Abstract
Despite the importance of CNS blood vessels, the molecular mechanisms that regulate CNS angiogenesis and blood−brain barrier (BBB) formation are largely unknown. Here we analyze the role of Wnt/β-catenin signaling in regulating the formation of CNS blood vessels. First, through the analysis of TOP-Gal Wnt reporter mice, we identify that canonical Wnt/β-catenin signaling is specifically activated in CNS, but not non-CNS, blood vessels during development. This activation correlates with the expression of different Wnt ligands by neural progenitor cells in distinct locations throughout the CNS, including Wnt7a and Wnt7b in ventral regions and Wnt1, Wnt3, Wnt3a, and Wnt4 in dorsal regions. Blockade of Wnt/β-catenin signaling in vivo specifically disrupts CNS, but not non-CNS, angiogenesis. These defects include reduction in vessel number, loss of capillary beds, and the formation of hemorrhagic vascular malformations that remain adherent to the meninges. Furthermore, we demonstrate that Wnt/β-catenin signaling regulates the expression of the BBB-specific glucose transporter glut-1. Taken together these experiments reveal an essential role for Wnt/β-catenin signaling in driving CNS-specific angiogenesis and provide molecular evidence that angiogenesis and BBB formation are in part linked.
Keywords: blood vessel, blood-brain barrier, Wnt, glut-1, transport
Angiogenesis, the process by which new blood vessels are generated from existing blood vessels, is critical to ensure the supply of oxygen and nutrients to many tissues throughout the body. This process is especially important for the CNS as the neural tissue is extremely sensitive to hypoxia and ischemia. The blood vessels in the brain form a specialized structure, termed the blood brain barrier (BBB), which limits the flow of molecules and ions from the blood to the brain (1, 2). This BBB is critical to maintain brain homeostasis and protect the CNS from toxins and pathogens. CNS endothelial cells which form the BBB differ from endothelial cells in non-neural tissue, in that they are highly polarized cells held together by tight junctions that limit the paracellular flow of molecules and ions (1, 2). In addition, CNS endothelial cells also express specific transporters, both to provide selective transport of essential nutrients across the BBB into the brain and to efflux potential toxins from the brain (1, 2). Transplantation studies have demonstrated that the properties of the BBB are not intrinsic to the endothelial cells but induced by signals from the CNS parenchyma (3, 4). This has led to a two step model for BBB induction, described as angiogenesis followed by barriergenesis (2). In this model, angiogenesis in the CNS proceeds by the same mechanisms as angiogenesis in non-neural tissues with the formation of leaky vessels, which are then induced to form the barrier by interactions with neural cells. Recent evidence, however, suggests that BBB formation occurs early during embryogenesis (5), raising the possibility that distinct molecular mechanisms may regulate CNS angiogenesis to tightly couple this process to BBB formation.
We have identified that several effectors of Wnt signaling are highly enriched in brain endothelial cells compared with the peripheral endothelial cells of the liver and lung, suggesting that Wnts may specifically regulate CNS vessel formation and/or function. Wnts are a highly conserved gene family that encode lipid modified secreted proteins; there are 19 known members of this gene family in mice (7). During canonical Wnt signaling, binding of Wnt ligands to Frizzled/LRP receptor complexes causes a stabilization of β-catenin, which is normally degraded by Axin/GSK-3/APC complexes. Stabilized β-catenin is then able to translocate to the nucleus and through interactions with the TCF/LEF-1 complexes, regulates the expression of specific genes (7). These genes are involved in cell proliferation, differentiation, adhesion, morphogenesis, and other processes that are involved in the development, homeostasis, and disease of many tissues and organs.
Several studies have implicated Wnt/β-catenin signaling in regulating angiogenesis. In cell culture, endothelial cells have been shown to express Wnt ligands, receptors, and secreted modulators, and Wnt5a has been shown to regulate endothelial cell survival, proliferation, and gene expression (8–10). These studies have used endothelial cells immortalized from several different tissues which implies that Wnt signaling may regulate general endothelial cell function. Our genomic data, however, suggests that canonical Wnt/β-catenin signaling may specifically act on CNS vessels. Interestingly, Wnts have also been implicated in regulating angiogenesis in the placenta and gonads in vivo (11, 12). Both the placenta and gonads are tissues where blood tissue barriers are critical to their physiological function.
Here we analyze the function of Wnt signaling in regulating the formation of cerebral vessels in vivo. First, using TOP-GAL Wnt reporter mice, we show that canonical Wnt signaling is indeed activated in CNS endothelial cells, but not in non-neural endothelial cells, during development. We demonstrate that Wnt is a potent migration signal for CNS endothelial cells, and that inhibition of Wnt/β-catenin signaling in vivo leads to severe CNS specific angiogenesis defects without affecting non-CNS angiogenesis. Lastly, we demonstrate that Wnt/β-catenin signaling regulates the expression of the BBB-specific transporter glut-1. Taken together, these data suggest an important role for Wnt signaling in the formation of specialized CNS blood vessels and provide a molecular link between CNS angiogenesis and induction of BBB properties.
