Abstract
Substance P (SP) is a potent modulator of monocyte/macrophage function. The SP-preferring receptor neurokinin-1 receptor (NK1R) has two forms: a full-length NK1R (NK1R-F) isoform and a truncated NK1R (NK1R-T) isoform, which lacks the terminal cytoplasmic 96-aa residues. The distribution of these receptor isoforms in human monocytes is not known. We previously identified an interaction among SP, NK1R, and HIV viral strains that use the chemokine receptor CCR5 as a coreceptor, suggesting crosstalk between NK1R and CCR5. The purpose of this study was to determine which form(s) of NK1R are expressed in human peripheral blood monocytes and to determine whether SP affects proinflammatory cellular responses mediated through the CCR5 receptor. Human peripheral blood monocytes were found to express NK1R-T but not NK1R-F. SP interactions with NK1R-T did not mobilize calcium (Ca2+), but SP mobilized Ca2+ when the NK1R-F was transfected into monocytes. However, the NK1R-T was functional in monocytes, as SP enhanced the CCR5 ligand CCL5-elicited Ca2+ mobilization, a response inhibited by the NK1R antagonist aprepitant. SP interactions with the NK1R-T also enhanced CCL5-mediated chemotaxis, which was ERK1/2-dependent. NK1R-T selectively activated ERK2 but increased ERK1 and ERK2 activation by CCL5. Activation of NK1R-T elicited serine phosphorylation of CCR5, indicating that crosstalk between CCL5 and SP may occur at the level of the receptor. Thus, NK1R-T is functional in human monocytes and activates select signaling pathways, and the NK1R-T-mediated enhancement of CCL5 responses does not require the NK1R terminal cytoplasmic domain.
Keywords: chemokines, MAPKs, calcium mobilization, receptor phosphorylation
INTRODUCTION
Substance P (SP), an undecapeptide, is a member of the tachykinin peptide family. SP is an important neuroimmune modulator, in particular, with respect to the immune functions of monocytes/macrophages [1,2,3]. SP is a potent proinflammatory mediator and plays an important role in inflammation and viral infections such as HIV infection [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18].
The biologic responses to SP are mediated by the SP-preferring neurokinin-1 receptor (NK1R), which is a G-protein-coupled receptor (GPCR) characterized by seven transmembrane domains and is a member of the pertussis toxin-insensitive Gq/11 family [19]. NK1R has been identified on immune cells [4, 5, 20,21,22,23,24,25], including monocytes/macrophages [5, 26], neutrophils [27], T and B lymphocytes [22, 28, 29], and mast cells [30]. In vivo studies with NK1R antagonists and gene-targeted disruption of NK1R protected the lung from inflammatory injury, demonstrating that SP proinflammatory responses are mediated through binding to NK1R [6, 31].
Two isoforms of NK1R have been identified. The human NK1R gene, through retention of introns in its genomic structure, produces splice variants: a full-length form (NK1R-F) composed of 407 aa and a naturally occurring splice variant with a truncated C-terminus {truncated NK1R (NK1R-T) [10, 32, 33]}, which has a 311-aa sequence with a short carboxyl-terminal sequence, extending only 7 aa residues after the end of the seventh transmembrane segment. The remaining amino acid sequence of the NK1R-T isoform is identical to that of NK1R-F [32]. The binding and signaling properties of NK1R-T or NK1R mutants have been compared with those of NK1R-F expressed in different cell systems, and differences have been observed [21, 32, 34,35,36,37,38]. When NK1R-T and NK1R-F were cloned and expressed in COS cells, NK1R-T binding affinity was tenfold less than NK1R-F [32]. Furthermore, NK1R-T elicited only weak electrophysiological responses in Xenopus oocytes (100-fold less) as compared with NK1R-F [32]. In the THP-1 cell line, we demonstrated differences in SP-induced calcium (Ca2+) mobilization between NK1R-F and NK1R-T [21]. The implications of differential expression of NK1R isoforms in different cell types are not known, and these different isoforms may regulate diverse cellular functions.
NK1R-F is the predominant form expressed at sites in the human brain, whereas NK1R-T is widespread throughout the CNS and is the predominant form in peripheral tissues [39, 40]. Although the existence and expression of NK1R-F and NK1R-T have been documented in several cell types [32, 38, 39, 41], the differences in their expression and physiological function have not been studied in human peripheral blood monocytes. Several studies have identified NK1R in human cells of monocyte/macrophage lineage; however, it is less certain which form of the receptor is expressed in human monocytes [5, 9, 18, 21, 24, 42, 43]. It has been variously reported that human monocytes express NK1R, do not express NK1R, or express an atypical form of the receptor [5, 28, 44, 45].
We previously identified a critical interaction among SP, NK1R, and the HIV coreceptor CCR5 in human monocyte-derived macrophages [21, 46]. The NK1R antagonist CP-96,345 selectively inhibited M-tropic HIVs, which use CCR5 as a coreceptor for entry, but did not prevent infection by T-tropic HIVs, which use CXCR4 as a coreceptor [46]. Thus, there is evidence that SP enhances M-tropic HIV infection [8, 46, 47], which may result from crosstalk between the NK1R and CCR5 receptors. CCR5 is also a chemokine receptor, which regulates chemotaxis and cellular activation at inflammatory sites. The chemokine receptor CCR5 interacts with the ligands CCL5 (RANTES) as well as CCL3 (MIP-1α), CCL4 (MIP-1β), CCL8, and CCL7 [48,49,50,51]. There is limited information available about regulation of chemokines and their receptors by SP and the role of this regulation in immune cell function. SP may mediate these effects through enhancement of CCR5 receptor function. In this study, we determined which form(s) of NK1R are expressed in human peripheral blood monocytes and determined the ability of NK1R to interact with CCR5 by examining functional effects of SP on CCL5-mediated cellular responses.
MATERIALS AND METHODS
Reagents
Recombinant human (rh)CCL5 (#300-06) was obtained from PrepoTech, Inc. (Rocky Hill, NJ, USA). SP, EGTA, Na-orthovanadate, 4-(2-aminoethyl)-benzenesulfonyl fluoride, leupeptin, protease inhibitor cocktail, and phosphatase inhibitor cocktail were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rabbit polyclonal antibodies against Thr202/Tyr204-phosphorylated ERK1/2 (#9101) and ERK1/2 (#9102) were purchased from Cell Signaling Technology (Beverly, MA, USA). The acetoxymethyl ester (AM) of Fura-2, calcein-AM, Alexa Fluor 568 donkey anti-mouse IgG (#A10037), and FITC-conjugated donkey anti-rabbit IgG (#A21206) was obtained from Invitrogen (Carlsbad, CA, USA). A rabbit polyclonal anti-CCR5 (#PX164A) was purchased from Cell Sciences (Canton, MA, USA). A mouse monoclonal anti-CCR5 (#MAB1801) was purchased from R&D Systems (Minneapolis, MN, USA). A mouse polyclonal antitachykinin receptor 1 (#H00006869-A01), which recognizes NK1R-T and NK1R-F, was obtained from Novus Biologicals (Littleton, CO, USA). Polyclonal rabbit antiphosphoserine (#61-8100) and membrane-blocking solution were obtained from Zymed Laboratories (San Francisco, CA, USA). Polyclonal rabbit anti-NK1R (C-terminal, SC-15323) and A/G PLUS agarose were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The MAPK inhibitor PD 98059 was obtained from BioMol (Plymouth Meeting, PA, USA). SuperSignal ULTRA chemiluminescence substrate, dimethylpimelimidate, and bicinchoninic acid (BCA) reagents were obtained from Pierce (Rockford, IL, USA).
The U.S. Food and Drug Administration-approved NK1R antagonist, aprepitant (Emend®), manufactured by Merck and Co. (Whitehouse Station, NJ, USA), was purchased through the Children’s Hospital of Philadelphia Pharmacy (Philadelphia, PA, USA). The NK1R antagonist was extracted and purified by Dr. Ivan Alferiev (Children’s Hospital of Philadelphia). Briefly, aprepitant (Emend®) was extracted from a suspension in aqueous sodium carbonate with ethyl acetate, the organic phase was clarified on a cellulose CC-31 column, the solvent was evaporated, and the residue was crystallized from heptane/ethyl acetate and dried in vacuo. The product was uniform on TLC; HPLC analysis demonstrated a purity of 99%.
