Abstract
Migratory birds travel vast distances each year, finding their way by various means, including a remarkable ability to perceive the Earth's magnetic field. Although it has been known for 40 years that birds possess a magnetic compass, avian magnetoreception is poorly understood at all levels from the primary biophysical detection events, signal transduction pathways and neurophysiology, to the processing of information in the brain. It has been proposed that the primary detector is a specialized ocular photoreceptor that plays host to magnetically sensitive photochemical reactions having radical pairs as fleeting intermediates. Here, we present a physical chemist's perspective on the “radical pair mechanism” of compass magnetoreception in birds. We outline the essential chemical requirements for detecting the direction of an Earth-strength ≈50 μT magnetic field and comment on the likelihood that these might be satisfied in a biologically plausible receptor. Our survey concludes with a discussion of cryptochrome, the photoactive protein that has been put forward as the magnetoreceptor molecule.
Could a chemical reaction be used to detect the direction of the Earth's magnetic field? At first sight, most physical chemists would be skeptical. The energy of interaction of a molecule with a ≈50-μT magnetic field is >6 orders of magnitude smaller than the average thermal energy kBT, which in turn is 10–100 times smaller than the strength of a chemical bond. It therefore seems inconceivable that the position of a chemical equilibrium or the rate of an activated chemical reaction could be significantly altered by such a minuscule perturbation. Nevertheless, there is growing evidence that the striking ability of birds to detect the direction of the geomagnetic field (1) is based on a chemical reaction whose product yields depend on the orientation of the reactants within the field (2) (for reviews, see also refs. 3–8).
It has been known since the 1970s that certain chemical reactions do in fact respond to applied magnetic fields. The key species are radical pairs—pairs of transient radicals created simultaneously, such that the 2 electron spins, one on each radical, are correlated. They have the unique properties that their chemical fate is largely controlled by weak (≪kBT) magnetic interactions via their spin correlation and that the electron spins remain far enough from thermal equilibrium for long enough that the kBT objection is irrelevant. Experimental and theoretical studies of the “radical pair mechanism” over the last 30 years have given an extraordinary variety of information on the magnetic properties, kinetics, and dynamics of radicals and their reactions (reviewed in refs. 9–13). This field of endeavor has come to be called “spin chemistry.”
The radical pair mechanism [and a few rare variants (10)] is currently the only plausible way in which weak magnetic fields can affect chemical reactivity. Most experimental studies have been of photochemically formed organic radicals in solution. The effects are usually not dramatic—typically <50% change in reaction yields or radical lifetimes and, with few exceptions (14), are observed for magnetic field intensities (1 mT–10 T) considerably stronger than the Earth's (25–65 μT). Compared with these chemical studies, there are relatively few reliable examples of magnetic field effects on biological processes, the most notable by far being photosynthetic reaction center proteins where light absorption leads to radical pair formation by sequential electron transfer steps (reviewed in refs. 15–17).
Radical pair reactions were first proposed as a magnetoreceptor by Schulten (18, 19), prompted by the findings that avian magnetic orientation is light-dependent—suggesting a photochemical process—and is responsive to the inclination rather than the polarity of the geomagnetic field (20–23); unlike a magnetic compass needle, birds maintain their orientation when the ambient field vector is reversed (5).
Our aim here is to survey the radical pair mechanism in the context of the avian magnetic compass. We identify the key features required for efficient magnetoreception, comment on the likelihood that they could occur in a biologically plausible receptor, and evaluate the evidence for and against the radical pair hypothesis. Throughout, the focus is firmly on the primary reception mechanism: We make no significant attempt to review the behavioral studies that led to and have since supported the radical pair model, or to comment on (neuro) physiological aspects of magnetoreception, all of which have been covered authoritatively and extensively in recent reviews (3–5, 8, 24, 25). Nor do we presume here to debate the relative merits of the radical pair hypothesis versus proposals based on biogenic magnetite (Fe3O4) (26–32). We close with a discussion of cryptochrome, the photoreceptor protein that is presently the only molecule under consideration as the magnetoreceptor (2), and make suggestions for further research. We hope that the thoughts set out here will be of interest to both physical and biological scientists who may be inspired to contribute to the elucidation of this intriguing and incompletely understood sensory mechanism.
Key Features of a Radical Pair Magnetoreceptor
Chemistry.
Magnetically sensitive reactions almost always involve radicals, i.e., molecules that have an odd number of electrons and consequently an unpaired electron spin that may be found in one of 2 spin states: ↑ or ↓. A radical pair is a short-lived reaction intermediate comprising 2 radicals formed in tandem whose unpaired electron spins may be either antiparallel (↑↓, a singlet state, S) or parallel (↑↑, a triplet state, T). As each electron spin has an associated magnetic moment, the interconversion and chemical fates of the S and T states can be influenced by internal and external magnetic fields.
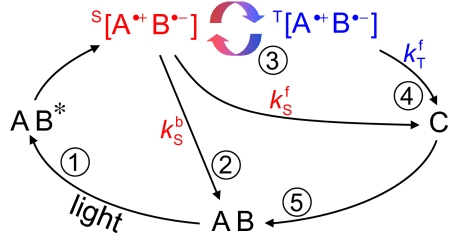
In chemical terms, the minimum requirement for a radical pair reaction to be sensitive to an external magnetic field is that at least one of the S and T states undergoes a reaction that is not open to the other, usually as a consequence of the imperative to conserve spin angular momentum. Fig. 1 shows what is probably the simplest reaction scheme possessing the essential chemical features required to form the basis of a magnetic compass. Imagining for now that the radicals are completely immobile, the individual reaction steps are as follows.
Fig. 1.
A simple reaction scheme that could form the basis of a compass magnetoreceptor. The spin-correlated radical pair is depicted in red for the singlet state and blue for the triplet. See Key Features of a Radical Pair Magnetoreceptor: Chemistry for further details.
A and B in Fig. 1 could be portions of the same molecule or distinct molecules held in close proximity by their surroundings (e.g., 2 cofactors or a cofactor and an amino acid residue in a protein). Species C is either the signaling state or leads to the signaling state via subsequent chemical transformations (which are not shown in Fig. 1). An applied magnetic field can alter the yield of C by regulating the competition between its formation (from the S and T states, step 4) and the regeneration of A B (exclusively from the S state, step 2). If the interconversion of S and T is hindered by the external magnetic field, then less C will be produced and correspondingly more radical pairs recombine directly to A B. The opposite follows if the field enhances S ↔ T interconversion. It is important to note that the external magnetic field is far too weak to initiate new radical reactions.
Variants on the reaction scheme in Fig. 1 are possible, and although the details of the chemistry may differ, the principles remain the same. Some of the more likely alternatives include: an excited triplet precursor state; electron transfer in the reverse direction (to form A•−B•+); and formation of the radical pair via sequential electron transfer steps (33). These and other possibilities are described in the supporting information (SI) Appendix together with comments on the nature of the reactions that could lead to C and subsequently convert it back to A B.