Results
Wnt Signaling Is Activated in CNS Vessels During Embryogenesis.
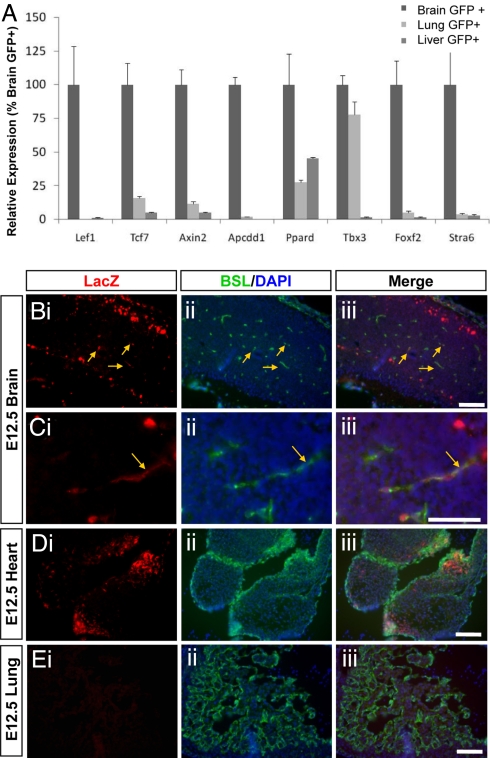
To confirm that Wnt signaling is indeed activated specifically in CNS vessels, we analyzed tissue sections from mice expressing the Wnt reporter TOP-GAL transgene. These transgenic mice express the lacZ gene under the control of Tcf promoters and thus synthesize lacZ only in cells in which canonical Wnt/β-catenin signaling is activated (13). During the development of the murine CNS, angiogenesis is initiated at E10, as endothelial cells from the perineural vascular plexus invade the underlying neural tissue. We therefore double stained tissue sections of E12.5 TOP-Gal mice with an anti-LacZ antibody and the vascular marker Bandeiraea simplicifolia lectin I (BSL) (Fig. 1). We observed activated Wnt signaling, as evidenced by anti-lacZ immunostaining, in many different tissues during embryogenesis. LacZ expression, however, co-localized with the vascular marker BSL only in the CNS, but not in peripheral tissues including the heart, liver, and lung (Fig. 1 B-E). These data demonstrate that Wnt signaling is specifically activated in CNS blood vessels during development.
Fig. 1.
Wnt signaling is activated specifically in brain endothelial cells. (A) GeneChip analysis of Wnt signaling components in purified endothelial cells. FACS analysis was used to purify endothelial cells from the brain, liver and lung of Tie2GFP mice, and gene expression was analyzed using Affymetrix microarray analysis. The expression of several molecules that have been demonstrated to be downstream of Wnt/β-catenin signaling are enriched in brain endothelial cells compared with the liver and lung samples. For each probe set values are normalized to brain endothelial cells sample. (B-E) Tissue sections from the cerebral cortex (B and C higher magnification), heart (D) and lung (E) of an E12.5 TOP-Gal transgenic mouse were stained with an anti-LacZ antibody to indicate Wnt activity (i), the vessel marker BSL and the nuclear stain DAPI (ii). In merged images (iii), yellow arrows point to co-localization of LacZ and BSL signals. Wnt activity is observed in blood vessels in the brain, but not heart or lung at E12.5. [Scale bar, 100 μm (B, D, E) and 50 μm (C).]
Active Wnt/β-Catenin Signaling in the CNS Vasculature Correlates with Wnt Expression in Neural Progenitors and Frizzled Expression in Blood Vessels.
