Abstract
Chronic ethanol consumption results in immunodeficiency. Previous work with chronic ethanol-fed mice has shown reduced splenic weight and cellularity, including reduced numbers of CD8+ T cells. However, antigen-specific CD8+ and CD4+ T cell responses in chronic ethanol-fed mice have been studied relatively little. We have used an attenuated Listeria monocytogenes strain DPL 1942 (LM ΔactA) to inoculate mice and subsequently used CD4+ and CD8+ immunodominant peptides of LM to measure the CD4+ and CD8+ T cell responses after chronic ethanol exposure. We found no major differences between control and ethanol-fed mice in the kinetics and persistence of antigen-specific CD4+ T cells in response to an immunodominant LM peptide, as measured by intracellular IFN-γ staining. In contrast to CD4+ responses, three methods of in vitro antigen presentation indicated that the primary response of CD8+ T cells to several different epitopes was reduced significantly in mice chronically fed ethanol. Antigen-specific CD8+ T cells were also reduced in chronic ethanol-fed mice during the contraction phase of the primary response, and memory cells evaluated at 29 and 60 days after inoculation were reduced significantly. BrdU proliferation assays showed that in vivo proliferation of CD8+ T cells was reduced in ethanol-fed mice, and IL-2-dependent in vitro proliferation of naive CD8+ T cells was also reduced. In conclusion, these results suggest that antigen-specific CD4+ T cell responses to LM are affected little by chronic ethanol consumption; however, antigen-specific CD8+ T cell responses are reduced significantly, as are in vivo and in vitro proliferation. The reduction of antigen-specific CD8+ T cells may contribute strongly to the immunodeficiency caused by ethanol abuse.
Keywords: CD8+ T cells, attenuated Listeria, ΔactA, intracellular bacteria
INTRODUCTION
Chronic excessive ethanol consumption in humans has long been known to increase the severity of several diseases including a prominent increase of infectious diseases such as pneumonia and tuberculosis [1,2,3,4,5,6,7,8,9,10]. Modeling of ethanol-induced immune dysfunction to explain the increased infectious diseases has included work with rodents after ethanol administration and study of the direct effects of ethanol on immune cells in vitro. Studies with acute and chronic ethanol-fed C57BL/6 and BALB/c mice have shown that there is a significant decrease in splenic cellularity, CD4+ T cell numbers, CD8+ T cell numbers, B cell numbers, and NK cell numbers [9, 11,12,13,14,15]. Several investigators have reported in work with rodent splenocytes or human peripheral blood mixed T cells that short-term or acute ethanol exposures resulted in decreased T cell proliferation in response to mitogens [16,17,18,19,20,21,22]. Although a lack of proliferative response to added IL-2 was commonly reported, the protocols varied widely, rendering comparisons difficult. In general, there was no consistent reduction in IL-2 production nor any change in IL-2R expression. Studies with chronic alcoholic patients [23] and mice fed a liquid ethanol diet up to 13 weeks [24] were reported to show a decrease in Th1 immunity and increased Th2 responses [23, 24]. Other studies with short-term ethanol-fed C57BL/6 mice have suggested that ethanol consumption increases cytokine imbalance to favor Th2 responses by enhancing Th2 and/or by suppressing Th1 function [14, 23,24,25].
Although cytokine imbalance studies and evaluation of the response to mitogens are of potential value, direct studies of the effects of chronic ethanol on the levels of antigen-specific CD4+ or CD8+ T cell responses have been lacking. Specifically, to model more accurately the changes occurring in chronic human alcoholics, a clear distinction between antigen-specific CD4+ and CD8+ T cell responses after chronic ethanol exposure is needed, including measurement of T cell expansion and contraction phases and the development of memory levels. As a clinically relevant model of cell-mediated immunity, we have chosen the T cell response to Listeria monocytogenes (LM), which has been characterized extensively in mice [26,27,28,29]. The early CD8+ T cell response to LM has been shown to be critical for development of sterilizing immunity [30]. Previous studies with virulent LM in ethanol-administered mice and rats consistently have shown increased numbers of bacteria in different organs and the inability to clear the infection compared with controls [7, 10, 31,32,33]. In the current work, for evaluation of CD8+ T cell responses, we have used an attenuated strain of LM, DPL 1942, which is unable to nucleate cellular actin (ΔactA) and is cleared within 2 days by water- and ethanol-administered mice. Attenuated LM ΔactA inoculation allows consistent evaluation of antigen-specific T cell responses in water- and chronic ethanol-fed mice, without the excess mortality of ethanol-fed mice demonstrated with the virulent LM [7]. A recombinant attenuated LM strain [LM ΔactA Leishmania homologue of the receptor for activated C kinase (LACK)], which expresses LACK antigen, was used to detect antigen-specific CD4+ T cell responses in BALB/c female mice. Two immunodominant epitopes, Listeriolysin O AA #91–99 (LLO91–99; GYKDGNEYI) of LM for specific CD8+ T cells [29] and LACK AA #158–173 (LACK158–173; FSPSLEHPIVVSG) of Leishmania major for specific CD4+ T cells, were used to assay the T cell responses [34]. Previous studies about the time course of antigen-specific CD8+ T cell responses in normal mice using LM ΔactA have shown that the antigen-specific response peaks at 7 days after inoculation, contracts by Day 12, and reaches early memory phase by Day 29 [26,27,28]. Here, we asked if the time course of the antigen-specific T cell response is similar in control and ethanol-consuming mice; whether the resulting number of antigen-specific T cells is reduced; whether cytokine production by T cells is reduced or altered, favoring a Th2/T cytotoxic 2 (Tc2) response in chronic ethanol-consuming mice following LM ΔactA inoculation; and whether T cells from chronic ethanol mice proliferate normally.
MATERIALS AND METHODS
Mice and ethanol administration
Female BALB/c mice were obtained from the National Cancer Institute (Frederick, MD, USA) at 5–7 weeks of age. Mice were housed in barrier facilities in the Animal Care Unit at the University of Iowa (Iowa City, IA, USA). Upon receipt, mice were acclimatized for at least 1–2 weeks, and each lot was divided randomly into two groups to maintain lot and age-matched water (control) and ethanol groups. Starting at ages 8–10 weeks and using a well-described protocol, pharmaceutical-grade ethanol was administered in double-distilled water at 10% (w/v) for 2 days, 15% for 5 days, and finally, 18% to BALB/c mice as the only source of drinking water [9, 11, 12, 35]. Ethanol-fed mice were kept at their final concentration throughout the remainder of the experiment, and the durations of ethanol exposures indicated were the number of weeks mice were exposed to the final ethanol concentration. The mice were fed laboratory chow (Teklad NIH-31-irradiated, Harlan, Indianapolis, IN, USA) ad libitum, and control mice were given the same double-distilled water used for mixing ethanol solutions. With this ethanol administration protocol, mice were visibly inebriated in the early morning following nocturnal drinking, and blood alcohol levels were as high as 400 mg/dL, with much lower levels later in the day [9]. All procedures were carried out under a protocol approved by the Animal Care Committee of the University of Iowa.
Bacterial preparation and infection
LM ΔactA and LM ΔactA LACK were the kind gifts of John T. Harty (University of Iowa). They were grown to log phase (OD 600: 0.06–––0.09) in tryptic soy broth (TSB) media. Bacteria were diluted to 5 × 105 cfu in 0.3 mL (LM ΔactA) or 1 × 107 cfu in 0.3 mL (LM ΔactA LACK) sterile saline (Hospira Inc., Lake Forest, IL, USA) and injected i.p. into control and ethanol-consuming mice. Serial dilutions of the same LM ΔactA cultures used for injections were plated in TSB agar culture plates and grown overnight at 37°C, and colonies were counted to determine independently the number of injected bacteria. LM ΔactA were not detectable in spleens or livers of control or ethanol-fed mice 2–3 days after inoculation, as determined by standard organ culture and cfu assays.