A well-studied precedent for this kind of magnetically sensitive radical pair chemistry is provided by the initial charge separation steps of bacterial photosynthetic energy conversion, which proceed via a series of radical ion pairs formed by sequential electron transfers along a chain of immobilized chlorophyll and quinone cofactors in a reaction center protein complex (15–17). Provided subsequent forward electron transfer is blocked, the recombination of the primary radical pair responds to magnetic fields in excess of ≈1 mT. In unblocked reaction centers, spin correlation can be transferred along the electron transport chain from the primary to the secondary radical pair (34–36), whose lifetime is also magnetically sensitive (37–39). Similar effects occur in plant photosystems. Other biological radical pair processes are much less well characterized (see SI Appendix).
Hyperfine Interactions.
We now turn to the essential magnetic properties of a radical pair magnetoreceptor, focusing first on detecting the presence of an external magnetic field and then its direction. An absolute requirement is that the 2 radicals, between them, have at least 1 hyperfine interaction, i.e., an intraradical coupling between the magnetic moment of the unpaired electron and the magnetic moment of an atomic nucleus such as 1H or 14N. Almost all biologically occurring radicals have several potentially suitable hyperfine interactions. Ample data on hyperfine tensors is available from electron paramagnetic resonance (EPR) spectroscopy (40, 41) and ab initio density functional calculations (42).
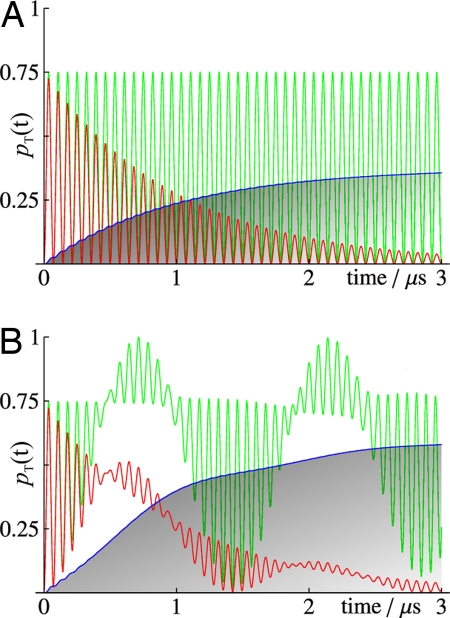
Hyperfine interactions are crucial because they drive the interconversion of the S and T states of the radical pair and allow it to be modified by an external magnetic field. S ↔ T interconversion is a coherent quantum mechanical process: Radical pairs oscillate between their S and T states at a variety of frequencies determined by the strengths of the hyperfine interactions. 1H and 14N hyperfine couplings in organic radicals are typically in the range 10–1,000 μT, corresponding to frequencies of 300 kHz to 30 MHz (the conversion factor is 28 kHz μT−1). The time scale for significant transformation of S into T and vice versa is thus typically 10 ns–1 μs. Sensitivity to an external magnetic field arises because the Zeeman interaction of the magnetic moments of the 2 electrons causes additional periodic S ↔ T interconversion. In a 50-μT field, this oscillation has a frequency of ≈1.4 MHz and a period of ≈700 ns. The calculated time dependence for a prototype radical pair is shown in Fig. 2.
Fig. 2.
Quantum mechanical spin dynamics simulations for the reaction in Fig. 1, performed as described in ref. 60. (A) In the absence of the magnetic field and with no recombination (green), the fraction of radical pairs that exist in the triplet state, pT(t), oscillates at the frequency of the hyperfine coupling (here 14 MHz). (B) When a weak magnetic field is introduced (green), pT(t) shows an additional, slower, modulation at the frequency of the Zeeman interaction (here 1.4 MHz). The radical pair reactions cause pT(t) to be exponentially damped (red) and allow the reaction product (species C in Fig. 1) to accumulate (blue). The applied magnetic field (50 μT) results in an increased transient conversion of the radical pair into the triplet state, causing C to be formed more rapidly and in higher yield. Faster recombination than shown here would allow scant time for the slow modulation arising from the Zeeman interaction to alter pT(t); the yield of C would then be much less affected by the field. For details of the calculation, see SI Appendix.
To act as a compass, a radical pair reaction must respond to the direction of the field and not just its intensity. This is unlikely to occur for solution-phase reactions where rapid molecular tumbling tends to average any anisotropic responses. The radicals must, at least partially, be immobilized and oriented and possess appropriate anisotropic magnetic interactions. As originally proposed by Schulten (18), the most likely source of anisotropy is the hyperfine interaction, i.e., the dependence of the electron–nuclear coupling on the orientation of the molecule in an external magnetic field. Almost every hyperfine interaction has an anisotropic component: As a consequence, S ↔ T interconversion, and therefore the reaction yield, should vary with the direction of the external field. This suggestion has been confirmed by numerical simulations (2, 33, 43–45).
Anisotropic magnetic field effects arising from hyperfine interactions have recently been observed experimentally (14). Somewhat related observations at higher magnetic fields, for radical pairs in solids (46, 47) and in viscous liquids (48–50), appear to have their origins in electron–electron dipolar interactions and g-tensor anisotropy. Additionally, time-resolved EPR (51–53) and solid state NMR (54) spectroscopies provide clear evidence for the involvement of anisotropic hyperfine interactions in the spin dynamics of photosynthetic radical pairs.
Ritz et al. (2) have suggested that the cells responsible for light-dependent magnetoreception are distributed around and aligned within the retina, in the manner of the visual rod and cone cells. In turn, the magnetoreceptor molecules would be oriented within the receptor cells perhaps by attachment to cytoskeletal proteins (55). Thus, cells at different retinal locations would make different angles to the Earth's magnetic field vector and so respond differently according to the anisotropy of the reactions of the radical pairs within them. The transduction of the magnetic compass information may “piggy-back” the visual reception pathway (2, 56), so that the bird literally sees the magnetic field as a “signal (or visual) modulation pattern,” reflecting the anisotropy of the radical pair reaction, and perhaps reminiscent of a “heads-up” display in an aircraft (2, 57).
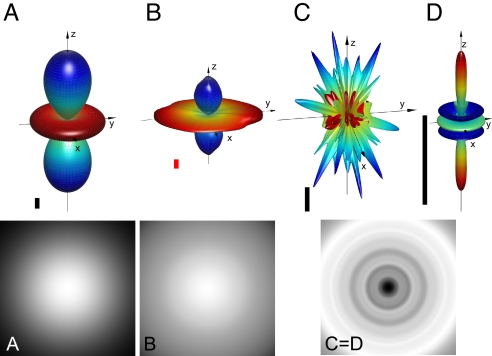
Fig. 3 shows the results of a few spin dynamics simulations of reaction yield anisotropies and the corresponding signal modulation patterns. Unless the radicals contain very few magnetic nuclei or possess some degree of molecular symmetry or are favorably disordered, the shape of the anisotropy can be complex. There is no clear picture of what would constitute the optimum sensory input for the bird; however, it seems reasonable to suppose that strongly anisotropic but relatively simple directional information would be favored. Simulations and experiments on solution-phase reactions suggest a few simple design features discussed in the section on cryptochromes (43, 58, 59).
Fig. 3.
