-Butyl Cyanoacrylate mixture casting in the right gastroepiploic artery is seen on post-embolic radiography (white arrow).
Analysis
The angiographic and clinical success rate, recurrent bleeding rate, procedure related complications and clinical outcomes were evaluated. Angiographic success was defined as when post-embolic angiography demonstrated cessation of extravasation with no backbleeding from collateral flow or non-opacification of pseudoaneurysm at the end of the procedure. Clinical success was defined as clinical improvement with complete cessation of bleeding after embolization without the need for emergent surgery or other interventional procedures. Cessation of bleeding was defined by clearing of the nasogastric aspirate by 24 hours after embolization or absence of bleeding on endoscopic examination as well as stabilization of the hemoglobin level within 48 hours of the embolization procedure.
Patients who met one of the following criteria were considered to have a coagulopathy: prothrombin ratio greater than 1.5, partial thromboplastin time greater than 45 seconds, or platelet count of less than 80,000/µL (2, 6, 7).
RESULTS
In all study patients, it was possible to deliver the NBCA mixture to the bleeding site; the angiographic success rate was 100% (32/32). The NBCA mixture was the only embolic material used to achieve angiographic success in 29 patients. Other embolic agents were used in combination with NBCA mixture in three patients, microcoils in two cases and gelatin sponge pieces in one case. Microcoils were used in combination with NBCA for proximal blockade of pseudoaneurysms in two cases during our early experience. Backbleeding from collateral flow after NBCA embolization occurred in one patient with massive gastric cancer bleeding and additional embolization of collateral flow from short gastric artery with gelatin sponge pieces could effectively stop the backbleeding.
Clinical success was achieved in 29 patients (91%). Recurrent bleeding after transcatheter embolization occurred in three patients (9%). However, a second TAE successfully stopped the rebleeding in two patients and emergency surgery was performed in one patient. In two patients with a second TACE, the causes of the bleeding were large duodenal ulcers in both. A repeat angiography revealed that the previously embolized vessels were not recanalized, and that other collateral vessels were responsible for the rebleeding at the same ulcer lesions that were previously embolized.
There was no evidence of symptomatic bowel infarction or other major complications directly related to NBCA embolization in any of the patients. Follow up endoscopic evaluations were performed in 20 patients between 1-105 days (mean 16 days) after the embolization and postembolization bowel ischemia was not observed in any of the patients.
Twenty-three of 32 patients were discharged after clinical improvement without surgery. Two patients underwent surgery for underlying gastric cancer. Seven patients expired during the hospital course due to other underlying conditions. Coagulopathy was present at the time of TAE in 18 patients (56%) and the clinical success rate in this group of patients was 83% (15/18).
DISCUSSION
Since Rosch et al. (8) introduced the technique of TAE as an alternative to surgery for the control of upper gastrointestinal bleeding in 1972, TAE has gained widespread acceptance for first-line treatment of acute nonvariceal upper gastrointestinal bleeding resistant to endoscopic therapy. TAE is generally preferred over surgery, especially in high-risk patients (3, 6, 9, 10). The efficacy of TAE in initial hemostasis of acute nonvariceal gastrointestinal bleeding has been reported to be 52-90%; however, relatively high rates of rebleeding have also been reported (3, 6, 7, 10-12).
A variety of embolic agents such as gelatin sponge pieces, microcoils, polyvinyl alcohol particles and autologous blood clots have been used in TAE for gastrointestinal bleeding. Some authors have reported the use of liquid adhesives for transcatheter embolization of gastrointestinal bleeding (11, 13-15). However, most interventional radiologists are reluctant to use this technique because of the potential risk of complicated ischemic injury and the difficulty with handling liquid adhesives. Liquid adhesives or glue have been used as embolic agents for nearly three decades, but experience with liquid adhesives outside of neurological interventions has been generally limited (16).