Preparation of human monocytes
Human peripheral blood monocytes were obtained from healthy adult donors through the Penn Center for AIDS Research Immunology Core (Philadelphia, PA, USA). Alternatively, monocytes were isolated from heparinized venous blood (10 U/ml) obtained from healthy adult donors following informed consent, in accordance with Institutional Review Board protocols at the Children’s Hospital of Philadelphia. In brief, heparinized blood was separated by centrifugation over lymphocyte separation medium (Organon Teknika Corp., Durham, NC, USA) [5]. The mononuclear cell layer was collected and resuspended in DMEM (Gibco Laboratories, Grand Island, NY, USA), plated onto gelatin-coated flasks for monocyte adherence, and incubated for 45 min in 5% CO2 at 37°C. The flasks were washed with DMEM to remove lymphocytes and nonadherent cells. Adherent monocytes were then detached by incubation with 5 mM EDTA in DMEM containing 20% FCS for 15 min at 37°C. Monocytes were collected, resuspended in DMEM, and cultured overnight to up-regulate CCR5 expression. These cell populations were >97% monocytes, as determined by nonspecific esterase staining and FACS analysis using mAb against CD14 (Leu-M3) and low-density lipoprotein specific for monocytes and macrophages.
RT
Total RNA was extracted from human monocytes and human embryonic kidney (HEK)293 cells using the RNeasy mini RNA kit (Qiagen, Valencia, CA, USA). Total RNA (1 ug) was subjected to RT. The final reaction mixture (20 ul) contained the following elements: 5 mM MgCl2, 1× RT buffer, 500 μM each dNTPs, 1 unit/ul recombinant RNasin, 10–15 units avian myeloblastosis virus RT (Promega, Madison, WI, USA), and 50 ng random primers. RT was performed at 42°C for 1 h. The reaction was terminated by holding the reaction mixture at 99°C for 5 min. One-tenth (2 μl) of the resulting cDNA was used as a template for real-time PCR amplification.
Real-time PCR primers
The PCR primers used for quantitative measurement of NK1R-F and NK1R-T mRNA were modified from the method of Caberlotto et al. [39]. The sequences of the primer pair used to amplify the NK1R-F were: (sense NK1R-F, forward) 5′-TCTTCTTCCTCCTGCCCTACATC-3′; (antisense NK1R-F, reverse-967) 5′-AGCACCGGAAGGCATGCTTGAAGCCCA-3′, which is specific for the NK1R-F sequence. The sequences of the primer pair for the NK1R-T were: (sense NK1R-F, forward) 5′-TCTTCTTCCTCCTGCCCTACATC-3′; (antisense NK1R-T, reverse-1083) 5′-TGGAGAGCTCATGGGGTTGGGATCCT-3′. The forward primer is identical to the one used by Caberlotto et al. [39]. However, the reverse primers for NK1R-F and NK1R-T are located further downstream from the forward primer to avoid potential nonspecific amplification as a result of the 50% sequence homology of reverse primers of NK1R-F and NK1R-T [40]. The sequences of the primer pair for GAPDH were: 5′-GGTGGTCTCCTCTGACTTCAACA-3 ′ (sense) and 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ (antisense) [52]. The specificity of the modified primer pair for NK1R-T was confirmed by SYBR Green dye dissociation curve and agarose gel electrophoresis [40]. The primers were resuspended in Tris-EDTA buffer and stored at −30°C.
Real-time PCR assay
The MyiQ iCycler system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for real-time PCR analysis. Thermal cycling conditions were designed as follows: initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. Fluorescent measurements were recorded during each annealing step. At the end of each PCR run, data were analyzed automatically by the system, and amplification plots were obtained. For each PCR, 2 μl cDNA template was added to 23 μl iQ™ SYBR Supermix (Bio-Rad Laboratories, Inc.) containing the primer pair for NK1R-F or -T or for GAPDH. A NK1R-F plasmid (a gift from Dr. Norma Gerard, Harvard University, Boston, MA, USA) was used to prepare standard curves and used as a specificity control for real-time PCR. The full-length plasmid was amplified by the primer pair for NK1R-F but not by the primer pair for NK1R-T. Using serial-diluted cDNA derived from pooled human brain tissue [40], the amplification efficiency of the NK1R-F and -T primer pairs was similar (103% for NK1R-T and 102% for NK1R-F, respectively), thus enabling use of the NK1R-F standards to measure NK1R-T expression levels. All amplification reactions were performed in duplicate, and the average comparative threshold cycle numbers of the duplicates were used to calculate the expression levels (copy numbers) of NK1R-F and -T using the standard curve generated with the NK1R-F plasmid. To control the integrity of the RNA and normalize NK1R mRNA levels in these samples, a GAPDH mRNA fragment in the RNA was also amplified using our established real-time RT-PCR with iQTM SYBR Green Supermix (Bio-Rad Laboratories, Inc.), as reported previously [52]. To generate a total RNA standard curve with GAPDH primers, a known amount of total RNA standard extracted from pooled human monocytes was reverse-transcribed and serial-diluted (ranging from 32 to 2000 ng), and the GAPDH fragment was amplified for 40 cycles. The human monocyte samples were amplified in the same plate with the standard under the identical conditions. The quantity (ng) of total RNA in the samples was calculated automatically by The MyiQ iCycler system based on the data obtained from the total RNA standard curve. All amplification reactions were performed in duplicate, and an average RNA quantity (ng) of the duplicates was used to normalize the NK1R-F and -T mRNA levels. The expression levels of NK1R-F and NK1R-T cDNA were normalized using endogenous cDNA, GAPDH. To normalize the NK1R-F and -T mRNA levels, the NK1R-F and -T copy numbers in cell samples were divided by the total RNA quantity (ng) determined by the GAPDH real-time RT-PCR in the same sample. The NK1R-F and NK1R-T mRNA levels are expressed as the mean copy number of NK1R mRNA/ng total RNA.
Immunofluorescence staining of NK1R-T and NK1R-F in human monocytes
Differential expression of human NK1R-T and NK1R-F was evaluated using antibodies directed against a common domain (aa 140–244) present in NK1R-T and NK1R-F (Novus Biologicals, #H00006869-A01) or the C-terminal of NK1R (aa 325–407; Santa Cruz Biotechnology, SC-15323). Monocytes and HEK293 cells transfected with NK1R-F were plated onto poly-L-lysine-coated glass slides, fixed in 2% paraformaldehyde, permeabilized with 0.2% Triton X-100, and blocked with 5% donkey serum in PBS with 5% BSA. The cells were incubated with normal rabbit IgG (1:500 dilution in blocking buffer) or rabbit anti-C-terminal NK1R (1:500) overnight at 4°C. The cells were washed and incubated with FITC-conjugated donkey anti-rabbit IgG (1:500 dilution) for 2 h at room temperature, rinsed, and blocked in 5% donkey serum in PBS with 5% BSA prior to incubation with normal mouse IgG (1:500 dilution) or the anti-mouse Novus NK1R antibody (1:500) overnight at 4°C. Fluorescence visualization was achieved using Alexa 568-conjugated donkey anti-mouse IgG (1:500) and nuclear counterstaining with 4′,6-diamidino-2-phenylindole (DAPI; Vectashield, Vector Laboratories, Burlingame, CA, USA, #H-1200). Slides were examined on an Olympus BX61 microscope; images were acquired and analyzed using a Hammamatsu ORCA-ER digital camera and Volocity software (Improvision, Waltham, MA, USA).
Ca2+ measurements
Cytosolic-free Ca2+ concentrations were determined in Fura-2-loaded monocytes, which were incubated with 10 uM acetoxymethyl ester of Fura-2 in HEPES buffer at 37°C for 5 min, then diluted 10 times in HEPES buffer, and incubated a further 20 min at 37°C. Cells were washed and resuspended in buffer at a concentration of 5 × 106 cells/ml. Prior to use, cells were resuspended in fresh buffer at a concentration of 2 × 106 cells/200 ul. The kinetics of fluorescence changes were monitored in a 96-well plate at excitation wavelengths of 340 nm and 380 nm and an emission wavelength of 510 nm in a Flexstation fluorescence plate reader. Triton X-100 (1%) was added to measure Fmax (to calculate maximal Ca2+ concentration), and excess EGTA was added to measure Fo (to calculate minimal Ca2+). Cytosolic Ca2+ was calculated by the ratio method of Grynkiewicz et al. [53]. Changes in peak cytosolic Ca2+ levels (calculated from resting to maximal intracellular Ca2+ concentrations) were used to compare Ca2+ levels under different experimental conditions.
For experiments in which monocytes were transfected with NK1R-F, Ca2+ measurements were carried out in single cells as described previously [21]. Monocytes were plated on poly-D-lysine-coated glass coverslips (Sigma Chemical Co.) in six-well plates. The coverslips were sized to fit a homeothermic perfusion chamber platform of an inverted Zeiss microscope. The cells were loaded with 2 μM Fura-2 acetoxymethylester as described previously [21]. The cells were then superfused with HBSS and supplemented with 1% FBS and 1.25 mM CaCl2 at 37°C at a flow rate of 1.5 ml/min. The excitation was performed at 334 and 380 nm with two narrow band-pass filters.
