Abstract
To determine if Neu is dominant over transforming growth factor β (TGF-β), we crossed mouse mammary tumor virus (MMTV)-Neu mice with MMTV-TGF-β1S223/225 mice expressing active TGF-β1 in the mammary gland. Bigenic (NT) and Neu-induced mammary tumors developed with a similar latency. The bigenic tumors and their metastases were less proliferative than those occurring in MMTV-Neu mice. However, NT tumors exhibited less apoptosis and were more locally invasive and of higher histological grade. NT mice exhibited more circulating tumor cells and lung metastases than Neu mice, while NT tumors contained higher levels of phosphorylated (active) Smad2, Akt, mitogen-activated protein kinase (MAPK), and p38, as well as vimentin content and Rac1 activity in situ than tumors expressing Neu alone. Ex vivo, NT cells exhibited higher levels of P-Akt and P-MAPK than Neu cells. These were inhibited by the TGF-β inhibitor-soluble TGF-β type II receptor (TβRII:Fc), suggesting they were activated by autocrine TGF-β. TGF-β stimulated migration of Neu cells into surrounding matrix, while the soluble TGF-β inhibitor abrogated motility and invasiveness of NT cells. These data suggest that (i) the antimitogenic and prometastatic effects of TGF-β can exist simultaneously and (ii) Neu does not abrogate TGF-β-mediated antiproliferative action but can synergize with TGF-β in accelerating metastatic tumor progression.
Transforming growth factor β (TGF-β) is a multifunctional cytokine involved in regulating cell growth, motility, differentiation, apoptosis, immune functions, and matrix remodeling (reviewed in references 25 and 26). The TGF-β ligands initiate cellular signaling pathways by activation of a heteromeric complex of transmembrane receptor serine-threonine kinases (27). TGF-β binds to the TGF-β type II receptor (TβRII), which recruits and phosphorylates the type I receptor (TβRI), known as Alk5. The activated TβRI kinase then transmits signals to cytosolic target proteins (50). The role of TGF-β in the biology of mammary epithelial cells is complex. The three mammalian TGF-β isoforms, TGF-β1, -2, and -3, are expressed at all stages of murine postnatal mammary gland development except lactation (45). They are widely accepted as potent inhibitors of mammary epithelial cell proliferation. Transgenic mouse mammary tumor virus mice that overexpress active TGF-β1 in mammary epithelium (MMTV/TGF-β) exhibit ductal hypoplasia (42). Overexpression of active TGF-β transgene in the mammary gland results in resistance to TGF-α- or carcinogen-induced mammary cancers (41) as well as enhanced stem cell senescence (23). Inhibition of autocrine TGF-β by expression of a dominant-negative TβRII mutant results in accelerated lobuloalveolar mammary development (17) and enhanced propensity for carcinogen-induced mammary cancers (6). Finally, decreased expression of TβRII in women with mammary epithelial hyperplasia has been shown to confer an increased risk for the subsequent development of breast cancer (16).
A dual role of TGF-β in tumorigenesis is underscored by observations that advanced human breast cancers show increased expression of TGF-β. Additionally, most human breast cancer cell lines are resistant to the antiproliferative effects of TGF-β, and TGF-β can promote the progression of breast cancer cells (reviewed in references 12 and 49). The molecular mechanisms by which tumor cells switch TGF-β function from tumor suppressor to tumor promoter may be dictated by other oncogenic signals also present in transformed cells. For example, TGF-β1 and Ras cooperate to induce proliferation and epithelial-to-mesenchymal transition (EMT) and have been shown to enhance survival of cancer and noncancer cells (20, 21, 35, 36, 39). Ha-Ras-overexpressing mammary tumor cells secrete high levels of TGF-β (24). Inhibition of TGF-β signaling using a dominant-negative TβRII in Ras-transformed cells impairs primary tumor and metastasis formation and prevents EMT (35, 44). Additionally, metastasis of Ras-induced skin tumors was enhanced by elevated levels of activated Smad2, a direct downstream effector of TGF-β signaling (34). Therefore, in early and late phases of transformation, TGF-β secretion by cancer and/or stromal cells enhances Ras-induced transformation.
In this report, we have examined the dominance of the Neu (erbB2) proto-oncogene over TGF-β and, second, determined whether Neu synergizes with TGF-β to accelerate mammary tumor progression. The Neu proto-oncogene product is a member of the erbB family of transmembrane receptor tyrosine kinases, which also includes the epidermal growth factor receptor (EGFR; erbB1), erbB3, and erbB4. Binding of ligands to EGFR, erbB3, and erbB4 induces the formation of homodimeric and heterodimeric, kinase-active complexes into which Neu is recruited as a preferred partner. Even though Neu is unable to interact directly with receptor ligands, it can amplify signaling pathways induced by this receptor network, which include phospholipase Cγ-1 (PLCγ-1), Ras-Raf-MEK-mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K)-Akt-p70S6 kinase, PAK-JNNK-JNK, SAPKs, and STATs (reviewed in references 37 and 52). In addition, Neu can multimerize and become activated in a ligand-independent fashion when present in cells at high density. Indeed, MMTV-Neu transgenic mice develop stochastic multifocal metastatic mammary cancers with a median latency of approximately 6 months (18).
As indicated above, TGF-β can suppress TGF-α-induced transformation in the mammary gland (41). Furthermore, TGF-β can also suppress EGF-induced mitogenesis in epithelial cells (7). These results imply that TGF-β is dominant over the EGFR. Because of the ability of Neu to signal with increased potency to Ras/MAPK (52), we speculated that overexpression of Neu may prevent TGF-β-mediated growth inhibition and potentially accelerate Neu-induced tumors. In this report, tumors in MMTV-Neu × MMTV-TGF-β1S223/225 bitransgenic mice were shown to proliferate more slowly but exhibited increased cell survival, local invasion, intravasation, and metastases than MMTV-Neu tumors. These data suggest that although Neu did not prevent TGF-β-mediated inhibition of mitogenesis, it synergized with this cytokine in accelerating mammary tumor progression.
MATERIALS AND METHODS
Studies in MMTV-Neu/MMTV-TGF-β1S223/225 mice.
All mice were derived from MMTV-TGF-β1S222/225 and MMTV-Neu (Jackson Laboratories, Bar Harbor, Maine) P1 founders, both on pure FVB backgrounds. Hemizygous MMTV-TGF-β1S223/225 and MMTV-Neu mice were intercrossed to generate progeny of the following genotypes: MMTV-Neu−/MMTV-TGF-β1S223/225− (wild type [WT]), MMTV-Neu+/MMTV-TGF-β1S223/225− (Neu), MMTV-Neu−/MMTV-TGF-β1S223/225+ (TGF-β), and MMTV-Neu+/MMTV-TGF-β1S223/225+ (NT). Mice were genotyped by PCR analysis of genomic DNA as previously described (31). Only age-matched virgin female mice were analyzed. Mice were monitored weekly by palpation to determine the presence of mammary tumors.
