Abstract
Angiogenesis, the synthesis of new blood vessels from preexisting vessels, plays a critical role in normal wound healing and tumor growth. HKa (cleaved high molecular weight kininogen) is an endogenous inhibitor of angiogenesis formed by the cleavage of kininogen on endothelial cells. Ferritin is a protein principally known for its central role in iron storage. Here, we demonstrate that ferritin binds to HKa with high affinity (Kd 13 nM). Further, ferritin antagonizes the antiangiogenic effects of HKa, enhancing the migration, assembly, and survival of HKa-treated endothelial cells. Effects of ferritin were independent of its iron content. Peptide mapping revealed that ferritin binds to a 22-aa subdomain of HKa that is critical to its antiangiogenic activity. In vivo, ferritin opposed HKa's antiangiogenic effects in a human prostate cancer xenograft, restoring tumor-dependent vessel growth. Ferritin-mediated regulation of angiogenesis represents a new angiogenic regulatory pathway, and identifies a new role for ferritin in cell biology.
Keywords: iron, kininogen
Angiogenesis, the process of making new blood vessels from preexisting vessels, plays a key role in physiologic processes ranging from wound healing to tumor growth. Angiogenesis is a carefully orchestrated process that is regulated by the balance between pro- and antiangiogenic factors. When this balance is altered, pathologic angiogenesis occurs. For example, during malignancy, many tumors alter the local levels of angiogenic factors, induce blood vessel formation, and thereby facilitate growth and dissemination of tumor cells (1).
High molecular weight kininogen (HK) is a plasma protein that serves as a cofactor in the intrinsic coagulation cascade and is an inhibitor of cysteine proteases (2). In addition to these activities inherent in the intact protein, proteolytic cleavage of HK by kallikrein produces 2 molecules with additional bioactivities: the nonapeptide bradykinin, a potent peptide hormone that mediates NO release, vasodilation, and pain (3), and 2-chain high molecular weight kininogen, HKa.
HKa is markedly different in structure and function from its parent protein, HK. It exhibits an altered conformation (4) and receptor binding (5). Most strikingly, HKa acquires biological properties not found in HK, exerting antiangiogenic effects on endothelial cells in vitro and in vivo (6, 7). In addition, HKa reduces the invasion and metastasis of osteosarcoma, breast, and lung cancer in mouse models (8, 9).
Ferritin is a 24-subunit intracellular protein that stores iron in a nontoxic yet bioavailable form (10). Ferritin also exists in extracellular compartments, such as the serum (11). Serum ferritin has low iron content and a distinctive subunit composition (10). Although levels of serum ferritin are used as a measure of total body iron (12), serum ferritin is also profoundly affected by acute and chronic inflammation, conditions under which it may rise 10- to 100-fold (13). In addition, serum ferritin levels are elevated in many forms of malignancy including neuroblastoma, Hodgkin's lymphoma, intestinal, liver, lung, ovarian, pancreatic, stomach, and breast cancers (10, 14). These elevations are independent of changes in body iron stores (12).
Ferritin is a binding partner of HK, and may be an important physiological regulator of its activity (15, 16). Ferritin exhibits specific and saturable binding to HK, with a Kd of ≈140 nM (17). Binding of ferritin to HK inhibits the cleavage of HK by kallikrein, thus reducing production of bradykinin (16) and its attendant proinflammatory effects. In addition, HK and ferritin colocalize at sites of inflammation, where ferritin inhibits the cleavage of HK by tryptase and elastase (17).
Ferritin binds to the light chain of HK, a region that is preserved in HKa, the antiangiogenic cleavage product of HK (16, 18). The presence of a shared ferritin target sequence in HK and HKa prompted us to ask whether ferritin can bind to HKa and regulate its antiangiogenic activity.
Results
Ferritin Exhibits High-Affinity Binding to HKa.
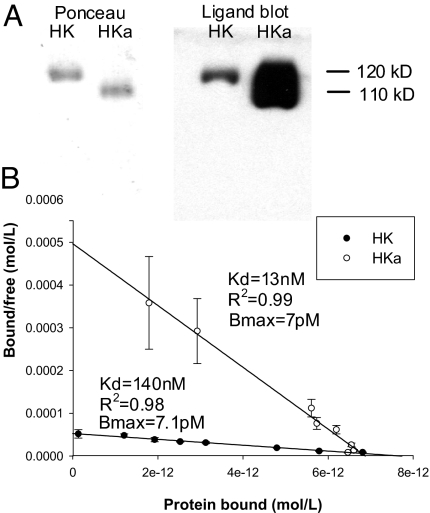
We first tested whether ferritin would bind to HKa by using a ligand blotting technique. As seen in Fig. 1A, ferritin bound both HK and HKa. Interestingly, although Ponceau staining verified equal loading of HK and HKa, ferritin binding was more intense in the HKa lane compared with the HK lane, suggesting preferential binding of ferritin to HKa.
Fig. 1.
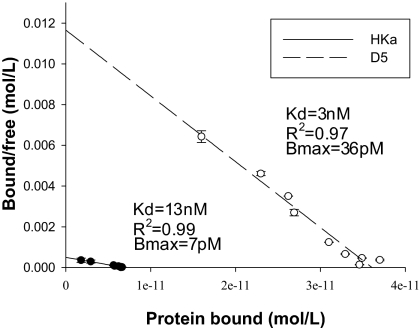
Binding of HKa to immobilized ferritin. (A) HK (120 kDa) and HKa (110 kDa) were subjected to SDS-PAGE and analyzed for ferritin binding activity by ligand blotting with a human spleen ferritin followed by visualization with a HRP-anti-Ft antibody. Left panel shows the Ponceau stain of the membrane and the Right panel shows the subsequent ligand blot. (B) Biotinylated HKa or HK was added in increasing amounts to immobilized ferritin, and Kd and Bmax were determined by Scatchard analysis. Shown are the means and standard deviations of three independent experiments.