Spin dynamics simulations of anisotropic reaction yields for model radical pairs performed as described in refs. 58 and 66 using the reaction scheme of Fig. 1. (Upper) Polar plots. (Lower) The corresponding signal modulation patterns for a bird looking directly along the Earth's magnetic field vector. The heights of the vertical scale bars in the upper images correspond to singlet yields of 2% (black) or 0.2% (red). (A and B) The simulations demonstrate that a relatively simple orientation dependence of the reaction yield (A) can be obtained from radical pairs containing a small number of hyperfine interactions or from more complex radicals when a few symmetry-related hyperfine interactions dominate (B). (C and D) More intricate anisotropy patterns (C) can be dramatically simplified if the radical pairs are axially rotationally disordered (D). The signal modulation pattern for C is identical to that for D and is only shown once. Note that in all cases the reaction yield is invariant to exact reversal of the magnetic field vector, i.e., the response is that of an inclination compass rather than a polarity compass. See SI Appendix for details of the calculations.
Kinetics.
The principal kinetic requirement for a sensitive magnetoreceptor is that the geomagnetic field has sufficient time to modulate the S ↔ T interconversion of the radical pair (Fig. 2) (60). This condition is met if the spin correlation of the 2 electrons persists for more than ≈1 μs, a requirement that constrains both the reaction kinetics and the molecular dynamics. The rate constants kSb + kSf and kTf (Fig. 1) must therefore, ideally, be ≈106 s−1 and fairly similar to one another (say within a factor of 10) to allow significant competition between the S and T reaction pathways. Such values are certainly achievable, e.g., in electron transfer proteins where rates can be tuned over many orders of magnitude via the reaction Gibbs energy, the reorganization energy and, especially, the donor–acceptor distance (61). The change in the reaction yield produced by a ≈50-μT field grows sigmoidally as a function of the radical pair lifetime, leveling out at ≈1 μs (see SI Appendix). There is therefore no advantage, and probably serious disadvantages (as a result of spin relaxation, see below), in having a radical pair lifetime much in excess of 1 μs.
Approximate structural constraints can be derived from the simple theory of electron transfer reactions (61, 62). First, for the lifetime of the radical pair to be ≈1 μs (to allow enough time for S ↔ T interconversion, without excessive spin relaxation), the edge-to-edge separation re of the 2 radicals in the magnetically sensitive radical pair should be less than ≈1.5 nm. Second, to achieve a high quantum yield of radical pairs, a plausible lower limit on the forward electron transfer rate would be ≈109 s−1, implying re ≤ 1.0 nm for each of the electron donor–acceptor pairs involved in the formation of the magnetoreceptive radical pair. The derivation of these constraints is given in the SI Appendix.
Geometry.
The separation of the 2 radicals not only has a strong influence on electron transfer rates but also determines the strength of the (exchange and dipolar) spin–spin interactions between the 2 unpaired electrons (63–65). The interconversion of S and T states by a ≈50-μT magnetic field is only possible when the interradical interactions are not much stronger than 50 μT. Given that the dipolar coupling has a magnitude of 500 μT at a radical–radical center-to-center separation rc of 1.8 nm and only falls to 50 μT at rc = 3.8 nm (58), it is not immediately obvious how this requirement can be reconciled with the much smaller separations just estimated from electron transfer rates. Theory suggests that the inhibitory effects of the exchange and dipolar interactions may partially cancel for immobilized protein-bound radicals when rc = 2.0 ± 0.2 nm (58). Such a center-to-center separation would be compatible with the above constraint on the edge-to-edge separation (re ≤ 1.5 nm) provided the radicals had diameters of more than ≈0.5 nm. The relative orientation of the radicals within the pair may also be important; simulations indicate that alignment of anisotropic hyperfine interactions could be a factor in optimizing the magnitude and shape of the reaction-yield anisotropy (43, 66). The experimental evidence that the effects of weak magnetic fields are quenched by strong interradical interactions is reviewed briefly in the SI Appendix.
Disorder and Motion.
As mentioned above, a critical requirement for a functional compass is that the radical pairs must be aligned within the host receptor cells which, in turn, must be spatially ordered within the bird's body (2). We focus here on the former. Rotational disorder would cause the anisotropic responses of differently oriented radical pairs to cancel one another, potentially reducing the directional sensitivity of the receptor. However, perfect ordering, as in a crystal, is not necessary. The compass function would be preserved if the magnetoreceptor molecules tended to be aligned along a common cell-fixed axis (the “director”) and had a reasonably large order parameter. Such alignment would largely preserve components of the magnetic response that are cylindrically symmetric with respect to the director axis, and cancel all others, so simplifying the orientational dependence of the reaction yield and resulting, perhaps, in a more efficient compass (Fig. 3). This point is illustrated by a recent study of an aligned radical pair whose lifetime was found to have a rather straightforward dependence on the orientation of the molecule in an applied magnetic field, as a consequence of static orientational averaging around the long axis of the rod-like molecule (14).
There are also strict constraints on the dynamics of the radical pair. First, rapid molecular rotation tends to average anisotropic magnetic interactions. For hyperfine and Zeeman interactions of ≈500 μT and ≈50 μT, respectively, motions with characteristic correlation times shorter than ≈1 μs would seriously attenuate the anisotropy of the reaction yield. The other consequence of molecular motion is that it causes the electron spins to relax, i.e., to equilibrate thermally with their surroundings, with concomitant loss of spin correlation. The criterion for a significant magnetic field effect is that relaxation times should be longer than the radical pair lifetime, which was estimated above to be ≈1 μs. This requires rotational motion either to be slower than ≈1 μs or faster than ≈100 ps. The latter, known as the “extreme narrowing limit,” seems implausibly fast for a molecule large enough to host a suitable radical pair and would anyway lead to efficient averaging of anisotropic magnetic interactions. Examples of slowly relaxing spin-correlated radical pairs at physiological temperatures are mentioned briefly in the SI Appendix.
In the light of the above, we may conclude that the ordering and motional constraints can be simultaneously satisfied if the radical pairs are uniaxially ordered with a high-order parameter and if all relevant motions are slower than ≈1 μs.
Evidence for Radical Pair Magnetoreception
We have presented a rather long list of magnetic, structural, kinetic, and dynamic requirements that must be fulfilled if a radical pair is to operate as a compass sensor. Using well-founded theory and experimental precedents, we have argued that all these conditions are individually plausible, both chemically and physically. The crucial question is whether these constraints can be satisfied simultaneously in a biologically credible magnetoreceptor. We discuss first the extent to which the available evidence supports a radical pair mechanism and then evaluate how well the “cryptochrome hypothesis” stands up against the various criteria.
Much of the evidence in favor of a photochemical radical pair magnetoreceptor is circumstantial. Schulten's original proposal (18) was inspired by 2 behavioral features of the avian magnetic compass: the requirement for light at wavelengths below ≈565 nm (23) and the invariance to reversal of the field direction (21). However, it is conceivable that light has an entirely different physiological effect: Rather than a direct involvement in magnetoreception, it could possibly affect the birds' motivation to act upon magnetic compass information derived from a separate, light-independent mechanism (7). Moreover, although radical pairs cannot form the basis of a polarity compass, one can speculate about magnetite-based receptors that would be consistent with inclination compass behavior (29, 67, 68).