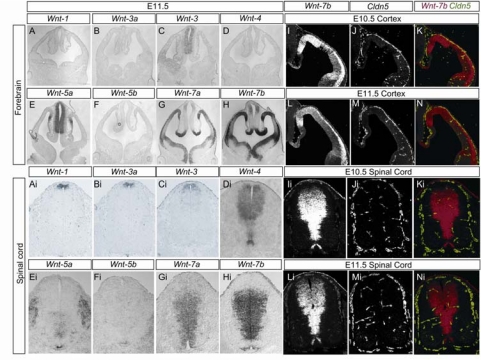
To identify which Wnt ligands may signal to CNS vessels, we next performed in situ hybridization studies using probes for various Wnt ligand mRNAs. We found that several canonical Wnt ligands were expressed by neural progenitors in the ventricular zone of the developing mouse CNS. In particular, vascular Wnt activation temporally correlated with the expression of Wnt7a and Wnt7b in the developing forebrain and in the ventral and intermediate spinal cord; Wnt 4 in the dorsal and intermediate spinal cord; and Wnt 1, Wnt 3, and Wnt3a throughout the dorsal neural tube (Fig. 2). As our analysis of TOP-GAL Wnt reporter mice demonstrated Wnt/β-catenin activity in endothelial cells throughout the CNS, this reporter activity is likely activated by different Wnt ligands expressed in spatially distinct regions of the CNS. In addition, neural progenitor cells in some CNS regions also expressed Wnt ligands that act through non-canonical signaling, including Wnt 5a and Wnt 5b (Fig. 2). These ligands may also be able to activate canonical β-catenin signaling depending on the Frizzled receptor type(s) expressed by the endothelial cells (14). Conversely, CNS endothelial cells express the Wnt receptors Frizzled 4, Frizzled 6, and Frizzled 8, as identified by microarray analysis of purified endothelial cells (supporting information (SI) Fig. S1). Frizzled 6 expression is highly enriched in CNS endothelial cells compared to the endothelial cells of the liver and lung (Fig. S1). Analysis of Wnt7b/Claudin 5 double fluorescent in situ hybridizations demonstrate that the capillary bed is largely formed in regions of the developing forebrain and spinal cord with high Wnt7b expression(Fig. 2 I-N, Ii-Ni). Taken together, these data suggest that canonical Wnt/β-catenin signaling mediates endothelial-neural progenitor cellular interactions in the developing CNS.
Fig. 2.
Expression of Wnts in the developing mouse CNS. In situ hybridizations demonstrating Wnt ligand expression in the developing forebrain (A-H) and spinal cord (Ai-Hi) of E11.5 mice. Canonical Wnt ligands Wnt7a and Wnt7b are expressed by neural progenitors in the ventricular zone in the ventral-lateral spinal cord and cortical forebrain, whereas canonical Wnt ligands Wnt1, Wnt3, and Wnt3a are expressed by neural progenitors in the ventricular zone of the dorsal spinal cord and the hindbrain. Non-canonical Wnt ligands Wnt4, Wnt5a, and Wnt5b are also expressed by neural progenitors located in spatially distinct regions of the spinal cord and cortex. Double fluorescent in situ hybridizations in the developing forebrain (E10.5 I-K, E11.5 L-N) and spinal cord (E10.5 Ii-Ki, E11.5 Li-Ni) with Wnt7b (I, L, Ii, Li) and Claudin 5 (J, M, Ji, Mi) and merged (K, N, Ki, Ni) demonstrate that claudin 5 positive vessels vascularize Wnt7b positive regions of the developing CNS.
β-Catenin Is Required for CNS Vessel Formation in Vivo.
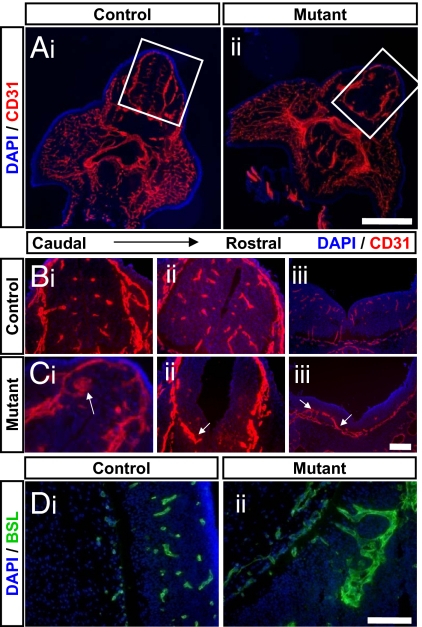
To determine whether β-catenin is required for CNS vessel formation, endothelial-specific β-catenin knockout mice were generated by using β-cateninflox/flox and Tek-cre mice. Tek-cre mice express cre recombinase in endothelial cells throughout the body and therefore we used this method to delete β-catenin, an essential component of canonical Wnt signaling, from all vessels. β-cateninflox/flox; Tek-cre mice have been previously generated and die by E12.5 displaying mild patterning defects in the large vessels of the vitalline, umbilical cord, and head. The tissue capillary beds of these mutants appear normal, however, their CNS vasculature has not yet been examined (15). We therefore next examined the vascular pattern of E11.5 endothelial-specific β-catenin mutants (β-cateninflox/flox; Tek-cre genotype) and litter mate controls (β-cateninflox/+; Tek-cre and β-cateninflox/flox genotypes) by immunostaining tissue sections with an anti-CD31 antibody to label all endothelial cells. We observed an overtly normal vascular pattern in non-neural tissues, including the liver, lung, skin, jaw, and tail, in all genotypes examined, but found major vascular defects in the CNS of all mutant mice examined(Figs. 3 and Figs. S2 and S3). Virtually no capillaries formed throughout the developing forebrain and spinal cord of the mutant mice. Furthermore, the perineural vascular plexus was significantly