To confirm and quantify the differential ferritin binding between HK and HKa observed in the ligand blot, we conducted a solid phase binding assay. Both HK and HKa demonstrated specific and saturable binding to ferritin (Fig. 1B). Scatchard analysis of the HKa curve revealed a Kd of 13 nM (R2 = 0.99). This represents a 10-fold higher affinity over the HK/ferritin interaction (Kd of 140 nM, R2 = 0.98). Both HK and HKa demonstrate a similar Bmax (7 pM for HKa, 7.1 pM for HK), indicating that HK and HKa interact with ferritin in similar stochiometric ratios. Thus, ferritin binds more tightly to HKa than to HK.
Ferritin Blocks Effects of HKa on Endothelial Cell Viability.
We next tested whether ferritin would affect the activity of HKa and in particular whether ferritin would impede the antiangiogenic effects of HKa.
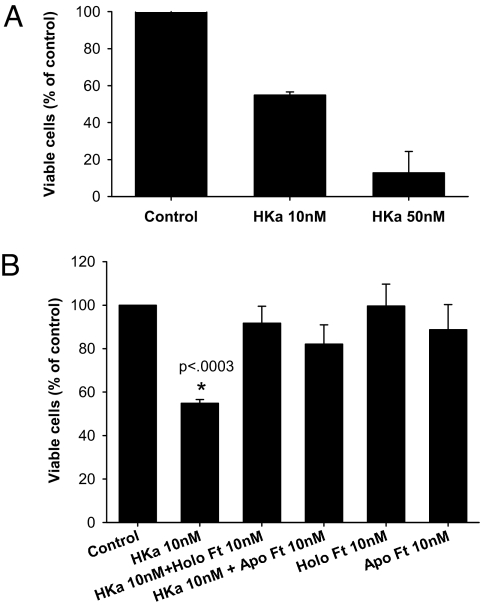
We initially assessed the effect of ferritin and HKa on the viability of endothelial cells. HKa exerts its antiproliferative effects on subconfluent, actively proliferating endothelial cells on a provisional extracellular matrix such as vitronectin (19). We therefore seeded human umbilical vein endothelial cells (HUVEC) onto vitronectin-coated plates at subconfluent density and then either left them untreated or treated them with HKa. As seen in Fig. 2A, treatment with HKa led to a dose-dependent inhibition of endothelial cell viability, with an IC50 of ≈10 nM, consistent with previous results (20). To test whether ferritin would block this effect of HKa, cells were treated with 10 nM HKa and 10 nM Ft, alone or in combination. As seen in Fig. 2B, HKa decreased the viability of endothelial cells to 55 ± 1.7% of control (P < 0.0003). Cotreatment with HKa and ferritin at a 1:1 molar ratio (Ft:HKa) markedly increased cell viability to 91.7 ± 8.8% of control. Treatment with ferritin alone did not alter the viability of endothelial cells (99.6 ± 11.4% of control). Therefore, ferritin, while not altering endothelial cell proliferation itself, blocks the HKa-induced reduction in endothelial cell viability.
Fig. 2.
Effect of HKa and ferritin on HUVEC viability. Viability was assessed by using an MTS assay after 48-h treatment with the indicated agents. Values represent means and standard deviation of 4 independent experiments. *, P < 0.0003, statistically significant difference from all other groups.
Effects of Ferritin on HKa Activity Are Not Iron Dependent.
Ferritin can store up to 4500 atoms of iron, and is principally known for its iron storage activity (10). To test whether iron contributes to the ability of ferritin to antagonize HKa activity, we compared apoferritin (ferritin from which >99% of iron had been removed) to holoferritin (iron-containing ferritin) for its ability to block the antiproliferative activity of HKa. As seen in Fig. 2B, apoferritin and holoferritin were equivalent in their ability to restore endothelial cell viability to control levels, demonstrating that the presence of iron within ferritin does not contribute to the ability of ferritin to block HKa-induced reduction in endothelial cell viability.
Ferritin Antagonizes the Proapoptotic Effects of HKa.
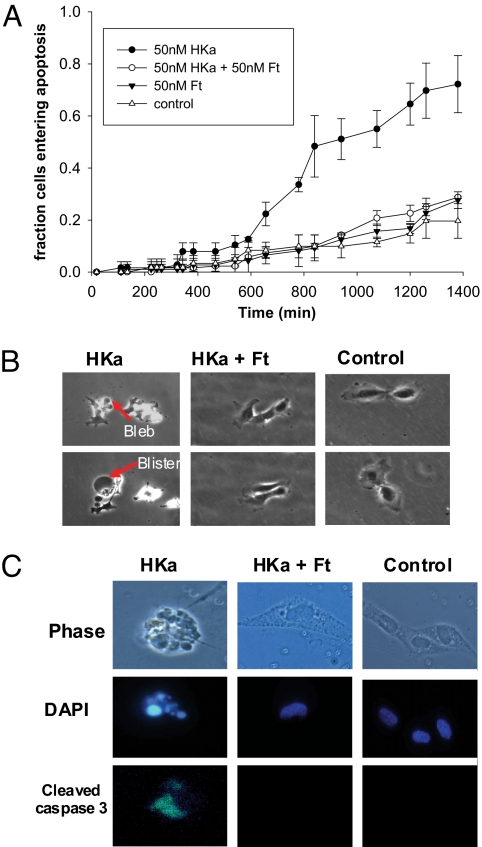
To test whether ferritin increases endothelial cell viability by inhibiting HKa-induced apoptosis, cells were treated with 50 nM HKa and ferritin alone or in combination, and the number of cells entering apoptosis over time was determined by using time-lapse microscopy (Fig. 3A). Time of entry of each cell to apoptosis was defined as the appearance of the first apoptotic bleb (Fig. 3B). After 10 h, HKa-treated cells began to undergo apoptosis. By 23 h, nearly 80% of HKa-treated cells had entered apoptosis as compared with ∼30% of HKa/Ft cotreated cells (Fig. 3A). The fraction of HKa/Ft cotreated cells entering apoptosis was not significantly different from control or cells treated with Ft alone at any time point.