Histological analysis.
Mammary glands were harvested and immediately fixed in 10% formalin (VWR Scientific). Hematoxylin-stained whole-mount preparations of right no. 4 mammary glands were prepared as described previously (32). Paraffin-embedded glands were sectioned (5 μm), rehydrated, and stained with Mayer's hematoxylin-and-eosin B-phloxine (Sigma, St. Louis, Mo.). Detection of apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) analysis was performed with the Apoptag detection kit (Serologicals Corp., Norcross, Ga.) according to the manufacturer's instructions. Immunohistochemistry and cytochemistry were performed as previously described (30) with the following antibodies: proliferating cell nuclear antigen (PCNA; Neomarkers, Freemont, Calif.), Akt and S473 P-Akt (Upstate Biotechnology, Lake Placid, N.Y.), vimentin (v9), and p65/RelA (Santa Cruz Biotechnology, Santa Cruz, Calif.), E-cadherin (Transduction Laboratories), and smooth-muscle actin (SMA; Zymed).
In situ analysis of active Rac.
Paraffin-embedded tumor sections were rehydrated, treated with 0.01% trypsin for 5 min, and then blocked for 30 min in 10% fetal bovine serum (FBS). Sections were incubated in Pak-1 binding domain (PBD)-glutathione S-transferase (PBD-GST) fusion protein (Upstate Biotechnology) in phosphate-buffered saline-Tween (PBST) or with GST alone as a control for 15 min at room temperature, followed by a GST antibody (Santa Cruz Biotechnology; diluted 1:1,000) and a Cy3-conjugated antirabbit immunoglobulin G (IgG) (Molecular Probes, Eugene, Oreg.).
Western analysis.
Mammary glands and tumors were harvested and homogenized as previously described (32). Total protein (20 μg) was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Western analyses were performed as described previously (32) with the following antibodies: HER2/ErbB2 (NeoMarkers); total MAPK and T202/Y204 P-MAPK (Promega, Madison, Wis.); ErbB3 (C-17), E-cadherin (H-108), vimentin (H-84), integrin β1 (M-106), pan-cytokeratin (H-240), α-actinin (H-300), Smad4 (B-8), p65/RelA (C-20), and p38Mapk (Santa Cruz Biotechnology); total Akt and S473 P-Akt (Upstate Biotechnology); Smad2/3, phospho-Smad2, and Rac-1 (Transduction Laboratories, Lexington, Ky.); SMA (Zymed, Inc.); and phospho-p38 (Cell Signaling, Beverly, Mass.).
Northern analysis and RNA in situ hybridization.
Mammary glands were collected and homogenized in Trizol (Invitrogen Life Technologies, Carlsbad, Calif.). Total cellular RNA was harvested according to the manufacturer's instructions. Northern analyses were performed as described previously (31) with (per milliliter) 106 cpm of a [α-32P]dCTP random primer-labeled BamHI fragment of simian TGF-β1 cDNA or rabbit β-globin cDNA comprising sequences from the 5′-untranslated exon of the MMTV-TGF-β1 transgene (42). For RNA in situ hybridization, the rabbit β-globin cDNA sequence was subcloned into pBluescript SK(−) (Stratagene), the plasmids were linearized, and the sense and antisense riboprobes were synthesized from the T3 or T7 transcription sites by using 5 U of T3 or T7 RNA polymerase (Promega). Paraffin sections were probed with sense or antisense digoxigenin-labeled riboprobes. Hybridization of the riboprobe was visualized immunohistochemically with an alkaline phosphatase-conjugated antidigoxigenin antibody (Boehringer-Mannheim).
Intravasation assay.
Circulating tumor cells in mice were quantified as described by Wyckoff et al. (51). One milliliter of blood was collected by heart puncture and centrifuged (3,000 rpm [1,643 × g] in a Beckman GS-6R centrifuge at 4°C for 5 min). The serum/buffy coat layers were plated in 1 ml of Dulbecco's modified Eagle's medium (DMEM)-F12 (50:50)-10% FBS. After 24 h, the plates were washed with PBS to remove erythrocytes and nonadherent cells, and fresh DMEM-F12-10% FBS was replenished. After 7 days, colonies were stained with hematoxylin and counted.
Isolation and culture of PMTCs.
Primary tumor cells (PMTCs) were isolated and cultured as follows. Tumors were digested at 37°C for 4 h in 3-mg/ml collagenase A (Sigma) in PBS (pH 7.4). The cell suspension was plated on dishes coated with growth factor-reduced Matrigel (BD Biosciences Pharmingen) in PTC medium—2.5% DMEM-F12 (50:50; Gibco BRL) and 50-ng/ml insulin (Clonetics)—and cultured at 37°C in 5% CO2.
Transwell invasion assays.
Wild-type primary mammary epithelial cells (PMECs), Neu, or NT PMTCs or 4T1 cells (104 each) were labeled with the lipophilic dye Sp-DiOC18 (3) (Molecular Probes) according to the manufacturer's instructions. Labeled cells were seeded in the upper chamber of transwells fitted with Matrigel-coated, 8-μm-pore-size polycarbonate filters (Corning, Inc., Life Sciences, Acton, Mass.). Lower chambers contained 2.5% serum with or without 20 nM Fc:TβRII (provided by Philip Gotwals, Biogen, Cambridge, Mass.), 2-ng/ml human recombinant TGF-β1 (R&D Systems, Minneapolis, Minn.), 10 μM LY294002, 10 μM SB202190 (both from Calbiochem, San Diego, Calif.), and 10 μM U-0126 (Promega). After 24 h, cells were scraped from upper filter surfaces, and the cells on the lower surfaces were photographed under fluorescence microscopy. Fluorescence was quantified with Scion Image software.
TGF-β reporter assays.
NMuMG cells (0.5 × 106 per well) were transfected with pCAGA-Lux (2 μg) as described previously (13). After 24 h, cells were treated for 24 h with 2-ng/ml TGF-β1 or with serum-free medium conditioned by Neu PMTCs, NT PMTCs, or WT PMECs, each in the presence or absence of 20 nM Fc:TβRII. Firefly and Renilla reniformis luciferase activities were determined by using the Promega dual-luciferase assay system, and the data were normalized utilizing the ratio of firefly to R. reniformis luciferase as previously described (13).
RESULTS
TGF-β decreases both proliferation and apoptosis in NT mammary glands.