thickened, suggesting that endothelial cells stalled in the meninges, unable to invade the CNS parenchyma (Fig. 3). In each mutant examined, we observed large malformed vessels that did invade the CNS parenchyma. These vessels, however, did not form discrete tubes or capillary networks, but instead remained as large aggregates of endothelial cells that were often associated with hemorrhage. The number and extent of these vascular malformations varied between animals and in different regions of the developing neural tube. In addition, the thickness of the neuroectodermal cell layer was significantly decreased in the mutants, leading to an increased ventricular volume. This defect is likely secondary to the vascular defects, as the conditional mutants have β-catenin deleted specifically within endothelial cells. The malformations consisted of multiple layers of CD31+ endothelial cells, instead of single cell tubes as seen in the capillary beds of control animals. In some cases, these aggregates formed layered tubes with lumens; whereas, in other cases, they appeared as multicellular balls with no discernable lumen (Fig. S3). In many cases the aggregates remained attached to the meninges, forming extended contacts with this vascular plexus. In most cases, the aggregates recruited pericytes, often surrounded by a layer of these mural cells (Fig. S3 A and B). The malformations were often associated with hemorrhage, which ranged from small leaks to massive bleeding into the parenchyma (Fig. S3 C and D). Interestingly, apparently normal capillary beds formed in the posterior regions of the cortex of these conditional mutants (Fig. S2), suggesting a unique mechanism for angiogenesis in this region of the CNS. Unfortunately, due to the early embryonic lethality of these mice, a complete map of β-catenin independent vessels remains unknown.
Fig. 3.
Conditional depletion of β-catenin in endothelial cells leads to CNS-specific vascular defects. (A) Cross sections of E11.5 (ii) endothelial-specific β-catenin mutants (β-cat flox/flox; Tek-cre) and (i) litter mate controls were stained with the nuclear marker DAPI (blue) and an antibody against the vascular marker CD31 (red). Normal vasculature was observed in peripheral tissues of both genotypes, whereas, angiogenesis defects were observed in the CNS of the endothelial-specific β-catenin mutants. White boxes outline developing neural tube. (Scale bar, 500 μm.) (B and C) Cross-sections of developing neural tube of an E11.5 (C) endothelial-specific β-catenin mutants and (B) litter-mate taken along the rostral to caudal axis (i-iii), were stained with the nuclear marker DAPI (blue) and an antibody against the vascular marker CD31 (red). The CNS of the endothelial-specific β-catenin mutants demonstrated a decrease in vascular density, a loss of capillary beds and the presence of malformed vessels (white arrows). (Scale bar, 100 μm.) (D) Sagittal sections through the developing neural tube of an E11.5 (ii) endothelial-specific β-catenin mutants and (i) litter-mate were stained with the nuclear marker DAPI (blue) and the vascular marker BSL (green). Large aggregates of endothelial cells were observed in the endothelial-specific β-catenin mutants. (Scale bar, 100 μm.)
Taken together, the above findings demonstrate that β-catenin is required for the proper formation of CNS vessels, but not vessels in non-neural tissues. β-catenin functions not only as a transducer of Wnt signaling, but also as a component of the adherens junctions that join all endothelial cells. The vascular malformations observed in the endothelial-specific β-catenin mutants express other adherens junctions components at cellular junctions including α-catenin, γ-catenin and ve-cadherin as well as tight junction components zo-1, occludin, and claudin 5 (Fig. S4, data not shown), suggesting that the defect is not due to incomplete junction protein expression. Although the expression of these proteins is unaffected, due to the cellular disorder of the malformations observed in these mice, the junctional components are also disordered. Adherens junctions connect endothelial cells in all tissues; however, the phenotype in the endothelial-specific β-catenin mutants is specific to the CNS matching the activation of Wnt signaling observed in the TOP-GAL mice.
Blockade of Wnt Signaling Inhibits CNS Angiogenesis.