Cell harvest and culture
LM ΔactA-inoculated water control or ethanol-consuming mice were killed at different time-points, and the spleens were harvested. Spleens were homogenized between glass slides and suspended in enriched RPMI media, which contained sterile-filtered RPMI 1640 (Invitrogen, Carlsbad, CA, USA), 10% FCS (Invitrogen), 1% 200 mM L-glutamine (Invitrogen), 0.1% 50 mM 2-ME, and 0.1% 50 mg/mL gentamycin (Sigma-Aldrich, St. Louis, MO, USA). The total number of splenocytes/spleen was determined by counting cells in a hemocytometer.
In vitro antigen presentation
The APC present in whole splenocytes, purified and activated normal B cells, or P815 cells (a mastocytoma cell line, ATCC #TIB-64™) were used as APC. B cells were purified from whole splenocytes using a B cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). B cells were cultured with LPS at 1 μg/mL at 37°C overnight for activation. After overnight culture, B cells were washed 2× with PBS and then reconstituted at 2 × 106/mL. LLO peptide (10 μM; LLO91–99, was added to 2 × 106 B cells/mL and incubated at 37°C for 1 h. Peptide-loaded B cells were washed 2× with PBS. For P815 assay, 10 μM LLO91–99 peptide was added to 5 × 106 P815 cells/mL and incubated at 37°C for 1 h followed by two washes to load P815 cells. CD8+ T cells were enriched using the CD8α+ cell isolation kit (Miltenyi Biotec). Activated B cells or P815 cells loaded with the LLO91–99 peptide were used as APC at the specified ratios. Purified CD8+ T cells and peptide-loaded APC were cultured for 6 h in the presence of Brefeldin-A, and the responding CD8+ T cells were determined by intracellular IFN-γ staining. In some assays, peptide at a concentration of 10−7M (LLO91–99, p60217–225, p60449–457) or 25 μg/mL (LACK158–173) was added directly to whole splenocytes in culture to assay antigen-specific CD8+ or CD4+ T cells, respectively, after presentation by homologous, splenic APC.
Detection of antigen-specific cells
Fresh splenocytes or purified CD8+ T cells were stained with the LLO91–99-loaded class I MHC pentamer (Proimmune, UK), according to the manufacturer’s instructions, to detect antigen-specific CD8+ T cells. As a second method for determining antigen-specific T cells, splenocytes or T cells were presented peptide at 37°C in the presence of Brefeldin A (BD Biosciences/PharMingen, San Diego, CA, USA) for 6 h, permeabilized, and stained intracellularly for IFN-γ to detect antigen-specific CD8+ or CD4+ T cells as described below. Class I-specific immunodominant LLO91–99 peptide and subdominant p60219–225 and p60449–457 peptides (Biosynthesis, Inc., Lewisville, TX, USA) were used for antigen-specific CD8+ T cells, and class II-specific LACK158–173 peptide (Biosynthesis, Inc.) was used for measuring antigen-specific CD4+ T cells by intracellular cytoplasmic staining.
Surface marker staining, intracellular staining, antibodies, and analytic flow cytometry
For staining of surface markers, cells were incubated with fluorochrome-conjugated mAb at room temperature. After 30 min, the cells were washed with PBS + 0.1% sodium azide and then fixed with 1% formaldehyde in PBS + 0.1% sodium azide.
For intracellular staining, first, surface markers were stained as just described. The cells were then washed 2× with staining buffer [0.1% heat-inactivated FCS (Invitrogen) in PBS+0.1% sodium azide] and incubated with cytofix/cytoperm (BD Biosciences/PharMingen) for 20 min at 4°C. Cells were washed 2× with perm/wash buffer (BD PharMingen) and then stained with fluorochrome-conjugated mAb for intracellular staining by incubating for 30 min at 4°C. Cells were washed 2× with perm/wash buffer and then fixed in staining buffer. Antigen-specific CD8+ and CD4+ T cells were analyzed using a BD FACSCalibur flow cytometer, as described previously in detail [9, 36]. For detection of low-frequency events, up to 106 cells were analyzed for each condition. All antibodies used in the current work were anti-mouse mAb. FITC-conjugated anti-CD4, -CD8, and -CD44 mAb; PE-conjugated anti-CD8, IFN-γ, and CD122/IL-2Rβ mAb; PerCP-conjugated anti-CD4 and -CD8 mAb; and allophycocyanin-conjugated anti-CD4 and -CD8 and BrdU mAb were all purchased from BD Biosciences.
Secreted cytokines
Splenocytes (2×106) were cultured with CD8-specific LLO91–99 peptide for 8 and 22 h, and supernatants were collected and frozen at –73°C. Supernatants were analyzed using a mouse Th1/Th2 cytokine cytometry bead array kit exactly as described by the manufacturer (BD Biosciences/PharMingen). Cytokines were analyzed using a BD FACSCalibur flow cytometer.
BrdU proliferation assays
Control and ethanol-fed mice were inoculated with LM ΔactA (5×105cfu/0.3 mL); BrdU (Sigma-Aldrich, Steinheim, Germany; 5 mg in 0.5 mL PBS) was administered at the same time i.p., and in some experiments, BrdU was administered 5 days after LM ΔactA inoculation. After BrdU administration, BrdU was also added in some experiments, as indicated, to drinking water of controls and to ethanol in water, both at 1.5 mg/mL. Mice were killed 7 days post-LM ΔactA inoculation, and spleens were harvested. Whole splenocytes were cultured for 6 h in the presence of Brefeldin A, permeabilized, and stained for intracellular BrdU exactly as instructed by the manufacturer’s manual (BD Biosciences/PharMingen). Stained splenocytes were analyzed using a BD FACSCalibur flow cytometer.
In vitro proliferation
T cells from naive control or chronic ethanol-fed BALB/c female mice were used for in vitro proliferation assays. Single-cell suspensions of splenocytes were depleted of B220+ cells by positive selection using magnetic separation beads and columns (Miltenyi Biotec) to enrich for T cells, which were then stained with FITC-CD44 and PE-CD8 antibody. CD8+CD44low (naïve phenotype) T cells were sort-purified by standard methods using a BD FACSDiva cell sorter. Sort-purified CD8+ T cells were cultured with soluble anti-CD3 mAb at 1 μg/mL, IL-12p70 (eBioscience, San Diego, CA, USA) at 6 U/mL, IFN-α (Accurate Chemical and Scientific Corp., Westbury, NY, USA) at 100 U/mL, and various concentrations of IL-2 (PharMingen) as indicated for 3 days. IL-12p70 and IFN-α were used in these experiments to achieve optimum proliferation, as determined in control experiments not shown. After 3 days, cells were pulsed with tritiated 3H-thymidine and harvested after 6 h. Proliferation of cells, as determined by the uptake of 3H-thymidine, was analyzed by liquid scintillation counting by standard methods (PerkinElmer Life and Analytical Sciences, Shelton, CT, USA).
Statistics
All results are expressed as mean ± se. The Mann-Whitney nonparametric test was performed for comparison of water and ethanol groups. A two-tailed unpaired t-test (Welch corrected) was also performed if sufficient numbers were present to evaluate normality to evaluate the significance of the mean difference between water and ethanol groups. One-way ANOVA was performed to determine statistical significance when more than two groups were present. All P values shown are two-tailed, and P values less than 0.05 (P<0.05) were considered significant. All statistical analyses were performed using the InStat program, Version 3.0a (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Reduced splenic weight, splenic cellularity, and T cell numbers
Consistent with previously published data for noninoculated C57BL/6 mice [9, 15], the splenic weight, splenic cellularity, and total CD8+ and CD4+ T cell numbers were reduced significantly in 6-week ethanol-fed female BALB/c mice that were inoculated with LM ΔactA 7 days prior (Fig. 1). Spleen weights, cellularity, and CD8+ T cell numbers were reduced significantly in mice consuming ethanol for as little as 4 weeks and as long as 44 weeks prior to inoculation. An increased CD4/CD8 ratio was demonstrated previously for C57BL/6 chronic ethanol-fed mice [9]; however, the CD4/CD8 ratios in the current BALB/c ethanol-administered mice were not different from controls at a statistically significant level.
Fig. 1.