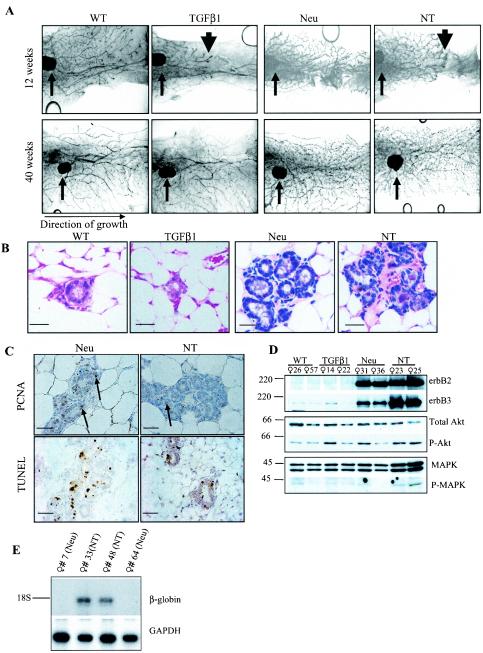
Expression of the Neu proto-oncogene in the mammary epithelium of MMTV-Neu transgenic mice (hereafter referred to as Neu mice) results in ductal hyperplasia, precocious lobuloalveolar development, and tumor formation. Approximately 60% of tumor-bearing MMTV-Neu mice develop lung metastases (18). To determine the effect of combined expression of Neu and TGF-β1 transgenes on mammary development, glands from virgin Neu/TGF-β1 mice (NT mice) were examined at 12 weeks of age. Whole mounts of Neu mammary glands demonstrated the previously described precocious alveolar budding of primary ducts (18). As reported, glands in MMTV-TGF-β1 mice displayed delayed ductal outgrowth with normal side branching (42) (Fig. 1A). Bigenic NT mammary glands displayed a combined phenotype characterized by both delayed ductal outgrowth and enhanced alveolar budding. By 40 weeks, the epithelium of TGF-β1 and NT mammary glands had permeated the entire fat pad, suggesting that the delayed ductal progression mediated by TGF-β1 is transient. At 40 weeks, the glands of NT mice were histologically similar to those in Neu mice, containing multiple acinar-like structures (Fig. 1B). Proliferation and apoptosis were measured by PCNA immunohistochemistry (IHC) and TUNEL analysis, respectively. The percentage of PCNA-positive nuclei in NT samples (1.9% ± 0.77%) was less than what was seen in Neu samples (4.8% ± 1.9%; n = 3; 10 ×400 fields per sample). This lower rate of cell proliferation in NT mammary glands was accompanied by a lower level of apoptosis in situ (5.2% ± 1.3% in Neu versus 0.7% ± 0.1% in NT; n = 3) (Fig. 1C). Western analysis confirmed MMTV-Neu transgene expression in Neu and NT mammary glands. Neu mammary glands exhibited a higher content of erbB3 than wild-type and TGF-β1 glands. Interestingly, erbB3 content was higher in lysates from NT than Neu mammary glands. Levels of total Akt and MAPK were approximately similar, and there were no obvious differences in the content of active Akt and MAPK among the four genotypes (Fig. 1D). Northern analysis using a rabbit β-globin cDNA (42) confirmed TGF-β1 transgene expression in mammary glands of NT mice but not in those of Neu mice (Fig. 1E).
FIG.1.
TGF-β decreases both proliferation and apoptosis in NT mammary glands. (A) Whole-mount hematoxylin-stained inguinal mammary glands from virgin female mice at 12 and 40 weeks of age (n = 3 per time point). Small arrows indicate centrally located lymph node in the no. 4 gland. Large arrowheads demonstrate the delayed progression of ductal epithelium through the mammary fat pads of TGF-β1 and NT mice. (B) Hematoxylin-and-eosin-stained sections of inguinal mammary glands harvested at 40 weeks of age. Scale bars = 50 μm. (C) (Upper panels) PCNA IHC in no. 4 glands harvested from virgin females at 40 weeks of age. PCNA-positive cells are indicated with arrows (n = 5). (Lower panels) TUNEL analysis of no. 4 glands harvested from virgin females at 40 weeks of age. Scale bars = 50 μm. (D) Western analysis of mammary gland lysates at 40 weeks of age. Molecular masses are shown to the left in kilodaltons. The antibodies used are shown to the right. (E) Northern analysis of total RNA extracted from mammary glands at 40 weeks of age. The cDNA probes used are shown to the right. GAPDH, mouse GAPDH.
TGF-β1 is functional in bigenic tumors but does not affect Neu-induced tumor latency.
Neu and NT mice were palpated weekly to detect the presence of mammary tumors. Tumor latency was similar in mice of both genotypes (224 versus 225 days; Fig. 2A). One hundred percent of Neu mice and NT mice developed tumors by 294 days, with approximately equal numbers of tumors per mouse (3.3 ± 1.3 versus 2.4 ± 1.3, respectively; P = 0.4 [Students t test]). Mice were sacrificed 100 days after initial tumor palpation, at which time total primary tumor volume per mouse was determined (Fig. 2B). Although Neu and NT tumors became apparent after similar latencies, NT mice carried significantly less cumulative tumor volume than Neu mice (2,454 ± 1,304 mm3 versus 5,431 ± 2,087 mm3, respectively; P = <0.0001). By histological analysis, 7 of 10 NT tumors were poorly differentiated with a high histological grade, prominent nuclear pleomorphism, evidence of local invasion, and, in some cases, a peritumoral stromal reaction. On the other hand, 7 of 10 Neu tumors were of low histological grade with features of papillary or glandular differentiation but more mitotic figures than the NT tumors (Fig. 2C to H).
FIG.2.
Active TGF-β1 overexpression does not affect Neu-induced tumor latency but increases malignancy. (A) The number of tumor-free days is shown for Neu and NT mice (n = 15; P = 0.47). (B) Total tumor volumes for each Neu and NT mouse (100 days following initial tumor palpation) are shown, along with the average total tumor volume per mouse (n = 15; mean ± standard deviation). Tumor volume was calculated with the formula volume = [(length)/2 × (width)2]. (C to H) Hematoxylin-and-eosin-stained sections of tumors harvested from Neu (C to E) or NT (F to H) mice 100 days following initial tumor palpation. Representative samples from three independent mice per genotype are shown. Panels F, G, and H show poorly differentiated carcinoma. Panels D and E show well-differentiated carcinoma. Panels F and G show highly vascularized invasive NT tumors. Arrows in panel F indicate areas in which the tumor has invaded the basement membrane into surrounding stroma. Scale bars, 100 μm. (I) Western analysis of proteins extracted from tumors 100 days after initial tumor palpation. The mouse identification number and genotype are indicated at the top. Molecular masses are shown to the left in kilodaltons. The antibodies used are shown at right. SMA, smooth muscle actin. (J) E-cadherin and vimentin IHC of representative tumors with immunoblot data in panel I. E-cadherin remains localized at cell junctions and tumor cells are vimentin negative. The vimentin staining in tumor 33 is limited to stromal elements.