To further test whether the CNS-specific vascular defects in the endothelial specific β-catenin mutant were due to impaired Wnt signaling, and not other functions of β-catenin, we next examined the consequence of delivering a Wnt inhibitor to developing embryos. In this experiment, pregnant mice at 9 days of gestation were injected with adenoviruses encoding a soluble Frizzled 8-Fc fusion (Ad-sFz8-Fc) or a control Fc (Ad-Fc). After systemic injection, adenoviruses are taken up by liver cells, which then express the molecules encoded by the viruses (16). A soluble Frizzled-8 ectodomain was used to bind extracellular Wnt ligands and Fc fusions were used to ensure delivery across the placenta. We observed many vascular malformations in the forebrains, but not non-neural tissue, of animals injected with Ad-sFz8-Fc but not when they were injected with the control Ad-Fc (Fig. S5). These malformations closely resembled those observed in the endothelial-specific β-catenin mutants, consisting of thickened tubes with multiple layers of endothelial cells and frequent meningeal attachment (Fig. S5 E and F). The phenotype produced by Ad-sFz8-Fc was less severe than in the endothelial-specific β-catenin mutants most likely because the amount of Ad-sFz8-Fc we could systemically deliver was limited by toxicity due to systemic (rather than specifically endothelial) Wnt signaling inhibition. This method inhibits Wnt-Frizzled interactions in all tissues, and thus vascular defects may be secondary to other developmental defects of inhibiting Wnt signaling. However, the similarity of the phenotype observed after Ad-sFz8-FC injection and endothelial-specific β-catenin depletion, suggests that the vascular defects are due to Wnt activity on CNS endothelial cells.
Wnt7a and Wnt7b Are Required for Normal CNS Angiogenesis.
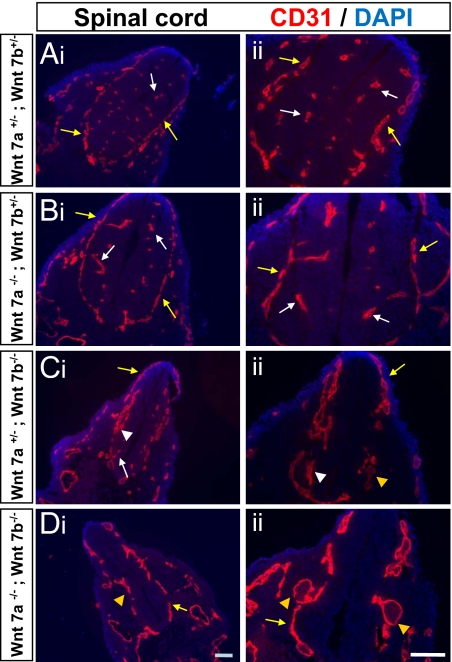
Which Wnts regulate CNS angiogenesis? Because Wnt7a and Wnt7b have the broadest expression pattern in ventral regions of the developing CNS, we next examined the vascular pattern of Wnt7a knockout, Wnt7b knockout, and Wnt7a;Wnt7b double knockout mice. Wnt7a knockout mice are viable and exhibit a normal vascular pattern at all ages tested including E10.5 and E12.5 (Fig. 4, data not shown). Wnt 7b knockout mice die by E11.5, and therefore due to the early lethality of this mutation we examined the spinal cord, which is vascularized before the forebrain during development. The ventral spinal cord of E10.5 Wnt7b knockout embryos displayed a decrease in capillary density, with vascular malformations that remained attached to the meningeal surface. In addition, the vascular plexus was thickened in many areas, similar to what was observed in the endothelial-specific β-catenin mutants (Fig. 4). The Wnt7a;Wnt7b double knockout mice also exhibited ventral vascular malformations and showed an even more severe thickening of the vascular plexus that often displayed extremely large dilations (Fig. 4). Due to the early lethality and thinning of the neural tissue in Wnt7b knockout mice, it is difficult to determine the extent of the capillary bed loss, however, the presence of vascular malformations and thickened vascular plexus is consistent with the defects observed in the endothelial-specific β-catenin mutants. In addition, as with the endothelial-specific β-catenin mutants, apparently normal vasculature was observed in the hindbrain of the Wnt7b knockout mice (data not shown).
Fig. 4.
Abnormal vasculature in the CNS of Wnt 7 mutants. (A-D) Coronal tissue sections of the E10.5 spinal cord in Wnt7a, Wnt7b double heterozygotes (A: Wnt 7a+/−; Wnt 7b+/−), Wnt 7a mutants (B: Wnt 7a−/− ;Wnt 7b+/−), Wnt 7b mutants (C: Wnt 7a+/− ;Wnt 7b−/−), and Wnt7a, Wnt 7b double mutants (D: Wnt 7a−/− ;Wnt 7b−/−) were stained with the nuclear marker DAPI (blue) and the vascular marker CD31 (red). Normal capillary beds were observed in the wild-type and Wnt7a mutants, whereas vascular malformations and thickened vascular plexus were observed in the Wnt7b mutants, and large vascular plexus dilations were observed in double mutants. Normal capillaries and normal vascular plexus are indicated with white and yellow arrows respectively, whereas vascular malformations and abnormal vascular plexus are indicated with white and yellow arrow heads respectively. (Scale bar, 100 μm.)