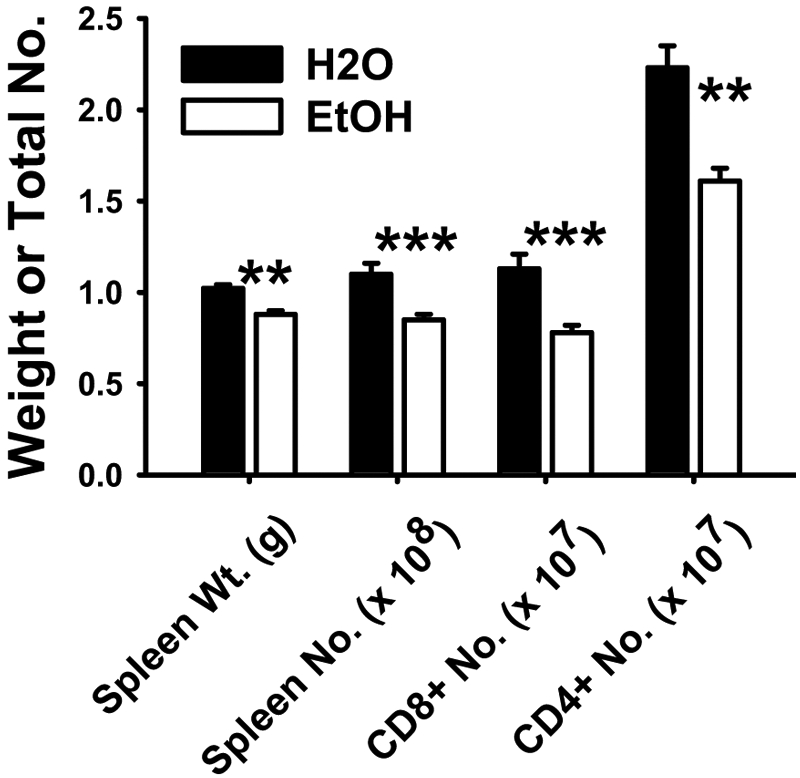
Splenic weight, total cellularity, and T cell numbers in chronic ethanol-consuming mice. Six-week ethanol-administered and matched water control BALB/c female mice were inoculated with LM ΔactA. Mice were killed 7 days post-LM ΔactA inoculation, and spleens were harvested. Spleen weights, total splenocytes/spleen, total number of CD8+ and CD4+ T cells/spleen were measured. N(H2O) = 12; N(EtOH) = 12; **, P < 0.01; ***, P < 0.001.
Antigen-specific CD8+ T cell responses
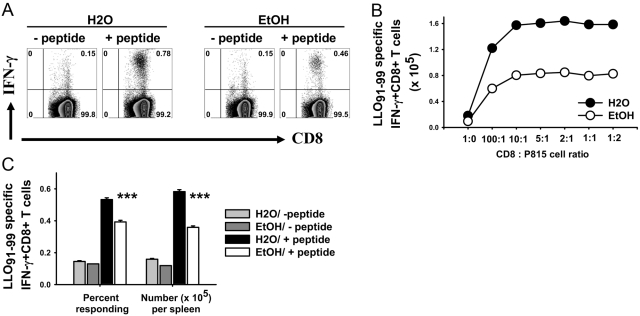
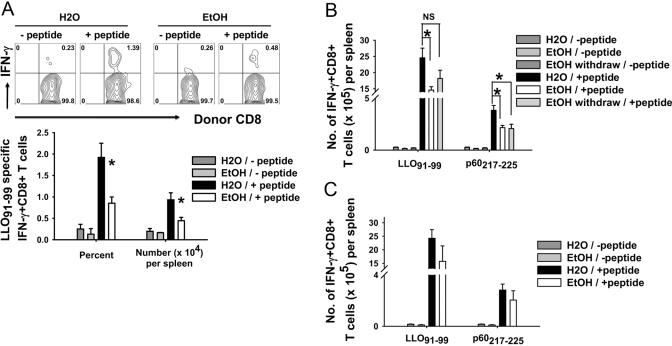
Figure 2A shows the flow cytometry pattern of responding CD8+ T cells to P815 cells loaded with or without the LLO91–99 peptide. Figure 2B demonstrates the titration of various CD8:P815 ratios on total CD8+ antigen-specific cells. Responding CD8+ T cells were significantly lower in ethanol-fed mice (Fig. 2C), as total responding cells per spleen and as the percentage responding in vitro. Inoculation of bacterial doses as high as 2 × 107 (40-fold) did not result in any major difference in the response ratio of control and ethanol-fed mice as compared with Figure 2 and Figure 3 (dose comparison not shown), although there was some variation in absolute response levels.
Fig. 2.
Antigen presentation assay. (A) MACS-purified CD8+ T cells from 7-day-inoculated, 32-week ethanol-consuming or control BALB/c female mice were cultured with the LLO91–99 peptide-loaded P815 cell at a 5:1 ratio in the presence of Brefeldin A for 6 h. As a control, CD8+ T cells were cultured with P815 cells not loaded with peptide. Flow cytometry data of one water- and ethanol-administered group in the absence or presence of the LLO91–99 peptide. (B) CD8+ T cells from 7-day-inoculated, 28-week ethanol-consuming and matched control BALB/c female mice were cultured with LLO91–99 peptide-loaded P815 cells at different ratios in the presence of Brefeldin A for 6 h at 37°C. Responding CD8+ T cells were analyzed by intracellular IFN-γ staining. Control wells contained no P815 cells. (C) Statistical analysis of the data shown in A from all mice in the groups; N(H20) = 9; N(EtOH) = 6; ***, P < 0.001.
Fig. 3.
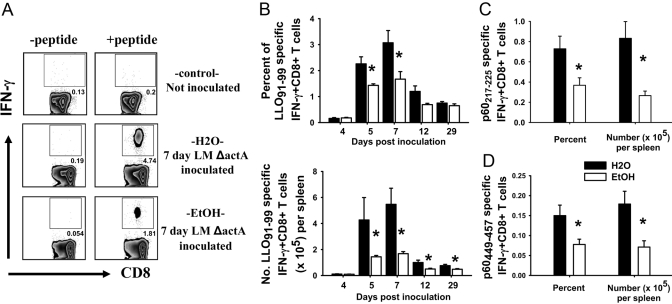
Antigen-specific CD8+ T cell responses. (A) Representative flow cytometry plots of noninoculated and water- and ethanol-administered CD8+ T cells with or without the LLO91–99 peptide at 7 days post-LM ΔactA LACK inoculation. Flow cytometry plots are gated on CD8+ T cells. (B–D) BALB/c female mice consuming ethanol and water for 4–44 weeks were inoculated with LM ΔactA or LM ΔactA LACK; spleens were harvested at different days postinoculation and splenocytes cultured 6 h. Time course of LLO91–99-specific CD8+ T cells as percent responding (B, upper) and total number per spleen (B, lower) in water- and chronic ethanol-consuming mice as determined by intracellular IFN-γ staining. Four days, N(H2O) = 4; N(EtOH) = 6. Five days, N(H2O) = 3; N(EtOH) = 4. Seven days, N(H20) = 7; N(EtOH) = 6. Twelve days, N(H2O) = 8; N(EtOH) = 8. Twenty-nine days, N(H2O) = 8; N(EtOH) = 7. (C) p60217–225-specific and (D) p60449–457-specific CD8+ T cell responses at 7 days post-LM ΔactA LACK inoculation; N(H2O) = 7; N(EtOH) = 6; *, P < 0.05.