Probably the most convincing experimental test of the radical pair model has involved subjecting caged migratory birds to weak radio frequency fields (2). Magnetic fields oscillating at frequencies in the range 1–100 MHz, with or without a static field present, are expected to alter the yields of radical pair reactions by modifying the spin dynamics (69). This has been observed for reactions of organic radicals in solution when the frequency of the applied field matches a S ↔ T interconversion frequency arising from hyperfine and/or Zeeman interactions (70–72). Such resonances are diagnostic for radical pairs: Magnetite particles of sufficient size to function as a compass are too large to reorient in magnetic fields fluctuating as rapidly as 1 MHz (73, 74).
Linearly polarized radio frequency fields 100 times weaker than the Earth's field (≈500 nT), with frequencies of 7.0 MHz or 1.315 MHz, are sufficient to disrupt the migratory orientation of caged European robins (75, 76). Moreover, disorientation is found when the angle between the Earth's field and the radio frequency field is 24° or 48°, but not when the 2 fields are collinear. The birds are also disoriented by a broadband (0.1–10 MHz) 85-nT radio frequency field (75). These remarkable observations appear to exclude a number of the more obvious artifacts: it seems unlikely, for example, that the motivation of freely moving birds would be disturbed to different extents after rotation of the radio frequency field axis. That the radio frequency field appears to have little effect on the birds' orientation when it is parallel to the Earth's field can be understood if one of the radicals has (effectively) no hyperfine interactions (14, 66). Bearing in mind that the periodic S ↔ T interconversion induced by a ≈500-nT Zeeman interaction has a period of ≈70 μs, sensitivity to such a feeble radio frequency field implies either a surprisingly long-lived spin-correlation or highly efficient signal transduction or both. In a very recent study, European robins exposed to radio frequency fields of various intensities and frequencies showed an extraordinarily sensitive response at 1.315 MHz in the local geomagnetic field (46 μT), which shifted to 2.630 MHz when the birds had been preconditioned to a 92-μT field (77). These resonances, at the frequency of the electron Zeeman interaction, were interpreted in terms of a radical pair in which one of the radicals was devoid of hyperfine interactions.
A remarkable aspect of the avian compass sense is the “functional window:” a 20–30% increase or decrease in the intensity of the ambient magnetic field is sufficient to disorient caged birds (78). In the context of radical pair magnetoreception, this unusual property could be related to the field-dependent changes in S ↔ T interconversion that are known to arise from energy-level degeneracies (44). As an example, simulations are shown in the SI Appendix that reveal a strong dependence of the form and amplitude of the reaction yield anisotropy on the magnetic field intensity in the range 10–100 μT (66). Robins are able to adapt relatively quickly to magnetic fields outside their normal functional window (79). This ability would have a natural interpretation in the radical pair model: The bird must learn to recognize a new anisotropy pattern.
A number of quantitative theoretical studies, most of which have been mentioned above, have tested selected aspects of the radical pair mechanism (2, 33, 43, 44, 57, 58, 66, 80). However, for the most part, disorder, motion and spin relaxation have all been ignored. Numerical simulations generally support the idea of a radical-pair-based compass and have shed some light on the conditions required for significant reaction yield anisotropies. In favorable cases, the orientational dependence is usually no more than a ≈50% variation in the reaction yield (43, 44, 58). Under more realistic conditions, changes of 1–10% seem more likely. Whether such a response is sufficient for a viable compass requires rather more insight into the signal transduction mechanisms than currently exists. The best that can be done at this stage is to estimate very approximately how many magnetoreceptors would be required to overcome stochastic fluctuations in the number of complexes formed between the signaling state and its signaling partner(s) (81). Such calculations suggest that a reaction yield anisotropy of 1–10% might be adequate (58).
Finally, it has recently been established, as a proof-of-principle, that a photochemical reaction can act as a magnetic compass (14). Green-light irradiation of a triad model compound composed of linked carotenoid, porphyrin, and fullerene groups produced a radical pair whose lifetime was both sensitive to magnetic fields <50 μT and that responded anisotropically. Frozen solutions at low temperatures were used in these experiments to immobilize the molecules, which were either aligned in a liquid crystalline solvent or probed anisotropically using photoselection with plane-polarized light. Clearly, rather different means of restricting molecular motion would be required for an in vivo magnetoreceptor.
Cryptochromes as Magnetoreceptors
It was the suggestion, in 2000, that cryptochromes could host magnetically sensitive radical pairs that set in motion the work reviewed in this Perspective (2). Nine years later, they are still the only contenders as chemical magnetoreceptors. Cryptochromes are 50- to 70-kDa blue-light photoreceptor flavoproteins, first identified in plants in 1993 (82) and since found in bacteria, insects, and animals (83–85). They contain 2 noncovalently bound chromophores: a redox-active flavin adenine dinucleotide (FAD) and a light-harvesting cofactor. Their functions, not all of which require light activation, include entrainment of circadian clocks and, in plants, regulation of growth and development. Unlike their evolutionary ancestors, the DNA photolyases (86), to which they show high sequence homology and structural similarity, cryptochromes do not repair double-stranded DNA. Here, we focus narrowly on the extent to which cryptochromes appear to meet the criteria outlined above. Others, better qualified than ourselves, have weighed the emerging physiological and biological evidence for their involvement in magnetoreception (3–5, 55, 87, 88).
Compared with photolyases, relatively little is known about the photocycles of cryptochromes, and most of that comes from studies of plant proteins (for reviews, see refs. 83, 85, 87, and 89). In contrast to photolyases, the photoactive forms of cryptochromes seem to contain the FAD chromophore in its fully oxidized redox state (90–92). Absorption of blue light leads to the formation of protein-bound flavin and tryptophan (and sometimes tyrosine) radicals (90, 93–95) apparently by a route similar to photoactivation in photolyase (95–97). In this reaction, which appears to play no part in the function of photolyase, photoexcited FAD is reduced to the flavosemiquinone radical (FAD•− or its protonated neutral form FADH•) by a sequence of intraprotein electron transfers along a conserved chain of 3 tryptophan residues (the “Trp-triad”) that culminates in the oxidation of the terminal Trp residue to form a TrpH•+ radical that can deprotonate to form the neutral Trp• radical (94, 98, 99). (See the SI Appendix for the structures of the redox states of flavins and of the Trp-triad.) The FADH• (or FAD•−) form of the protein accumulates under continuous blue-light irradiation both in vitro and in cryptochrome-expressing insect cells, and is thought to be the signaling state (91, 92). Corresponding to species C in Fig. 1, the flavosemiquinone state of the protein is presumably stabilized by independent reduction of the Trp or Tyr counterradical. The detailed mechanism of cryptochrome signaling is presently unclear, but is presumed to involve conformational changes in the protein that promote interaction with downstream signaling partners (83, 87).
