Abstract
Hypersensitivity pneumonitis is an interstitial lung disease that is characterized by alveolitis, granuloma formation, and in some patients, fibrosis. Using the Saccharopolyspora rectivirgula animal model of Farmer’s lung disease, our laboratory has demonstrated that neutrophils play a critical role in IFN-γ production during the acute phase of the disease. As IFN-γ is necessary for granuloma formation, it is important to identify the factors that lead to neutrophil recruitment during disease. To begin to identify the pattern recognition receptors (PRRs) that initiate chemokine production, leading to neutrophil recruitment following S. rectivirgula exposure, we examined the role of MyD88 and TLR2. Our results demonstrate that neutrophil recruitment, as measured by flow cytometry and the myeloperoxidase assay, was abolished in the absence of MyD88 following S. rectivirgula exposure. The decrease in neutrophil recruitment was likely a result of a significant decrease in production of neutrophil chemokines MIP-2 and keratinocyte-derived chemokine. These results suggest that S. rectivirgula interacts with PRRs that are upstream of the MyD88 pathway to initiate cytokine and chemokine production. In vitro studies suggest that S. rectivirgula can interact with TLR2, and stimulation of adherent cells from TLR2 knockout (KO) mice with S. rectivirgula resulted in a significant decrease in MIP-2 production. However, TLR2 KO mice did not have a reduction in neutrophil recruitment compared with wild-type mice following S. rectivirgula exposure. The results from our studies suggest that one or more PRR(s) upstream of MyD88 are necessary for neutrophil recruitment following S. rectivirgul a exposure.
Keywords: chemokines, IFN-γ, TLR2
INTRODUCTION
Hypersensitivity pneumonitis (HP), or extrinsic allergic alveolitis, is an interstitial lung disease that develops following repeated exposure to inhaled environmental antigens [1–5]. The disease is characterized by alveolitis and granuloma formation, and with continued exposure to the inciting agent, some patients develop chronic, irreversible fibrosis [1, 3]. Once a patient progresses to the chronic form of the disease, the long-term prognosis is poor. The environmental antigens that induce HP include organic dusts, vapors, fungi, bacteria, and molds as well as simple chemical compounds [6, 7]. Exposure to these airborne antigens may occur in occupational and residential settings, and the different types of HP are frequently named after the occupation or activity that results in exposure to the inciting agent. Farmer’s lung disease is one of the most common types of HP and is caused by repeated inhalation of the thermophile Saccharopolyspora rectivirgula, which is commonly found in moldy hay [3].
One of the animal models used to study HP is the well-characterized S. rectivirgula mouse model [8, 9]. Mice intranasally inoculated with S. rectivirgula for 3 days/week for 3 weeks develop an alveolitis that is initially neutrophilic but becomes more lymphocytic in the days following exposure. By the 3rd week of S. rectivirgula exposures, mice develop granulomas composed of macrophages and T cells surrounded by fibroblasts. The development of granulomas in HP is dependent on the Th1 cytokine IFN-γ; IFN-γ knockout (KO) mice exposed to S. rectivirgula develop alveolitis but not granuloma formation [10, 11]. Our previous studies demonstrated that innate immune cell IFN-γ production is sufficient for granuloma formation following exposure to S. rectivirgula. Additionally, neutrophils were identified as a source of IFN-γ, and depletion of neutrophils in mice prior to S. rectivirgula exposure resulted in a significant decrease in the level of IFN-γ produced in the lungs [12]. These results suggest that neutrophils play a critical role in the development of HP, and therefore, it is important to identify the mechanisms that lead to neutrophil recruitment into the lung following S. rectivirgula exposure.
Regulation of neutrophil recruitment is mediated by the expression of proinflammatory cytokines, adhesion molecules, and chemokines. Within the chemokine family, the Arg-Leu-Glu+ CXC subfamily contains members responsible for neutrophil migration [13]. Members of this subfamily include IL-8/CXCL8 and growth-related oncogene α,β (GRO-α,β) in humans and MIP-2 (functional homologue of human IL-8), kertainocyte-derived chemokine (KC; murine homologue of GRO-α), and LPS-induced CXC chemokine in mice. These chemokines act by binding to their cognate receptors CXCR1 or CXCR2 on the surface of neutrophils in humans; until recently, only CXCR2 had been identified in mice [14]. Numerous models have demonstrated that the production of these chemokines is necessary for neutrophil recruitment into inflamed tissues.