Figure 3A shows the flow cytometry pattern of the CD8+ T cell response with or without the LLO91–99 peptide in noninoculated (control) and 7-day LM ΔactA LACK-inoculated control and ethanol-fed mice. The response of LLO91–99-specific CD8+ T cells for control BALB/c female mice was maximum at Day 7, significant contraction occurred by Day 12, and residual, antigen-specific cells were present at Day 29 (Fig. 3B), similar to the time course for normal mice described by others [26, 27, 37]. For mice consuming ethanol for 4–6 weeks or longer, the contraction phase was different, such that the slope of contraction was flatter as compared with water controls. The frequency and total numbers of LLO91–99-specific CD8+ T cells from ethanol-fed mice were reduced at Days 5, 7, 12, and 29 after LM ΔactA inoculation (Fig. 3B). Similar results were obtained for the kinetics of antigen-specific CD8+ T cells in mice consuming ethanol for 19 weeks and 44 weeks before inoculation (data not shown). Antigen-specific CD8+ T cells were present even at 60 days (late memory phase) postinoculation in control and ethanol-fed mice; similar to the earlier times, the antigen-specific CD8+ T cells were comparatively reduced in ethanol-fed mice (60 days not shown). Similar to the immunodominant epitope (LLO91–99), CD8+ T cell responses to subdominant epitopes (p60217–225 and p60449–457) were also reduced significantly at Day 7 post-LM ΔactA LACK inoculation in the ethanol-fed mice (Fig. 3, C and D).
Antigen-specific CD4+ T cell responses
In contrast to the result with CD8+ T cells, the antigen-specific CD4+ T cell response at 7 days after LM ΔactA LACK inoculation was similar between control and ethanol-fed mice (Fig. 4A). In some experiments with long ethanol exposures (>40 weeks ethanol), there was a trend toward reduced total antigen-specific CD4+ T cells per spleen in ethanol-fed mice; however, no statistical significance was obtained. Soluble IFN-γ in the supernatant, produced by antigen-specific CD4+ T cells in response to two subdominant peptides added together (LLO189–200+LLO216–227), was also similar between control and ethanol-fed mice (Fig. 4B). The IFN-γ detected in the supernatants was clearly produced by antigen-specific CD4+ T cells, as no IFN-γ was detected in CD4+-depleted cultures in parallel experiments (data not shown). Comparable results with no reduction of antigen-specific CD4+ T cells in mice chronically fed ethanol were obtained in numerous trials of C57BL/6 mice using the immunodominant CD4 epitope (LLO190–201; data not shown).
Fig. 4.
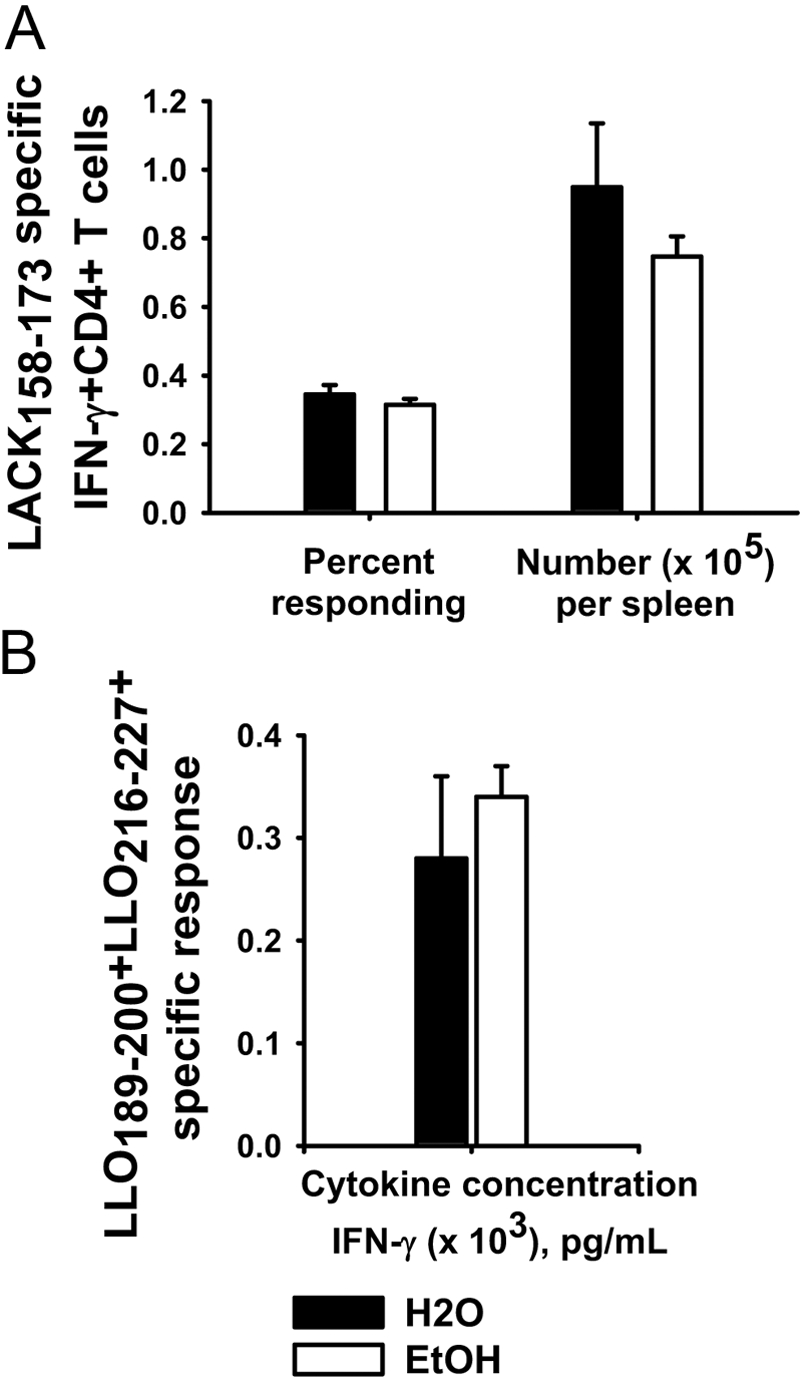
Antigen-specific CD4+ T cells. Chronic ethanol- or water-administered BALB/c female mice were inoculated with 1 × 107 cfu LM ΔactA LACK. Mice were killed at 7 days after inoculation, and antigen-specific CD4+ T cell responses were measured in splenocyte cultures. (A) Frequency and total number of LACK158–173-specific CD4+ T cells were measured by intracellular cytoplasmic staining for IFN-γ. (B) Splenocytes were incubated at the same time with two CD4-specific subdominant peptides (LLO189–200+LLO216–227) for 96 h, and supernatants were collected and analyzed for IFN-γ; N(H2O) = 4; N(EtOH) = 4.
Cytokine production by CD8+ T cells after LLO91–99 peptide stimulation
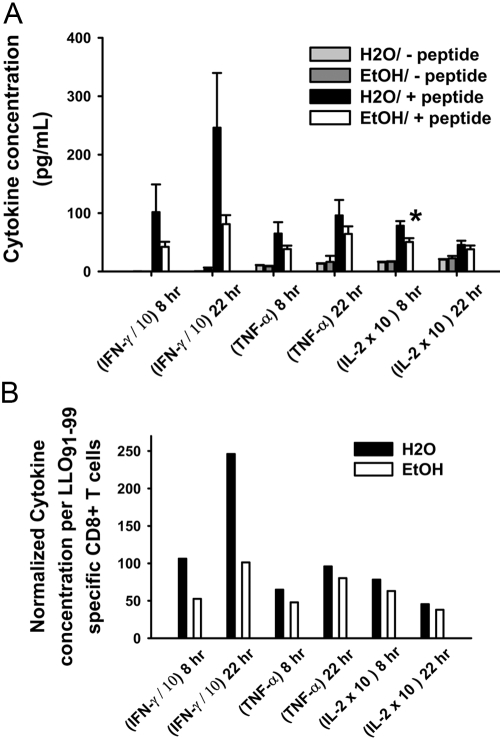
Supernatants from whole splenocyte cultures of inoculated mice that were stimulated with or without the LLO91–99 peptide for 8 and 22 h were analyzed for Tc1 cytokines (TNF-α, IFN-γ, and IL-2) and Tc2 cytokines (IL-4 and IL-5) to determine any Tc1 to Tc2 skewing. Tc1 and Tc2 cytokines were at background levels in supernatants from cultures without the LLO91–99 peptide (Fig. 5A). In the presence of the LLO91–99 peptide, Tc2 cytokines were not detectable in peptide-stimulated cells from control or ethanol-fed mice in any experiments. Tc1 cytokines were produced by cells from control and ethanol-fed groups, albeit less by cells from most ethanol-administered groups (Fig. 5A), during the primary response (7 days). As antigen-specific CD8+ T cells were reduced already, reduced production of these cytokines was expected. IFN-γ production was reduced even when normalized to cytokine production on an antigen-specific, per-cell basis (Fig. 5B), suggesting a functional defect in antigen-specific CD8+ T cells in addition to their decreased numbers. TNF-α, IFN-γ, and IL-2 levels were lower for all 4-, 19-, or 44-week ethanol-fed mice compared with their age-matched controls.