The photochemically formed FAD–Trp radical pairs in cryptochromes from both the model plant Arabidopsis thaliana (AtCry) and the migratory garden warbler Sylvia borin (gwCry) have lifetimes (several milliseconds in vitro) that could be compatible with magnetic sensitivity, although there is no direct evidence yet that these species are spin correlated. Time-resolved EPR studies of the photoactivation reaction in 2 photolyases have found long-lived (>10 μs at 5 °C) electron spin polarization patterns that are characteristic of spin-correlated FAD–Trp and FAD–Tyr radical pairs (86, 100, 101). Similar observations, of a radical pair comprising the FADH• radical and the neutral radical form of the terminal Trp residue of the Trp-triad, have recently been reported for a Cry-DASH protein (102). Furthermore, a small (3–4%) effect of a relatively strong magnetic field (39 mT) on the radical pair recombination kinetics has been found for Escherichia coli photolyase (103). The magnetic sensitivity appears to arise from competition on a microsecond time scale between spin-selective back electron transfer in [FAD•− TrpH•+] and spin-independent deprotonation to produce the much longer lived (≈100 μs) radical pair [FAD•− Trp•] which is not magnetically sensitive presumably because of spin relaxation. The structural and photochemical similarity to photolyases suggests that cryptochromes may exhibit similar magnetic responses.
Further evidence for the involvement of cryptochromes has come from experiments on plants (104). Enhanced cryptochrome-mediated blue-light responses in A. thaliana seedlings grown in a 500-μT magnetic field have been observed, including a ≈30% inhibition of hypocotyl (stem) growth. If plants, in which magnetoreception has no apparent function and which presumably lack highly evolved receptors, are sensitive to magnetic fields, then other cryptochrome-containing species may be also.
Evidence that the photochemistry may be more complex than presented above, has come recently from a behavioral assay that shows that cryptochrome is necessary for light-dependent magnetic responses in Drosophila, although the wavelengths required are compatible with the action spectrum of the reduced form of the protein (105). The notion that insect cryptochromes do not contain a fully oxidized FAD in vivo (and may therefore be unable to generate radical pairs) seems to be supported by mutation studies in which the Trp-triad is disrupted (106). Fluorescence and EPR measurements, however, indicate that animal (human and Drosophila) cryptochromes are photoreduced from the fully oxidized state in insect cells, allowing accumulation of the flavosemiquinone radical state of the proteins (107).
Theoretical considerations indicate that photoactive flavoproteins may be suitable as magnetoreceptors. Although FAD–Trp radical pairs have ≈15 magnetic nuclei (mostly 1H, and a few 14N) with isotropic hyperfine coupling constants larger than ≈100 μT, simulations suggest that the anisotropy of the reaction yield is determined principally by 2 nitrogens (N5 and N10, see SI Appendix for numbering scheme) in the FAD radical that have relatively large, axial and collinear hyperfine tensors (43, 66). In the absence of such dominant interactions, it is likely that the anisotropic contributions of the various hyperfine interactions would tend to cancel one another, destroying much of the directional information.
Three geometrical constraints were derived above from considerations of the electron transfer kinetics and the likely magnitudes of the interradical interactions: re ≤ 1.5 nm and rc = 2.0 ± 0.2 nm for the magnetically sensitive radical pair, and re ≤ 1.0 nm for the electron donor–acceptor pairs involved in its formation. The distances between the FAD and the 3 constituents of the Trp-triad electron transfer chain (W400, W377, W324) in AtCry1 (108) (no crystal structure is yet available for an animal cryptochrome) are given in the SI Appendix. The 2 constraints on the terminal pair [FADH• W324•+] are satisfied (re = 1.47 nm and rc = 1.90 nm). Moreover, each of the donor–acceptor pairs that precede it, [FAD W400], [W400•+ W377] and [W377•+ W324], have edge-to-edge distances (0.45, 0.48, and 0.51 nm, respectively) well below 1.0 nm. However, these distances may differ in avian cryptochromes, and FAD-Tyr magnetosensitive radical pairs, with different re and rc, are also possible.
With the center-to-center separations in AtCry1, both the primary and secondary radical pairs, [FADH• W400•+] and [FADH• W377•+], are expected to have strong exchange interactions [estimated at >50 T and >100 mT, respectively (58)] and hence no appreciable S ↔ T interconversion or response to the geomagnetic field. Consequently, the tertiary pair [FADH• W324•+] should be formed in a pure spin state with maximum spin correlation. Although the exchange and dipolar interactions are not negligible in [FADH• W324•+] [≈500 μT (58)], they seem to be of appropriate magnitudes partially to cancel one another's effects, allowing the Earth's field to modulate S ↔ T interconversion. Other, at present hypothetical, sources of magnetic responses in cryptochromes are discussed in the SI Appendix.
To conclude, the limited information currently available suggests that cryptochrome radical pairs could, in principle, form the basis of a compass magnetoreceptor. Probably the physicochemical aspect about which there is most uncertainty is the mechanism and degree of molecular alignment and immobilization.
Future Work
The riddle of the avian magnetic compass is being teased apart, yet even after 4 decades' endeavor, a considerable shroud of mystery remains. In the following, we make a few suggestions for further work. The list has a physical chemistry bias and is far from comprehensive.
Animal behavior experiments are of crucial importance. Radio frequency fields have hitherto only been used to disrupt the magnetic compass sense. Such experiments cannot exclude the possibility that disorientation arises from effects on the animals' motivation rather than on the primary magnetoreception events. Theory predicts that weak static and radio frequency fields of similar strength can induce similar changes in product yield. It might therefore be possible to condition animals to respond, in the absence of the Earth's field, to the presence and orientation of a radio frequency field of suitable strength. Such observations would be difficult to explain without the involvement of radical pairs. A skeptic would need to conjecture a light-induced, highly magnetically sensitive radical pair reaction that did not supply the birds with directional information but did affect their moti vation, a possibility that seems to us somewhat less probable than a light-dependent radical pair magnetoreceptor.
Theoretical and computational studies have an important role to play in the prediction and interpretation of the response of radical pairs to weak magnetic fields. These calculations are challenging, especially for biologically plausible radicals most of which contain a plethora of internal magnetic interactions. Whereas in strong fields, semiclassical approaches allow reliable simulations to be performed at a cost that rises only polynomially with the number of magnetic nuclei, current approaches for weak fields scale exponentially. A reliable method for low-field calculations with polynomial scaling would be a significant development (109). Now that a model system has been shown to respond to <50-μT fields (14), it ought to be possible to explore experimentally as well as theoretically the design constraints imposed on a chemical compass by molecular disorder and motion. Efforts to produce a model system that functions at physiological temperatures are already under way.
Finally, experiments to test the cryptochrome hypothesis must continue. Spectroscopic measurements, both in vitro (90) and in vivo (77, 107), will provide insight into the photocycles of the proteins and reveal the identity and properties of radical pair intermediates. Characterization of the influence of static and time-dependent magnetic fields on isolated proteins (103) and Cry-mediated processes in living systems (105) will be equally important.
The physical chemistry underlying the avian magnetic compass has matured much in the last 10 years, yet there remain many intriguing questions to be addressed. We hope that this short survey will encourage more physical scientists to join us in this endeavor.
Supplementary Material
Acknowledgments.