Fig. 3.
Effect of HKa and ferritin on endothelial cell apoptosis. (A) Time-lapse microscopy was performed on HUVECs treated with 50 nM HKa and 50 nM ferritin, alone or together. The number of cells entering apoptosis, as identified by the formation of apoptotic blebs, was counted over a period of 24 h and compared with control cells with no treatment. Results are the mean and standard deviation of 2 independent experiments. Apoptosis in HKa-treated cells was statistically different from all other groups after the 11-h time point (P < 0.001); all other treatment groups were not statistically different from each other at any time point. The rate of apoptosis in control cells is similar to that observed in HUVEC cells plated on vitronectin (36). (B) Phase contrast images of HKa, HKa + ferritin, and control-treated cells during the time-lapse microscopy experiment. Top row, cells at 10 h; Bottom row, cells at 12 h. (C) Cell treated with HKa and ferritin, alone or together, were stained after 24 h for the presence of the apoptotic marker cleaved caspase 3. DAPI staining was used to identify fragmented nuclei. Shown are representative individual cells exhibiting caspase 3 activation and nuclear fragmentation.
To further confirm that cell death was specifically the result of apoptosis, we analyzed cells for the presence of activated caspase 3 and nuclear fragmentation, biochemical hallmarks of apoptosis (21, 22). HKa-treated endothelial cells exhibited activated caspase 3 and nuclear fragmentation, as measured by immunohistochemistry and DAPI staining, respectively (Fig. 3C), indicating that apoptosis was the mechanism of endothelial cell death. Consistent with results obtained by using time-lapse microscopy, ferritin reduced the number of apoptotic cells from 59% in the presence of HKa alone to 23.5% in the combined presence of ferritin and HKa, as measured by staining for activated caspase 3. Thus, ferritin opposes the proapoptotic effects of HKa.
Ferritin Antagonizes the Antimigratory Effects of HKa.
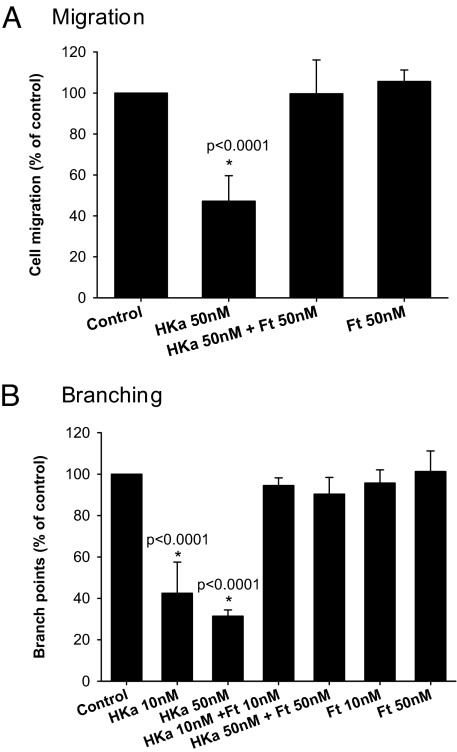
HKa has also been shown to inhibit endothelial cell migration, another key early step in angiogenesis (7). To test whether ferritin modulates this activity of HKa, we used a transwell system with bFGF as the chemoattractant and analyzed migration after 5 h (Fig. 4A). HKa reduced the number of migrating cells to 47.2 ± 12.2% of control (P < 0.0001, difference from control) whereas HKa/Ft cotreatment restored migration to 99.6 ± 16.1% of control. Ferritin treatment alone did not alter migration, with cells migrating at 105.7 ± 7.7% of control levels. To ensure that the effect seen was the result of changes in cell migration and not differences in viability, the total number of viable cells was assessed. There was no difference in the number of viable cells under any experimental condition (data not shown), consistent with the time-lapse microscopy, which also indicated no effects of HKa on viability at this early (5-h) time point (Fig. 3A). Thus, ferritin inhibits the antimigratory effect of HKa.
Fig. 4.
Ferritin blocks HKa-dependent inhibition of endothelial cell migration and assembly into vessel-like structures. (A) HUVECs treated with HKa and ferritin alone or together were allowed to migrate for 5 h toward bFGF. The values represent means and standard deviation of 4 independent experiments. *, P < 0.0001, significant difference from all other treatments. Differences between all other groups were not statistically significant. There was no effect of any treatment on viability at this timepoint (data not shown). (B) HUVECs were allowed to differentiate for 7 h into 2D tube-like structures and the number of branch points per 100 viable cells was counted. Values are the means and standard deviation of 3 independent experiments. *, P < 0.0001, HKa at 10 and 50 nM were significantly different from all other groups.