Fig. 5.
Cytokine production after CD8-specific LLO91–99 peptide restimulation. (A) BALB/c female mice consuming ethanol in water for 4 or 6 weeks were inoculated with LM ΔactA, and spleens were harvested 7 days postinoculation. Whole splenocytes (2×106) were cultured with 10−7M LLO91–99 peptide for 8- and 22-h durations. Supernatants were analyzed using a Th1/Th2 cytokine bead assay. (B) Values from the above experiments were normalized to evaluate cytokines produced per antigen-specific CD8+ T cells. For normalization, ethanol cytokine values were multiplied by an antigen-specific CD8+ T cell ratio of water and ethanol; i.e., ratio = percent antigen-specific CD8+ T cells (H2O/ethanol). IFN-γ values were divided by 10, and IL-2 values were multiplied by 10 to fit in the same graph; N(H2O) = 7; N(EtOH) = 8; *, P < 0.05.
In vivo proliferation of CD8+ T cells
BrdU was administered to inoculated mice for 7 days, 2 days, or 1 day (Fig. 6), and the relative proliferation of CD8+ T cells was determined. The percentage of and the total BrdU+CD8+ T cell numbers were significantly lower in ethanol-fed mice compared with their controls (Fig. 6A). Proliferation of antigen-specific CD8+ T cells, as determined by double staining for LLO91–99-specific MHC I pentamer or intracellular IFN-γ and BrdU, was also reduced in ethanol-fed mice compared with their respective controls (Fig. 6B).
Fig. 6.
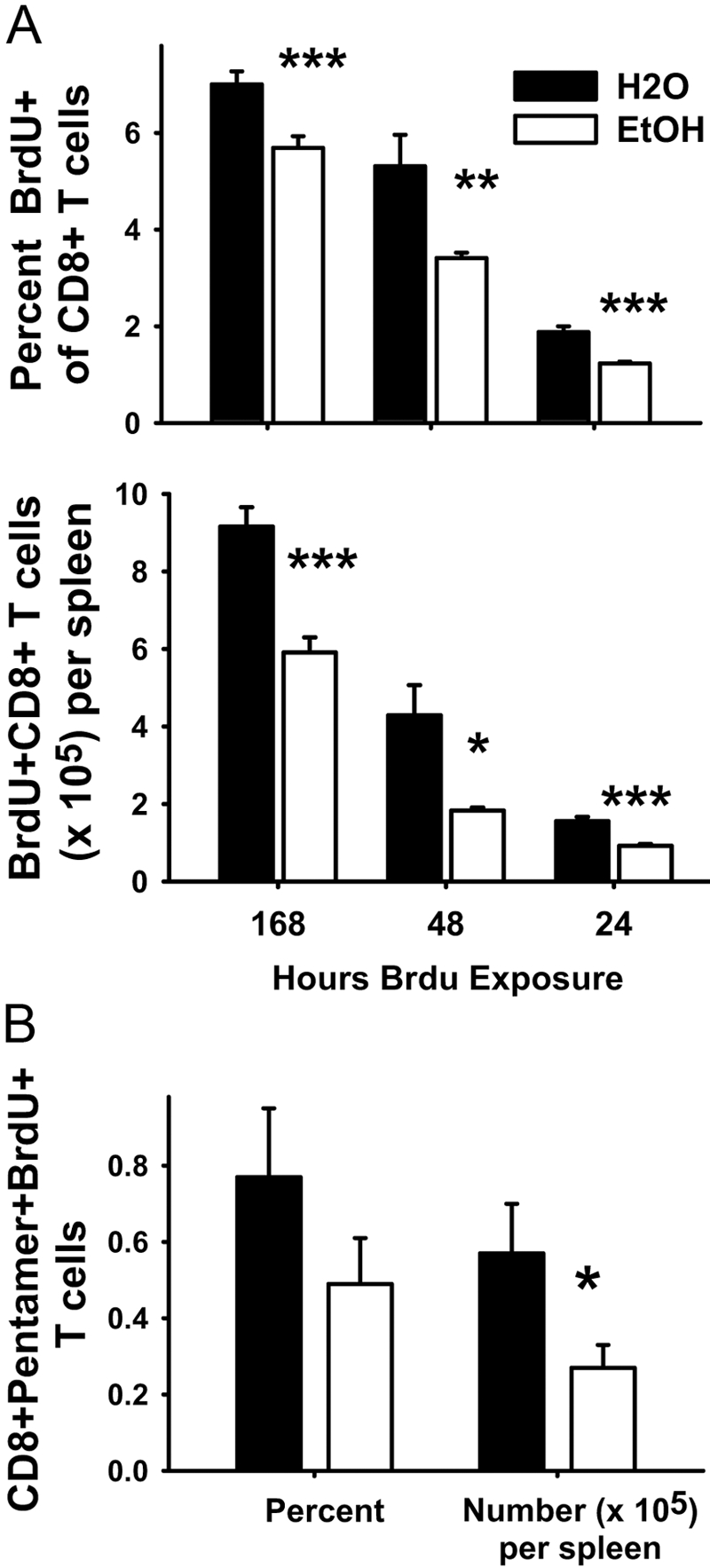
In vivo CD8+ T cell proliferation assays. (A) In vivo total CD8+ T cell proliferation assays. (Upper panel) Percent BrdU+CD8+ T cells and (lower panel) numbers of BrdU+CD8+ T cells per spleen. Ethanol- and water-administered BALB/c female mice (9–32 weeks) were inoculated with LM ΔactA i.p. One hundred sixty-eight hours: BrdU was injected with LM ΔactA and added to the drinking water or ethanol of water and ethanol mice, which were killed 7 days after LM ΔactA inoculation and BrdU injection; N(H2O) = 8; N(EtOH) = 8. Forty-eight hours: BrdU was injected 5 days after LM ΔactA inoculation and then added to drinking water or ethanol of water and ethanol mice, which were killed 7 days after LM ΔactA inoculation and 2 days after BrdU injection; N(H2O) = 4; N(EtOH) = 5. Twenty-four hours: BrdU was injected 5 days after LM ΔactA inoculation, and mice were killed the next day, i.e., 6 days after LM ΔactA inoculation. BrdU was not added in drinking water or ethanol; N(H2O) = 4; N(EtOH) = 6. Intracellular cytoplasmic staining was performed for BrdU. (B) In vivo antigen-specific CD8+ T cell proliferation. Ethanol (14 weeks)-administered BALB/c female mice and water mice were inoculated with LM ΔactA. Five days after inoculation, BrdU was administered as described for the 48-h exposure (A), and splenocytes were harvested at the peak of the response (Day 7). Whole splenocytes were surface-stained with PerCP-conjugated CD8 mAb and APC-conjugated LLO91–99-loaded class I MHC pentamer and intracellularly stained for incorporated BrdU; N(H20) = 4; N(EtOH) = 4; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CD122 expression and in vitro proliferation of T cells
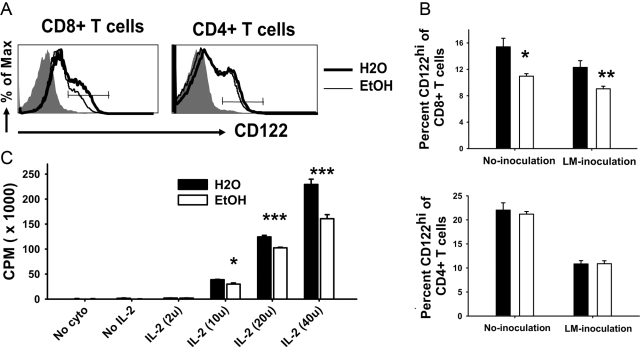
CD122 (IL-2Rβ) is present on CD8+ T cells and is important for their proliferation and survival. The frequency of CD8+CD122hi T cells in noninoculated or LM ΔactA-inoculated mice was reduced significantly in ethanol-fed mice (Fig. 7, A and B). IL-2-dependent, in vitro proliferation of CD8+CD44low T cells from naïve mice was also reduced significantly in the cells from chronic ethanol mice (Fig. 7C). In contrast and consistent with the normal antigen-specific CD4+ T cell response in ethanol-fed mice (Fig. 4), CD122 expression on CD4+ T cells from chronic ethanol-fed mice was normal (Fig. 7, A and B). Additionally, multiple proliferation assays have been carried out for CD4+ T cells, and no difference between control and ethanol-fed mice has been seen to date (data not shown).