Western analysis using erbB2 and erbB3 antibodies confirmed that Neu and erbB3 contents were not altered in the NT bigenic tumors. Smad4 and Smad2/3 proteins were detected at equal levels in tumors derived from NT or Neu mice, but Smad2 phosphorylation was higher in NT tumors (Fig. 2I). NT tumor extracts contained higher levels of active Akt, active MAPK, and active p38Mapk than Neu tumors as measured by Western analysis utilizing phosphospecific antibodies. Levels of total Akt, MAPK, and p38 were the same in tumors of both genotypes. E-cadherin contents remained approximately equal in Neu and NT tumor lysates, while expression of vimentin, a marker of fibroblasts or of epithelial cells that have undergone EMT, was enhanced in NT tumors. By IHC, E-cadherin localized to cell-cell junctions in tumors of both genotypes. Tumor cells did not stain with vimentin (Fig. 2J) or SMA antibodies (not shown). However, NT tumors displayed increased vimentin staining, which was specific to tumor stroma (Fig. 2J), providing evidence against transmesenchymal differentiation in NT tumor cells.
TGF-β1 expression increases angiogenesis, intravasation, and metastasis of Neu-induced tumors.
Expression of CD31, a marker of endothelial cells, was examined by IHC of tumor sections (Fig. 3A). The increased abundance of CD31-positive structures in NT tumors compared to that in Neu tumors suggested increased vascularization. PCNA IHC suggested that a threefold-lower percentage of cancer cells were proliferating in NT tumors than in Neu tumors (Fig. 3B), which may account for the smaller tumor volume observed in NT mice. However, TUNEL analysis suggested that NT tumors underwent a fivefold-lower rate of cell death (Fig. 3C).
FIG.3.
TGF-β1 increases vascularization and cell survival in tumors but reduces cell proliferation. (A) CD31 IHC in tumor vasculature. Corresponding 4′,6′-diamidino-2-phenylindole (DAPI)-counterstained nuclei are shown at left (n = 5). (B) (Right panels) PCNA IHC in tumor cells. Scale bars, 50 μm. (Left panel) The index of PCNA-positive nuclei was calculated with the formula [(number of PCNA+ nuclei)/(total nuclei)]. n = 30 fields (six random fields of five tumors per genotype). The data point for each field is shown, along with the mean ± standard deviation. (C) TUNEL analysis of tumors. Scale bars, 100 μm. (Left panel) Index of TUNEL-positive nuclei was calculated by using the formula [(no. of TUNEL+ nuclei)/(total nuclei)]. n = 9 fields (3 random fields of 3 tumors per genotype). The data point for each field is shown, along with the mean ± standard deviation.
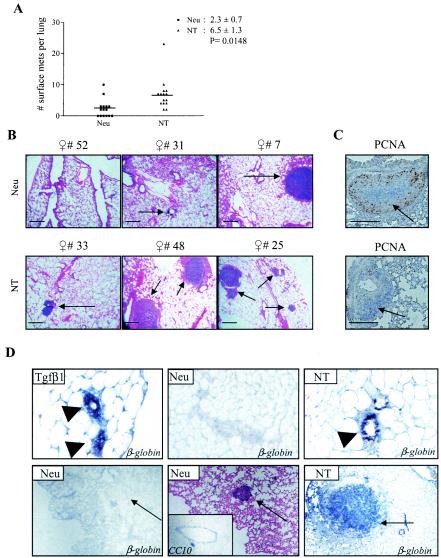
The effect of TGF-β1 overexpression on the metastatic potential of Neu-induced tumor was determined by quantifying the number of lung surface metastases 100 days after initial primary tumor palpation. Whereas 60% of all Neu mice developed lung surface metastases, 100% of NT mice did so (Fig. 4A). The average number of lung surface metastases in NT mice was 2.8 times the number found in Neu mice. No microscopic lung metastases were identified in Neu mice in which surface metastases were absent. Microscopic lung metastases were present in all NT mice (Fig. 4B). PCNA IHC suggested that, like the primary tumors, NT lung metastases were less proliferative than their Neu counterparts (Fig. 4C). Tumor cell intravasation was measured by collecting blood via atrial puncture and culturing the serum and buffy coat layers (30). After 1 week, blood from wild-type mice produced no colonies (data not shown). However, blood from Neu and NT mice grew an average of 0.6 ± 0.3 and 2.8 ± 0.8 colonies per mouse, respectively (n = 10 per group; P = 0.013). RNA in situ hybridization demonstrated that NT but not Neu cells in lung metastases expressed the TGF-β1S223/225 transgene (Fig. 4D), suggesting that they had not occurred as a consequence of silencing the TGF-β transgene.
FIG. 4.
TGF-β1 increases mammary tumor lung metastases in MMTV-Neu mice. (A) The number of lung surface metastases per mouse (n = 15) is shown, along with the mean ± standard deviation. Significance was calculated by using the unpaired Student's t test. (B) Low-power magnification of hematoxylin-and-eosin-stained sections of lungs harvested from mice 100 days after initial tumor palpation. Representative pictures of three individual mice per genotype are shown. Lung metastases are indicated with arrows. Scale bars, 200 μm. (C) Representative PCNA IHC in lung metastases. (D) In situ hybridization of a digoxigenin-labeled rabbit β-globin cDNA fragment to RNA in paraffin-embedded mammary gland or lung sections from TGF-β1 and NT mice. Arrowheads indicate mammary epithelium. Arrows indicated mammary tumor-derived lung metastases.
TGF-β1 increases invasion of Neu-expressing tumor cells.
PMTCs were purified from Neu and NT tumors that were harvested from mice 100 days after initial tumor palpation. Cells were embedded in growth factor-reduced Matrigel, a combination of extracellular matrix (ECM) proteins that form a three-dimensional matrix. Growth was monitored over a period of 10 days. Cultures derived from Neu tumors grew into large acinar cystic structures (Fig. 5A). These structures deposited the laminin-5, suggesting epithelial polarization (data not shown). In the presence of exogenous TGF-β1, growth of the acini was inhibited, although they developed projections into the surrounding matrix. In contrast, NT tumor-derived cells grew as solid structures with invasive structures into the Matrigel in the absence of added TGF-β (Fig. 5A) and maintained laminin-5 expression (data not shown). When plated with the TGF-β inhibitor Fc:TβRII (described in reference 30), NT-derived PMTCs grew as solid round structures lacking any projections, suggesting that the increased secretion of TGF-β accounted for the enhanced invasiveness.