We thank our colleagues, collaborators, and coworkers, without whom we would not have been in a position to write this perspective: Margaret Ahmad, Olga Efimova, Devens Gust, Sue-Re Harris, Kevin Henbest, Ilya Kuprov, Miriam Liedvogel, Kiminori Maeda, Henrik Mouritsen, Haruko Okamoto, Thorsten Ritz, Alex Robinson, Erik Schleicher, Christiane Timmel, Nicola Wagner-Rundell, Stefan Weber, Roswitha Wiltschko, and Wolfgang Wiltschko. We are grateful to the Engineering and Physical Sciences Research Council and the EMF Biological Research Trust for financial support and to the Oxford Supercomputing Centre for CPU time. C.T.R. thanks Somerville College, Oxford, for a Junior Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711968106/DCSupplemental.
References
- 1.Wiltschko W. Über den Einfluss statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula) Z Tierpsychol. 1968;25:537–558. [PubMed] [Google Scholar]
- 2.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnsen S, Lohmann KJ. Magnetoreception in animals. Phys Today. 2008;61:29–35. [Google Scholar]
- 4.Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opin Neurobiol. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Wiltschko R, Wiltschko W. Magnetoreception. BioEssays. 2006;28:157–168. doi: 10.1002/bies.20363. [DOI] [PubMed] [Google Scholar]
- 6.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 7.Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nat Rev Neurosci. 2005;6:703–712. doi: 10.1038/nrn1745. [DOI] [PubMed] [Google Scholar]
- 8.Ritz T, Dommer DH, Phillips JB. Shedding light on vertebrate magnetoreception. Neuron. 2002;34:503–506. doi: 10.1016/s0896-6273(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 9.Brocklehurst B. Magnetic fields and radical reactions: Recent developments and their role in Nature. Chem Soc Rev. 2002;31:301–311. doi: 10.1039/b107250c. [DOI] [PubMed] [Google Scholar]
- 10.Steiner UE, Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem Rev. 1989;89:51–147. [Google Scholar]
- 11.Timmel CR, Henbest KB. A study of spin chemistry in weak magnetic fields. Philos Trans R Soc London Ser A. 2004;362:2573–2589. doi: 10.1098/rsta.2004.1459. [DOI] [PubMed] [Google Scholar]
- 12.Woodward JR. Radical pairs in solution. Prog React Kinetic Mech. 2002;27:165–207. [Google Scholar]
- 13.Brocklehurst B, McLauchlan KA. Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int J Radiat Biol. 1996;69:3–24. doi: 10.1080/095530096146147. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, et al. Chemical compass model of avian magnetoreception. Nature. 2008;453:387–390. doi: 10.1038/nature06834. [DOI] [PubMed] [Google Scholar]
- 15.Hoff AJ. Magnetic-field effects on photosynthetic reactions. Q Rev Biophys. 1981;14:599–665. doi: 10.1017/s0033583500002481. [DOI] [PubMed] [Google Scholar]
- 16.Volk M, Ogrodnik A, Michel-Beyerle ME. In: Anoxygenic Photosynthetic Bacteria. Blankenship RE, Madigan MT, Bauer CE, editors. The Netherlands: Kluwer, Dordrecht; 1995. pp. 595–626. [Google Scholar]
- 17.Boxer SG, Chidsey CED, Roelofs MG. Magnetic-field effects on reaction yields in the solid-state—an example from photosynthetic reaction centers. Annu Rev Phys Chem. 1983;34:389–417. [Google Scholar]
- 18.Schulten K, Swenberg CE, Weller A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z Phys Chem NF. 1978;111:1–5. [Google Scholar]
- 19.Schulten K, Windemuth A. Biophysical Effects of Steady Magnetic Fields. Berlin: Springer; 1986. pp. 99–106. [Google Scholar]
- 20.Wiltschko W, Merkel FW. Orientierung zugunruhiger Rotkehlchen im statischen Magnetfeld. Verh der Dtsch Zoolog Ges. 1966;59:362–367. [Google Scholar]
- 21.Wiltschko W, Wiltschko R. Magnetic compass of European robins. Science. 1972;176:62–64. doi: 10.1126/science.176.4030.62. [DOI] [PubMed] [Google Scholar]
- 22.Merkel FW, Fromme HG. Untersuchungen über das Orientierungsvermögen nächtlich ziehender Rotkehlchen, Erithacus rubecula. Naturwissenschaften. 1958;45:499–500. [Google Scholar]
- 23.Wiltschko W, Wiltschko R. Light-dependent magnetoreception in birds: The behaviour of European robins, Erithacus rubecula, under monochromatic light of various wavelengths and intensities. J Exp Biol. 2001;204:3295–3302. doi: 10.1242/jeb.204.19.3295. [DOI] [PubMed] [Google Scholar]
- 24.Deutschlander ME, Phillips JB, Borland SC. The case for light-dependent magnetic orientation in animals. J Exp Biol. 1999;202:891–908. doi: 10.1242/jeb.202.8.891. [DOI] [PubMed] [Google Scholar]
- 25.Frost BJ, Mouritsen H. The neural mechanisms of long distance animal navigation. Curr Opin Neurobiol. 2006;16:481–488. doi: 10.1016/j.conb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 27.Walker MM. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. J Theor Biol. 2008;250:85–91. doi: 10.1016/j.jtbi.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Walker MM, Dennis TE, Kirschvink JL. The magnetic sense and its use in long-distance navigation by animals. Curr Opin Neurobiol. 2002;12:735–744. doi: 10.1016/s0959-4388(02)00389-6. [DOI] [PubMed] [Google Scholar]
- 29.Kirschvink JL, Gould JL. Biogenic magnetite as a basis for magnetic-field detection in animals. Biosystems. 1981;13:181–201. doi: 10.1016/0303-2647(81)90060-5. [DOI] [PubMed] [Google Scholar]
- 30.Fleissner G, et al. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol. 2003;458:350–360. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- 31.Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. A novel concept of Fe-mineral-based magnetoreception: Histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften. 2007;94:631–642. doi: 10.1007/s00114-007-0236-0. [DOI] [PubMed] [Google Scholar]
- 32.Solov'yov IA, Greiner W. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys J. 2007;93:1493–1509. doi: 10.1529/biophysj.107.105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solov'yov IA, Chandler DE, Schulten K. Magnetic field effects in Arabidopsis thaliana cryptochrome-1. Biophys J. 2007;92:2711–2726. doi: 10.1529/biophysj.106.097139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hore PJ. In: Advanced EPR Applications in Biology and Biochemistry. Hoff AJ, editor. Amsterdam: Elsevier; 1989. pp. 405–440. [Google Scholar]
- 35.Hulsebosch RJ, Borovykh IV, Paschenko SV, Gast P, Hoff AJ. Radical pair dynamics and interactions in quinone-reconstituted photosynthetic reaction centers of Rb. sphaeroides R26: A multifrequency magnetic resonance study. J Phys Chem B. 1999;103:6815–6823. [Google Scholar]
- 36.Tang J, Utschig LM, Poluektov O, Thurnauer MC. Transient W-band EPR study of sequential electron transfer in photosynthetic bacterial reaction centers. J Phys Chem B. 1999;103:5145–5150. [Google Scholar]
- 37.Dzuba SA, et al. Control of radical pair lifetimes by microwave irradiation. Application to photosynthetic reaction centres. Chem Phys Lett. 1996;253:361–366. [Google Scholar]