Fig. 7.
IL-2R expression and in vitro proliferation of CD8+ T cells. (A) Histogram of CD122 (IL-2Rβ chain) expression on CD8+ and CD4+ T cells from control and 9- to 32-week ethanol-administered BALB/c female mice, as analyzed by flow cytometry. Shaded gray is the isotype control; thick black line is water; and thin line is ethanol CD8+ or CD4+ T cells. A line across the histogram denotes CD122hi populations. (B) Total percentages of CD8+CD122hi (left) and CD4+CD122hi (right) T cells are shown as bar graphs. Some mice were inoculated with 5 × 105 cfu LM ΔactA to generate immune CD8+ T cells. No inoculation, N(H2O) = 8; N(EtOH) = 6. LM ΔactA inoculation, N(H2O) = 12; N(EtOH) = 16. (C) In vitro proliferation of splenic CD8+CD44low T cells. Sort-purified CD8+CD44low T cells from 19-week, ethanol-consuming and control mice were cultured with soluble anti-CD3 mAb, IL-12p70, IFN-α, and increasing concentrations of IL-2 for 3 days. After 3 days, cells were pulsed with 3H-thymidine for 6 h, and the uptake of 3H-thymidine was measured using standard liquid scintillation analysis. One of two similar experiments. No cyto, No added cytokines; No IL-2, no IL-2 added; and IL-2 (2u), units IL-2 added. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Secondary antigen-specific CD8+ T cell responses
The primary responses of CD8+ T cells are inhibited in ethanol-consuming mice (Fig. 1), and the total number of resulting memory cells appears to remain low (Fig. 3). As a further test of memory cell numbers, we performed an adoptive transfer study with cells from 8-week, ethanol-fed and control mice inoculated with LM 44 days before transfer and maintained on their respective diets until transfer. Cells were transferred only to normal control mice, after which, the recipient mice were inoculated with LM. The secondary response to the dominant LLO91–99 peptide of donor Thy1.2+CD8+ T cells from ethanol-fed mice was reduced significantly in comparison with Thy1.2+CD8+ T cells from control mice (Fig. 8A), consistent with the reduction observed previously in 29-day memory cells of chronic ethanol mice (Fig. 3).
Fig. 8.
Secondary CD8+ T cell responses to LM ΔactA LACK reinoculation. (A) Thy 1.2 BALB/c control and 8-week ethanol-administered mice were inoculated with LM and continued on their respective diets. Forty-four days later, 5 × 106 MACS-purified, splenic CD8+ T cells were adoptively transferred to Thy1.1 water mice i.v. Recipient Thy1.1 water mice were inoculated with LM ΔactA LACK 1 day later, and the secondary response of donor-immune Thy1.2+CD8+ T cells was analyzed by intracellular cytoplasmic staining for IFN-γ on Day 5 after inoculation. Thy 1.1 recipients received water source cells N(H2O) = 4 or ethanol source cells N(EtOH) = 6. (B) Water- and 46-week chronic ethanol-administered mice were inoculated with LM ΔactA LACK. Twenty days following inoculation, some ethanol-administered mice were withdrawn from ethanol for 15 days. All three groups—H2O, EtOH, and EtOH-withdrawn mice—were reinoculated with LM ΔactA LACK, and the secondary CD8+ T cell responses were measured on Day 5. N(H2O) = 4; N(EtOH) = 5; N(EtOH withdrawn) = 5. (C) Normal water mice (no ethanol history) were inoculated with LM ΔactA LACK. Sixteen days postinoculation, some water mice were started on EtOH. After 5 weeks of EtOH administration, mice were reinoculated with LM ΔactA LACK, and the secondary CD8+ T cell responses were measured on Day 5 by intracellular cytoplasmic staining for IFN-γ. N(H2O) = 5; N(EtOH) = 3; *, P < 0.05. NS, Not significant.
Chronic ethanol-fed mice have reduced LLO91–99- and p60217–225-specific, secondary CD8+ T cell responses to LM ΔactA LACK when compared with normal controls; 3 weeks ethanol withdrawal did not reverse the ethanol effects, although there was some variability (Fig. 8B and not shown). LACK158–173-specific CD4+ T cell responses were similar among all three groups (data not shown).
All of the above findings resulted from examination of mice inoculated during chronic ethanol exposure. Using an alternative protocol, control mice were inoculated with LM, followed by extended ethanol drinking and a secondary challenge. LM ΔactA LACK-immune control mice that were subsequently administered ethanol for 5 weeks and rechallenged with LM ΔactA LACK trended to a lower CD8+ T cell response in the ethanol-administered group but did not show statistically significant reductions of LLO91–99-, p60217–225-, and p60449–457-specific CD8+ T cells nor of LACK158–173-specific CD4+ T cells (Fig. 8C, and data not shown).
DISCUSSION
The adverse effect of ethanol consumption on immune function has been studied in various models to understand the mechanisms involved. We have used a chronic ethanol-in-water protocol [35] to understand the effects of chronic ethanol feeding on adaptive immunity and especially T cells. Consistent with previous reports [9, 15, 36], splenic weights, total cellularity, and CD8+ T cell numbers were reduced significantly in chronic ethanol-fed mice. A major finding was that antigen-specific CD8+ T cell responses to three-peptide epitopes of Listeria were all reduced significantly in mice chronically consuming ethanol. These reductions in the ethanol source CD8+ T cell responses are in the same ratio to control responses using three different APC systems for the in vitro assay, and it is clear that APC impairment in the in vitro assay does not explain the results. Instead, the sharp reduction in the antigen-specific CD8+ T cells reflects compromised CD8+ T cell responses after in vivo inoculation of the ethanol-consuming mice.
Several key time-points in the antigen-specific CD8+ T cell response after LM ΔactA inoculation were evaluated by LLO91–99-specific MHC class I pentamer staining (data not shown) and IFN-γ intracellular staining after restimulation with the LLO91–99 peptide. Both readouts demonstrated a similar reduction in the CD8+ T cell response in ethanol-fed mice at all time-points when inoculation occurred after 4 weeks of ethanol exposure or greater. Similar to data for normal mice published by others [26, 27], the maximum response of LM ΔactA LLO91–99-specific CD8+ T cells was seen at Day 7, contraction by Day 12, and measurable early memory phase was present at Day 29 for control and chronic ethanol-fed mice; the antigen-specific response was still detectable after 60 days. The contraction of antigen-specific CD8+ T cell responses in ethanol-fed mice was flattened in slope, suggesting a slower relative decay rate when compared with control mice, and the total number of antigen-specific CD8+ T cells was reduced significantly in ethanol-fed mice at all time-points (Fig. 3).
We also evaluated the antigen-specific CD4+ T cell response to the LACK158–173 peptide in chronic ethanol-fed BALB/c mice and in contrast to CD8+ T cell responses, were unable to find any significant differences compared with controls. However, a trend toward reduced total numbers of antigen-specific CD4+ T cells in BALB/c mice administered ethanol for more than 40 weeks was observed (data not shown), and it is possible that some deterioration of CD4+ T cell function will be found to occur in long-term ethanol exposure protocols.