FIG.5.
TGF-β1 induces motility and invasiveness in Neu tumor cells. (A) PMTCs from Neu or NT mice were cultured in serum-reduced Matrigel supplemented with or without TGF-β1 (2 ng/ml) or Fc:TβRII (20 nM). Representative microphotographs are shown. Scale bars, 200 μm. (B and C) Western analysis of Neu and NT PMTCs incubated in 2.5% serum in the presence of 2-ng/ml TGF-β1, 20 nM Fc:TβRII, 10 μM U-0126, 10 μM SB202190, or 10 μM LY294002 where indicated. The antibodies used are indicated to the right, and molecular masses are indicated to the left. (D) (Left panel) Transwell assays using WT PMECs or 4T1 cells. (Middle and right panels) Transwell assays with WT PMECs, Neu PMTCs, or NT PMTCs. A total of 104 fluorescently labeled cells were plated in the upper chamber of transwells fitted with Matrigel-coated polycarbonate filters (8-μm pores). Medium containing 2.5% FBS with or without 2-ng/ml TGF-β1, 20 nM Fc:TβRII, 10 μM LY294002, or 10 μM SB202190 was placed in the lower chamber. After 24 h, the number of WT PMECs migrating to the lower side of the filter was given the value of 1, such that migration of 4T1 cells, Neu PMTCs, or NT PMTCs is represented as a multiple of WT PMECs. Values shown are the average (± standard deviation) of triplicate transwells in three independent experiments. *, P < 0.01; **, P < 0.001; @, P < 0.001. (E) Rac1 Western analysis of mammary tumor lysates. (F) In situ analysis of Rac1 activity using a PBD-GST fusion protein or with GST, followed by GST IHC as described in Materials and Methods. DAPI-counterstained nuclei are shown below each corresponding panel. (G) NMuMG cells transfected with pCAGA-Lux were treated for 24 h with 2-ng/ml TGF-β1 or with medium conditioned by Neu PMTCs, NT PMTCs, or WT PMECs, each in the presence or absence of 20 nM Fc:TβRII, and luciferase activity was measured as described in Materials and Methods. The ratio of firefly to R. reniformis luciferase activities in untreated NMuMG cells was given a value of 1. Bars represent the mean (± standard deviation) luciferase activities of three experiments each using duplicate wells for each condition compared to untreated NMuMG cells. *, P < 0.001 (Student's t test).
PMTCs from Neu and NT tumors expressed both E-cadherin and keratin 14 (Fig. 5B) and exhibited epithelial morphology. In addition, they all contained equal levels of fibronectin and (low) vimentin by immunoblot analyses (data not shown), suggesting absence of transmesenchymal differentiation in culture. TGF-β1 was added to cell cultures after 24 h of serum starvation to determine Smad2 activation. In Neu cells, Smad2 phosphorylation was increased by TGF-β, and this increase was blocked by Fc:TβRII. In NT cells, however, Smad2 phosphorylation was constitutive, suggesting that it was being autoactivated by transgenic TGF-β. Basal levels of phosphorylation of MAPK and Akt were also higher in NT cells than in Neu cells, and both were reduced by Fc:TβRII (Fig. 5B). Addition of the MEK1 inhibitor U0126 impaired basal and TGF-β-induced phosphorylation of MAPK in Neu and NT cells while having no effect on the phosphorylation of p38 (Fig. 5C, left panel). Addition of p38 or PI3K inhibitors (SB202190 and LY294002, respectively) impaired basal and TGF-β-induced phosphorylation of p38 or Akt, respectively (Fig. 5C, right panel).
In transwell invasion assays, wild-type primary mammary epithelial cells (PMECs) did not migrate in response to 2.5% serum, and this rate of migration was not increased by added TGF-β1 (Fig. 5D). In contrast, transwell migration of 4T1 mammary tumor cells through ECM to the opposite sides of filters was increased by TGF-β1 and reduced by Fc:TβRII. In response to 2.5% serum, NT cells migrated with a significantly higher frequency than Neu cells (Fig. 5B). TGF-β1 stimulated transwell migration of Neu cells to levels comparable to those of NT cells in the absence of added ligand. Addition of Fc:TβRII interfered with TGF-β1-induced migration in Neu cells, but NT cell migration was modestly affected. The inability of Fc:TβRII to affect NT migration may be attributed to the addition of the TGF-β inhibitor to the lower chamber of the transwell system, whereas the NT cells are being exposed to endogenously synthesized TGF-β1S222/225 in the upper chamber. The p38 inhibitor SB202190 impaired TGF-β-induced motility of Neu and NT cells, suggesting p38 is required for this effect. LY294002 (Fig. 5D, right panel) and U0126 (data not shown) had no effect on cell motility.
Because of the increased motility of NT-derived PMTCs and the reported ability of TGF-β to induce Rac activity (3), we evaluated Rac1 expression and activity. Levels of expression of Rac1 were similar in Neu and NT tumor samples (Fig. 5E). Paraffin-embedded tumor sections were incubated with a PBD-GST fusion protein containing the PBD for active GTP-bound Rac1 and GST. Tissue-bound PBD-GST was detected by GST IHC. NT tumor sections bound a greater amount of PBD-GST than Neu tumor sections or NT tumor sections incubated with control GST (Fig. 5F), suggesting that in situ Rac activity was higher in TGF-β1-overexpressing tumors.
Finally, we verified that active TGF-β1 was being produced in NT cells by adding serum-free medium conditioned by Neu or NT cells to NMuMG cells transfected with a Smad-dependent p(CAGA)12-luciferase reporter (10) (Fig. 5G). Media conditioned by wild-type PMECs or exogenous TGF-β1 were used as negative and positive controls, respectively. Medium from NT cells elicited significantly greater luciferase activity than medium from Neu cells, suggesting higher levels of active TGF-β1 in medium conditioned by cells from the bigenic tumors.
TGF-β1 increases survival of Neu-expressing tumor cells.