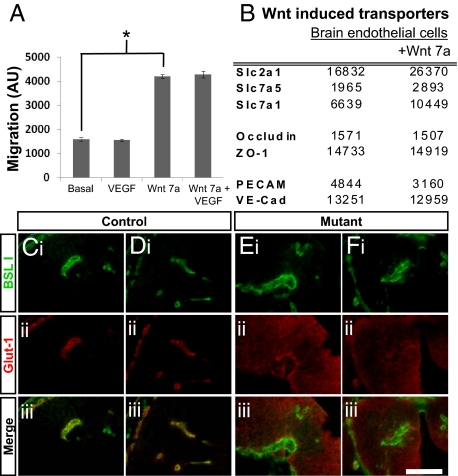
Because we observed similar malformed vessels following conditional depletion of β-catenin, delivery of Wnt inhibitors, and in Wnt mutants, we conclude that Wnt/β-catenin signaling is required for the formation of CNS vessels during embryogenesis. This is consistent with our genomic data in which several genes downstream of Wnt/β-catenin signaling are enriched in CNS endothelial cells compared with endothelial cells in non-neural tissues (Fig. 1A). The presence of vascular malformations that fail to invade the CNS after disruption of Wnt signaling suggests that Wnt may be a potent factor stimulating migration of endothelial cells into the CNS. To test whether this is the case, we measured the ability of Wnt7a to elicit CNS endothelial cell migration in vitro across a fibronectin-coated transwell system. Indeed, Wnt7a, but not VEGF, induced a strong migration of a mouse brain endothelial cell line (bEND3.0 cells) across the filter (Fig. 5A). These results demonstrate that Wnts are a potent migration factor for CNS endothelial cells.
Fig. 5.
Wnt7a regulates CNS endothelial cell migration and expression of the BBB-specific transporter glut-1 (slc2a1). (A) Measurement of mouse brain endothelial cell (bEND3.0) migration across a fibronectin coated filter to a basal media, basal media containing VEGF, basal media containing Wnt, or basal media containing both VEGF and Wnt. Wnt7a is a potent migration factor for CNS endothelial cells. Error bars represent standard error of the mean. *<0.001 as analyzed by a two tailed standard T-test not assuming normal variance. (B) Table of GeneChip values, given in arbitrary units, for primary cultures of mouse brain endothelial cells grown in basal medium, or basal medium with Wnt7a. Wnt7a up-regulates expression of transcripts encoding molecular transporters (slc2a1, slc7a1, and slc7a5) but not tight junction molecules (Occludin, ZO-1) or pan-endothelial cell adhesion molecules (PECAM, VE-Cad). (C-F) Cross sections of the developing CNS of E11.5 endothelial-specific β-catenin mutants (E and F) and litter-mate controls (C and D) were stained with vascular marker BSL I (i, green) and an antibody directed against the BBB-specific glucose transporter glut-1 (ii, red). Glut-1 specifically stains vascular endothelial cells in the control animals, whereas glut-1 does not stain the vascular endothelial cells in the endothelial-specific β-catenin mutants but instead stains CNS parenchymal cells (iii, merged images). (Scale bar,100 μm.)
Wnt/β-Catenin Signaling Regulates BBB-Specific Properties of CNS Endothelial Cells.
The fact that Wnt/β-catenin signaling regulates angiogenesis in the CNS, but not in other tissues, raises the possibility that this molecular signal imparts tissue-specific properties on the CNS endothelial cells to tightly couple CNS angiogenesis and BBB formation. CNS endothelial cells which form the BBB are characterized by the formation of tight junctions, and the expression of a variety of transporters both to provide selective transport of essential nutrients across the BBB into the brain and to efflux potential toxins from the brain. Does Wnt/β-catenin signaling regulate specific properties of the BBB? To test whether Wnt signaling might regulate specific components of the BBB, we next used Affymetrix microarrays to examine the transcriptional profile of purified primary CNS endothelial cells cultured in the presence or absence of recombinant Wnt7a. Wnt7a increased the expression of several BBB-specific influx transporters, including slc2a1 (glut-1), slc7a1 (CAT1), and slc7a5 (TA1), but not tight junction molecules including occludin and ZO-1 or pan-endothelial molecules including PECAM and VE-cadherin (Fig. 5B). To test the relevance of this in vivo, we examined the expression of glut-1 in the endothelial-specific β-catenin mutants and wild-type littermates. In the CNS of wild-type embryos, glut-1 displays specific expression in the vascular endothelial cells, whereas this expression is lost in endothelial cells in the endothelial-specific β-catenin mutants (Fig. 5 C-F). Interestingly, the endothelial-specific β-catenin mutants exhibited an up-regulation of glut-1 in the CNS parenchyma, likely a secondary response to lack of glucose transport from the blood as the conditional mutation of β-catenin is specific to the vascular endothelial cells. Along with the in vitro studies, these results demonstrate that Wnt/β-catenin regulates endothelial cell expression of the BBB-specific transporter glut-1.