Several mechanistic possibilities exist for the reduction in antigen-specific CD8+ T cell responses in the chronic ethanol-fed mice. Altered APC functions, including migration, up-regulation of costimulatory molecules, and production of effector cytokines, have been described in the context of various ethanol-exposure models [19, 25, 36, 38,39,40]. It is possible, therefore, that there is some contribution of altered APC function in vivo to the reduction of antigen-specific CD8+ T cells in the current work. However, it is clear that the CD8+ T cells of the chronic ethanol-fed mice have intrinsic defects. These cells do not proliferate normally in vitro, even when purified as naïve (from noninoculated mice) CD8+ T cells (Fig. 7C), ruling out altered antigen processing/presentation for the effect. Antigen-specific memory cells raised in ethanol-fed mice did not expand at a control level, even when transferred to a control host (Fig. 8A), and as discussed below, the antigen-specific cells from ethanol-fed mice do not produce normal levels of IFN-γ (Fig. 5) in response to antigenic challenge in vitro.
BrdU incorporation studies were performed to examine the proliferative response of CD8+ T cells in vivo. BrdU was administered over the entire 7 days of the primary response or in other protocols as only a short pulse of BrdU before euthanasia. Similar results were obtained with all different BrdU time pulses, consistent with reduced proliferation in ethanol-fed mice, in contrast to preferential apoptosis. Direct ex vivo evaluation of apoptosis by Annexin V staining of freshly isolated splenocytes also did not reveal any differences between CD8+ and CD4+ T cells from control and ethanol-fed mice (data not shown). These results strongly point to reduced proliferation as the basis for lower CD8+ T cell responses, although a role for apoptosis in vivo cannot be excluded entirely, given the rapid removal of apoptotic cells by macrophages.
Previous studies of chronic alcoholics have shown that the ability of peripheral blood T cells from chronic alcoholics with liver disease to bind IL-2 was reduced when compared with healthy individuals [41], which is in contrast to studies in rats that showed no effect on the ability of IL-2R to bind IL-2 [21]. Proliferation and IL-2 production by T cells from acute ethanol-administered mice with added burn injury were reduced significantly when compared with controls [42], as was proliferation of T cells from fetal alcohol exposure rats [43]. In the current work, we have examined in vitro proliferation of sort-purified CD8+CD44low T cells from noninoculated mice in an assay designed to test the proliferative response of CD8+ T cells to IL-2, and IL-2-dependent proliferation was reduced in naïve CD8+ T cells from chronic ethanol-fed mice at all concentrations (Fig. 7). Previous studies evaluated low-affinity IL-2R (IL-2Rα chain/CD25) on T cells and found no difference between the ethanol and control groups [18, 22, 44]. We measured high-affinity IL-2Rβ (CD122) to evaluate the expression of the IL-2R; interestingly, ex vivo staining demonstrated reduced expression of the IL-2Rβ chain on CD8+ T cells from chronic ethanol-fed mice, a change not present on CD4+ cells from the same mice. It thus appears that one likely mechanism of the reduction of CD8+ T cell responses by chronic ethanol consumption is reduced induction/expression of the high-affinity IL-2R on naïve CD8+ T cells after antigen exposure and subsequently, reduced proliferative capacity of those cells. An additional feature of CD8+ T cell dysfunction in ethanol-fed mice is suggested by the reduction of IFN-γ secretion even after normalization to the number of antigen-specific CD8+ T cells (Fig. 5). Some degree of dysregulation thus may exist in the antigen-specific effector cells; we have recently demonstrated a similar result in pulmonary CD8+ T cells from chronic ethanol-fed mice responding to influenza A virus [45].
The secondary responses of LM ΔactA-immune CD8+ T cells from ethanol-consuming mice after transfer to normal control mice were reduced significantly when compared with LM ΔactA-immune CD8+ T cells transferred from control mice. This result is consistent with the transfer of a smaller proportion of memory cells from ethanol-fed mice, as implied by the reduced total number of memory cells assayed previously in such mice 29 days after inoculation (Fig. 3). However, the immune CD8+ T cells from donor control mice expanded two- to threefold more after inoculation of the recipients when compared with immune CD8+ T cells from ethanol-fed donor mice. Thus, an intrinsic defect of memory CD8+ T cells generated in mice during chronic ethanol consumption is not excluded and will be the subject of future studies in this laboratory. In a different protocol, the secondary response of CD8+ T cells in LM-immune ethanol-fed mice was measured after withdrawal from ethanol; the secondary CD8+ T cell response was not rescued by such withdrawal (Fig. 8B). The above results indicate that the decreased primary CD8+ T cell response resulting from chronic ethanol ingestion at the time of antigenic exposure results in a reduced memory pool that does not recover numerically, even after ethanol is stopped. In a separate memory protocol, subsequent chronic ethanol feeding up to 6 weeks had little effect on the functional ability of previously generated LM ΔactA LACK memory CD8+ T cells to mount a secondary response upon reinoculation with the LM ΔactA LACK; however, there was a trend toward a total reduced number of specific CD8+ T cells (Fig. 8C). Future long-term studies will be required to determine whether longer periods of ethanol exposures will affect the functional ability or numbers of memory CD8+ T cells originally generated in the absence of ethanol.
In conclusion, our results show that the antigen-specific CD8+ T cell primary response to LM peaks at Day 7, contracts by Day 12, and reaches early memory phase by Day 29 for control and ethanol-fed mice; however, the response curve is flattened, and total numbers are reduced in the ethanol mice. In contrast, antigen-specific CD4+ T cell responses are not reduced in the chronic ethanol-fed mice. The total Tc1 cytokine production in response to in vitro antigen was reduced in chronic ethanol-fed mice, but Tc1 to Tc2 skewing was not seen in our experimental model. Memory CD8+ T cell numbers were also reduced by chronic ethanol feeding; reduction of secondary CD8+ antigen-specific cell numbers appeared to be dependent on the initial reduction occurring during the primary response of CD8+ T cells in the ethanol-fed mice. The reduction in the number of antigen-specific CD8+ T cells seen in chronic ethanol-fed mice was accompanied by reduced expression of IL-2Rβ and reduced proliferation in vivo and in vitro, but the in vivo decrease of antigen-specific cells may not be limited to this mechanism.
Acknowledgments
This work was supported by the Department of Pathology at the University of Iowa and an Interactive Research Program grant including National Institutes of Health Grants AA 011405 (R. T. C.), AA 014400 (T. J. W.), AA 014406 (Annette J. Schlueter), and AA 014418 (Zuhair K. Ballas) at the University of Iowa and AA012450 (Thomas R. Jerrells) at the University of Nebraska (Lincoln, NE, USA). We thank Drs. John T. Harty and Vladimir P. Badovinac at the University of Iowa for kind gifts of LM ΔactA strains and consultations.