The emitted fluorescence was filtered (520 nm), captured with an Attofluor charged-coupled device video camera (512×480 pixel resolution), digitized (256 gray levels), and analyzed with Attofluor ratio vision (Version 6.00) software (Atto Instrument, Rockville, MD, USA). The intracellular Ca2+ concentration was calculated by comparing the ratio of fluorescence at each pixel with an in vitro two-point calibration curve. The Ca2+ concentration presented is that obtained by averaging the values of all pixels of a cell body. Data points were collected at 5-s intervals.
Transfection of NK1R by nucleofection
HEK293 cells were purchased from American Type Culture Collection (Rockville, MD, USA) and grown in DMEM, supplemented with 10% FBS, glutamine, and antibiotics at 37°C in 5% CO2. A NK1R-F plasmid (a gift from Dr. N. Gerard) was introduced into HEK293 cells as described previously [21] and using Nucleofector II and cell line Nucleofection Kit V (Program Q-001), according to the manufacturer’s instructions (Amaxa, Gaithersburg, MD, USA). Transfected cells were selected with antibiotic (G418, 1 mg/ml) for 4–6 weeks. The antibiotic-resistant cells were further selected for by limited dilution in 96-well plates for another 3–5 weeks. The antibiotic-resistant clones were selected for further characterization by real-time RT-PCR quantitation of NK1R mRNA and by flow cytometry staining of the surface expression of NK1R. The expression levels of NK1R-F mRNA in HEK293 were 345 copies/ng RNA. Using flow cytometry, cell surface expression of NK1R in these cell lines was confirmed (data not shown).
For human peripheral blood monocytes, monocytes (5×106 cells) were suspended in 100 ul nucleofector solution. The NK1R-F gene containing plasmid (0.5 ug) was used for each nucleofection. Control monocytes were mock-transfected with an empty plasmid, pcDNA 3.0. The human monocyte nucleofection kit was used following the manufacturer’s protocol (Nucleofector Program #Y-01). Transfected cells then were transferred to six-well plates and incubated for 48 h before measuring Ca2+ mobilization.
Monocyte chemotaxis
Monocytes were suspended in DMEM + 0.2% BSA and incubated with calcein-AM for 30 min at 37°C. Cells were washed and resuspended in DMEM + 0.2% BSA at a concentration of 2 × 106 cells/ml and pretreated with 10−7M SP or buffer (DMEM+0.2% BSA) for 10 min at 37°C. For inhibitor experiments, cells were pretreated with buffer or the NK1R antagonist aprepitant (10−6M) for 15 min at 37°C. Following pretreatment with buffer, buffer + aprepitant, SP, or SP + aprepitant calcein-labeled monocytes (2×105) were placed in the upper compartment of the chemotaxis chamber (Neuroprobe, Cabin John, MD, USA). A filter (5 um pore-size) separated the upper and lower compartments. The lower compartment contained rhCCL5 at desired concentrations diluted in DMEM + 0.2% BSA. SP and/or aprepitant were not added to the lower compartment. The cells were incubated at 37°C for 90 min in humidified air with 5% CO2. The cells remaining in the upper chamber were removed, and 50 μl 5 mM EDTA was added to the top chamber to promote monocyte detachment in the filter. The cell plate was incubated for 60 min at 4°C and centrifuged, and the filter was removed. Monocyte migration was determined fluorometrically using a Flex Station fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA) at an excitation wavelength of 494 nm and an emission wavelength of 515 nm. The number of monocytes migrating was calculated from a standard curve prepared from calcein-loaded monocytes.
Measurement of ERK1/2 phosphorylation
Monocytes (8×106 cells/ml) were incubated with buffer, SP (10−7M), CCL5 (100 ng/ml), or SP (10−7M) + CCL5 (100 ng/ml) or pretreated with the NK1R antagonist aprepitant (10−7M) and then incubated with SP or SP + CCL5 for 10 min at 37°C. The cells were then harvested, and cell lysates were prepared as described above and run on 4–12% SDS-PAGE gels at a protein concentration of 30 ug/lane. ERK1/2 activation was determined by immunoblotting of cell lysates using a phospho-specific antibody for ERK1/2 (Thr202/Tyr204). Equal loading of ERK1/2 was confirmed by reprobing membranes using an antibody that recognizes phosphorylated and nonphosphorylated forms of ERK1/2, and ERK1/2 activation was quantitated by densitometry analysis of the immunoreactive bands using ImageJ software, Version 1.32j [National Institutes of Health (NIH), Bethesda, MD, USA]. Densitometry was measured in arbitrary densitometry units (ADU).
Immunoprecipitation (IP) of CCR5
Monocytes (10×106 cells/well) were incubated with buffer, SP (10−7M), CCL5 (100 ng/ml), or SP (10−7M) + CCL5 (100 ng/ml) or pretreated with aprepitant (10−7M) and then incubated with SP + CCL5 for 10 min at 37°C. Cell pellets were lysed in IP buffer and vortexed for 20 min at 4°C to solubilize the membrane fraction. The IP buffer consisted of 10 mM Hepes, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1 mM Na-orthovanadate, 20 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 0.2% Nonidet P-40, 5 μg/ml leupeptin, Sigma phosphatase inhibitor cocktail, and Sigma protease inhibitor cocktail. Protein concentrations of the cell lysates were determined by the BCA protein assay kit according to the manufacturer’s instructions (Pierce). Cell lysates (250 μg protein) were incubated with anti-CCR5 (1 μg/200 ug cell lysates, Cell Sciences) overnight at 4°C and then with A/G PLUS agarose for 2 h at 4°C. The IgG agarose pellet was washed four times with lysis buffer, resuspended in 45 μl 2× SDS-PAGE sample buffer, and boiled for 5 min at 95°C to elute the immune complex. The samples were run on a 4–12% gradient SDS-PAGE at 100 V for 4 h. Serine phosphorylation of CCR5 was determined by Western blot analysis using a phosphoserine antibody. Total CCR5 levels were determined by Western blot analysis using a different CCR5 antibody (R&D Systems) directed to a different epitope of the receptor.
Statistical analysis
The results are expressed as means ± se (n). Data were analyzed by Student’s t-test for two group comparisons or ANOVA for multiple comparisons. The Tukey-Kramer multiple comparisons post-test was used to evaluate the significance between experimental groups. Differences were considered significant when P < 0.05.
RESULTS
Human monocytes express NK1R-T but not NK1R-F
Using RT-PCR, we examined the expression of NK1R-F and NK1R-T mRNA in isolated human peripheral blood monocytes obtained from healthy donors. NK1R mRNA for NK1R-T was detected in human monocytes at an average of 1876 ± 171 copies/ng RNA (mean±se, n=14). NK1R-F mRNA was not detected in any of the donor samples examined, indicating that only NK1R-T mRNA was present in human peripheral blood monocytes.
Differential protein expression of NK1R-F and NK1R-T in human peripheral blood monocytes was evaluated next using an antibody directed against a common domain of NK1R-T and NK1R-F (Novus Biologicals) and an antibody directed against the C-terminal of NK1R (Santa Cruz Biotechnology). The Novus NK1R antibody recognizes NK1R-F and NK1R-T, as it was produced against a common domain (aa 140–244), present in NK1R-T and NK1R-F. In contrast, the C-terminal NK1R antibody (Santa Cruz Biotechnology) was raised against a recombinant protein corresponding to amino acid residues 325–407 in the terminal sequence of the cytoplasmic domain of NK1R and detects only the NK1R-F. As shown in Figure 1, monocytes stained positively with the antibody, which recognizes NK1R-F and NK1R-T (Fig. 1A) but did not bind the anti-C-terminal antibody (Fig. 1B). In contrast, HEK293 cells transfected with NK1R-F stained positively when antibodies directed against the common domain of NK1R (Fig. 1E) or the C-terminal NK1R were used (Fig. 1F). Thus, in contrast to transfected HEK cells (NK1R-F), monocytes express NK1R-T but not NK1R-F (see Fig. 1, D and H). In summary, using mRNA and protein analysis, our studies demonstrate that human monocytes express NK1R and only express the NK1R-T.
Fig. 1.
Immunofluorescence of NK1R in human monocytes and HEK293 cells transfected with NK1R-F. Monocytes (A–D) and HEK293 cells transfected with NK1R-F (E–H) were fixed, permeabilized, and immunostained with an antibody that recognizes NK1R-T and NK1R-F (red, A and E) and an antibody that recognizes the C-terminal of NK1R and only reacts with NK1R-F (green, B and F). The cells were mounted in Vectashield with DAPI (blue, C and G) and imaged using fluorescence microscopy. Merged images for monocytes and transfected HEK cells are shown in D and H, respectively (original magnification, ×400) The data are representative of three independent experiments and donors. Rabbit and mouse IgG were used as negative controls (data not shown).