Ferritin Restores the Ability of Hka-Treated Endothelial Cells to Form Vessel-Like Structures (Tubes).
The ability of endothelial cells to assemble into vessel-like structures (tubes) is an important aspect of blood vessel formation that is sensitive to inhibition by HKa (23). To investigate whether ferritin blocks the inhibitory effect of HKa on tube formation, endothelial cells were incubated with matrigel, which induces the cells to assemble into branching tube-like structures (24, 25), in the presence of HKa, ferritin, or the combination of HKa plus ferritin. After 7 h, tube formation was quantified in a standard assay that measures the number of branch points per 100 viable cells. As seen in Fig. 4B, HKa reduced the number of branch points per 100 viable cells to 42.5 ± 15% (P < 0.0001) and 31.4 ± 3% (P < 0.0001) of control at 10 nM and 50 nM levels, respectively. Cotreatment of ferritin with HKa restored branch points to 94.5 ± 3.7% and 90.4 ± 7.9% of control at 10 nM HKa/10 nM Ft and 50 nM HKa/50 nM Ft levels, respectively. Ferritin alone did not alter the number of branch points formed (95.7 ± 6.3% and 101.3 ± 9.7% of control at 10 nM and 50 nM levels, respectively).
Ferritin Binds to the Antiangiogenic Domain of HKa.
Our observations that ferritin inhibits the antiangiogenic activity of HKa at multiple levels suggested that ferritin might exert its effects by direct binding to the antiangiogenic site of HKa. HKa is a multidomain protein consisting of 5 domains organized into a heavy chain (domains 1–3) and a light chain (domains 5 and 6). These domains remain linked by a disulfide bond following proteolytic removal of bradykinin (contained in domain 4) from the parent protein, HK. The antiangiogenic region of HKa has been mapped to domain 5 (7).
To test the possibility that ferritin inhibits the antiangiogenic activity of HKa by direct binding to domain 5, we created recombinant glutathione-s-transferase (GST) fusion proteins comprising different domains of HK and tested their binding to ferritin by using a ligand blot. These experiments demonstrated that ferritin binds to D4–6-GST and D5-GST, but not to D6-GST or GST alone [supporting information (SI) Fig. S1].
To verify this finding, we conducted a solid phase binding assay by using purified domain 5 freed of the GST tag. As seen in Fig. 5, D5 also exhibited specific and saturable binding to ferritin, with Kd of 3 nM (R2 = 0.97). Domain 5, therefore, binds ferritin with a higher affinity than either HK or HKa. Interestingly, whereas both proteins exhibit high affinity ferritin binding, domain 5 binding saturates at approximately 5 times the level of HKa (D5 Bmax = 36 pM, HKa Bmax = 7 pM) (Fig. 5). This indicates that domain 5 binds ferritin at a higher stoichiometric ratio than HKa or HK (≈5:1 stoichiometric ratio D5:Ft vs. 1:1 stoichiometric ratio HK/HKa:Ft). Further, this finding reveals that ferritin contains multiple binding sites for domain 5 of HK/HKa.
Fig. 5.
Ferritin binds domain 5 of HK/HKa. Scatchard analysis of binding of biotinylated D5 or HKa to immobilized ferritin. Shown are the means and standard deviations of 3 independent experiments.
Ferritin Binds the Antiproliferative, Proapoptotic Region Within Domain 5.
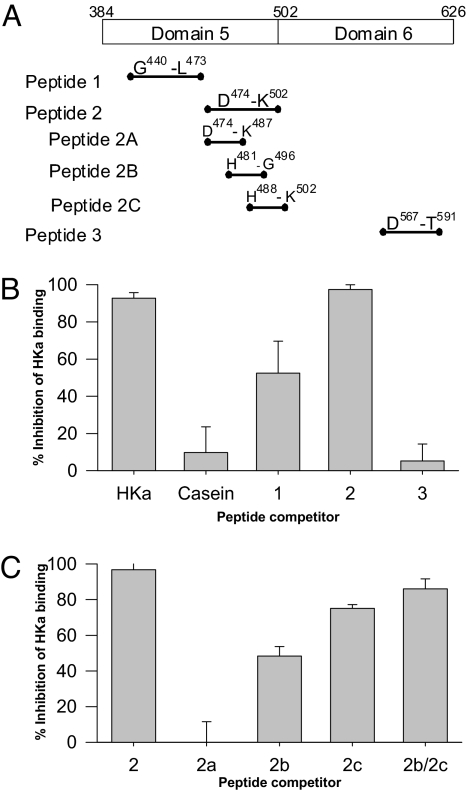
To further narrow the ferritin binding site, synthetic peptides were used as competitors. We initially created 3 peptides (peptides 1–3, Fig. 6A). Peptides 1 and 2 span portions of the histidine-glycine (HG) (K438–D492) and histidine-glycine-lysine (HGK) (H493–K520) rich regions (respectively) within domain 5, and peptide 3 is the prekallikrein binding region within domain 6 (see Table S1 for amino acid sequences of all peptides). Each of these regions has a physiologic function: Peptide 1 encompasses a region responsible for the antiadhesive function of HKa (8), peptide 2 spans an antiproliferative/proapoptotic and cell binding region of HKa (20, 26), and peptide 3 comprises the prekallikrein binding region (18). The HGK region (contained in peptide 2) has also been recognized to inhibit the adhesion, invasion, and metastasis of cancer cells (9).
Fig. 6.