Our data demonstrates that Wnt/β-catenin signaling is essential for CNS angiogenesis and the expression of BBB-specific transport molecules. This provides evidence that angiogenesis and BBB formation are at least in part linked.
Discussion
Wnt/β-Catenin Signaling Is Required for CNS, but Not Non-CNS, Angiogenesis, and at Least Some Aspects of BBB Formation.
Here we have used 3 methods to demonstrate that Wnt/β-catenin signaling is required specifically for the formation of CNS vessels in vivo. First, we demonstrate that conditional depletion of β-catenin, an essential component of canonical Wnt signaling, in all endothelial cells leads to severe CNS-specific angiogenesis defects. Second, we demonstrate that delivery of a soluble Frizzled ectodomain, a molecule which binds and inhibits Wnt ligands, produces a similar phenotype. Finally, we demonstrate that mutations in Wnt7 genes result in CNS-specific vascular malformations. Because inhibiting canonical Wnt signaling at multiple different steps in the pathway produces the same phenotype, we conclude these CNS-specific angiogenesis defects are due to inhibition of Wnt/β-catenin signaling and not other functions of these molecules. The fact that these angiogenesis defects are CNS-specific demonstrates that distinct molecular mechanisms regulate CNS angiogenesis.
An important question for future studies will be to identify the relevant ligand-receptor pairs. Interestingly, Norrin signaling through Frizzled 4 has been implicated in regulating retinal angiogenesis (17). Our data demonstrates that Wnt/β-catenin signaling is required for angiogenesis throughout the CNS and suggests that different Wnt/Frizzled ligand-receptor pairs may mediate this response in spatially distinct regions of the CNS. Specifically, Wnt7a and Wnt7b are expressed in the forebrain and ventral neural tube, whereas Wnt 1, Wnt3, Wnt3a, and Wnt4 are expressed in dorsal regions of the neural tube. In fact, our analysis of Wnt7a and Wnt7b mutants have identified that these genes are required for normal angiogenesis in ventral regions of the CNS, with Wnt7b likely more important. Frizzled6 is a likely candidate receptor as it is highly enriched in CNS blood vessels.
Our findings implicate neural stem cells and progenitors as important sources of angiogenic signals in the developing CNS, as we found that the expression of Wnt ligands in the ventricular zone of the neural tube correlates with the activation of β-catenin in the CNS vasculature. The interaction between neural stem cells and vascular cells has been hypothesized by the close association of these two cell populations in what is termed a “vascular niche” for neural stem cells (18). Reciprocal signals between these two cell populations have been observed in vitro. Endothelial cells signal to neural stem cells regulating their self-renewal and differentiation into neurons (19), whereas neural stem cells have been implicated in regulating the resistance of endothelial tight junctions (20). The dramatic failure of embryonic brain growth that occurred when we blocked Wnt/β-catenin signaling is likely explained not only by a lack of blood flow due to suppression of angiogenesis but quite possibly also the loss of endothelial signals required to support neural stem cell survival and proliferation.
How does Wnt signaling interact with other signaling systems that have previously been shown to regulate angiogenesis? VEGF, neuropilin-1, integrin αv, and integrin β8 have been implicated in regulating the formation of CNS vessels (21–24). Mice deficient in these genes show CNS angiogenesis phenotypes similar to those observed in the endothelial-specific β-catenin mutants, suggesting that these molecules may interact with Wnt/β-catenin signaling. One striking difference, however, is that both VEGF/Nrp1 signaling and integrins are used for angiogenesis in many tissues throughout the body (25–27), whereas Wnt/β-catenin signaling appears to be specifically required for angiogenesis in the CNS.
An intriguing possibility is that the same signal that promotes angiogenesis into the CNS also drives morphogenesis into barrier forming vessels. It appears that this is at least in part the case as we have identified that Wnt7a regulates expression of the BBB-specific transporter glut-1 in purified endothelial cells in vitro, and β-catenin is necessary for its expression in vivo. Standard hypotheses describe BBB formation as a two step model in which angiogenesis is followed by barriergenesis (2). In this model, CNS vessels are first generated as leaky vessels, and then later in development are induced by neural cells to form a tight seal. Our findings suggest the possibility that the formation of leaky CNS blood vessels may be incompatible with developmental viability and that Wnt/β-catenin signaling functions to tightly couple angiogenesis to barriergenesis. Consistent with this, Wnts have also been implicated in regulating angiogenesis in the placenta and gonads in vivo (11, 12), both tissues where blood tissue barriers are critical to their physiological function.
Targeting Pathogenic Angiogenesis.