NK1R-T does not mobilize Ca2+ in human peripheral blood monocytes but does enhance CCL5-mediated Ca2+ mobilization
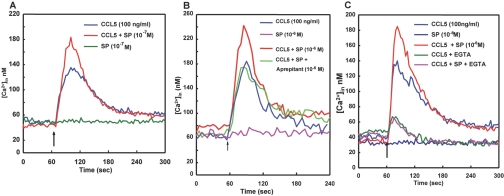
To determine whether NK1R-T in human monocytes had functional relevance, we determined the ability of SP to induce changes in cytosolic Ca2+. The addition of SP (10−7M) did not trigger a Ca2+ response in isolated monocytes, indicating that NK1R-T is not capable of eliciting a Ca2+ response in human monocytes (Fig. 2A). Although NK1R-T did not mobilize Ca2+ independently, we determined whether the NK1R-T expressed in human monocytes was capable of interacting with other members of the GPCR family. Crosstalk between members of the GPCR family enhances (primes) or inhibits (desensitizes) cellular responses. We previously demonstrated a priming effect of SP on CCR5-mediated Ca2+ responses in transfected HEK cells and the monocytic cell line THP-1, but it is not known whether SP can prime CCR5-mediated cellular functions in primary cells [21, 54]. We determined the effect of SP on the CCR5 ligand CCL5-mediated Ca2+ mobilization. As shown in Figure 2A, CCL5 (100 ng/ml) elicited a rapid transient increase in cytosolic Ca2+. SP, at a concentration that did not elicit Ca2+ mobilization (10−7M), significantly increased peak cytosolic Ca2+ levels triggered by CCL5 by 149 ± 16% of control (CCL5 alone; mean±se, n=10, P<0.02, Fig. 2A). Even at higher concentrations, i.e.,10−6M, SP per se did not mobilize Ca2+ in human monocytes but was able to significantly enhance peak cytosolic Ca2+ levels elicited by CCL5 (Fig. 2B). The priming effect of SP on CCL5-mediated Ca2+ mobilization was not significantly different when 10−6 M SP was used as compared with 10−7 M SP. There was a 149 ± 16% increase in peak cytosolic Ca2+ levels in response to CCL5 in cells pretreated with 10−7 M SP as compared with CCL5 alone, and pretreatment with 10−6 M SP produced a 150 ± 5% increase in peak cytosolic Ca2+ in response to CCL5 as compared with CCL5 alone [n=15, P=not significant (NS)]. Pretreatment with aprepitant (10−6 M) for 30 min ablated SP enhancement of CCL5-mediated Ca2+ mobilization, indicating that the priming effect was mediated through NK1R (Fig. 2B). However, pretreatment of monocytes with aprepitant (30 min) did not alter the CCL5-elicited peak cytosolic Ca2+ levels as compared with cells not pretreated with aprepitant [106±11% increase in peak cytosolic Ca2+ levels in aprepitant+CCL5-treated monocytes as compared with CCL5-treated monocytes (controls); mean±se, n=8, P=NS], indicating that short-term pretreatment with the NK1R antagonist does not interfere with CCL5 signaling.
Fig. 2.
(A) SP (10−7M) pretreatment enhances CCL5-mediated Ca2+ mobilization in human monocytes. An intracellular Ca2+ ([Ca2+]in) increase was measured in Fura-2-loaded monocytes following the addition of SP (10−7 M) or CCL5 (100 ng/ml). Changes in cytosolic Ca2+ were monitored at the dual excitation wavelengths at 340 and 380 nm and the emission wavelength of 510 nm (see Materials and Methods). The arrow indicates addition of CCL5 or SP. For experiments examining the effects of SP pretreatment on CCL5-elicited Ca2+ mobilization, SP (10–7 M) was added 5 min prior to the addition of CCL5 (representative experiment of n=9). (B) SP (10−6M) pretreatment enhances CCL5-mediated Ca2+ mobilization in human monocytes via NK1R. An intracellular Ca2+ increase was measured in Fura-2-loaded monocytes as described in A. Cells were activated by CCL5 (100 ng/ml; indicated by an arrow), stimulated by SP (10–6 M; arrow), pretreated with SP (10–6 M) and activated by CCL5 (100 ng/ml; arrow), and pretreated with SP (10–6 M) plus aprepitant (10–6 M) followed by stimulation with CCL5 (100 ng/ml; arrow; representative experiment of n=15). (C) SP (10−6M) priming of CCL5-mediated Ca2+ mobilization is the result of increased uptake of extracellular Ca2+. Intracellular Ca2+ increase was measured in Fura-2-loaded monocytes as described in A. Fura-2-loaded monocytes were pretreated with buffer or SP (10−6M) and incubated for 5 min at 37°C in the presence of 1.25 mM Ca2+ or 5 mM EGTA, followed by the addition of CCL5 (100 ng/ml), as indicated by the arrow (representative experiment of n=10).
In the presence of 5 mM EGTA, where cytosolic Ca2+ changes are derived only from intracellular stores, there was no difference in peak cytosolic Ca2+ elevations in response to CCL5 plus SP as compared with CCL5 alone [110±12%, percent increase in peak cytosolic Ca2+ levels pretreated with 10−7 M SP (CCL5+SP) as compared with increase in peak cytosolic Ca2+ levels in response to CCL5 alone (control), mean±se, n=8, P=NS]. These results indicate that the enhanced Ca2+ responses in the presence of SP were the result of increased uptake of extracellular Ca2+ (Fig. 2C).
SP also enhanced Ca2+ mobilization elicited by another CCR5 ligand, CCL3. Pretreatment of human monocytes with SP, prior to the addition of CCL3, increased peak cytosolic Ca2+ levels 149 ± 10% as compared with peak cytosolic Ca2+ levels in response to CCL3 alone (control, n=4, P<0.01). Thus, SP priming of Ca2+ mobilization is not limited to CCL5, but SP also enhances Ca2+ mobilization in response to another CCR5 agonist, CCL3.
To determine whether the inability of SP to mobilize Ca2+ independently in human monocytes was a result of the absence of expression of NK1R-F, a NK1R-F gene-containing plasmid was introduced into isolated human monocytes. SP, at a concentration that did not elicit Ca2+ mobilization (10−6M) in monocytes lacking NK1R-F, stimulated a transient increase in intracellular Ca2+ in monocytes expressing NK1R-F (Fig. 3). These results indicate that the NK1R-F is required for SP-induced Ca2+ mobilization.
Fig. 3.
Expression of NK1R-F is required for a SP-induced Ca2+ increase in human monocytes. Intracellular Ca2+ measurements in monocytes transfected with NK1R-F were carried out in single cells as described in Materials and Methods. SP (10−6 M) did not induce Ca2+ responses in Fura-2-loaded, mock-transfected monocytes (▴, monocytes) but induced Ca2+ responses in monocytes transfected with a NK1R-F gene-containing plasmid (□, monocytes+NK1R-F). Each data point on the Ca2+ curves corresponds to mean values measured in six to eight individual cells. The data shown are representative of three independent transfection experiments.
SP pretreatment enhances CCL5-mediated chemotaxis
To ascertain the functional relevance of NK1R-T in human monocytes, we determined the effect of SP on proinflammatory signaling triggered by the chemokine CCL5, which elicited directed monocyte migration over a bell-shaped concentration curve (0.01–100 ng/ml; Fig. 4A). The peak chemotactic activity occurred at concentrations between 1 and 10 ng CCL5/ml. Chemotaxis was decreased significantly at 100 ng CCL5/ml, indicating that chemotaxis to CCL5 follows a biphasic response characteristic of chemoattractants.
Fig. 4.

