Abstract
T lymphocytes play a key role in adaptive immunity and are activated by interactions of their T cell receptors (TCR) with peptides (p) derived from antigenic proteins bound to MHC gene products. The repertoire of T lymphocytes available in peripheral organs is tuned in the thymus. Immature T lymphocytes (thymocytes) interact with diverse endogenous peptides bound to MHC in the thymus. TCR expressed on thymocytes must bind weakly to endogenous pMHC (positive selection) but must not bind too strongly to them (negative selection) to emerge from the thymus. Negatively selecting pMHC ligands bind TCR with a binding affinity that exceeds a sharply defined (digital) threshold. In contrast, there is no sharp threshold separating positively selecting ligands from those that bind too weakly to elicit a response. We describe results of computer simulations and experiments, which suggest that the contrast between the characters of the positive and negative selection thresholds originates in differences in the way in which Ras proteins are activated by ligands of varying potency. The molecular mechanism suggested by our studies generates hypotheses for how genetic aberrations may dampen the digital negative selection response, with concomitant escape of autoimmune T lymphocytes from the thymus.
Keywords: positive feedback, Ras activation, signal transduction, T cell antigen receptor, thymic development
T lymphocytes (T cells) orchestrate adaptive immunity, and misregulation of their activity can lead to autoimmune diseases. T cell activation requires sufficiently strong binding of TCR expressed on T cell surfaces to pathogen-derived peptide-MHC (pMHC) complexes on the surface of antigen-presenting cells. TCR genes rearrange stochastically to generate a clonally distributed TCR repertoire that can recognize diverse pMHC complexes. Weak binding of TCRs to endogenous pMHC complexes confers self-tolerance.
The diverse but self-tolerant TCR repertoire is shaped by positive and negative selection of immature T cells (thymocytes) in the thymus (1, 2). Thymic epithelial and stromal cells, as well as hematopoietically derived macrophages and dendritic cells, display pMHC complexes representing endogenous peptide products of the organism's genome. During positive selection, thymocytes bearing TCR that interact weakly with endogenous pMHC complexes receive survival signals that also promote differentiation. During negative selection, thymocytes expressing TCRs that bind too strongly to endogenous pMHC molecules are deleted by apoptosis, thereby aiming to eliminate T cells capable of autoimmune responses.
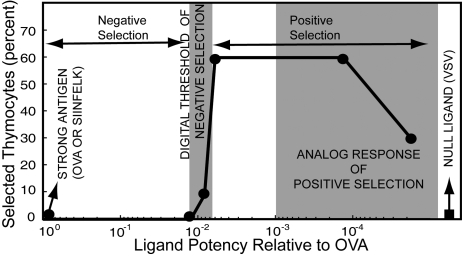
Daniels et al. (3) reported remarkable results concerning the TCR-pMHC binding characteristics that result in positive or negative selection in fetal thymic organ culture. A central finding (Fig. 1) is that the negative selection threshold is very sharply defined: a 1.2-fold difference in affinity of the TCR-pMHC complex separates the weakest negative selecting ligand from the strongest positive selector. In contrast, positive selection occurs to varying degrees over a broad range of ligand potency (i.e., the positive selection window is graded). Differences in amplitudes and spatial locations of signaling intermediates have led to hypotheses regarding differential signal propagation stimulated by ligands that mediate positive and negative selection (2). However, the mechanism that underlies a graded range for positive selection and a sharp threshold for negative selection is not known.
Fig. 1.
The fraction of CD8 single positive thymocytes selected by various pMHC ligands (adapted from Daniels et al. [3]). Ligand potency is the peptide concentration required to induce CD69 expression in 50% of the double-positive thymocytes, normalized to the antigenic peptide SIINFELK or OVA. Ligand potency of the weakest negative selector and strongest positive selector differ by a factor of ≈1.2.
We propose a mechanistic description based on computer simulations of a model that integrates diverse experimental data and known TCR-regulated signaling events. In lymphocytes, a key signaling intermediate, Ras (4), is primarily activated by two families of Ras guanine exchange factors (GEFs), RasGRP (Ras guanyl nucleotide release protein) (5) and SOS (Son of Sevenless) (6). Our findings indicate that weak stimulation of the TCR by positively selecting ligands activates Ras primarily by RasGRP, whereas only strong ligands can target SOS to the membrane. RasGRP-mediated Ras activation increases gradually as the stimulatory potency of ligands increases (an “analogue” response), which may underlie the graded increase in the fraction of selected thymocytes with ligand potency (Fig. 1). Beyond a sharply defined threshold of ligand potency, there is a large increase in Ras activation due to positive feedback regulation of Ras activation by SOS (6, 7). This sharp increase in Ras activation may separate ligands that stimulate positive and negative selection. Positive feedback regulation of Ras activation by SOS also results in a fluctuation-mediated bimodal activation of Ras for negatively selecting signals; that is, the response is “digital” in that cells are either “on” or “off.” Consistent with this prediction, in our experiments strong stimulation of thymocytes led to bimodal responses only when the SOS pathway was engaged. We predict that certain mutations to key signaling components would abolish the sharp potency boundary separating positive and negative selectors.