Ferritin binds within the HGK rich region of domain 5 of HK/HKa. (A) Schematic representation of synthetic HK peptides used as competitors for binding of HKa to immobilized ferritin. (B) Nonbiotinylated HKa (positive control), casein (negative control), or the indicated peptides were added in 50 molar excess with biotinylated HKa to immobilized ferritin and biotinylated HKa binding was assessed. (C) Percent inhibition of biotinylated HKa binding to immobilized ferritin by peptides 2, 2A, 2B, 2C, and the combination of 2B and 2C. Shown are the means and standard deviation of 3 independent experiments.
These peptides were used as competitors of binding of biotinylated HKa to ferritin in the solid phase binding assay. As shown in Fig. 6B, peptide 3 at a 50-fold molar excess is a poor competitor, reducing HKa binding by 5 ± 9%. Peptide 1 exhibits an intermediate level of competition, reducing HKa binding by 52 ± 17% at a 50-fold molar excess. Peptide 2 is an excellent competitor, reducing HKa binding by 96 ± 1.7%, even at a 10-fold molar excess. The ability of peptide 2 to strongly compete for ferritin binding localizes the minimal ferritin binding region to this 28-aa domain.
To further narrow the ferritin binding domain of HKa, 3 additional peptides spanning the region represented in peptide 2 were created: peptide 2A (N-terminal region), 474DHGHKHKHGHGHGK487; peptide 2B (central region), 481HGHGHGKHKNKGKKNG496; and peptide 2C (C-terminal region), 488HKNKGKKNGKHNGWK502 (Fig. 6A). Peptide 2A had no inhibitory effect on HKa binding to ferritin (50-fold molar excess, 0 ± 11% inhibition). Peptides 2B and 2C, used independently, inhibited HKa binding by 48 ± 5.3% and 75 ± 2% (respectively). Used together, peptides 2B and 2C inhibited binding by 86 ± 5.6%. Therefore, the ferritin binding region is contained within the 22-aa C-terminal region of peptide 2 represented by peptides 2B and 2C.
Ferritin Antagonizes the Antiangiogenic Effects of HKa in a Tumor Angiogenesis Model.
Because these results indicated that ferritin blocks the antiangiogenic effects of HKa through specific binding to the antiangiogenic domain of HKa, we tested whether HKa and ferritin would exhibit similar effects on angiogenesis in vivo. Although HKa has not been directly implicated in tumor angiogenesis, both HKa (9) and ferritin have been linked to malignant processes (27). We therefore asked whether HKa would inhibit tumor angiogenesis, and whether ferritin could override this effect. PC3 prostate cancer cells were mixed with growth factor-reduced matrigel and either HKa, ferritin, the combination of HKa and ferritin, or saline as a control, and the cell suspension was injected s.c. into the flanks of athymic nude mice. After 10 days, microvessel density was assessed by quantifying 2 standard measures of in vivo angiogenesis: the density of angiogenic “hot spots” per tumor section (the number of blood vessel clusters per tumor section at 100× magnification, reported as number of hot spots/mm2 tumor cross-sectional area), and vessel density within the hot spot (the number of vessels seen at 400× magnification within a hot spot) (28).
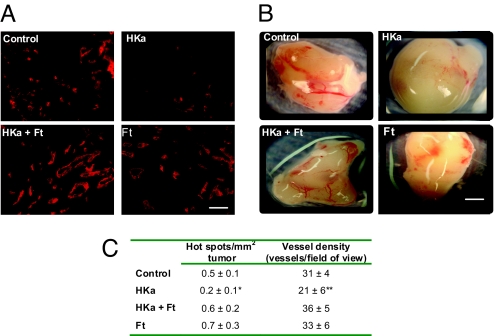
Fig. 7A shows representative images of blood vessels within a hot spot in each treatment group whereas Fig. 7B shows representative images of the entire tumor following resection from the mouse flank. Quantification of hot spot density, as displayed in Fig. 7C, revealed that untreated tumors in control mice had 0.5 ± 0.1 hot spots/mm2. As anticipated from its antiangiogenic effects in vitro, HKa decreased the number of hot spots in vivo to 0.2 ± 0.1 hot spots/mm2. Ferritin was able to block the antiangiogenic activity of HKa, resulting in a mean hot spot density of 0.6 ± 0.2, a statistically significant increase when compared with HKa alone (P = 0.0003). Ferritin alone had no effect on hot spot density. Similar results were obtained when vessel densities within the hot spots were quantified: HKa alone reduced vessel density from 31 ± 4 in controls to 21 ± 6 in HKa-treated mice, and this decrease was blocked in the presence of ferritin, which restored vessel density to 36 ± 5, a statistically significant increase when compared with HKa alone (P = 0.0001). Ferritin alone did not affect vessel density. Antiangiogenic effects of HKa were not secondary to cytotoxic effects on the tumor itself, because HKa did not affect proliferation of PC3 prostate cancer cells in vitro (data not shown), and because at this early time point, tumors in all treatment groups were similar in size, with an average tumor volume of 53 ± 21 mm3.
Fig. 7.
Inhibition of angiogenesis by HKa in PC3 xenografts is blocked by ferritin. (A) PC3 prostate cancer cells mixed with 400 nM HKa, 400 nM HKa + 400 nM Ft, 400 nM Ft, or saline control were injected s.c. in the flank of athymic nude mice. Tumors were resected 10 days postinoculation and stained with anti-CD-31 to identify blood vessels. Mean blood vessel density was calculated for each treatment type. (Scale bar, 45 μm.) (B) Gross images of the tumors after resection from the mouse flank. (Scale bar, 1.5 mm.) (C) Quantification of blood vessel density from all tumors. Means and 95% confidence interval are shown. *, P = 0.0003, difference from Hka + ferritin; **, P = 0.0001, difference from Hka + ferritin.