N-Butyl Cyanoacrylate is a well known liquid adhesive used in the vascular system; the US Food and Drug Administration recently approved its use for cerebral arteriovenous malformations in 2000. A localized vascular occlusion mimicking a surgical ligation can be achieved within seconds as the NBCA polymerizes on exposure to the anions in blood (17). Unlike temporary embolic agents, vascular occlusion using NBCA is permanent and cannot be removed by reversal of vessel flow. NBCA is also easy to deliver through a microcatheter because of its low viscosity. However, its lack of radiopacity and its rapid polymerization time make it difficult to use. The addition of iodized oil to NBCA can opacify the agent and polymerization time can be tempered with an increased ratio of iodized oil to NBCA. Stoesslein et al. reported that the mixture of NBCA and iodized oil at a mixing ratio of 1:3-1:4 provided optimal embolic material with a polymerization time of 7.5-11.5 seconds with excellent contrast definition reported in their experimental study in 1982 (18). However, the in vivo polymerization time for NBCA appears to be more rapid than the in vitro time because of the higher body temperature compared to room temperature and a greater presence of anions intravascularly (19). Pollak and white suggested that the estimated in vivo polymerization time for the NBCA to iodized oil mixtures of between 1:1 and 1:4 were 1-4 seconds, with a linear relationship between time and mixture ratio (16). In the present study, we mixed NBCA with iodized oil in ratios varying from 1:1 to 1:3, and we determined the appropriate amount and dilution of NBCA depending on the size and shape of the vessel and its distribution after test injection with contrast material to the target of embolization. However, we used the 1:3 mixing ratio in most cases because the 1:3 ratio was thought to provide a useful polymerization time for embolization in most patients with GI bleeding. The test injection took only one or two minutes and did not cause a significant time delay during emergency procedures.
Among the variety of embolic agents, coils are usually preferred for embolization of pseudoaneurysms. Superselective catheterization and stable catheter positioning are required for safe release and ideal coil embolization. However, sometimes it is difficult to selectively place the catheter close to and beyond the pseudoaneurysm in small or tortuous vessels (20). Furthermore, when there are many efferent arteries originating from the pseudoaneurysm, or many collateral vessels coming from the adjacent arteries, the procedure becomes time consuming and technically difficult to carry out with coils. Although catheterization may be possible, coils may not be delivered because of the tortuosity of vessels (20). However, because of its low viscosity, NBCA can be injected through a microcatheter into small arteries and collateral circulation, which are difficult to embolize with a coil. Even though it is impossible to advance the microcatheter to the bleeding point, when the microcatheter is tightly engaged in the bleeding artery, it is possible to deliver NBCA selectively to the bleeding site without embolizing innocent vessels.
In the upper gastrointestinal tract there are rich anastomoses between adjacent arteries; this had made backbleeding a problem with embolization procedures. For effective hemostasis, embolization of both feeding arteries and potential collateral channels is required. However, gelatin sponge pieces or polyvinyl alcohol particles have a chance to embolize only the feeding artery and sometimes post-embolic angiography reveals backbleeding from collateral channels that are usually more complex and difficult to access (16). Superselection of those collateral feeders is very time consuming and can occasionally result in failed catheterization and embolization. In this series, backbleeding from collateral channels did not occur except in one case of massive gastric cancer bleeding. This suggests that NBCA can effectively embolize all potential collateral channels together with the main feeder vessels with only a single injection. In cases of life threatening bleeding, the choice of NBCA as an embolization material can be lifesaving by minimizing the risk of backbleeding, which can occur with the use of other embolic materials. Toyoda et al. reported that the time for TAE, using NBCA, was significantly faster than for TAE procedures that do not use NBCA. This is important particularly in cases of massive bleeding that require urgent hemostasis (11).