(A) CCL5-mediated chemotaxis in human monocytes is concentration-dependent. SP pretreatment potentiates CCL5-mediated chemotaxis. CCL5-directed migration was assessed in Transwell migration assays using calcein-labeled human monocytes, which were pretreated with buffer or SP (10–7 M) for 15 min prior to chemotaxis measurements. Different concentrations of CCL5 (0.01–100 ng/ml) were placed in the bottom wells, and the number of monocytes that migrated over the 90-min incubation period was determined fluorometrically as described in Materials and Methods. Values are mean ± se, n = 5–8 separate monocyte preparations; *, CCL5 versus CCL5+SP: P < 0.01 at 0.01 ng/ml CCL5, P < 0.01 at 0.1 ng/ml CCL5, P < 0.05 at 1 ng/ml CCL5, P = NS at 10 ng/ml CCL5, P < 0.01 at 100 ng/ml CCL5; 0.01 ng/ml CCL5 (C0.01) versus C0.01 + SP, P < 0.01; 0.1 ng/ml CCL5 (C0.1) versus C0.1 + SP, P < 0.01; 1 ng/ml CCL5 (C1) versus C1 + SP, P < 0.05; 10 ng/ml CCL5 (C10) versus C10 + SP, P = NS; 100 ng/ml CCL5 (C100) versus C100 + SP (10–7 M), P < 0.01. (B) Potentiation of CCL5-mediated chemotaxis by SP is regulated via NK1R. Monocytes were pretreated in the absence or presence of the NK1R antagonist aprepitant (Aprep; 10−6M) for 30 min prior to the addition of CCL5 (100 ng/ml) or CCL5 (100 ng/ml) + SP (10–7 M). CCL5-directed chemotaxis was measured as described in A. Values are mean ± se, n = 5, *, P < 0.02; CCL5 (100 ng/ml) versus CCL5 + SP (10–7 M), **, P < 0.01; CCL5 + SP versus CCL5 + SP + aprepitant (10−6M).
Pretreatment with SP (10−7 M) enhanced CCL5-directed migration over the bell-shaped response curve with significant increases attained at 0.01, 0.1, 1, and 100 ng/ml (Fig. 4A). The shape of the response curve to CCL5 was not altered by SP pretreatment, which alone, did not affect random migration, indicating a direct effect on CCL5-mediated signaling [110±15% of control (buffer alone), mean±se,n=14, P=NS]. As SP was not present in the lower chamber, the enhancement in chemotaxis was the result of priming and not a chemotactic response to SP, and SP-elicited enhancement of CCL5-mediated chemotaxis was inhibited by the NK1R antagonist aprepitant (10−6M), indicating that the effect of SP was through SP:NK1R-T interactions (Fig. 4B). Pretreatment with aprepitant (10−6M) had no significant effect on CCL5-directed chemotaxis (Fig. 4B) nor did pretreatment with aprepitant affect random migration [99±7% of control (buffer alone), mean±se,n=11,P=NS], indicating that aprepitant did not directly affect CCL5-directed chemotaxis.
SP pretreatment enhances CCL5-mediated activation of the MAPK ERK
SP activates the MAPK ERK in other cell types [55, 56], but it is not known which form(s) of NK1R activates ERK and whether ERK has a role in the potentiating effects of SP on CCL5-directed chemotaxis. As shown in Figure 5A, at a concentration (10−7 M) that did not mobilize Ca2+, SP triggered phosphorylation of ERK2 but not ERK1. Activation of ERK2 by SP was ablated by pretreatment with aprepitant (10−6 M), indicating that SP-induced activation of ERK2 was mediated through NK1R-T. In contrast, treatment of monocytes with CCL5 (100 ng/ml) triggered phosphorylation of ERK1 and ERK2 (Fig. 5B). When monocytes were incubated with SP + CCL5 together, phosphorylation of ERK1 and ERK2 was increased significantly as compared with with CCL5 alone. The SP-mediated enhancement in ERK1 and ERK2 activation was inhibited by pretreatment with aprepitant (Fig. 5B). Pretreatment with aprepitant had no significant effect on CCL5-elicited ERK1 and ERK2 activation [CCL5+aprepitant=98.5±20% of control (CCL5 alone), mean±se, n=5, P=NS]. These results indicate involvement of the SP:NK1R-T interaction with activation and phosphorylation of ERK1 as well as ERK2.
Fig. 5.
Activation of the MAPK ERK in monocytes by SP and CCL5. (A) Activation of ERK2 by NK1R-T. Monocytes were incubated for 10 min with buffer alone, SP (10–7 M), or SP, following pretreatment with aprepitant (10–6 M) for 30 min. (Upper) ERK1/2 activation was determined in cell lysates by immunoblotting with antibodies specific to phosphorylated ERK1/2 (Phos-ERK1/2). Equal protein loading was determined by Western blot analysis for total ERK1/2. Representative Western blots. (Lower) Densitometry analysis of SP-mediated activation of ERK2. Values are expressed as mean ± se (n=7 separate monocyte experiments) and are expressed in ADU. Statistical significance for phosphorylated ERK2: *, P <0.003, SP versus buffer; **, P < 0.001, SP + aprepitant versus SP. (B) Activation of ERK1 and ERK2 by CCL5: effect of SP. Monocytes were incubated for 10 min with SP (10–7 M), CCL5 (100 ng/ml), CCL5 + SP, or CCL5 + SP following pretreatment with aprepitant (10–7 M) for 30 min. (Upper) ERK1/2 activation was determined in cell lysates as described in A (representative Western blot from four different monocyte experiments). (Lower) Densitometry analysis of CCL5 and SP-mediated activation of ERK1 and ERK2. Values are expressed as mean ± se (n=4) and are expressed in ADU. Statistical significance for phosphorylated ERK1 (pERK1): *, P < 0.05, CCL5 versus buffer; **, P < 0.001, CCL5 + SP versus CCL5 and CCL5 + SP versus SP; ***, P < 0.01, CCL5 + SP + aprepitant versus CCL5 + SP. Statistical significance for phosphorylated ERK2: *, P < 0.01, CCL5 versus buffer; ‡, P < 0.01, SP versus buffer; **, P < 0.01, CCL5 + SP versus CCL5 and CCL5 + SP versus SP; ***, P < 0.05, CCL5 + SP + aprepitant versus CCL5 + SP.
Role of ERK in SP-mediated enhancement of chemotaxis
Pretreatment of monocytes with the MEK1/2 inhibitor PD 98059 (20 μM) significantly attenuated the enhancing effect of SP on CCR5-mediated chemotaxis (Fig. 6). In contrast, pretreatment with PD 98059 had no significant effect on CCL5 (100 ng/ml)-mediated chemotaxis itself, indicating a selective role for ERK1/2 in the potentiating effects of SP on CCR5-mediated chemotaxis. Pretreatment with PD 98059 had no significant effect on monocytes random migration, indicating a selective effect on SP priming [104±7% of control (buffer alone), mean±se, n=7, P=NS]. Similar results were also obtained when monocytes were pretreated with another MEK1/2 inhibitor UO126 (data not shown). Thus, SP signaling through NK1R-T activates discrete signaling pathways involved in ERK2 activation. Although NK1R-T cannot directly activate ERK1, similar to Ca2+ mobilization, SP:NK1R-T interactions enhanced ERK1 activation by other GPCR ligands, i.e., CCL5. This enhancement in ERK1/2 activation was associated with the potentiating effect of SP on CCL5-directed migration. Thus, ERK1/2 activation is not required for CCL5-mediated chemotaxis, but ERK1/2 activation is required for the potentiation of CCL5-mediated chemotaxis induced by SP.
Fig. 6.

Role of ERK in SP-mediated enhancement of CCL5-directed chemotaxis. CCL5-mediated chemotaxis was determined as described in Figure 4. Monocytes were pretreated for 15 min with buffer or the MEK1/2 inhibitor PD 98059 (PD; 20 uM) prior to pretreatment with buffer or SP (10−7M). Monocyte chemotaxis was measured in response to CCL5 (100 ng/ml). Values are mean ± se, n = 5, *, P < 0.01, CCL5 versus CCL5 + SP; **, P < 0.01, CCL5 + SP versus CCL5 + SP + PD.