Initial Signaling Events.
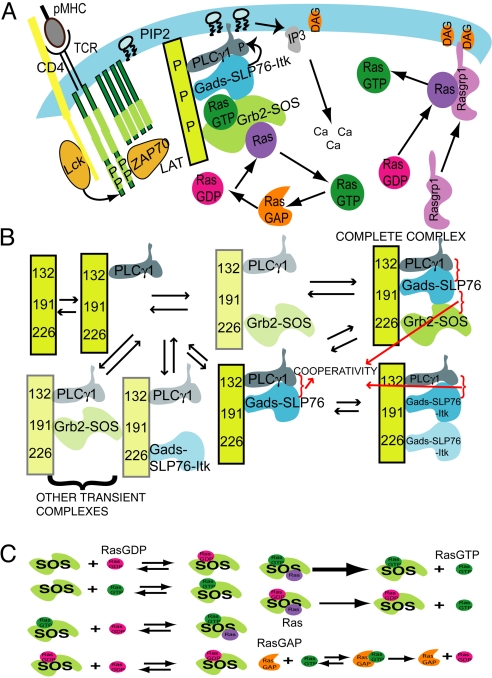
During TCR-pMHC engagement, the Src kinase Lck, bound to the CD4 or CD8 coreceptor, is recruited to the TCR complex and activated (Fig. 2A). Lck can phosphorylate the immunotyrosine activation motifs (ITAMs) within the CD3- and ζ-chains of the TCR complex (8). A doubly phosphorylated ITAM can bind a Syk kinase, ZAP70, which is subsequently activated by being phosphorylated by Lck or is transautophosphorylated by an ITAM-bound ZAP70 molecule (9, 10). Ligands that bind the TCR with a longer half-life result in more efficient ITAM phosphorylation and hence greater amounts of activated ZAP70 molecules. Daniels et al. (3) reported a continuous increase of active ZAP70 as thymocytes were stimulated with ligands of greater potency (3). Therefore, we use the amount of active ZAP70 as the surrogate for ligand potency.
Fig. 2.
Cartoons of the signaling network. (A) Schematic representation of the simulated network. (B) Schematic representation of the important signaling complexes assembled by the adaptor molecule LAT. Stable complexes (due to cooperative interactions) are represented by darker shades. The complete complex is indicated. (C) The reactions of the positive feedback loop associated with SOS-mediated Ras activation.
Assembly of the LAT Signalsome.
Activated ZAP70 can phosphorylate tyrosines on an adaptor molecule called linker for activation of T cells (LAT) (11). Human and murine LAT contain nine conserved tyrosines which, when phosphorylated, can bind SH2 domains of several proteins to assemble a signaling complex (signalosome) (Fig. 2B). Mutation experiments have shown that only three membrane distal tyrosines (denoted by Y132, Y171, and Y191) are necessary and sufficient for calcium signaling and can partially reconstitute Erk activation (12, 13). Restoration of wild-type active Erk levels requires addition of both Y226 and Y110 (12, 13). Because both Y226 and Y110 bind Grb2 (14), we subsume these tyrosines into one (denoted Y226). This reduction of the size of the computational model should not affect qualitative results. On the basis of mutation experiments, we assume that Y191, Y171, and Y226 are independently phosphorylated, whereas phosphorylation of Y132 requires prior phosphorylation of at least one other tyrosine (12).
Experimental results (12–14) suggest the following minimal model (Fig. 2 A and B) for the way in which the LAT signalosome is assembled: (i) PLCγ1 binds to pY132. (ii) Gads can bind to pY171 or pY191, and weakly to pY226 (14). (iii) Gads bound to pY191 or pY171 recruits SLP-76. SLP-76 binds PLCγ1, stabilizing the resulting complex (14, 15). (iv) Phosphorylated SLP-76 (by ZAP70) is associated with the kinase Itk and the guanine exchange factor Vav (15, 16). Itk, a tyrosine kinase, activates PLCγ1. SLP-76 is strongly associated with Gads (17). Therefore, we subsume Itk, SLP-76, and Gads into one species. Because we focus on Ras activation (reasons described below), we do not include Vav in our model. (v) Grb2's SH2 domain can bind to phosphorylated Y171, Y191, and Y226 (12–14). Grb2 binds most strongly to pY191 if other proteins are not bound to LAT (14). If Gads is bound to pY191 or pY171 and Grb2 is bound to pY226, mutual interactions cooperatively stabilize the resulting complex (12, 14). (vi) We assume that Grb2 and SOS are constitutively associated because SOS' C-terminal proline-rich regions bind SH3 domains of Grb2 with high affinity (18). Experimental facts indicate that pY171 and pY191 have similar functions, so we subsume both into one (referred to as Y191).