Discussion
HKa is a recently discovered inhibitor of angiogenesis that targets endothelial cells (29). HKa has been shown to inhibit angiogenesis in in vivo assays of angiogenesis, such as the chick chorioallantoic membrane (CAM) and corneal micropocket assays (6, 8, 30), but has not been shown to inhibit tumor angiogenesis. In this manuscript, we demonstrate that HKa inhibits angiogenesis induced by prostate cancer cells in a tumor xenograft, substantially reducing tumor vascularity as measured both by number of vascular hot spots (blood vessel clusters per tumor area) and density of vessels within those hot spots (Fig. 7).
To explore the role of ferritin in modulating the effects of HKa on angiogenesis, we first examined the binding of ferritin to both HK and HKa. Our binding studies revealed that ferritin interacts not only with HK, as we have demonstrated (15, 16), but also with HKa (Fig. 1). We therefore tested whether ferritin modulates the antiangiogenic activity of HKa, a protein structurally and functionally distinct from HK (4, 5).
By using primary endothelial cells and well-established assays of angiogenesis, we demonstrate that ferritin effectively antagonizes 3 antiangiogenic activities of HKa: induction of apoptosis, inhibition of migration, and inhibition of tube formation. Inhibition of endothelial cell migration (Fig. 4A) and assembly into branching tube-like structures (Fig. 4B) occurred within 5–7 h of HKa treatment. Ferritin antagonized both of these antiangiogenic processes (Fig. 4 A and B). At ≈10 h, HKa-treated cells began to enter apoptosis, a process that ultimately results in a profound decrease in overall survival of HKa-treated cells (Fig. 3). Ferritin also completely inhibited this process (Fig. 3). The ability of ferritin to prevent the antiangiogenic effects of HKa was also observed in vivo: Ferritin blocked the antiangiogenic effect of HKa in tumor xenografts, restoring tumor-dependent assembly of blood vessels to control levels (Fig. 7). Collectively, these findings suggest that the interplay between HKa and ferritin, 2 endogenous proteins, contributes to regulation of angiogenesis, particularly during tumor growth.
Although ferritin restored migration, assembly, and survival of HKa-treated endothelial cells and preserved angiogenesis in the presence of HKa in vivo, ferritin alone did not affect any of these parameters, indicating that its effects were mediated through direct antagonism of HKa. In further support of this direct interaction model, we localized the ferritin binding domain to a critical antiangiogenic region within domain 5 of HKa (Figs. 5 and 6). This region has a high content of basic amino acid residues, particularly histidines and lysines, suggesting that charge interactions contribute to the binding of HKa to ferritin. However, because the his + lys content of a noncompeting peptide (peptide 2A—64% his + lys residues) was similar to that of the most effective competitor (peptide 2C—53% his + lys residues), additional interactions must also come into play. Structural studies will be required to fully delineate the binding interface between these 2 proteins.
The ability of ferritin to antagonize the antiangiogenic effects of HKa identifies a new role for ferritin in physiologic processes. Studies of ferritin have largely focused on its role as an iron storage protein. However, holoferritin and apoferritin were equally active in inhibiting the effects of HKa on endothelial cell viability (Fig. 2), indicating that antagonism of HKa is an iron-independent function of ferritin. These results are consistent with a model in which ferritin regulates HKa by surface interactions that do not involve the internal iron core of ferritin.
Many of the molecular and cellular details of the interaction between HKa and ferritin remain to be clarified. In particular, the domain of ferritin involved in this interaction is unknown. Ferritin contains 2 types of subunits, termed H and L. Serum ferritin is L-rich, similar to the spleen ferritin used in these experiments (31); however, all natural ferritins contain a mixture of subunits, so our results do not exclude a role for H ferritin in HKa binding.
The physiological site of the interaction between HKa and ferritin also requires further study. We have shown that ferritin retards (but does not entirely prevent) the production of HKa at sites of inflammation (17). Here, we demonstrate that the HKa produced despite this first ferritin blockade is susceptible to a second level of inhibition, in which ferritin directly blocks the activity of HKa. Indeed, ferritin binds with 10-fold greater affinity to HKa than it binds to uncleaved HK (Fig. 1), suggesting that HKa represents a critical target of ferritin. But where do ferritin and HKa colocalize, and at what stoichiometry? Although circulating levels of uncleaved HK exceed those of serum ferritin, basal concentrations of HKa in the serum are low (32), and may be comparable to those of ferritin: approximately 0.2 nM for ferritin (33) vs. 0.1 nM for HKa [as measured by a stable metabolite of HKa's coproduced product, bradykinin (34)]; further, because ferritin increases in inflammation or malignancy, the molar ratio of ferritin to HKa may rise to 10:1 or even 100:1 under these pathological conditions. Thus, it is possible that ferritin could effectively inhibit the activity of HKa if these proteins interacted in the circulation. Perhaps more likely, the interaction between these proteins could take place on the endothelial cell surface, because HKa is produced following docking and cleavage of HK on endothelial cells (5). In this case, locally produced HKa may recruit ferritin from the circulation or adjacent cellular sources to the endothelial cell surface, making ferritin an effective inhibitor of this reaction even at low serum ferritin concentrations.
We propose that ferritin, which is elevated during inflammation and malignancy, may play a role in regulating the levels of angiogenesis in these conditions. By antagonizing the antiangiogenic effects of HKa, ferritin may allow the proangiogenic effects of bradykinin and other biologically active proangiogenic molecules to predominate, thus shifting the balance toward blood vessel formation.