- 38.van Dijk B, Gast P, Hoff AJ. Control of radical pair lifetime by a switched magnetic field. Phys Rev Lett. 1996;77:4478–4481. doi: 10.1103/PhysRevLett.77.4478. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk B, Carpenter JKH, Hoff AJ, Hore PJ. Magnetic field effects on the recombination kinetics of radical pairs. J Phys Chem B. 1998;102:464–472. [Google Scholar]
- 40.Gerson F, Huber W. Electron Spin Resonance Spectroscopy of Organic Radicals. Weinheim, Germany: Wiley-VCH; 2003. [Google Scholar]
- 41.Hoff AJ. Advanced EPR. Applications in Biology and Biochemistry. Amsterdam: Elsevier; 1989. [Google Scholar]
- 42.Improta R, Barone V. Interplay of electronic, environmental, and vibrational effects in determining the hyperfine coupling constants of organic free radicals. Chem Rev. 2004;104:1231–1253. doi: 10.1021/cr960085f. [DOI] [PubMed] [Google Scholar]
- 43.Cintolesi F, Ritz T, Kay CWM, Timmel CR, Hore PJ. Anisotropic recombination of an immobilized photoinduced radical pair in a 50-μT magnetic field: A model avian photomagnetoreceptor. Chem Phys. 2003;294:385–399. [Google Scholar]
- 44.Timmel CR, Cintolesi F, Brocklehurst B, Hore PJ. Model calculations of magnetic field effects on the recombination reactions of radicals with anisotropic hyperfine interactions. Chem Phys Lett. 2001;334:387–395. [Google Scholar]
- 45.Canfield JM, Belford RL, Debrunner PG. Calculations of Earth-strength steady and oscillating magnetic field effects in coenzyme B-12 radical pair systems. Mol Phys. 1996;89:889–930. [Google Scholar]
- 46.Bube W, Michel-Beyerle ME, Haberkorn R, Steffens E. Sensitized charge carrier injection into organic-crystals studied by isotope effects in weak magnetic fields. Chem Phys Lett. 1977;50:389–393. [Google Scholar]
- 47.Groff RP, Suna A, Avakian P, Merrifield RE. Magnetic hyperfine modulation of dye-sensitized delayed fluorescence in organic crystals. Phys Rev B. 1974;9:2655–2660. [Google Scholar]
- 48.Boxer SG, Chidsey CED, Roelofs MG. Anisotropic magnetic interactions in the primary radical ion-pair of photosynthetic reaction centers. Proc Natl Acad Sci USA. 1982;79:4632–4636. doi: 10.1073/pnas.79.15.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutta R, Basu S, Chowdhury M. Magnetic-field effect on the diphenylhexatriene dimethylaniline exciplex system - anisotropic effects in viscous media. Chem Phys Lett. 1991;182:429–434. [Google Scholar]
- 50.Sengupta T, Aich S, Basu S. Isotropic and anisotropic magnetic field effect on the exciplex between all-s-trans-1,6-diphenylhexa-1,3,5-triene and 1,4-dicyanobenzene. J Phys Chem B. 1999;103:3784–3790. [Google Scholar]
- 51.Link G, et al. Structure of the P700+ A1− radical pair intermediate in photosystem I by high time resolution multifrequency electron paramagnetic resonance: Analysis of quantum beat oscillations. J Am Chem Soc. 2001;123:4211–4222. doi: 10.1021/ja003382h. [DOI] [PubMed] [Google Scholar]
- 52.Weber S, Ohmes E, Thurnauer MC, Norris JR, Kothe G. Light-generated nuclear quantum beats—A signature of photosynthesis. Proc Natl Acad Sci USA. 1995;92:7789–7793. doi: 10.1073/pnas.92.17.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fursman CE, Hore PJ. Distance determination in spin-correlated radical pairs in photosynthetic reaction centres by electron spin echo envelope modulation. Chem Phys Lett. 1999;303:593–600. [Google Scholar]
- 54.Jeschke G, Matysik J. A reassessment of the origin of photochemically induced dynamic nuclear polarization effects in solids. Chem Phys. 2003;294:239–255. [Google Scholar]
- 55.Mouritsen H, et al. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc Natl Acad Sci USA. 2004;101:14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heyers D, Manns M, Luksch H, Gűntűrkűn O, Moutitsen H. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE. 2007;2:e937. doi: 10.1371/journal.pone.0000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K, Mattern E, Ritz T. On the use of magnets to disrupt the physiological compass of birds. Phys Biol. 2006;3:220–231. doi: 10.1088/1478-3975/3/3/007. [DOI] [PubMed] [Google Scholar]
- 58.Efimova O, Hore PJ. Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys J. 2008;94:1565–1574. doi: 10.1529/biophysj.107.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodgers CT, Norman SA, Henbest KB, Timmel CR, Hore PJ. Determination of radical re-encounter probability distributions from magnetic field effects on reaction yields. J Am Chem Soc. 2007;129:6746–6755. doi: 10.1021/ja068209l. [DOI] [PubMed] [Google Scholar]
- 60.Timmel CR, Till U, Brocklehurst B, McLauchlan KA, Hore PJ. Effects of weak magnetic fields on free radical recombination reactions. Mol Phys. 1998;95:71–89. doi: 10.1080/09553000050176270. [DOI] [PubMed] [Google Scholar]
- 61.Moser CC, Keske JM, Warncke K, Farid RS, Dutton PL. Nature of biological electron transfer. Nature. 1992;355:796–802. doi: 10.1038/355796a0. [DOI] [PubMed] [Google Scholar]
- 62.Moser CC, Dutton PL. Engineering protein-structure for electron-transfer function in photosynthetic reaction centers. Biochim Biophys Acta. 1992;1101:171–176. [PubMed] [Google Scholar]
- 63.Verhoeven JW. On the role of spin correlation in the formation, decay, and detection of long-lived, intramolecular charge-transfer states. J Photochem Photobiol C. 2006;7:40–60. [Google Scholar]
- 64.O'Dea AR, Curtis AF, Green NJB, Timmel CR, Hore PJ. Influence of dipolar interactions on radical pair recombination reactions subject to weak magnetic fields. J Phys Chem A. 2005;109:869–873. doi: 10.1021/jp0456943. [DOI] [PubMed] [Google Scholar]
- 65.Wasielewski MR. Energy, charge, and spin transport in molecules and self-assembled nanostructures inspired by photosynthesis. J Org Chem. 2006;71:5051–5066. doi: 10.1021/jo060225d. [DOI] [PubMed] [Google Scholar]
- 66.Rodgers CT. University of Oxford (Oxford): 2007. D Phil thesis. [Google Scholar]
- 67.Davila AF, Fleissner G, Winklhofer M, Petersen N. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys Chem Earth. 2003;28:647–652. [Google Scholar]
- 68.Solov'yov IA, Greiner W. Iron-mineral-based magnetoreceptor in birds: Polarity or inclination compass? Eur Phys J D. 2008 doi: 10.1140/epjd/e2008-00118-y. [DOI] [Google Scholar]
- 69.Timmel CR, Hore PJ. Oscillating magnetic field effects on the yields of radical pair reactions. Chem Phys Lett. 1996;257:401–408. [Google Scholar]
- 70.Woodward JR, Timmel CR, McLauchlan KA, Hore PJ. Radio frequency magnetic field effects on electron-hole recombination. Phys Rev Lett. 2001;87 doi: 10.1103/PhysRevLett.87.077602. 077602. [DOI] [PubMed] [Google Scholar]
- 71.Henbest KB, Kukura P, Rodgers CT, Hore PJ, Timmel CR. Radio frequency magnetic field effects on a radical recombination reaction: A diagnostic test for the radical pair mechanism. J Am Chem Soc. 2004;126:8102–8103. doi: 10.1021/ja048220q. [DOI] [PubMed] [Google Scholar]
- 72.Rodgers CT, Henbest KB, Kukura P, Timmel CR, Hore PJ. Low-field optically detected EPR spectroscopy of transient photoinduced radical pairs. J Phys Chem A. 2005;109:5035–5041. doi: 10.1021/jp050765z. [DOI] [PubMed] [Google Scholar]