Ras Activation.
Ras, a membrane-bound signaling protein, exists in inactive (GDP-bound) or active (GTP-bound) states (4). In T cells, Ras is primarily activated by two families of GEFs, RasGRP (5) and SOS (18). A third family, RasGRF2, is believed to be dispensable for normal thymocyte development (19), hence we do not include it. RasGRP is activated by diacylglycerol (DAG), which is produced by active PLCγ1 cleaving PIP2 on the cell membrane. SOS is recruited to the membrane via the binding of Grb2 to LAT (11). The catalytic domain of SOS binds RasGDP, thereby facilitating nucleotide release and subsequent binding of abundantly present GTP. SOS has an “allosteric pocket” distal from the catalytic domain that can bind nucleotide-associated Ras (6, 7). The basal catalytic rate of Ras activation by SOS is very low. If RasGDP binds to the distal site, the catalytic rate is approximately five times larger than the basal rate. However, if RasGTP is bound to the allosteric pocket, Ras activation occurs at approximately 75 times the basal rate (6, 7). Thus, there is a positive feedback loop associated with SOS-mediated Ras activation (Fig. 2C).
Active Ras is the Readout of Signaling.
Positive selection requires ERK activation (2). ERK and other MAPK pathways, especially the JNK pathway, are involved in normal negative selection (2). However, some experiments suggest that negative selection may take place in the absence of Erk (20) but not JNK (21). Because activation of both the ERK and JNK pathways requires Ras activation (22), we chose active Ras as the readout of signaling. Strong (and transient) activation of ERK correlates with negative selection, whereas weak (and sustained) activation of ERK is required for positive selection (2). We therefore associate strong and weak Ras signals with negative and positive selection, respectively. Many mechanisms can explain how a strong signal is turned off more rapidly than weaker signals (23, 24), and so we did not model how signals are turned off.
Results
Only Strong Ligands Can Generate Fully Phosphorylated LAT Molecules Efficiently.
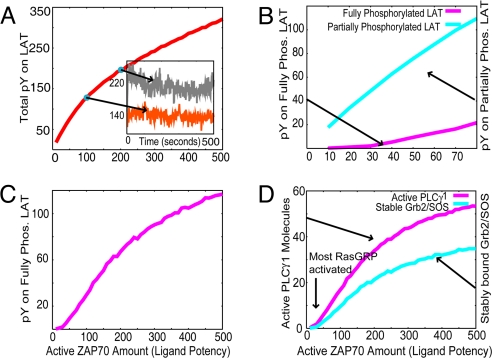
Our calculations show that the total number of phosphotyrosines associated with LAT increases with ligand potency, as measured by amount of active ZAP70 [Fig. 3A and supporting information (SI)]. Daniels et al. (3) reported an approximately twofold difference in phosphorylated LAT levels between the weakest negative selector and the strongest positive selector and similar differences in the levels of active ZAP70. The Inset in Fig. 3A shows that our calculations yield similar results. However, the sharpness of the increase in total phosphotyrosines on LAT depends on the rate at which ZAP70 phosphorylates its substrate (See SI).
Fig. 3.
Various quantities relevant to the assembly of the LAT signalosome as a function of ligand potency. Each data point represents the average of 100 stochastic simulations, taken after 20 min. (A) Total phosphotyrosine (pY) associated with LAT. Inset shows the total phosphotyrosine on LAT as a function of time for two ligand potencies, as obtained from a single stochastic simulation, representing a single lymphocyte. (B) Phosphotyrosine on fully and partially phosphorylated LAT at low ligand potencies. (C) Total phosphotyrosine associated with fully phosphorylated LAT molecules. (D) Activated PLCγ1 molecules bound to LAT and stably bound Grb2/SOS. The latter is defined as Grb2/SOS stably bound to complexes containing PLCγ1 and Gads (B).
An important subtlety is revealed by our computer simulations. Stimulation with weak ligands results in very few fully phosphorylated LAT molecules, and most LAT molecules are partially phosphorylated (Fig. 3 B and C). Because of multisite phosphorylation (25) and cooperativity (phosphorylation of Y132 requires another tyrosine to be phosphorylated), the number of fully phosphorylated LAT molecules is a weak sigmoidal function (Hill coefficient of approximately 2) of the ligand potency (Fig. 3 B and C). The sharpness of the response depends on the rates for phosphorylation and dephosphorylation of LAT tyrosines (see SI). The qualitative difference in the amount of fully phosphorylated LAT molecules as ligand potency is varied has important consequences for assembly of the LAT signalosome when thymocytes are stimulated by weak (positively selecting) and strong (negatively selecting) ligands.