SP signaling through NK1R-T triggers phosphorylation of the CCR5 receptor
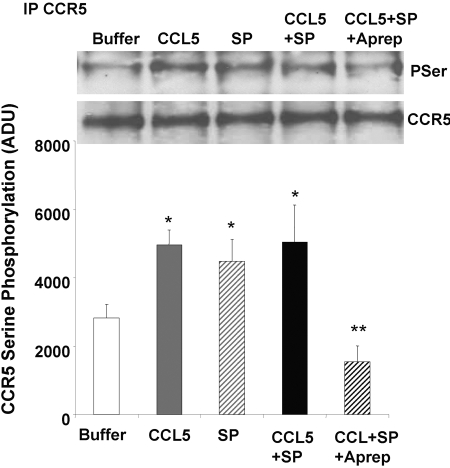
The enhancing effect of SP on CCL5-mediated signaling may occur at the level of the CCR5 receptor through alterations in phosphorylation of the receptor. To determine whether SP:NK1R-T interactions altered phosphorylation of CCR5, monocytes were treated with buffer alone, CCL5 (100 ng/ml), SP (10−7 M), or CCL5 + SP for 10 min and the CCR5 receptor immunoprecipitated. Serine phosphorylation of the receptor was determined by immunoblotting using a phosphoserine-specific antibody. As shown in Figure 7, there was little serine phosphorylation of the CCR5 receptor in monocytes treated with buffer alone. As expected, the CCR5 ligand CCL5 triggered significant phosphorylation of the CCR5 receptor. SP also triggered phosphorylation of CCR5, and the combination of CCL5 + SP did not significantly alter CCR5 phosphorylation as compared with SP or CCL5 treatment alone. Serine phosphorylation of CCR5 in response to CCL5 + SP was inhibited by pretreatment with aprepitant (Fig. 7), which did not inhibit CCL5-mediated phosphorylation of CCR5 (CCL5=5412±363 ADU vs. CCL5+aprepitant=4934±398 ADU, n=4, P=NS). Aprepitant pretreatment did, however, inhibit SP-mediated phosphorylation of CCR5 (SP=4737±368 ADU vs. SP+aprepitant=2110±328 ADU, n=4, P<0.001), indicating CCL5 + SP-elicited phosphorylation was dependent on SP:NK1R-T interactions. Similar results were obtained when serine-phosphorylated proteins were immunoprecipitated using the phosphoserine antibody and CCR5 identified by Western blotting, according to the method of Grimm at al [57] (data not shown). These results suggest that SP interactions with NK1R-T result in crosstalk with CCR5 and SP-mediated phosphorylation of the CCR5 receptor.
Fig. 7.
SP signaling through NK1R triggers phosphorylation of the CCR5 receptor. Monocytes were incubated for 10 min with CCL5 (100 ng/ml), SP (Aprep, 10−7 M), CCL5 + SP, or CCL5 + SP following pretreatment with aprepitant (Aprep, 10−7M). The CCR5 receptor was immunoprecipitated, and serine phosphorylation (PSer) of the receptor was determined by Western blotting with a phospho-specific antibody. Equal protein loading was determined by Western blot analysis for total CCR5. Representative Western blot from five separate monocyte preparations. Densitometry analysis of SP and CCL5-mediated phosphorylation of CCR5. Values are expressed as mean ± se (n=5) in ADU. The addition of CCL5 (100 ng/ml) or SP (10−7M) to isolated monocytes triggered serine phosphorylation of CCR5 (*, buffer versus CCL5, P<0.005; *, buffer versus SP, P<0.05; buffer versus CCL5+SP, P<0.05). Treatment with CCL5 + SP did not produce additional phosphorylation (CCL5 versus CCL5+SP, P=NS). Serine phosphorylation of CCR5 was inhibited by pretreatment with (10−6 M; CCL5+SP versus CCL5+SP+aprepitant, **, P<0.02).
DISCUSSION
SP is an important immune regulator of monocyte and macrophage cell function. A role for SP in the regulation of macrophage function is well established [8, 46]. The function of SP in monocyte cellular responses is, however, less well defined. In this study, using mRNA and protein analysis, we demonstrate that human peripheral blood monocytes express NK1R-T but not the NK1R-F form of the NK1R receptor. This NK1R-T lacks the last 96-aa sequence at the C-terminal end. The remaining amino acid sequence of this NK1R-T isoform is identical to that of the NF1R-F [32]. Although the C-terminus of NK1R is not involved directly in coupling of the receptor with specific G-proteins, alterations in the C-terminus may impact on G-protein interactions with the cytoplasmic domains of the receptor, modify NK1R signaling pathways, and alter ligand-binding affinity [38]. Previous studies from our group [21] and others [32, 35,36,37] have led us to propose that the cytoplasmic domain between 311 and 324/337 aa residues is critical for Ca2+ mobilization, and in the absence of this domain, SP will not elicit Ca2+ mobilization. The physiological concentration range of SP is between 10−9 M and 10−6M [9]. Thus, at physiologically relevant concentrations, SP did not mobilize Ca2+ in human monocytes (Fig. 2). Although, previous studies have reported that SP does mobilize Ca2+ in monocytes via a non-NK1 receptor [45], the concentration of SP used in those studies was 1000-fold greater (100 uM) than the concentration used in our studies and is likely to be the result of nonspecific effects. Our studies indicate that the NK1R-T lacks the capacity to mobilize Ca2+ in response to SP. Furthermore, transfection of NK1R-F into human monocytes results in cytosolic Ca2+ increases in response to SP, indicating that the signaling pathways required for NK1R-mediated Ca2+ mobilization are present in human monocytes. These results are consistent with previous studies in which we demonstrated that NK1R-F occupancy mobilizes Ca2+ in differentiated THP-1 cells, indicating that the C-terminal sequence in NK1R is required for SP-induced Ca2+ responses [21].
The NK1R-T, however, was functional. Although SP:NK1R-T interactions did not mobilize Ca2+, SP did enhance CCL5-elicited Ca2+ mobilization in monocytes. In addition, aprepitant, a NK1R antagonist, inhibited the priming effect of SP on CCL5-elicited Ca2+ mobilization, indicating that the priming effect of SP was mediated via the NK1R-T receptor. The priming effect of SP on CCL5-elicited Ca2+ mobilization was rapid (within minutes) and also occurred when SP and CCL5 were added simultaneously (data not shown), indicating that the priming effect mediated through NK1R-T did not require de novo protein synthesis. SP enhanced Ca2+ mobilization elicited by CCL5 and CCL3, chemokines that are ligands for the CCR5 receptor. NK1R and CCR5 are members of the GPCR family, and there is extensive crosstalk between constituents of this receptor family. Heterologous interactions or crosstalk between GPCRs result in loss of function (desensitization) or alternatively, a gain or enhancement of function, which is often associated with increased intracellular Ca2+ mobilization [58, 59]. Our studies establish that there is positive crosstalk, as demonstrated by SP-enhanced Ca2+ mobilization by two CCR5 ligands, CCL5 and CCL3.
CCL5 and CCL3 binding to the CCR5 receptor elicits a transient mobilization of Ca2+ from intracellular stores and the uptake of extracellular Ca2+ [60]. In the presence of EGTA, where cytosolic Ca2+ is derived solely from intracellular stores, SP did not prime CCR5-mediated Ca2+ mobilization, indicating that the enhancement in cytoplasmic Ca2+ concentrations was the result of increased uptake of extracellular Ca2+ and not release of intracellular Ca2+ stores. In neutrophils, SP also primed Ca2+ mobilization mediated by the chemokine IL-8, and this enhancement was dependent on extracellular Ca2+ uptake [61]. Thus, although SP does not mobilize Ca2+ in human monocytes, SP signaling through NK1R-T does enhance CCL5-mediated Ca2+ mobilization through increased extracellular Ca2+ uptake, indicating the NK1R-T is functional in human monocytes, and nanomolar concentrations of SP enhance CCL5-mediated cellular responses.
The finding that SP primes CCL5- and CCL3-elicited Ca2+ mobilization suggests that activation of NK1R-T modulates CCR5-mediated cellular responses. SP augments or potentiates the inflammatory response in macrophages and microglial cells [11, 14, 62], but little is known about whether signaling through NK1R-T also modulates inflammatory responses in monocytes. Chemotaxis or directed migration is an important component of the immune response, and our studies demonstrate that SP significantly enhanced CCL5-mediated chemotaxis (Fig. 4). The priming effect of SP was mediated through the NK1R-T receptor, as pretreatment with the NK1R antagonist aprepitant significantly inhibited SP priming. These studies demonstrate that SP, through interactions with NK1R-T, amplifies the inflammatory response by enhancing multiple receptor-mediated responses.
SP might enhance CCL5-mediated cellular responses at several different levels. SP:NK1R-T interactions could increase cell surface CCR5 receptor expression, decrease CCL5-mediated receptor internalization, modify ligand-binding affinity, affect assembly of G-protein signaling complexes, and/or interact with selective signal transduction pathways [58, 59]. The potentiating effects of SP on CCL5-directed migration may be the result of differential activation of a common signaling pathway. Indeed, unlike Ca2+ signaling, the NK1R-T activates signaling pathways required for activation of the MAPK ERK. Similar results were observed in a human colonic epithelial cell line, where SP (0.1 nM–10 uM) did not elicit increases in intracellular Ca2+, but SP activated ERK [63]. Our studies also show that CCL5 and SP elicited differential activation of the MAPK ERK1/2 in human monocytes (Fig. 5). CCL5 triggered phosphorylation of ERK1 and ERK2, and SP, at a concentration that did not mobilize Ca2+, activated ERK2 only. However, the addition of CCL5 and SP together resulted in enhanced phosphorylation of ERK1 and ERK2. Although ERK1/2 was activated by CCL5, it was not required for CCL5-directed migration, as an inhibitor of ERK activation had no effect on CCL5-mediated chemotaxis (Fig. 6 and ref. [64]). In contrast, the potentiating effects of SP on CCL5-directed migration were ERK1/2-dependent, indicating a role for ERK1/2 only in SP-mediated potentiation of CCL5-directed migration. The activation of this common signaling pathway by SP and CCL5 may lead to altered cellular distribution of ERK1/2 and the phosphorylation of a distinct group of substrates. Our results suggest that crosstalk between CCL5 and SP signaling pathways is at the level of the ERK or upstream of the MAPKs, possibly at the level of the receptors [64, 65].