The expression of these chemokines, as well as other cytokines involved in inflammation, can be induced by stimulation through pattern recognition receptors (PRRs), which make up a family of signaling receptors that recognize pathogen-associated molecular patterns (PAMPs), conserved structures found almost exclusively on microbes. Activation of PRRs by microbial products is key to activation of the innate and adaptive immune systems (reviewed in ref. [15]). The best-known family of PRRs is the TLRs, which are Type I transmembrane proteins containing amino-terminal leucine-rich repeats (LRR) that are responsible for binding to PAMPs and a carboxy-terminal Toll/IL-1R domain that is responsible for signaling. There have been 13 TLRs identified to date in mice and 10 in humans. TLR1, -2, -4, -5, and -6 are expressed on the cell surface, whereas TLR3, -7, -8, and -9 are found intracellularly in endosomes [16–19]. Binding of PAMPs to TLRs leads to the recruitment of adaptor proteins to the receptor complex and induction of a signaling cascade that results in the activation of numerous proinflammatory genes. Of the five adaptor proteins used by TLRs to transduce signals, MyD88 is the most commonly used adaptor protein; only TLR3 and -4 are not completely dependent on it. Stimulation of the MyD88 pathway leads to activation of the MAPK and NF-κB signaling pathways leading to production of proinflammatory cytokines such as TNF-α, IL-1β, IL-12, IL-6, and IL-8. The importance of MyD88 in cytokine gene expression is highlighted by studies using mice deficient in MyD88, and MyD88 KO mice are highly susceptible to infection with Staphylococcus aureus, Pseudomonas aeruginosa, and Toxoplasma gondii [20–22]. These mice exhibit deficient neutrophil recruitment following infection and significantly increased bacterial loads compared with wild-type (WT) littermate controls. The decrease in neutrophil recruitment in the infected MyD88 KO mice correlated with a decrease in the levels of IL-1β, KC, and MIP-2, suggesting that MyD88 is necessary for induction of these cytokines. The receptors upstream of MyD88 that predominantly recognize bacterial components include TLR2, -4, -5, and -9. TLR2 plays a critical role in recognizing pathogens; it can bind to a variety of ligands, such as peptidoglycan (PGN) from gram-positive bacteria and lipotechoic acids and lipoproteins from gram-positive and gram-negative bacteria, mycobacteria, and yeast zymosan [23, 24]. The broad ligand specificity of TLR2 is likely a result of its ability to form heterodimers with other receptors such as TLR1 and TLR6 [25]. The heterodimer TLR2/TLR1 recognizes triacetylated bacterial lipoproteins produced mainly by gram-negative bacteria, and TLR2/TLR6 recognizes diacetylated lipoproteins produced mainly by gram-positive bacteria [26]. S. rectivirgula, although originally classified as a fungus, is a gram-positive member of the actinomycetes family containing many of the ligands that can potentially interact with TLR2, and therefore, it is reasonable to suggest that TLR2 may play a role in the disease. The importance of TLR2 in host defense against other gram-positive bacteria has been demonstrated using TLR2 gene-deficient (TLR2 KO) mice, which are susceptible to infection with S. aureus or Streptococcus pneumoniae, demonstrating increased bacterial growth and reduced inflammatory responses [20, 27].
In the present study, we examine the kinetics of chemokine induction following exposure to S. rectivirgula and determine the role of MyD88 and TLR2 in chemokine induction and neutrophil recruitment during HP.