Efficient Recruitment of PLCγ1 and Activation of RasGRP Does Not Require Fully Phosphorylated LAT, But Stable Grb2/SOS Recruitment Does.
LAT molecules phosphorylated only on the Y132 and the Y191 sites can support an incomplete signalosome consisting of PLCγ1 and Gads/SLP76 that is sufficient for activation of PLCγ1 (Fig. 2B). Thus, weak ligands that result in mostly partially phosphorylated LAT molecules (Fig. 3B) can activate PLCγ1 (Fig. 3D). The hydrolysis of PIP2 by activated PLCγ1 generates DAG (in addition to inositol Tris-phosphate), which is required for bringing RasGRP to the membrane and its GEF activity. Because PIP2 is present at high local concentrations in lipid rafts where LAT resides, a relatively small amount of activated PLCγ1 can activate most of the RasGRP molecules in the simulation volume (Fig. 3D). Thus, RasGRP's GEF activity is stimulated by weak ligands.
In contrast to RasGRP, the GEF activity of Grb2/SOS is not stimulated efficiently by weak ligands. Grb2/SOS can bind independently to pY171, pY226, and pY191, but these interactions are weak. Because of multiple cooperative interactions, the most stable binding of Grb2/SOS to LAT occurs when Grb2/SOS is bound to pY226, Gads/SLP76 is bound to pY191, and PLCγ1 is bound on pY132 (Fig. 2B) (12, 14). Therefore, strong binding of Grb2/SOS to LAT requires that LAT is fully phosphorylated, and stably recruited Grb2/SOS molecules and fully phosphorylated LAT track each other as a function of ligand potency (Fig. 3 B and D).
These results suggest that SOS will be recruited efficiently to the membrane only when thymocytes are stimulated by strong (negatively selecting) ligands. This is consistent with the results of Daniels et al. (3), who found that negatively selecting ligands stimulate efficient recruitment of Grb2/SOS to the plasma membrane, whereas positively selecting ligands do not.
The results depicted in Fig. 3 show that there is a separation in the range of signal strengths over which RasGRP can act but SOS cannot. This conclusion is robust to variations in parameters over wide ranges (see tables in SI). The main requirement for this effect is the cooperativity in the formation of the LAT signalsome that experiments suggest (14). Note that the reported clustering of LAT due to SOS molecules binding more than one Grb2 molecule (26), which we have not included in this model, would lead to further enhancement of these cooperative effects.
Graded Increase in Ras Activation for Weakly Stimulatory Ligands May Underlie the Analogue Threshold for Positive Selection.
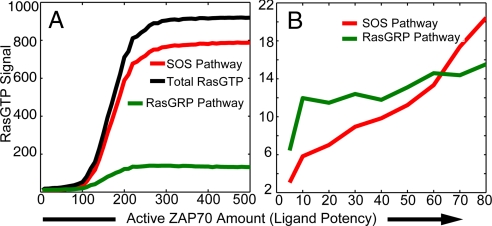
Fig. 4 shows our computer simulation results for Ras activation (readout for signaling). For weakly stimulatory ligands that generate small amounts of activated ZAP70, activated Ras levels are low, and most of the Ras molecules are activated by RasGRP and not SOS (Fig. 4B). This is because weakly stimulatory ligands can activate the GEF activity of RasGRP but not SOS (Fig. 3). RasGRP-mediated Ras activation results in a graded response to increasing ligand potency because its catalytic function is not known to be subject to positive feedback regulation (7).
Fig. 4.
The number of RasGTP molecules is shown as a function of ligand potency at 20 min. Each curve represents the average of 100 stochastic simulations and represents the mean behavior of a population of cells. (A) The number of active Ras molecules generated by RasGRP (green), SOS (red), and the total amount (black). (B) The response for weakly stimulatory ligands is shown in amplified form to show that most RasGTP molecules are generated via RasGRP in this regimen and that the response is graded.
These results suggest that signaling stimulated by weak positively selecting ligands is mediated by Ras activation via RasGRP. Furthermore, the observed graded positive selection window (5) may be because RasGRP-mediated signaling increases in analogue fashion as ligand potency increases (Fig. 4 A and B).
A Sharp Increase in Ras Activation Beyond a Threshold Ligand Potency May Underlie the Digital Negative Selection Threshold.
We find that there is a sharp increase in RasGTP level when ligand potency exceeds a threshold and that this is due to SOS-mediated Ras activation (Fig. 4A). The sharp threshold is the result of positive feedback regulation of SOS' GEF activity. The feedback loop ignites a high rate of RasGTP production when a threshold amount of SOS has been targeted to the membrane by fully phosphorylated LAT molecules and a sufficient number of them have RasGTP bound to the allosteric site.