References
- Breitkopf K, Hass S, Wiercinska E, Singer M V, Dooley S. Anti-TGF-β strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S–131S. doi: 10.1097/01.alc.0000189284.98684.22. [DOI] [PubMed] [Google Scholar]
- Cook R T. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook R T, Zhu X, Coleman R A, Ballas Z K, Waldschmidt T J, Ray N B, LaBrecque D R, Cook B L. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:175–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Happel K I, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- Lewohl J M, Wixey J, Harper C G, Dodd P R. Expression of MBP, PLP, MAG, CNP, and GFAP in the human alcoholic brain. Alcohol Clin Exp Res. 2005;29:1698–1705. doi: 10.1097/01.alc.0000179406.98868.59. [DOI] [PubMed] [Google Scholar]
- MacGregor R R, Louria D B. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Saad A J, Saad R D, Jerrells T R. Ethanol ingestion increases susceptibility of mice to Listeria monocytogenes. Alcohol Clin Exp Res. 1993;17:75–78. doi: 10.1111/j.1530-0277.1993.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Simanowski U A, Homann N, Knuhl M, Arce L, Waldherr R, Conradt C, Bosch F X, Seitz H K. Increased rectal cell proliferation following alcohol abuse. Gut. 2001;49:418–422. doi: 10.1136/gut.49.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Coleman R A, Zhu X, Alber C, Ballas Z K, Waldschmidt T J, Cook R T. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Jerrells T R, Sibley D. Effects of ethanol on cellular immunity to facultative intracellular bacteria. Alcohol Clin Exp Res. 1995;19:11–16. doi: 10.1111/j.1530-0277.1995.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Meadows G G, Blank S E, Duncan D D. Influence of ethanol consumption on natural killer cell activity in mice. Alcohol Clin Exp Res. 1989;13:476–479. doi: 10.1111/j.1530-0277.1989.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Meadows G G, Wallendal M, Kosugi A, Wunderlich J, Singer D S. Ethanol induces marked changes in lymphocyte populations and natural killler cell activity in mice. Alcohol Clin Exp Res. 1992;16:474–479. doi: 10.1111/j.1530-0277.1992.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Shellito J E, Olariu R. Alcohol decreases T-lymphocyte migration into lung tissue in response to Pneumocystis carinii and depletes T-lymphocyte numbers in the spleens of mice. Alcohol Clin Exp Res. 1998;22:658–663. doi: 10.1111/j.1530-0277.1998.tb04308.x. [DOI] [PubMed] [Google Scholar]
- Starkenburg S, Munroe M E, Waltenbaugh C. Early alteration in leukocyte populations and Th1/Th2 function in ethanol-consuming mice. Alcohol Clin Exp Res. 2001;25:1221–1230. [PubMed] [Google Scholar]
- Zhang H, Meadows G G. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- Brodie C, Domenico J, Gelfand E W. Ethanol inhibits early events in T-lymphocyte activation. Clin Immunol Immunopathol. 1994;70:129–136. doi: 10.1006/clin.1994.1020. [DOI] [PubMed] [Google Scholar]
- Chang M P, Norman D C, Makinodan T. Immunotoxicity of alcohol in young and old mice. I. In vitro suppressive effects of ethanol on the activities of T and B immune cells of aging mice. Alcohol Clin Exp Res. 1990;14:210–215. doi: 10.1111/j.1530-0277.1990.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Kaplan D R. A novel mechanism of immunosuppression mediated by ethanol. Cell Immunol. 1986;102:1–9. doi: 10.1016/0008-8749(86)90320-5. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defense. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Jerrells T R, Peritt D, Marietta C, Eckardt M J. Mechanisms of suppression of cellular immunity induced by ethanol. Alcohol Clin Exp Res. 1989;13:490–493. doi: 10.1111/j.1530-0277.1989.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Jerrells T R, Perritt D, Eckardt M J, Marietta C. Alterations in interleukin-2 utilization by T-cells from rats treated with an ethanol-containing diet. Alcohol Clin Exp Res. 1990;14:245–249. doi: 10.1111/j.1530-0277.1990.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Chang M P, Norman D C. Mechanism of ethanol-mediated immunosuppression in mice: ethanol suppresses T-cell proliferation without affecting IL2 production and IL2 receptor expression. Int J Immunopharmacol. 1992;14:707–719. doi: 10.1016/0192-0561(92)90134-7. [DOI] [PubMed] [Google Scholar]
- Spies C D, von Dossow V, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schröder T, Höflich C, Sinha P. Altered cell-mediated immunity and increased postoperative infection rate in long term alcoholic patients. Anesthesiology. 2004;100:1088–1100. doi: 10.1097/00000542-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Krolewiecki A J, Leon S, Scott P A, Nolan T J, Schad G A, Abraham D. Effect of chronic ethanol consumption on protective T-helper 1 and T-helper 2 immune responses against the parasites Leishmania major and Strongyloides stercoralis in mice. Alcohol Clin Exp Res. 2001;25:571–578. [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol. 2004;33:241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Badovinac V P, Porter B B, Harty J T. Programmed contraction of CD8+ T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Badovinac V P, Messingham K A N, Hamilton S E, Harty J T. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Badovinac V P, Porter B B, Harty J T. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- Geginat G, Schenk S, Skoberne M, Goebel W, Hof H. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J Immunol. 2001;166:1877–1884. doi: 10.4049/jimmunol.166.3.1877. [DOI] [PubMed] [Google Scholar]
- Ladel C H, Flesch I E, Arnoldi J, Kaufmann S H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- Pavia C S, Harris C M, Kavanagh M. Impaired bactericidal activity and host resistance to Listeria monocytogenes and Borrelia burgdorferi in rats administered an acute oral regimen of ethanol. Clin Diagn Lab Immunol. 2002;9:282–286. doi: 10.1128/CDLI.9.2.282-286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N B, Coleman R A, Wiechert S C, Hu L, Jerrells T R, Barry R A, Cook R T. Murine immune responses to Listeria following chronic exposure to ethanol. Alcohol Clin Exp Res. 2003;27:35A. [Google Scholar]
- Ray N B, Wiechert S C, Coleman R A, Cook R T. Innate immune responses of alcohol-exposed mice and macrophage-like cells following infection with Listeria monocytogenes. Alcohol Clin Exp Res. 2004;28:37A. [Google Scholar]
- Saklani-Jusforgues H, Fontan E, Soussi N, Milon G, Goossens P L. Enteral immunization with attenuated recombinant Listeria monocytogenes as a live vaccine vector: organ-dependent dynamics of CD4 T lymphocytes reactive to a Leishmania major tracer epitope. Infect Immun. 2003;71:1083–1090. doi: 10.1128/IAI.71.3.1083-1090.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R T, Schlueter A J, Coleman R A, Tygrett L, Ballas Z K, Jerrells T R, Nashelsky M B, Ray N B, Haugen T H, Waldschmidt T J. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion—absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Coleman R A, Alber C, Ballas Z K, Waldschmidt T J, Ray N B, Krieg A M, Cook R T. Chronic ethanol ingestion by mice increases expression of CD80 and CD86 by activated macrophages. Alcohol. 2004;32:91–100. doi: 10.1016/j.alcohol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty J T. The generation and modulation of antigen-specific memory CD8+ T cell responses. J Leukoc Biol. 2006;80:16–23. doi: 10.1189/jlb.0206118. [DOI] [PubMed] [Google Scholar]
- Lau A H, Abe M, Thomson A W. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Edsen-Moore M R, Fan J, Ness K J, Marietta J R, Cook R T, Schlueter A J. Effects of chronic ethanol feeding on murine dendritic cell numbers, turnover rate, and dendropoiesis. Alcohol Clin Exp Res. 2008;32:1309–1320. doi: 10.1111/j.1530-0277.2008.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness K J, Fan J, Wilke W W, Coleman R A, Cook R T, Schlueter A J. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso F J, Iglesias-Osma C, Ciudad J, Lopez A, Pastor I, Torres I, Orfao A. Alcoholic liver cirrhosis is associated with a decreased expression of the CD28 costimulatory molecule, a lower ability of T cells to bind exogenous IL-2, and increased soluble CD8 levels. Cytometry. 2000;42:290–295. [PubMed] [Google Scholar]
- Choudhry M A, Messingham K A N, Namak S, Colantoni A, Fontanilla C V, Duffner L A, Sayeed M M, Kovacs E J. Ethanol exacerbates T cell dysfunction after thermal injury. Alcohol. 2000;21:239–243. doi: 10.1016/s0741-8329(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Jerrells T R, Weinberg J. Influence of ethanol consumption on immune competence of adult animals exposed to ethanol in utero. Alcohol Clin Exp Res. 1998;22:391–400. [PubMed] [Google Scholar]
- Chang M P, Yamaguchi D T, Yeh M, Taylor A N, Norman D C. Mechanism of the impaired T-cell proliferation in adult rats exposed to alcohol in utero. Int J Immunopharmacol. 1994;16:345–357. doi: 10.1016/0192-0561(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Meyerholz D K, Edsen-Moore M, McGill J, Coleman R A, Cook R T, Legge K L. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181:641–648. doi: 10.4049/jimmunol.181.1.641. [DOI] [PMC free article] [PubMed] [Google Scholar]