CCL5 and CCL3 stimulate serine phosphorylation and activation of the CCR5 receptor [57, 66,67,68]. A possible mechanism of crosstalk between the two GPCRs is at the level of the CCR5 receptor through regulation of phosphorylation. Our studies demonstrate that SP elicited serine phosphorylation of CCR5, which was NK1R-T-dependent. The current study did not identify specific serine residues but does demonstrate that SP-mediated phosphorylation was mediated by NK1R-T. SP:NK1R interaction is capable of transactivation of other GPCRs. SP:NK1R interactions trigger transactivation of the epidermal growth factor receptor, which mediates SP-induced mitogenic responses in human glioma cells, human colonocytes, and the U-373 MG cell line [56, 69, 70]. Whether NK1R-T is active in these other cell types is not known. Further studies are required to ascertain whether crosstalk is mediated through receptor:receptor heterodimerization in human monocytes and whether SP interaction with NK1R-T alters CCR5-binding affinity or cell surface expression.
In summary, the results of the present study demonstrate that human peripheral blood monocytes express only the NK1R-T, which is functional and is capable of crosstalk with other GPCRs. At physiological relevant concentrations of SP, SP interaction with NK1R-T enhanced CCL5-directed monocyte migration. The SP-mediated increase in CCL5-elicited cellular responses was associated with enhanced Ca2+ signaling and ERK1/2 activation, indicating that NK1R-T is capable of activating selective signaling pathways in human monocytes. Finally, NK1R-T interaction with SP elicited serine phosphorylation of CCR5, suggesting that crosstalk between CCL5 and SP-mediated signaling occurs at the level of the CCR5 receptor.
Acknowledgments
This investigation was supported by NIH grants RO1MH049981 and PO1MH076388 (to S. D. D.) and RO1GM064552 (to L. E. K.). We thank Kathryn Matthias and Kayma Freeman for their technical assistance.
References
- Ho W Z, Evans D L, Douglas S D. Substance P and human immunodeficiency virus infection: psychoneuroimmunology. CNS Spectr. 2002;7:867–874. doi: 10.1017/s1092852900022483. [DOI] [PubMed] [Google Scholar]
- Schaffer M, Beiter T, Becker H D, Hunt T K. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg. 1998;133:1107–1116. doi: 10.1001/archsurg.133.10.1107. [DOI] [PubMed] [Google Scholar]
- O'Connor T M, O'Connell J, O'Brien D I, Goode T, Bredin C P, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- Bost K L. Tachykinin-mediated modulation of the immune response. Front Biosci. 2004;9:3331–3332. doi: 10.2741/1484. [DOI] [PubMed] [Google Scholar]
- Ho W Z, Lai J P, Zhu X H, Uvaydova M, Douglas S D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Bozic C R, Lu B, Hopken U E, Gerard C, Gerard N P. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Maggi C A. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Ho W Z, Cnaan A, Li Y H, Zhao H, Lee H R, Song L, Douglas S D. Substance P modulates human immunodeficiency virus replication in human peripheral blood monocyte-derived macrophages. AIDS Res Hum Retroviruses. 1996;12:195–198. doi: 10.1089/aid.1996.12.195. [DOI] [PubMed] [Google Scholar]
- Schratzberger P, Reinisch N, Prodinger W M, Kahler C M, Sitte B A, Bellmann R, Fischer-Colbrie R, Winkler H, Wiedermann C J. Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J Immunol. 1997;158:3895–3901. [PubMed] [Google Scholar]
- Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- Lee H R, Ho W Z, Douglas S D. Substance P augments tumor necrosis factor release in human monocyte-derived macrophages. Clin Diagn Lab Immunol. 1994;1:419–423. doi: 10.1128/cdli.1.4.419-423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Fiebich B L, Busse-Grawitz M, Hull M, Berger M, Bauer J. Effects of substance P and selected other neuropeptides on the synthesis of interleukin-1 β and interleukin-6 in human monocytes: a re-examination. J Neuroimmunol. 1996;67:77–81. doi: 10.1016/0165-5728(96)00034-3. [DOI] [PubMed] [Google Scholar]
- Lotz M, Vaughan J H, Carson D A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Kincy-Cain T, Bost K L. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–2339. [PubMed] [Google Scholar]
- Delgado A V, McManus A T, Chambers J P. Production of tumor necrosis factor-α, interleukin 1-β, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37:355–361. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Marriott I, Mason M J, Elhofy A, Bost K L. Substance P activates NF-κB independent of elevations in intracellular calcium in murine macrophages and dendritic cells. J Neuroimmunol. 2000;102:163–171. doi: 10.1016/s0165-5728(99)00182-4. [DOI] [PubMed] [Google Scholar]
- Ho W Z, Stavropoulos G, Lai J P, Hu B F, Magafa V, Anagnostides S, Douglas S D. Substance P C-terminal octapeptide analogues augment tumor necrosis factor-α release by human blood monocytes and macrophages. J Neuroimmunol. 1998;82:126–132. doi: 10.1016/s0165-5728(97)00175-6. [DOI] [PubMed] [Google Scholar]
- Bardelli C, Gunella G, Varsaldi F, Balbo P, Del Boca E, Bernardone I S, Amoruso A, Brunelleschi S. Expression of functional NK1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-κB pathway. Br J Pharmacol. 2005;145:385–396. doi: 10.1038/sj.bjp.0706198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja A M, Rogers D F. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Ho W Z, Douglas S D. Substance P and neurokinin-1 receptor modulation of HIV. J Neuroimmunol. 2004;157:48–55. doi: 10.1016/j.jneuroim.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Lai J P, Ho W Z, Kilpatrick L E, Wang X, Tuluc F, Korchak H M, Douglas S D. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci USA. 2006;103:7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J P, Douglas S D, Ho W Z. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lai J P, Douglas S D, Rappaport E, Wu J M, Ho W Z. Identification of a δ isoform of preprotachykinin mRNA in human mononuclear phagocytes and lymphocytes. J Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Lai J P, Zhan G X, Campbell D E, Douglas S D, Ho W Z. Detection of substance P and its receptor in human fetal microglia. Neuroscience. 2000;101:1137–1144. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- Lai J P, Douglas S D, Zhao M, Ho W Z. Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J Immunol Methods. 1999;230:149–157. doi: 10.1016/s0022-1759(99)00120-9. [DOI] [PubMed] [Google Scholar]
- Lucey D R, Novak J M, Polonis V R, Liu Y, Gartner S. Characterization of substance P binding to human monocytes/macrophages. Clin Diagn Lab Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A, McLennan G, Betts W H, Murphy G A, Scicchitano R. Activation of human neutrophils by substance P: effect on fMLP-stimulated oxidative and arachidonic acid metabolism and on antibody-dependent cell-mediated cytotoxicity. Immunology. 1989;68:359–364. [PMC free article] [PubMed] [Google Scholar]
- Payan D G, Brewster D R, Missirian-Bastian A, Goetzl E J. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984;74:1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz A M, Scicchitano R, Dazin P, Bienenstock J, Payan D G. Distribution of substance P receptors on murine spleen and Peyer’s patch T and B cells. J Immunol. 1987;139:749–754. [PubMed] [Google Scholar]
- Shanahan F, Denburg J A, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- Hegde A, Zhang H, Moochhala S M, Bhatia M. Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis. J Leukoc Biol. 2007;82:678–685. doi: 10.1189/jlb.0407217. [DOI] [PubMed] [Google Scholar]
- Fong T M, Anderson S A, Yu H, Huang R R, Strader C D. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- Zhang Y, Berger A, Milne C D, Paige C J. Tachykinins in the immune system. Curr Drug Targets. 2006;7:1011–1020. doi: 10.2174/138945006778019363. [DOI] [PubMed] [Google Scholar]
- Richardson M D, Balius A M, Yamaguchi K, Freilich E R, Barak L S, Kwatra M M. Human substance P receptor lacking the C-terminal domain remains competent to desensitize and internalize. J Neurochem. 2003;84:854–863. doi: 10.1046/j.1471-4159.2003.01577.x. [DOI] [PubMed] [Google Scholar]