Negative selection correlates with high levels of signaling and positive selection with low levels. Fig. 4 shows that ligands that induce high and low levels of Ras activation are separated by a sharply defined boundary. This suggests that the observed sharp boundary between positive and negative selectors may result from “digital” Ras activation by SOS.
Extensive parameter sensitivity analyses (SI) show that the qualitative results shown in Fig. 4 hold for wide ranges of parameters, provided some basic conditions are met: (i) a positive feedback loop in catalytic Ras activation by SOS, and (ii) Ras activation by RasGRP be sufficiently weaker than the largest rate at which SOS activates Ras.
Bimodal and Unimodal Signaling in Thymocytes.
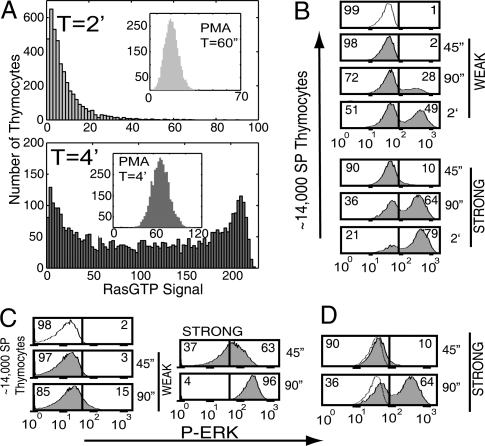
Fig. 5A shows histograms of Ras activity from 4,000 stochastic computer simulations of stimulation by a strong ligand. Each simulation (or trajectory) corresponds to a different cell. The simulations predict that, initially, most cells exhibit low levels of Ras activity. At later time points, a bimodal pattern of signaling is predicted as a second population of cells exhibiting much higher levels of active Ras emerges; that is, thymocytes are “on” or “off.” Bimodality is the consequence of positive feedback regulation of SOS resulting in a bifurcation, whereby a single stable solution transforms into two stable solutions and an unstable solution when stimulus exceeds a threshold value (27). Positive feedback is one of the biological motifs that are known to show bistability and switch-like responses (28). In the bistable region, because of fluctuations, different trajectories (cells) correspond to one of the two stable solutions.
Fig. 5.
Analogue and digital signaling in thymocytes. (A) Histogram of 4,000 computer simulation calculations of the RasGTP signal after “TCR stimulation” by a stimulus of 70 ZAP70 molecules. At 2 min the cells display a unimodal distribution of Ras levels (Top). At 4 min a bimodal distribution emerges. Inset shows the unimodal distribution of Ras activation after addition of 500 molecules of DAG with no ZAP70 (i.e., no TCR stimulation). RasGAP concentration was reduced from that in A to account for their dynamic regulation (23). (B) Analysis of ERK phosphorylation by flow cytometry in CD4 single-positive thymocytes that were stimulated WEAK or STRONG through their TCR for the indicated time points. Cells show an initial shift of the distribution, followed by a bimodal distribution with cells divided between those with low Erk activation and those with high Erk activation. For strong stimulation the bimodality occurs very fast. Numbers indicate the percentage of cells on either side of the divider. (C) Thymocytes stimulated and analyzed as in B, except that stimulation occurs via PMA, a DAG analogue, at a WEAK or STRONG dose. The distribution of cells shifts in an analogue manner to the right at both weak and strong stimulation. (D) Enlargement of “Strong Stimulation” data in B above to show details of the bimodal distribution of cells and the analogue shift at early times. The dotted line represents the ERK phosphorylation in unstimulated thymocytes.
We tested these predictions by carrying out experiments with double-positive and single-positive thymocytes (described in Methods). Single-cell assays for RasGTP levels are technically not feasible; so, we monitored ERK phosphorylation by FACS in single thymocytes, downstream of the Ras-RAF-MEK-ERK pathway. We focused our analyses on single-positive thymocytes because this subset expresses higher levels of TCR, and engagement of only the TCR on these cells effectively induces ERK phosphorylation. Fig. 5B shows that the expectations from the computer simulations are correct, with cells being either “on” or “off.” Similar experiments cross-linking both TCR and CD4 on double-positive thymocytes also demonstrate induction of digital ERK phosphorylation (data not shown).
The experimental results also imply that SOS is required for the bimodal activation of Erk. Stimulation of lymphocytes using phorbol myristate acetate (PMA) directly activates the RasGRP pathway and does not target SOS to the membrane. Fig. 5C shows that thymocytes exhibit a unimodal pattern of signaling when stimulated by PMA, a result that is true at all doses. This result is consistent with simulations (Fig. 5A, Inset) performed by increasing active levels of DAG (which stimulate RasGRP activity but not SOS). Note that the small analogue shift in the Erk signal in Fig. 5D is also observed in our computer simulations (Fig. 5A) and is a result of early analogue signaling due to RasGRP.