Coagulopathy has been associated with clinical failure after TAE (2, 6, 7). Encarnacion et al. reported that embolization was 2.9 times more likely to fail in patients with coagulopathy, and that death from bleeding after embolization was 9.6 times more likely to occur in this group (6). Schenker et al. also reported that embolization in patients with a coagulopathy at the time of intervention was 2.8 times more likely to fail, and the odds of dying were 3.4 times greater for patients with a coagulopathy (2). In cases of coil or polyvinyl alcohol particle embolization, occlusion of vessels or pseudoaneurysms is dependent on thrombosis rather than the embolic material itself and the coagulation state of the patient is important for the ultimate success of the embolization procedure. However, NBCA does not depend on the coagulation process for its therapeutic effect and therefore can be used effectively in patients with a coagulopathy. Eighteen of 32 patients (56%) in our study had a diagnosis of a coagulopathy prior to arrival to the angiographic suite; the angiographic success rate in this group of patients was 100% and the clinical success rate was 83% in spite of their coagulopathy. Considering that angiographic success rate of TAE in patients with coagulopathy has been previously reported to be less than 46%, NBCA appears to provide a more successful approach in patients with a coagulopathy (2, 6).
Transcatheter embolization with NBCA has the potential risk of complications including: embolization of the proximal segment without adequate embolization at the bleeding site due to rapid polymerization, distal embolization of untargeted sites due to too rapid injection of the NBCA mixture or an excessively prolonged polymerization time (21) and reflux of glue into another innocent vessel by overinjection. Complications related to NBCA embolization can be minimized by careful attention to the specific vascular anatomy and the information obtained from test injections with contrast material. In this series, none of these patients developed symptomatic bowel infarction or mesenteric ischemia after NBCA embolization. These results correlate well with the experience of Yamakado et al. (20) and Kish et al. (13) who similarly had no cases of organ infarction.
In order to carry out a precise and safe embolization and to reduce the possibility of complications with the use of NBCA as an embolic material, we recommend the following: 1) Pay careful attention to the specific vascular anatomy and the information obtained from the test injections with contrast media; 2) Use the wedged catheter technique with no pericatheter flow. It allows the interventional radiologists to precisely control the extent of glue embolization; 3) Perform a simulated injection with contrast media in the same condition before the actual NBCA injection with precise volume calculation.
The single use of a microcatheter, without flushing after NBCA injection, is highly recommended for precise embolization with the wedged technique because overinjection of NBCA can occur during flushing of the microcatheter. The intraluminal volume of microcathter is usually around 0.3 mL and this should be considered for precise volume estimation. For example, if initially 0.8 mL of NBCA mixture was drawn up in a 1-mL syringe and 0.1 mL was left in the syringe after injection, then 0.4 mL was injected into the embolized vessels and 0.3 mL was left in the discarded microcatheter. Adequate experience and training are also mandatory for successful NBCA embolization without major complications. Although most interventional radiologists are familiar with the use of coils and particles such as gelatin sponge pieces and polyvinyl alcohol, few have experience with liquid adhesives such as NBCA (16). NBCA is difficult to use initially, however after becoming familiar with its properties in the animal laboratory, it can be used safely for the care of patients.
In conclusion, TAE with NBCA is a highly effective and safe treatment modality for upper gastrointestinal bleeding, especially in cases when it is technically difficult to advance the microcatheter to the bleeding site and in patients with a coagulopathy.