Although our studies focused on tumor angiogenesis, activities of HKa and ferritin may have importance in other settings in which angiogenesis occurs, such as wound healing and diabetic retinopathy. Modulation of HKa activity represents an unanticipated role of ferritin in angiogenesis, and provides a new target for the promotion or inhibition of physiologic and pathologic blood vessel formation.
Materials and Methods
Cell Culture.
HUVECs were maintained in EGM-2 at 37°C, 5% CO2. Passages 2–8 were used for experiments. PC3 cells were grown in RPMI, 10% FBS, 1% penicillin/streptomycin at 37°C, 5% CO2 (see SI Text for additional details on this and other experimental procedures).
HUVEC Viability Assay.
Ninety-six-well plates were coated with vitronectin (2 μg/ml in PBS) for 1 h at 27°C. HUVECs were seeded into the coated plates at a density of 3000 cells/well in M199 basal media containing 10 ng/ml bFGF and 10 μM ZnCl2. Cells were treated with 10 nM HKa with or without 10 nM human spleen ferritin or human spleen apoferritin, 10 nM human spleen ferritin or human spleen apoferritin alone or a saline control. The cells were incubated for 48 h at 37°C, 5% CO2. Viability was assessed with the Promega CellTiter 96 AQueous Nonradioactive Cell Proliferation Assay.
Ferritin Preparation.
Apoferritin was prepared by dialyzing human spleen ferritin extensively against 30% wt/vol sodium dithionite, essentially as described (35). As determined by ferrozine assay, 99.3% of the iron was removed.
Caspase and DAPI Staining.
HUVECs seeded onto vitronectin-coated coverslips were treated with 10 nM HKa with or without 10 nM ferritin, 10 nM ferritin alone, or a saline control. After 24 h, cells were fixed, permeablized, and stained with DAPI or antibody to cleaved (activated) caspase 3.
Migration Assays and in Vitro Tube Formation.
The migration assay was an adaptation of the QCM Chemotaxis 96-well cell migration assay kit to a 24-well format using 8-μm 24-well polyethelene terephthalate (PET) membrane inserts. Tube formation was quantified by using the in vitro angiogenesis assay kit from Chemicon.
Ligand Blot.
Ferritin binding to immobilized proteins was analyzed by ligand blotting as described in ref. 15.
GST-Fusion Protein Production.
GST fused recombinant HK domains D4–6, D5 and D6 were produced and purified by first isolating the coding regions for HK domains via RT-PCR from human liver cDNA using reverse transcriptase. The product was subcloned into the pGEX-6P-1 bacterial expression vector (Amersham) and verified by sequencing. Escherichia coli (B834) was transformed with the vector and expression was induced with IPTG. Bacteria were lysed, and the supernatant applied to a Ni-NTA column in the absence of imidazole. The GST-rHK domains were eluted with 250 mM imidazole and dialyzed against 20 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.5 mM EDTA overnight. GST was removed by PreScission protease digestion followed by binding to glutathione-Sepharose.
Solid Phase Binding Assays.
Microtiter plates were coated with spleen ferritin (2.5 μg per well). HKa and domain 5 were biotinylated with sulfo-N-hydroxysuccinimido-long-chain-biotin (sulfo-NHS-LC-biotin) and the EZ-Link Sulfo-NHS-LC Biotinylation kit (Pierce) according to the manufacturer's instructions and added to ferritin-coated wells. Binding of HKa or domain 5 was detected by incubation with streptavidin-peroxidase, TMB substrate (3,3′, 5,5′-tetra-methylbenzidine; Pierce) and measurement of absorbance at 450 nm. To identify the ferritin binding site, synthetic peptides were synthesized by AnaSpec, San Jose, CA and used as competitors at 10- or 50-fold molar excess (see Table S1 for sequence of all peptides).
Effect of HKa and Ferritin on Tumor Angiogenesis.
Animal studies were performed under an institutionally approved protocol. PC3 prostate cancer cells were injected into the flanks of athymic nude mice together with 400 nM HKa, 400 nM HKa + 400 nM Ft, 400 nM Ft, or saline control. Tumors were resected 10 days later and stained with anti CD-31 to identify endothelial cells in blood vessels. Vessel density was assessed as described in ref. 28.
Statistical Analysis.
Results are reported as the mean and 95% confidence interval and were obtained by using a general linear model (1-way ANOVA) followed by pairwise comparisons with Bonferroni adjustments.
Supplementary Material
Acknowledgments.
We thank Julie Brown for cloning of GST fusion proteins. This work was supported by the National Institutes of Health Grants R01DK71892, (S.V.T.) and R37 DK42421 (to F.M.T.) and a predoctoral fellowship from the American Heart Association (L.G.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812010106/DCSupplemental.