We find that for the parameters used in Figs. 3 and 4, the bimodal response emerges at times longer than the 2- or 3-min time observed in experiments (Fig. 5B). However, simple changes in parameters can reduce the time at which bimodality emerges without changing qualitative behavior (see SI). Kuriyan and coworkers have noted that the in vivo rate of SOS' GEF activity may be much higher than that measured in vitro (29). A fivefold increase in this rate, in addition to reducing the RasGDP level (unknown in thymocytes) to 75 molecules/μm2 and a lower rate of DAG production, results in bimodality at ≈4 min (Fig. 5A). In addition, all our simulations start with no basal level of active Ras. The distribution of basal Ras activity in real cells would accelerate the time at which bimodality emerges.
In Silico “Mutation Experiments” Are in Accord with Available Data and Make Experimentally Testable Predictions.
We have carried out simulations of the model shown in Fig. 2 with various “in silico mutations” to help design experiments that can test whether digital Ras signaling predicates the sharp discrimination between ligands that stimulate positive and negative selection (Fig. 6). Parameter sensitivity analyses support our conclusions (see SI).
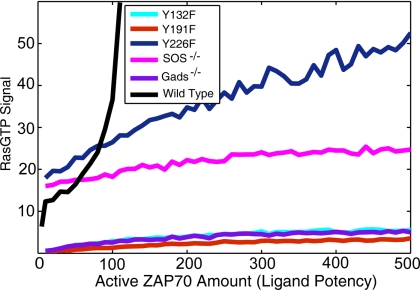
Fig. 6.
Total RasGTP production at a specific time obtained from computer simulations of models with various mutations. Each data point is obtained by averaging 30 stochastic simulations. Y132F, Y191F, and Y226F refer to mutations of the corresponding tyrosine to phenylalanine. Gads-/- and SOS-/- represent deletion of these molecules from the model. The wild type is shown for part of the ligand potency range for comparison.
The Y132F mutation prevents PLCγ1 binding to LAT, thereby inhibiting the activity of RasGRP. This also inhibits Ras activation by SOS because PLCγ1 is essential for forming complete LAT signalosomes, and the RasGRP pathway helps prime the SOS feedback loop (30). We expect, and prior experiments confirm, that both positive and negative selection are severely impaired in mice with this mutation (31). However peripheral T cells that do emerge participate in an uncontrolled lymphoproliferative syndrome (31). This could be due to altered signaling pathways or because TCRs with high affinity to endogenous pMHC that would normally be negatively selected are positively selected in mice with this mutation. T cells with such TCRs would be activated by endogenous pMHC expressed more abundantly in peripheral organs.
Gads plays an important role in activating PLCγ1 by recruiting SLP76-Itk to the signalosome and stabilizes the binding of both PLCγ1 and Grb2 to LAT (Fig. 2). Therefore, deletion of Gads from our model has a profound effect on Ras activation (Fig. 6 and SI) because it affects signaling by both Ras GEFs. In accord, both positive and negative selection is severely impaired in Gads-/- mice (32).
Gads bound on Y191 activates PLCγ1 on Y132 and stabilizes both PLCγ1 on Y132 and Grb2/SOS on Y226. Hence, mutating Y191 to phenylalanine has the effects associated with the Gads-/- phenotype (Fig. 6 and SI). In the Y226F mutation, positive selecting signals are unaffected, but negative selection seems to be abrogated (Fig. 6). Parameter sensitivity analysis (see SI), however, indicates a less severe phenotype, because negative selection may be possible at higher ligand potencies. Note that Y191 and Y226 in our model are proxies for multiple tyrosines on LAT, and results obtained by mutating them need to be interpreted with care.
Computer simulations of a model where SOS is deleted (Fig. 6) show that the sharp rise in signaling beyond a threshold potency of the ligand is abrogated. However, signaling levels are not affected much for weakly stimulatory ligands because the RasGRP pathway is unaffected. Thus, we predict that SOS deficiency should result in profound defects in negative selection but essentially normal positive selection. Although thymocyte development in SOS1-/- or SOS2-/- mice has not been examined, haplodeficiency of Grb2 (which recruits SOS) affects only negative selection (33). Our simulations also predict that blocking feedback regulation of the GEF activity of SOS in experiments such as those carried out by Daniels et al. (3) would abrogate the sharp boundary separating ligands that stimulate positive and negative selection (see SI).