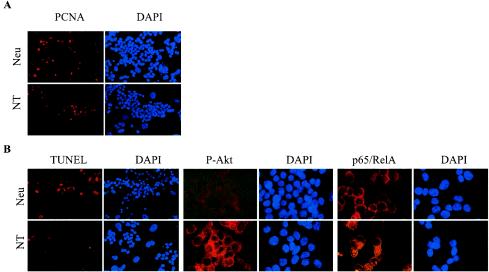
PCNA IHC of Neu and NT cells cultured in 10% serum demonstrated that NT cells proliferated more slowly than their Neu counterparts, similar to what is seen in vivo (18.6% ± 1.6% versus 4.3% ± 1.6%; n = six ×400 fields) (Fig. 6A). NT cells also displayed a 9.8-fold-lower rate of apoptosis than Neu cells when cultured in 0.5% serum for 3 days (Fig. 6B). Under the same conditions, P-Akt was detected in NT but not in Neu cells. Akt has multiple cytoplasmic targets, including IKKα (38, 46). Activated IKKα then phosphorylates a protein known as “inhibitor of κB” (IκB), leading to the nuclear localization of the transcription factor p65/RelA, a component of the NF-κB family (15). p65/RelA was detected by IHC in both Neu and NT cells (Fig. 6B). However, nuclear p65/RelA was found only in NT cells (37.8% ± 3.9%; n = six ×400 fields), whereas p65/RelA was restricted to the cytoplasm in Neu cells. This suggests that TGF-β1 overexpression may enhance cell survival in Neu-induced tumors by activating Akt/NF-κB signaling.
FIG. 6.
NT cells exhibit higher active Akt and reduced apoptosis. (A) Immunocytofluorescent detection of PCNA in Neu and NT PMTCs grown in 10% serum. 4′,6′-Diamidino-2-phenylindole (DAPI)-counterstained nuclei are shown to the right of each panel. PCNA+ cells: Neu, 18.6% ± 1.6% versus NT, 4.3% ± 1.6%; n = six ×400 fields. (B) Immunocytofluorescent detection of TUNEL-positive nuclei and P-Akt and p65/RelA expression in Neu and NT PMTCs grown in 0.5% serum for 3 days. DAPI-counterstained nuclei are shown to the right of each panel. Nuclear p65/RelA was found only in NT cells (37.8% ± 3.9%; n = six ×400 fields), whereas p65/RelA was restricted to the cytoplasm in Neu cells.
DISCUSSION
In a transgenic mouse model of breast cancer, we have examined whether Neu is dominant over TGF-β and whether Neu and TGF-β synergized to accelerate mammary tumor progression. We show herein that overexpression of active TGF-β1 in Neu-induced mammary tumors resulted in cancers that were both less proliferative and less apoptotic as well as more invasive and metastatic than tumors expressing the Neu transgene alone. This is the first report of an oncogene that in vivo is not only dominant over TGF-β but also synergizes with this “tumor suppressor” in the acceleration of tumor progression. In both tumor lysates and primary cultures of NT cells, Smad2 was constitutively phosphorylated, implying that autocrine TGF-β was operative in the bigenic tumors and cells. This progression of tumors to a more metastatic phenotype occurred even though TGF-β1-overexpressing cancer cells proliferated more slowly than their Neu counterparts, and the cumulative macroscopic tumor burden was lower in NT than in Neu mice. These data imply that, even in advanced cancers, TGF-β1 may be able to exert tumor-suppressing (antiproliferative) and tumor-promoting (invasion, motility, and survival) effects simultaneously. This is perhaps best shown in Fig. 5A in this report: large acini formed by Neu cells treated with TGF-β are much smaller than those in controls but exhibit long invasive projections into the surrounding matrix.
In addition to Smads, other signaling pathways have been implicated on TGF-β actions. These include MAPK, c-Jun NH2-terminal kinase (JNK), p38, PI3K, Akt, and Rho GTPases (reviewed in references 9 and 11). However, TGF-β does not induce these pathways in all cells and the cellular or biochemical determinants for such responses to occur are not known. Interestingly, in NT tumors or cells, levels of active MAPK, p38, Akt, and Rac1 were higher than in Neu tumors or cells. Treatment of the NT primary cultures with Fc:TβRII inhibited MAPK and Akt activity while impairing cellular invasion into the surrounding matrix, suggesting that all of these effects were autoinduced by the TGF-β1 transgene product. These data suggest that overexpression of Neu cooperates with TGF-β or sensitizes cells to the ability of TGF-β to activate signaling programs in addition to Smads that lead to enhanced tumor cell invasiveness and survival. Although the enhanced motility and branching of NT cells suggest that TGF-β and Neu were inducing EMT, the absence of mislocalization of E-cadherin and overexpression of vimentin, SMA, and fibronectin in tumor cells (Fig. 2J and data not shown) suggested otherwise. Thus, we surmise that the increase in vimentin and SMA content observed in NT tumor lysates (Fig. 2I) probably reflects an increase in tumor-associated fibroblasts in NT versus Neu tumors. Consistent with the effect of TGF-β on the production of ECM by surrounding fibroblasts, periductal (Fig. 1B) and peritumoral stromal reactions were commonly observed in NT glands and tumors, respectively.
There is substantial evidence that TGF-β can promote tumor cell survival. Blockade of TGF-β signaling with Fc:TβRII increases apoptosis in mammary tumors in MMTV/polyomavirus middle T antigen transgenic mice (30). Conversely, TGF-β can protect epithelial cells from stress-induced apoptosis (47). Melanoma cells engineered to overexpress TGF-β1 exhibit increased survival following injection into immunocompromised mice (4). In human breast cancer pathogenesis, the role and molecular regulation of apoptosis remains unclear. In ductal carcinoma in situ, a high apoptotic index is often associated with high mitotic rates, similar to what we observed in MMTV-Neu mice (14, 28) (Fig. 2). However, simultaneous overexpression of factors promoting proliferation and inhibiting apoptosis results in luminal filling of mammary acini ex vivo, while expression of either one by itself does not result in luminal filling (8). It is possible, therefore, that factors promoting tumor cell survival (such as TGF-β) can cooperate with proliferative signals, such as Neu, that are primarily involved in acinar expansion (33) to produce a more aggressive tumor phenotype. We have shown that TGF-β1-overexpressing tumor cells displayed enhanced survival and higher levels of active Akt (Fig. 2). In fact, the increased rate of cell survival by TGF-β overexpression was seen in proliferating mammary epithelial cells even prior to cancer formation (Fig. 1C), suggesting that TGF-β can enhance survival in cells before they acquire a tumorigenic phenotype. Consistent with this observation, tumor formation was not delayed in NT mice compared to Neu mice, even though proliferation occurred at a greatly reduced rate, presumably due to decreased cell death in mammary hyperplasias overexpressing TGF-β1.
Metastatic tumor progression is often associated with increased angiogenesis, and there was clear evidence of such in the NT compared to the Neu tumors (Fig. 3A). TGF-βs, particularly TGF-β1, regulates vessel formation by inducing VEGF secretion, facilitating FGF-mediated capillary sprouting, inhibiting endothelial cell migration, inducing smooth muscle cell differentiation, recruiting pericytes, increasing ECM production, and thus stabilizing new vessels (reviewed in reference 40). The cellular mechanisms by which active TGF-β1S223/225 alone or in synergy with Neu leads to this more angiogenic phenotype are beyond the scope of this report but could well be more critical to the observed metastatic phenotype than the tumor cell-autonomous mechanisms discussed here.