- Li H, Leeman S E, Slack B E, Hauser G, Saltsman W S, Krause J E, Blusztajn J K, Boyd N D. A substance P (neurokinin-1) receptor mutant carboxyl-terminally truncated to resemble a naturally occurring receptor isoform displays enhanced responsiveness and resistance to desensitization. Proc Natl Acad Sci USA. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm S K, Khitin L M, Smeekens S P, Grady E F, Payan D G, Bunnett N W. Identification of potential tyrosine-containing endocytic motifs in the carboxyl-tail and seventh transmembrane domain of the neurokinin 1 receptor. J Biol Chem. 1997;272:2363–2372. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- Sasakawa N, Sharif M, Hanley M R. Attenuation of agonist-induced desensitization of the rat substance P receptor by progressive truncation of the C-terminus. FEBS Lett. 1994;347:181–184. doi: 10.1016/0014-5793(94)00532-x. [DOI] [PubMed] [Google Scholar]
- Baker S J, Morris J L, Gibbins I L. Cloning of a C-terminally truncated NK-1 receptor from guinea-pig nervous system. Brain Res Mol Brain Res. 2003;111:136–147. doi: 10.1016/s0169-328x(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Hurd Y L, Murdock P, Wahlin J P, Melotto S, Corsi M, Carletti R. Neurokinin 1 receptor and relative abundance of the short and long isoforms in the human brain. Eur J Neurosci. 2003;17:1736–1746. doi: 10.1046/j.1460-9568.2003.02600.x. [DOI] [PubMed] [Google Scholar]
- Lai J-P, Cnaan A, Zhao H, Douglas S D. Detection of full length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J Neurosci Methods. 2008;168:127–133. doi: 10.1016/j.jneumeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennefather J N, Lecci A, Candenas M L, Patak E, Pinto F M, Maggi C A. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Simeonidis S, Castagliuolo I, Pan A, Liu J, Wang C C, Mykoniatis A, Pasha A, Valenick L, Sougioultzis S, Zhao D, Pothoulakis C. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-κ B site on its promoter. Proc Natl Acad Sci USA. 2003;100:2957–2962. doi: 10.1073/pnas.0530112100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood V C, Cruwys S C, Urban L, Kidd B L. Differential role of neurokinin receptors in human lymphocyte and monocyte chemotaxis. Regul Pept. 2000;96:17–21. doi: 10.1016/s0167-0115(00)00195-6. [DOI] [PubMed] [Google Scholar]
- Jeurissen F, Kavelaars A, Korstjens M, Broeke D, Franklin R A, Gelfand E W, Heijnen C J. Monocytes express a non-neurokinin substance P receptor that is functionally coupled to MAP kinase. J Immunol. 1994;152:2987–2994. [PubMed] [Google Scholar]
- Kavelaars A, Broeke D, Jeurissen F, Kardux J, Meijer A, Franklin R, Gelfand E W, Heijnen C J. Activation of human monocytes via a non-neurokinin substance P receptor that is coupled to Gi protein, calcium, phospholipase D, MAP kinase, and IL-6 production. J Immunol. 1994;153:3691–3699. [PubMed] [Google Scholar]
- Lai J P, Ho W Z, Zhan G X, Yi Y, Collman R G, Douglas S D. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci USA. 2001;98:3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas S D, Lai J-P, Tuluc F, Tebas P, Ho W-Z. Neurokinin-1 receptor antagonist (aprepitant) inhibits drug-resistant HIV-1 infection of macrophages in vitro. J Neuroimmune Pharmacol. 2007;2:42–48. doi: 10.1007/s11481-006-9059-6. [DOI] [PubMed] [Google Scholar]
- Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mueller A, Strange P G. The chemokine receptor, CCR5. Int J Biochem Cell Biol. 2004;36:35–38. doi: 10.1016/s1357-2725(03)00172-9. [DOI] [PubMed] [Google Scholar]
- Onuffer J J, Horuk R. Chemokines, chemokine receptors and small-molecule antagonists: recent developments. Trends Pharmacol Sci. 2002;23:459–467. doi: 10.1016/s0165-6147(02)02064-3. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Lai J P, Yang J H, Douglas S D, Wang X, Riedel E, Ho W Z. Quantification of CCR5 mRNA in human lymphocytes and macrophages by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2003;10:1123–1128. doi: 10.1128/CDLI.10.6.1123-1128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Lai J-P, Lai S, Tuluc F, Tansky M F, Kilpatrick L E, Leeman S E, Douglas S D. Differences in the length of the carboxyl terminus mediate functional properties of neurokinin-1 receptor. Proc Natl Acad Sci USA. 2008;105:12605–12610. doi: 10.1073/pnas.0806632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansky M F, Pothoulakis C, Leeman S E. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci USA. 2007;104:10691–10696. doi: 10.1073/pnas.0703394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagliuolo I, Valenick L, Liu J, Pothoulakis C. Epidermal growth factor receptor transactivation mediates substance P-induced mitogenic responses in U-373 MG cells. J Biol Chem. 2000;275:26545–26550. doi: 10.1074/jbc.M003990200. [DOI] [PubMed] [Google Scholar]
- Grimm M C, Newman R, Hassim Z, Cuan N, Connor S J, Le Y, Wang J M, Oppenheim J J, Lloyd A R. Cutting edge: vasoactive intestinal peptide acts as a potent suppressor of inflammation in vivo by trans-deactivating chemokine receptors. J Immunol. 2003;171:4990–4994. doi: 10.4049/jimmunol.171.10.4990. [DOI] [PubMed] [Google Scholar]
- Werry T D, Wilkinson G F, Willars G B. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+ Biochem J. 2003;374:281–296. doi: 10.1042/BJ20030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbie L A, Hill S J. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signaling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Mueller A, Mahmoud N G, Goedecke M C, McKeating J A, Strange P G. Pharmacological characterization of the chemokine receptor, CCR5. Br J Pharmacol. 2002;135:1033–1043. doi: 10.1038/sj.bjp.0704540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani C, Lombardi G, Collino M, Ferrara C, Cassone M C, Fantozzi R. Priming effects of substance P on calcium changes evoked by interleukin-8 in human neutrophils. J Leukoc Biol. 2001;69:1013–1018. [PubMed] [Google Scholar]
- Rasley A, Marriott I, Halberstadt C R, Bost K L, Anguita J. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J Immunol. 2004;172:5707–5713. doi: 10.4049/jimmunol.172.9.5707. [DOI] [PubMed] [Google Scholar]
- Bockmann S. Substance P (NK(1)) receptor expression by human colonic epithelial cell line Caco-2. Peptides. 2002;23:1783–1791. doi: 10.1016/s0196-9781(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Fine J S, Byrnes H D, Zavodny P J, Hipkin R W. Evaluation of signal transduction pathways in chemoattractant-induced human monocyte chemotaxis. Inflammation. 2001;25:61–67. doi: 10.1023/a:1007152903135. [DOI] [PubMed] [Google Scholar]
- Ottonello L, Montecucco F, Bertolotto M, Arduino N, Mancini M, Corcione A, Pistoia V, Dallegri F. CCL3 (MIP-1α) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. 2005;17:355–363. doi: 10.1016/j.cellsig.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Olbrich H, Proudfoot A E, Oppermann M. Chemokine-induced phosphorylation of CC chemokine receptor 5 (CCR5) J Leukoc Biol. 1999;65:281–285. doi: 10.1002/jlb.65.3.281. [DOI] [PubMed] [Google Scholar]
- Shen W, Li B, Wetzel M A, Rogers T J, Henderson E E, Su S B, Gong W, Le Y, Sargeant R, Dimitrov D S, Oppenheim J J, Wang J M. Down-regulation of the chemokine receptor CCR5 by activation of chemotactic formyl peptide receptor in human monocytes. Blood. 2000;96:2887–2894. [PubMed] [Google Scholar]
- Li B Q, Wetzel M A, Mikovits J A, Henderson E E, Rogers T J, Gong W, Le Y, Ruscetti F W, Wang J M. The synthetic peptide WKYMVm attenuates the function of the chemokine receptors CCR5 and CXCR4 through activation of formyl peptide receptor-like 1. Blood. 2001;97:2941–2947. doi: 10.1182/blood.v97.10.2941. [DOI] [PubMed] [Google Scholar]
- Koon H W, Zhao D, Na X, Moyer M P, Pothoulakis C. Metalloproteinases and transforming growth factor-α mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519–45527. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- Al-Sarraj A, Thiel G. Substance P induced biosynthesis of the zinc finger transcription factor Egr-1 in human glioma cells requires activation of the epidermal growth factor receptor and of extracellular signal-regulated protein kinase. Neurosci Lett. 2002;332:111–114. doi: 10.1016/s0304-3940(02)00939-4. [DOI] [PubMed] [Google Scholar]