Discussion
The sharp threshold in TCR-pMHC binding characteristics that defines the border between negative and positive selection is expected to suppress fluctuation-mediated escape of potentially autoreactive T cells. The graded response of thymocytes to positively selecting ligands determines diversity of the available T cell repertoire. Therefore, the mechanistic origin of these observations (3) is important for understanding how the T cell repertoire is selected and the consequences of its aberrant regulation.
Our study provides such a mechanistic description. Cooperative assembly of the LAT signalosome results in a separation in the range of ligand potencies that can stimulate the GEF activity of SOS and RasGRP. Weak ligands activate Ras primarily via RasGRP, whereas targeting SOS to the membrane requires strong ligands. Because RasGRP's GEF activity is not subject to feedback regulation, signaling increases in a graded manner with ligand potency when this path to Ras activation is dominant, and this underlies the analogue response of thymocytes to different weakly stimulatory positively selecting ligands. Because of positive feedback regulation of SOS, active Ras levels increase dramatically above a threshold level of stimulus. This underlies the sharply defined boundary separating ligands that stimulate positive and negative selection.
An alternative scenario is that the large increase in Ras production due to the feedback loop in SOS serves only to ignite downstream feedback loops that are necessary for the digital response. Candidates for such downstream signaling modules include the MAPK pathway (34), especially feedback to upstream signaling through cytoplasmic phospholipase 2 (cpla2). cpla2 is absent in mature lymphocytes, and this may be why our recent results show that feedback regulation of SOS underlies a digital response in mature T cells (27). Although cpla2 is present in thymocytes (35), the unimodal ERK response to PMA stimulation seen in our experiments (Fig. 5C) seems to suggest that digital ERK signaling is predicated upon digital Ras activation by SOS.
It has been suggested that compartmentalization of Ras signaling plays a role in the selection process; that is, positively selecting ligands stimulate RasGTP signals mainly at the Golgi, whereas negatively selecting ligands lead to high levels of RasGTP at the plasma membrane (3). TCR ligation leads to RasGRP translocation at both the plasma membrane and the Golgi, where it can be activated by DAG (30) and can activate Ras. SOS activates Ras only at the plasma membrane (36). Incorporation of these assumptions in our signaling model (see SI) leads to the result that positively selecting ligands stimulate Ras activation via RasGRP at the Golgi, whereas negatively selecting ligands induce Ras activity primarily at the plasma membrane (see SI).
Our proposed mechanism is consistent with diverse experimental data, including the following: RasGRP1 deficiency results in defective positive, but normal negative selection (37); Grb2-haplodeficient mice exhibit defects in negative, but not positive, selection (33); Grb2/SOS is recruited efficiently to the plasma membrane by negatively selecting ligands, but not positively selecting ligands (5); and deletion of Gads severely impairs both positive and negative selection (32). Tests of our proposal that specific mutations would abrogate the sharp boundary separating positive and negative selecting ligands would further enhance our understanding of signaling during T cell development.
Methods
Computational Studies.
We simulated the signaling pathway shown in Fig. 2 on a computer using the Gillespie algorithm (37), which accounts for the effects of stochastic fluctuations (details in SI). Noise and fluctuations have been shown to have important effects on biochemical networks involved in gene regulation and signaling (including T cell signaling) (28). Indeed, we show that intrinsic fluctuations lead to the emergence of bimodality in Ras activation and compare these predictions with experiments (see below).
The kinetic parameters used in the simulations are provided in the SI. Parameters for several reactions, particularly crucial ones measuring the catalytic conversion of RasGDP to RasGTP, are known. We have used biologically reasonable estimates for the unknown parameters and have tested the robustness of the qualitative results to wide variations (by a factor of 25) in the chosen values (see SI).
Experimental Studies.
To test some of the predictions of the computer simulations, we carried out experiments assaying signaling in thymocytes. Single-cell suspensions of primary thymocytes in DMEM were rested at 37 °C for at least 45 min before stimulation. Cells were stimulated through their TCR using anti-CD3 hamster antimouse antibody (2C11 clone, Harlan) followed by cross-linking with goat anti-Armenian hamster Ig (H+L) (Jackson Immunoresearch Laboratories; final concentration of 50 μg/ml). 2C11 was used at a final concentration of either 5 μg/ml (WEAK) or 25 μg/ml (STRONG). Alternatively, cells were stimulated with PMA, used at a 125-μg/ml WEAK or 250-μg/ml STRONG dose. Cells were fixed for 20 min with 150 μl fixation buffer (Cytofix/Cytoperm, BD Biosciences), washed twice in staining buffer (SB; Ca/Mg free PBS, 2 mM EDTA, 3% FCS), and permeabilized for 30 min on ice by drop-wise addition of 200 μl 100% methanol (at −20 °C) to a loosened cell pellet. The sample was rehydrated in SB × 30 min and washed two times in SB. Cells were stained for 45 min at room temperature in 50 μl SB containing 1 μl Phospho-p44/42 MAP Kinase (Thr-204/Tyr-204) antibody (Cell Signaling) and 1 μl Fc receptor block (2.4G2; BD Biosciences). Subsequently, cells were washed two times in SB and stained for 45 min at room temperature with 50 μl SB containing 1 μl 2.4G2, 1 μl APC-conjugated AffiniPure F(ab′)2 fragment donkey antirabbit IgG (Jackson Immunoresearch Laboratories), cell-surface staining for CD4 (anti-CD4-PE) and CD8 (anti-CD8-FITC, both BD Biosciences.) Cells were then washed two times in SB and directly analyzed by FACS. Cell-surface stainings were used to gate on ERK phosphorylation in specific thymocyte subsets.