In addition to contributing to the stabilization phase of tumor angiogenesis, TGF-β can induce motility and invasion of transformed cells. In line with this notion, NT cells were more motile, and NT tumors were more invasive than those overexpressing Neu alone. The increased invasion and motility may have been due, at least in part, to activation of Rac1, a member of the Rho family of small GTPases. Rho proteins regulate the actin cytoskeleton and complex formation at sites of focal adhesion in many cell types (19). Indeed, activation of the Neu receptor tyrosine kinase reorganizes the actin cytoskeleton and activates direct targets of Rac1 (1), which in turn regulate the morphogenic and migratory properties of cells (43). In response to growth factors, Rac activity induces membrane ruffling (22), which is required for cell migration. TGF-β can activate Rac1 (3), which can then activate p38 (3, 48, 53), a serine-threonine kinase also shown to be involved in TGF-β-mediated EMT and cell motility (5). Furthermore, GTPases such as Rac have been shown to contribute to TGF-β-mediated cellular and transcriptional responses (2, 29). Of note, active p38 and Rac1 were constitutively higher in NT cells and tumors, respectively, and the motility of NT PMTCs was abrogated by treatment with a small molecule inhibitor of p38 (Fig. 5). Therefore, based on these data, we speculate that Neu and TGF-β signaling converge at Rac1-p38, providing an adequate threshold of sustained activation that leads to an enhanced migratory phenotype. This heightened invasion and motility of NT cells correlated with an increase in the number of circulating tumor cells and lung metastases in NT versus Neu mice (Fig. 3).
Although the more invasive NT tumor cells produced more lung metastases overall, the NT cells at metastatic sites proliferated more slowly than metastatic cells from Neu tumors. This observation reinforces the notion that some tumors in advanced metastatic states may not be completely refractile to TGF-β-mediated antiproliferative action. The results presented herein further underscore the dual role of TGF-β as both tumor suppressor and tumor promoter. Although both effects exist simultaneously in this bitransgenic model, the autocrine effects of TGF-β on tumor cell survival, invasion, and metastases as a function of Neu overexpression predominate over its antiproliferative action. Taken together, these data suggest that Neu does not abrogate the antimitogenic effect of TGF-β. However, it synergizes with TGF-β in the induction of signaling programs that are dominant over TGF-β-induced inhibition of proliferation, thus accelerating metastatic tumor dissemination. If operative in human breast cancers, such cooperation would provide a basis for the combined use of HER2 (erbB2) and TGF-β inhibitors in patients bearing metastatic tumors with HER2 overexpression and evidence of TGF-β hyperactivity.
ADDENDUM IN PROOF
Recently, P. M. Siegel et al. reported the cross-breeding of mice expressing activated forms of the Neu receptor tyrosine kinase and activated TGF-β type I receptor in the mammary gland. These mice exhibited increased tumor latency and enhanced frequency of lung metastases compared to mice expressing the activated Neu transgene alone (Proc. Natl. Acad. Sci. USA 100:8430-8435, 2003).
Acknowledgments
This work was supported by R01 CA62212 (C.L.A.), Breast Cancer Specialized Program of Research Excellence (SPORE) grant P50 CA98131, and Vanderbilt-Ingram Cancer Center Support grant CA68485. M.E.S. is the recipient of a Career Development Award from the Vanderbilt Breast Cancer SPORE Grant.
REFERENCES
- 1.Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. [DOI] [PubMed] [Google Scholar]
- 2.Atfi, A., S. Djelloul, E. Chastre, R. Davis, and C. Gespach. 1997. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J. Biol. Chem. 272:1429-1432. [DOI] [PubMed] [Google Scholar]
- 3.Bakin, A. V., C. Rinehart, A. K. Tomlinson, and C. L. Arteaga. 2002. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 115:3193-3206. [DOI] [PubMed] [Google Scholar]
- 4.Berking, C., R. Takemoto, H. Schaider, L. Showe, K. Satyamoorthy, P. Robbins, and M. Herlyn. 2001. Transforming growth factor-beta1 increases survival of human melanoma through stroma remodeling. Cancer Res. 61:8306-8316. [PubMed] [Google Scholar]
- 5.Bhowmick, N. A., R. Zent, M. Ghiassi, M. McDonnell, and H. L. Moses. 2001. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 276:46707-46713. [DOI] [PubMed] [Google Scholar]
- 6.Bottinger, E. P., J. L. Jakubczak, D. C. Haines, K. Bagnall, and L. M. Wakefield. 1997. Transgenic mice overexpressing a dominant-negative mutant type II transforming growth factor beta receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7, 12-dimethylbenz-[a]-anthracene. Cancer Res. 57:5564-5570. [PubMed] [Google Scholar]
- 7.Coffey, R. J., Jr., N. J. Sipes, C. C. Bascom, R. Graves-Deal, C. Y. Pennington, B. E. Weissman, and H. L. Moses. 1988. Growth modulation of mouse keratinocytes by transforming growth factors. Cancer Res. 48:1596-1602. [PubMed] [Google Scholar]
- 8.Debnath, J., K. R. Mills, N. L. Collins, M. J. Reginato, S. K. Muthuswamy, and J. S. Brugge. 2002. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111:29-40. [DOI] [PubMed] [Google Scholar]
- 9.De Caestecker, M., E. Piek, and A. B. Roberts. 2000. Role of transforming growth factor-beta signaling in cancer. J. Natl. Cancer Inst. 92:1388-1402. [DOI] [PubMed] [Google Scholar]
- 10.Dennler, S., S. Itoh, D. Vivien, P. ten Dijke, S. Huet, and J. M. Gauthier. 1998. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 17:3091-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derynck, R., R. J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29:117-129. [DOI] [PubMed] [Google Scholar]
- 12.Dumont, N., and C. L. Arteaga. 2000. Transforming growth factor-beta and breast cancer: tumor promoting effects of transforming growth factor-beta. Breast Cancer Res. 2:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont, N., A. V. Bakin, and C. L. Arteaga. 2003. Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells. J. Biol. Chem. 278:3275-3285. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi, A., P. A. Holland, W. F. Knox, C. S. Potten, and N. J. Bundred. 1998. Evidence of significant apoptosis in poorly differentiated ductal carcinoma in situ of the breast. Br. J. Cancer 78:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 16.Gobbi, H., W. D. Dupont, J. F. Simpson, W. D. Plummer, Jr., P. A. Schuyler, S. J. Olson, C. L. Arteaga, and D. L. Page. 1999. Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J. Natl. Cancer Inst. 91:2096-2101. [DOI] [PubMed] [Google Scholar]