References
- 1.Hastings GS. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics. 2000;20:1160–1168. doi: 10.1148/radiographics.20.4.g00jl361160. [DOI] [PubMed] [Google Scholar]
- 2.Schenker MP, Duszak R, Jr, Soulen MC, Smith KP, Baum RA, Cope C, et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263–1271. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey DT, Burke DR, Reilly RS, McLean GK, Rosato EF. Angiography in poor-risk patients with massive nonvariceal upper gastrointestinal bleeding. Am J Surg. 1990;159:282–286. doi: 10.1016/s0002-9610(05)81218-8. [DOI] [PubMed] [Google Scholar]
- 4.Goldman ML, LAND WC, Bradley EL, Anderson RT. Transcatheter therapeutic embolization in the management of massive upper gastrointestinal bleeding. Radiology. 1976;120:513–521. doi: 10.1148/120.3.513. [DOI] [PubMed] [Google Scholar]
- 5.Parildar M, Oran I, Memis A. Embolization of visceral pseudoaneurysms with platinum coils and N-butyl cyanoacrylate. Abdom Imaging. 2003;28:36–40. doi: 10.1007/s00261-002-0021-7. [DOI] [PubMed] [Google Scholar]
- 6.Encarnacion CE, Kadir S, Beam CA, Payne CS. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505–508. doi: 10.1148/radiology.183.2.1561358. [DOI] [PubMed] [Google Scholar]
- 7.Aina R, Oliva VL, Therasse E, Perreault P, Bui BT, Dufresne MP, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12:195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- 8.Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 9.Lang EV, Picus D, Marx MV, Hicks ME. Massive arterial hemorrhage from the stomach and lower esophagus: impact of embolotherapy on survival. Radiology. 1990;177:249–252. doi: 10.1148/radiology.177.1.2399325. [DOI] [PubMed] [Google Scholar]
- 10.Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182:703–707. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]
- 11.Toyoda H, Nakano S, Kumada T, Takeda I, Sugiyama K, Osada T, et al. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996;11:252–258. doi: 10.1111/j.1440-1746.1996.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 12.Gomes AS, Lois JF, McCoy RD. Angiographic treatment of gastrointestinal hemorrhage: comparison of vasopressin infusion and embolization. AJR Am J Roentgenol. 1986;146:1031–1037. doi: 10.2214/ajr.146.5.1031. [DOI] [PubMed] [Google Scholar]
- 13.Kish JW, Katz MD, Marx MV, Harrell DS, Hanks SE. N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. J Vasc Interv Radiol. 2004;15:689–695. doi: 10.1097/01.rvi.0000133505.84588.8c. [DOI] [PubMed] [Google Scholar]
- 14.Slaba S, Nassar J, El Murr T, Saba M, Ghayad E. Distal glue embolization in a patient with gastrointestinal hemorrhage. J Radiol. 2002;83:656–658. [PubMed] [Google Scholar]
- 15.Goldman ML, Freeny PC, Tallman JM, Galambos JT, Bradley EL, 3rd, Salam A, et al. Transcatheter vascular occlusion therapy with isobutyl 2-cyanoacrylate (bucrylate) for control of massive upper-gastrointestinal bleeding. Radiology. 1978;129:41–49. doi: 10.1148/129.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Pollak JS, White RI., Jr The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001;12:907–913. doi: 10.1016/s1051-0443(07)61568-1. [DOI] [PubMed] [Google Scholar]
- 17.Dotter CT, Goldman ML, Rosch J. Instant selective arterial occlusion with isobutyl 2-cyanoacrylate. Radiology. 1975;114:227–230. doi: 10.1148/114.1.227. [DOI] [PubMed] [Google Scholar]
- 18.Stoesslein F, Ditscherlein G, Romaniuk PA. Experimental studies on new liquid embolization mixtures (histoacryl-lipiodol, histoacryl-panthopaque) Cardiovasc Intervent Radiol. 1982;5:264–267. doi: 10.1007/BF02565409. [DOI] [PubMed] [Google Scholar]
- 19.Widlus DM, Lammert GK, Brant A, Tsue T, Samphillipo MA, Jr, Magee C, et al. In vivo evaluation of iophendylate-cyanoacrylate mixtures. Radiology. 1992;185:269–273. doi: 10.1148/radiology.185.1.1523322. [DOI] [PubMed] [Google Scholar]
- 20.Yamakado K, Nakatsuka A, Tanaka N, Takano K, Matsumura K, Takeda K. Transcatheter arterial embolization of ruptured pseudoaneurysms with coils and n-butyl cyanoacrylate. J Vasc Interv Radiol. 2000;11:66–72. doi: 10.1016/s1051-0443(07)61284-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim BS, Do HM, Razavi M. N-butyl cyanoacrylate glue embolization of splenic artery aneurysms. J Vasc Interv Radiol. 2004;15:91–94. doi: 10.1097/01.rvi.0000099537.29957.13. [DOI] [PubMed] [Google Scholar]