- 73.Weiss BP, et al. Ferromagnetic resonance and low-temperature magnetic tests for biogenic magnetite. Earth Planet Sci Lett. 2004;224:73–89. [Google Scholar]
- 74.Kirschvink JL. Microwave absorption by magnetite: A possible mechanism for coupling nonthermal levels of radiation to biological systems. Bioelectromagnetics. 1996;17:187–194. doi: 10.1002/(SICI)1521-186X(1996)17:3<187::AID-BEM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 75.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- 76.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften. 2005;92:86–90. doi: 10.1007/s00114-004-0595-8. [DOI] [PubMed] [Google Scholar]
- 77.Ritz T, et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J. 2008 doi: 10.1016/j.bpj.2008.11.072. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiltschko R, Wiltschko W. Magnetic Orientation in Animals. Berlin: Springer; 1995. [Google Scholar]
- 79.Wiltschko W, Stapput K, Thalau P, Wiltschko R. Avian magnetic compass: Fast adjustment to intensities outside the normal functional window. Naturwissenschaften. 2006;93:300–304. doi: 10.1007/s00114-006-0102-5. [DOI] [PubMed] [Google Scholar]
- 80.Wang KF, Ritz T. Zeeman resonances for radical-pair reactions in weak static magnetic fields. Mol Phys. 2006;104:1649–1658. [Google Scholar]
- 81.Weaver JC, Vaughan TE, Astumian RD. Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature. 2000;405:707–709. doi: 10.1038/35015128. [DOI] [PubMed] [Google Scholar]
- 82.Ahmad M, Cashmore AR. HY4 gene of A-thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 83.Lin CT, Todo T. The cryptochromes. Genome Biol. 2005;6:220. doi: 10.1186/gb-2005-6-5-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Partch CL, Sancar A. Photochemistry and photobiology of cryptochrome blue-light photopigments: The search for a photocycle. Photochem Photobiol. 2005;81:1291–1304. doi: 10.1562/2005-07-08-IR-607. [DOI] [PubMed] [Google Scholar]
- 85.Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103:2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 86.Weber S. Light-driven enzymatic catalysis of DNA repair: A review of recent biophysical studies on photolyase. Biochim Biophys Acta. 2005;1707:1–23. doi: 10.1016/j.bbabio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Partch CL, Sancar A. Photochemistry and photobiology of cryptochrome blue-light photopigments: The search for a photocycle. Photochem Photobiol. 2005;81:1291–1304. doi: 10.1562/2005-07-08-IR-607. [DOI] [PubMed] [Google Scholar]
- 88.Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: A possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91:585–588. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- 89.Losi A. Flavin-based blue-light photosensors: A photobiophysics update. Photochem Photobiol. 2007;83:1283–1300. doi: 10.1111/j.1751-1097.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 90.Liedvogel M, et al. Chemical magnetoreception: Bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE. 2007;2:e1106. doi: 10.1371/journal.pone.0001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouly JP, et al. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- 92.Banerjee R, et al. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J Biol Chem. 2007;282:14916–14922. doi: 10.1074/jbc.M700616200. [DOI] [PubMed] [Google Scholar]
- 93.Giovani B, Byrdin M, Ahmad M, Brettel K. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat Struct Biol. 2003;10:489–490. doi: 10.1038/nsb933. [DOI] [PubMed] [Google Scholar]
- 94.Zeugner A, et al. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J Biol Chem. 2005;280:19437–19440. doi: 10.1074/jbc.C500077200. [DOI] [PubMed] [Google Scholar]
- 95.Aubert C, Mathis P, Eker APM, Brettel K. Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. Proc Natl Acad Sci USA. 1999;96:5423–5427. doi: 10.1073/pnas.96.10.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aubert C, Vos MH, Mathis P, Eker APM, Brettel K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature. 2000;405:586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 97.Lukacs A, Eker APM, Byrdin M, Brettel K, Vos MH. Electron hopping through the 15 Å triple tryptophan molecular wire in DNA photolyase occurs within 30 ps. J Am Chem Soc. 2008;130:14394–14395. doi: 10.1021/ja805261m. [DOI] [PubMed] [Google Scholar]
- 98.Byrdin M, et al. Intraprotein electron transfer and proton dynamics during photoactivation of DNA photolyase from E. coli: Review and new insights from an “inverse” deuterium isotope effect. Biochim Biophys Acta. 2004;1655:64–70. doi: 10.1016/j.bbabio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Kao YT, et al. Ultrafast dynamics and anionic active states of the flavin cofactor in cryptochrome and photolyase. J Am Chem Soc. 2008;130:7695–7701. doi: 10.1021/ja801152h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weber S, et al. Photoactivation of the flavin cofactor in Xenopus laevis (6-4) photolyase: Observation of a transient tyrosyl radical by time-resolved electron paramagnetic resonance. Proc Natl Acad Sci USA. 2002;99:1319–1322. doi: 10.1073/pnas.032469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gindt YM, et al. Origin of the transient electron paramagnetic resonance signals in DNA photolyase. Biochemistry. 1999;38:3857–3866. doi: 10.1021/bi981191+. [DOI] [PubMed] [Google Scholar]
- 102.Biskup T, et al. Direct observation of a photoinduced radical-pair intermediate in a cryptochrome DASH blue-light photoreceptor. Angew Chem Int Ed. 2009;48:404–407. doi: 10.1002/anie.200803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henbest KB, et al. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc Natl Acad Sci USA. 2008;105:14395–14399. doi: 10.1073/pnas.0803620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta. 2007;225:615–624. doi: 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- 105.Gegear RJ, Casselman A, Waddell S, Reppert SM. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature. 2008;454:1014–1018. doi: 10.1038/nature07183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kavakli IH, Sancar A. Analysis of the role of intraprotein electron transfer in photoreactivation by DNA photolyase in vivo. Biochemistry. 2004;43:15103–15110. doi: 10.1021/bi0478796. [DOI] [PubMed] [Google Scholar]
- 107.Hoang N, et al. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6:e160. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brautigam CA, et al. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:12142–12147. doi: 10.1073/pnas.0404851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuprov I, Wagner-Rundell N, Hore PJ. Polynomially scaling spin dynamics simulation algorithm based on adaptive state-space restriction. J Magn Reson. 2007;189:241–250. doi: 10.1016/j.jmr.2007.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.