- 17.Gorska, A. E., H. Joseph, R. Derynck, H. L. Moses, and R. Serra. 1998. Dominant-negative interference of the transforming growth factor beta type II receptor in mammary gland epithelium results in alveolar hyperplasia and differentiation in virgin mice. Cell Growth Differ. 9:229-238. [PubMed] [Google Scholar]
- 18.Guy, C. T., M. A. Webster, M. Schaller, T. J. Parsons, R. D. Cardiff, and W. J. Muller. 1992. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 89:10578-10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 20.Iglesias, M., P. Frontelo, C. Gamallo, and M. Quintanilla. 2000. Blockade of Smad4 in transformed keratinocytes containing a Ras oncogene leads to hyperactivation of the Ras-dependent Erk signalling pathway associated with progression to undifferentiated carcinomas. Oncogene 19:4134-4145. [DOI] [PubMed] [Google Scholar]
- 21.Janda, E., K. Lehmann, I. Killisch, M. Jechlinger, M. Herzig, J. Downward, H. Beug, and S. Grunert. 2002. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjoller, L., and A. Hall. 1999. Signaling to Rho GTPases. Exp. Cell Res. 253:166-179. [DOI] [PubMed] [Google Scholar]
- 23.Kordon, E. C., R. A. McKnight, C. Jhappan, L. Hennighausen, G. Merlino, and G. H. Smith. 1995. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev. Biol. 168:47-61. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann, K., E. Janda, C. E. Pierreux, M. Rytomaa, A. Schulze, M. McMahon, C. S. Hill, H. Beug, and J. Downward. 2000. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14:2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massague, J. 1998. TGF-beta signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 26.Massague, J. 1990. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 6:597-641. [DOI] [PubMed] [Google Scholar]
- 27.Massague, J., and Y. G. Chen. 2000. Controlling TGF-beta signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 28.Moreno, A., A. Figueras, B. Lloveras, A. Escobedo, E. Griera, A. Sierra, and A. Fabra. 2001. Apoptosis in ductal carcinoma in situ of the breast. Breast J. 7:245-248. [DOI] [PubMed] [Google Scholar]
- 29.Mucsi, I., K. L. Skorecki, and H. J. Goldberg. 1996. Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-beta1 on gene expression. J. Biol. Chem. 271:16567-16572. [DOI] [PubMed] [Google Scholar]
- 30.Muraoka, R. S., N. Dumont, C. A. Ritter, T. C. Dugger, D. M. Brantley, J. Chen, E. Easterly, L. R. Roebuck, S. Ryan, P. J. Gotwals, V. Koteliansky, and C. L. Arteaga. 2002. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J. Clin. Investig. 109:1551-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muraoka, R. S., A. E. Lenferink, B. Law, E. Hamilton, D. M. Brantley, L. R. Roebuck, and C. L. Arteaga. 2002. ErbB2/Neu-induced, cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 22:2204-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muraoka, R. S., A. E. Lenferink, J. Simpson, D. M. Brantley, L. R. Roebuck, F. M. Yakes, and C. L. Arteaga. 2001. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J. Cell Biol. 153:917-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muthuswamy, S. K., D. Li, S. Lelievre, M. J. Bissell, and J. S. Brugge. 2001. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oft, M., R. J. Akhurst, and A. Balmain. 2002. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat. Cell Biol. 4:487-494. [DOI] [PubMed] [Google Scholar]
- 35.Oft, M., K. H. Heider, and H. Beug. 1998. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8:1243-1252. [DOI] [PubMed] [Google Scholar]
- 36.Oft, M., J. Peli, C. Rudaz, H. Schwarz, H. Beug, and E. Reichmann. 1996. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10:2462-2477. [DOI] [PubMed] [Google Scholar]
- 37.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 39.Park, B. J., J. I. Park, D. S. Byun, J. H. Park, and S. G. Chi. 2000. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 60:3031-3038. [PubMed] [Google Scholar]
- 40.Pepper, M. S. 1997. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 8:21-43. [DOI] [PubMed] [Google Scholar]
- 41.Pierce, D. F., Jr., A. E. Gorska, A. Chytil, K. S. Meise, D. L. Page, R. J. Coffey, Jr., and H. L. Moses. 1995. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc. Natl. Acad. Sci. USA 92:4254-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce, D. F., Jr., M. D. Johnson, Y. Matsui, S. D. Robinson, L. I. Gold, A. F. Purchio, C. W. Daniel, B. L. Hogan, and H. L. Moses. 1993. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 7:2308-2317. [DOI] [PubMed] [Google Scholar]
- 43.Pollard, T. D. 2001. Genomics, the cytoskeleton and motility. Nature 409:842-843. [DOI] [PubMed] [Google Scholar]
- 44.Portella, G., S. A. Cumming, J. Liddell, W. Cui, H. Ireland, R. J. Akhurst, and A. Balmain. 1998. Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 9:393-404. [PubMed] [Google Scholar]
- 45.Robinson, S. D., G. B. Silberstein, A. B. Roberts, K. C. Flanders, and C. W. Daniel. 1991. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development 113:867-878. [DOI] [PubMed] [Google Scholar]
- 46.Romashkova, J. A., and S. S. Makarov. 1999. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 47.Shin, I., A. V. Bakin, U. Rodeck, A. Brunet, and C. L. Arteaga. 2001. Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol. Biol. Cell 12:3328-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uddin, S., F. Lekmine, N. Sharma, B. Majchrzak, I. Mayer, P. R. Young, G. M. Bokoch, E. N. Fish, and L. C. Platanias. 2000. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon alpha-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J. Biol. Chem. 275:27634-27640. [DOI] [PubMed] [Google Scholar]
- 49.Wakefield, L. M., and A. B. Roberts. 2002. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr. Opin. Genet. Dev. 12:22-29. [DOI] [PubMed] [Google Scholar]
- 50.Wrana, J. L., L. Attisano, R. Wieser, F. Ventura, and J. Massague. 1994. Mechanism of activation of the TGF-beta receptor. Nature 370:341-347. [DOI] [PubMed] [Google Scholar]
- 51.Wyckoff, J. B., J. G. Jones, J. S. Condeelis, and J. E. Segall. 2000. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 60:2504-2511. [PubMed] [Google Scholar]
- 52.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, S., J. Han, M. A. Sells, J. Chernoff, U. G. Knaus, R. J. Ulevitch, and G. M. Bokoch. 1995. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270:23934-23936. [DOI] [PubMed] [Google Scholar]