Supplementary Material
Acknowledgments.
We thank Dr. Ed Palmer for stimulating discussions. This work was supported by the National Institutes of Health Director's Pioneer Award and Grant 1-PO1-AI071195–01 (to A.K.C.), the Howard Hughes Medical Institute and the Rosalind Russell Medical Research Center for Arthritis (to A.W.), and the Sandler Foundation (to J.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805981105/DCSupplemental.
References
- 1.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: Timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 4.Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–294. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 5.Ebinu JO, et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 6.Sondermann H, et al. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Freedman TS, et al. A Ras-induced conformational switch in the Ras activator Son of sevenless. Proc Natl Acad Sci USA. 2006;103:16692–16697. doi: 10.1073/pnas.0608127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 9.Neumeister EN, et al. Binding of ZAP-70 to phosphorylated T-cell receptor zeta and eta enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins. Mol Cell Biol. 1995;15:3171–3178. doi: 10.1128/mcb.15.6.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: Analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–4933. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 14.Houtman JC, et al. Binding specificity of multiprotein signaling complexes is determined by both cooperative interactions and affinity preferences. Biochemistry. 2004;43:4170–4178. doi: 10.1021/bi0357311. [DOI] [PubMed] [Google Scholar]
- 15.Beach D, Gonen R, Bogin Y, Reischl IG, Yablonski D. Dual role of SLP-76 in mediating T cell receptor-induced activation of phospholipase C-gamma1. J Biol Chem. 2007;282:2937–2946. doi: 10.1074/jbc.M606697200. [DOI] [PubMed] [Google Scholar]
- 16.Bogin Y, Ainey C, Beach D, Yablonski D. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc Natl Acad Sci USA. 2007;104:6638–6643. doi: 10.1073/pnas.0609771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seet BT, et al. Efficient T-cell receptor signaling requires a high-affinity interaction between the Gads C-SH3 domain and the SLP-76 RxxK motif. EMBO J. 2007;26:678–689. doi: 10.1038/sj.emboj.7601535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chardin P, et al. Human Sos1: A guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz S, Santos E, Bustelo XR. RasGRF2, a guanosine nucleotide exchange factor for Ras GTPases, participates in T-cell signaling responses. Mol Cell Biol. 2007;27:8127–8142. doi: 10.1128/MCB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pages G, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 21.Rincon M, et al. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binetruy B, Smeal T, Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351:122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- 23.Lockyer PJ, Kupzig S, Cullen PJ. CAPRI regulates Ca(2+)-dependent inactivation of the Ras-MAPK pathway. Curr Biol. 2001;11:981–986. doi: 10.1016/s0960-9822(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 24.Balagopalan L, et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol Cell Biol. 2007;27:8622–8636. doi: 10.1128/MCB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markevich NI, Hoek JB, Kholodenko BN. Signaling switches and bistability arising from multisite phosphorylation in protein kinase cascades. J Cell Biol. 2004;164:353–359. doi: 10.1083/jcb.200308060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houtman JC, et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 27.Das J, et al. Digital signaling and hysteresis characterize Ras activation in lymphoid cells. Cell. 2008 doi: 10.1016/j.cell.2008.11.051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arkin A, Ross J, McAdams HH. Stochastic kinetic analysis of developmental pathway bifurcation in phage lambda-infected Escherichia coli cells. Genetics. 1998;149:1633–1648. doi: 10.1093/genetics/149.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gureasko J, et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol. 2008;15:452–461. doi: 10.1038/nsmb.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommers CL, et al. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoder J, et al. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291:1987–1991. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]
- 33.Gong Q, et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 34.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert JJ, et al. Antigen receptors on immature, but not mature, B and T cells are coupled to cytosolic phospholipase A2 activation: Expression and activation of cytosolic phospholipase A2 correlate with lymphocyte maturation. J Immunol. 1996;156:2054–2061. [PubMed] [Google Scholar]
- 36.Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 37.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie DT. Exact stochastic simulation of coupled chemical reactions. J Phys Chem. 1977;81:2340–2361. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.