The present findings have several implications for understanding and treating neurological diseases. First, we observed vascular malformations after disruption of Wnt/β-catenin signaling that are similar to the arteriovenous malformations observed in patients with Sturge-Weber syndrome (28). Both consist of disorganized aggregates of endothelial cells that are attached to meningeal surface that hemorrhage easily. Our findings therefore have implications for understanding the origin of, and developing new treatments for, these malformations that cause debilitating leptomeningeal angiomas. Second, our findings offer new approaches for disruption of pathologic angiogenesis, without harming mature vessels. Inhibition of Wnt/β-catenin signaling, or downstream targets, may prevent neovascularization in diabetic retinopathy and macular degeneration or disrupt the angiogenesis that drives growth of glioblastomas and other retinal or CNS tumors (29, 30).
Materials and Methods
Animals.
Homozygous Tie2GFP mice (strain 003658) were obtained from Jackson Laboratory and bred to maintain homozygosity. Wnt7a+/− (strain 001253) and Wnt7b+/− (strain 004693) mice were obtained from Jackson Laboratory, and interbred to generate embryos with different combinations of mutant alleles of these Wnt 7 genes. TOP-GAL transgenic mice were provided by Roel Nusse (Stanford, CA). Conditional β-catenin mutant mice (strain 004152) and Tek-Cre mice (strain 004128) were obtained from the Jackson Laboratory. Wild type C57bl6 and FVB mice were obtained from Charles River.
Adenoviral Injections.
Adenoviruses expressing FC and soluble Frizzled 8-FC fusions were generated as previously described (16). Pregnant C57bl6 mice, 9 days gestation, were administered 5 × 108 pfu of adenovirus via tail vein injection, and embryos were isolated 3 days later.
In Situ Hybridizations and Immunohistochemistry.
The in situ hybridizations were performed essentially as described previously (31) with a few modifications that include incubation of fixed embryonic tissues with proteinase K for 5 min. cDNAs were either amplified from purified endothelial cells or they were obtained from Open Biosystems. Double in situ hybridizations were performed as described previously (6). For Immunohistochemistry methods see SI Text.
Migration Assays.
A mouse brain endothelial cell line (bEND3.0 cells from ATCC) was grown in DMEM, with pen/strep, sodium pyruvate, glutamate, insulin, and 10% FCS. Upon reaching confluency, the cells were starved of serum for 5 h and then trypsinized and plated at 105 cells/insert in a BD BioCoat Angiogensis System:Endothelial Cell Migration Plate (BD Biosciences 354144) with basal media (bEND3.0 media without FCS) alone or containing 10 ng/ml VEGF (BD 354107), 0.5 μg/ml Wnt 7a (R&D 3008-WN), or both VEGF and Wnt in the wells beneath the insert. Cells were incubated for 20 h, and then stained with 4 μg/ml Calcein AM (BD 354216). Endothelial cell migration was calculated by determining the fluorescence in the bottom well using a fluorescence plate reader with bottom reading capabilities.
Supplementary Material
Acknowledgments.
Thanks to Mark Lee and Jenny Yuan for preparation of Ad Fz8-Fc adenovirus. This work was supported by grants from the National Institute of Neurological Disease (R01 NS045621; B.A.B.), the Myelin Repair Foundation (B.A.B.), NMSS (Grant RG3936A7 to B.A.B.), American Heart Association Graduate Fellowship (R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.C.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805165106/DCSupplemental.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 3.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail−chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 4.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 5.Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidi AU, Enomoto H, Milbrandt J, Roth KA. Dual fluorescent in situ hybridization and immunohistochemical detection with tyramide signal amplification. J Histochem Cytochem. 2000;48:1369–1375. doi: 10.1177/002215540004801007. [DOI] [PubMed] [Google Scholar]
- 7.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 9.Masckauchan TN, et al. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- 11.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 12.Jeays-Ward K, et al. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- 13.Hens JR, et al. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 14.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cattelino A, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 18.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 20.Weidenfeller C, Svendsen CN, Shusta EV. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J Neurochem. 2007;101:555–565. doi: 10.1111/j.1471-4159.2006.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raab S, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemostasis. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki T, et al. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 23.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerhardt H, et al. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 26.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Weis SM. Evaluating integrin function in models of angiogenesis and vascular permeability. Methods Enzymol. 2007;426:505–528. doi: 10.1016/S0076-6879(07)26021-5. [DOI] [PubMed] [Google Scholar]
- 28.Nathan N, Thaller SR. Sturge-Weber syndrome and associated congenital vascular disorders: A review. J Craniofac Surg. 2006;17:724–728. doi: 10.1097/00001665-200607000-00024. [DOI] [PubMed] [Google Scholar]
- 29.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 30.Noonan DM, Benelli R, Albini A. Angiogenesis and cancer prevention: A vision. Recent Results Cancer Res. 2007;174:219–224. doi: 10.1007/978-3-540-37696-5_19. [DOI] [PubMed] [Google Scholar]
- 31.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: In situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.