References
- 1.Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;2(Suppl 59):S340–S343. doi: 10.1016/s0753-3322(05)80070-8. [DOI] [PubMed] [Google Scholar]
- 2.Colman RW. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman RW, Hirsh J, Marder VJ, Salzman EW, editors. Philadelphia: J. B. Lippincott Company; 1998. pp. 1567–1583. [Google Scholar]
- 3.Sharma JN. The kallikrein-kinin system: From mediator of inflammation to modulator of cardioprotection. Inflammopharmacology. 2005;12:591–596. doi: 10.1163/156856005774382760. [DOI] [PubMed] [Google Scholar]
- 4.Weisel JW, et al. The shape of high molecular weight kininogen. Organization into structural domains, changes with activation, and interactions with prekallikrein, as determined by electron microscopy. J Biol Chem. 1994;269:10100–10106. [PubMed] [Google Scholar]
- 5.Guo YL, Colman RW. Two faces of high-molecular-weight kininogen (HK) in angiogenesis: Bradykinin turns it on and cleaved HK (HKa) turns it off. J Thromb Haemost. 2005;3:670–676. doi: 10.1111/j.1538-7836.2005.01218.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JC, et al. The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin. Proc Natl Acad Sci USA. 2002;99:12224–12229. doi: 10.1073/pnas.192668299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman RW, et al. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543–550. [PubMed] [Google Scholar]
- 8.Asakura S, et al. Inhibition of cell adhesion by high molecular weight kininogen. J Cell Biol. 1992;116:465–476. doi: 10.1083/jcb.116.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki M, et al. Effect of His-Gly-Lys motif derived from domain 5 of high molecular weight kininogen on suppression of cancer metastasis both in vitro and in vivo. J Biol Chem. 2003;278:49301–49307. doi: 10.1074/jbc.M308790200. [DOI] [PubMed] [Google Scholar]
- 10.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 11.Worwood M. Ferritin. Blood Rev. 1990;4:259–269. doi: 10.1016/0268-960x(90)90006-e. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH. The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol. 2006;1(Suppl 1):S9–S18. doi: 10.2215/CJN.01390406. [DOI] [PubMed] [Google Scholar]
- 13.Linder MC, et al. Serum ferritin: Does it differ from tissue ferritin? J Gastroenterol Hepatol. 1996;11:1033–1036. doi: 10.1111/j.1440-1746.1996.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 14.Torti SV, Torti FM. Iron and ferritin in inflammation and cancer. Adv Inorg Biochem. 1994;10:119–137. [PubMed] [Google Scholar]
- 15.Torti SV, Torti FM. Human H-kininogen is a ferritin-binding protein. J Biol Chem. 1998;273:13630–13635. doi: 10.1074/jbc.273.22.13630. [DOI] [PubMed] [Google Scholar]
- 16.Parthasarathy N, Torti SV, Torti FM. Ferritin binds to light chain of human H-kininogen and inhibits kallikrein-mediated bradykinin release. Biochem J. 2002;365:279–286. doi: 10.1042/BJ20011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffman LG, et al. Cleavage of high molecular weight kininogen by elastase and tryptase is inhibited by ferritin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L505–L515. doi: 10.1152/ajplung.00347.2007. [DOI] [PubMed] [Google Scholar]
- 18.Colman RW. Role of the light chain of high molecular weight kininogen in adhesion, cell-associated proteolysis and angiogenesis. Biol Chem. 2001;382:65–70. doi: 10.1515/BC.2001.011. [DOI] [PubMed] [Google Scholar]
- 19.Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599–2607. doi: 10.2174/138161206777698710. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JC, et al. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: Partial activity within domain 5. FASEB J. 2000;14:2589–2600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- 21.Hacker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 22.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, et al. The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Exp Cell Res. 2007;314:774–788. doi: 10.1016/j.yexcr.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian W, et al. Dihydrotanshinone I inhibits angiogenesis both in vitro and in vivo. Acta Biochim Biophys. 2008;40:1–6. doi: 10.1111/j.1745-7270.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 25.Mukai N, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2007;314:430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Hasan AA, et al. Mapping the cell binding site on high molecular weight kininogen domain 5. J Biol Chem. 1995;270:19256–19261. doi: 10.1074/jbc.270.33.19256. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg ED. The role of iron in cancer. Eur J Cancer Prev. 1996;5:19–36. [PubMed] [Google Scholar]
- 28.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 29.Guo YL, Wang S, Colman RW. Kininostatin, an angiogenic inhibitor, inhibits proliferation and induces apoptosis of human endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:1427–1433. doi: 10.1161/hq0901.095277. [DOI] [PubMed] [Google Scholar]
- 30.McCrae KR, et al. Inhibition of angiogenesis by cleaved high molecular weight kininogen (HKa) and HKa domain 5. Curr Cancer Drug Targets. 2005;5:519–528. doi: 10.2174/156800905774574039. [DOI] [PubMed] [Google Scholar]
- 31.Santambrogio P, Cozzi A, Levi S, Arosio P. Human serum ferritin G-peptide is recognized by anti-L ferritin subunit antibodies and concanavalin-A. Br J Haematol. 1987;65:235–237. doi: 10.1111/j.1365-2141.1987.tb02271.x. [DOI] [PubMed] [Google Scholar]
- 32.Berrettini M, et al. Detection of in vitro and in vivo cleavage of high molecular weight kininogen in human plasma by immunoblotting with monoclonal antibodies. Blood. 1986;68:455–462. [PubMed] [Google Scholar]
- 33.Heimburger DC, Weinsier RL. Handbook of Clinical Nutrition. St. Louis: Mosby; 1997. [Google Scholar]
- 34.Murphey LJ, et al. Quantification of BK1–5, the stable bradykinin plasma metabolite in humans, by a highly accurate liquid-chromatographic tandem mass spectrometric assay. Anal Biochem. 2001;292:87–93. doi: 10.1006/abio.2001.5073. [DOI] [PubMed] [Google Scholar]
- 35.Bauminger ER, et al. Mossbauer spectroscopic investigation of structure-function relations in ferritins. Biochim Biophys Acta. 1991;1118:48–58. doi: 10.1016/0167-4838(91)90440-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.