MATERIALS AND METHODS
Animals and S. rectivirgula exposure protocol
C57BL/6 female mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) at 6 weeks of age. MyD88 gene-deficient mice were bred at University of Tennessee Health Science Center (Memphis, TN, USA) and were in the sixth and seventh generation of backcross to C57BL/6 mice. TLR2 gene-deficient mice were obtained from Jackson Laboratories and were in the ninth generation backcross to C57BL/6 mice. All animals were housed in sterile micro-isolator cages with sterile food and water ad libitum and were maintained by the Division of Comparative Medicine at the University of Tennessee Health Science Center according to the guidelines of the Animal Welfare Act. The S. rectivirgula (strain designation A1313—ATCC) preparation was grown at 55°C in trypticase soy broth. The bacterial preparation was washed in endotoxin-free distilled water three times, followed by sonication and lyophilization. The lyophilized preparation was reconstituted with endotoxin-free saline at a concentration of 5 mg/ml and inoculated intranasally at the indicated amounts [11]. Although the preparation is sonicated, it is likely that there are still viable bacteria in the preparation. S. rectivirgula is a gram-positive thermophile and to our knowledge, has not been shown to possess LPS. The S. rectivirgula preparations used throughout these studies were tested in the Limulus amebocyte lysate assay (Cambrex, Walkersville, MD, USA) and found to have less than 0.1 EU/ml LPS.
Bronchoalveolar lavage (BAL) and lung cell isolation
Mice were anesthetized with isoflurane and intranasally inoculated with the indicated amount of S. rectivirgula. At 6 or 24 h post-exposure, the mice were killed and BAL performed by intratracheal injection of 1 ml PBS into the lungs with immediate vacuum aspiration. The amount of fluid recovered was routinely around 70%. Cells were recovered from BAL fluid (BALF) by centrifugation and counted using trypan blue dye exclusion. Protease inhibitors were added to the BALF, which was frozen at -80°C until used in a bead-based ELISA assay for cytokine and chemokine measurement. Lungs were perfused with PBS to remove blood and both lobes removed. Lung tissue was digested with collagenase (20 U/ml) and DNase I (40 μg/ml) for 60 min at 37°C. Cells were freed by disruption in a Stomacher tissue processor (Seward, UK) and then isolated by centrifugation on a discontinuous Percoll gradient. Mononuclear cells were isolated at the 40/80% interface following density gradient centrifugation and used in flow cytometry.
Flow cytometry
Flow cytometry was performed on isolated BAL and lung cells using fluorochrome-conjugated antibodies to Gr-1, Ly6G, CD11b, F4/80, CD45 (BD Biosciences, San Jose, CA, USA), and CXCR2 (R&D Systems Inc., Minneapolis, MN, USA). A minimum of 50,000 events/sample was collected on BD LSRII (BD Biosciences) using a live gate based on 4′,6-diamidino-2-phenylindole (DAPI) dye exclusion and analyzed using FACSDiva or FLoJo software.
Multiplex sandwich immunoassay
Chemokines present in unconcentrated BALF were measured using a MIP-2 ELISA (Biosource, Camarillo, CA, USA) or a multiplex sandwich immunoassay (BD Biosciences), per the manufacturer’s instructions. Fluorescence was measured using the Bio-Plex array reader (Bio-Rad Laboratories, Hercules, CA, USA) Cytokine standards ranging from 5 to 20,000 pg/ml were prepared to determine the concentration of cytokine in the samples. For data analysis, a curve fit was applied to the standards and the sample concentrations extrapolated from the standard curve using the Logistic 5PL model in the BioPlex Manager 3.0 software (Bio-Rad Laboratories).
Measurement of myeloperoxidase (MPO) activity
MPO activity was measured using the method of Koike et al. [28]. Lung tissue was removed from mice and homogenized at a final concentration of 50 mg/ml in hexadecyl trimethylammonium bromide buffer. The samples were centrifuged at 16,000 g for 20 min. The supernatants were mixed with 50 mmol phosphate buffer containing O-dianisidine hydrochloride and H2O2. The plates were read spectrophotometrically at 460 nm for 1–20 min on a Spectra Max 340 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Luciferase assay
HeLa cells were transfected to stably express TLR2 or TLR4/myeloid differentiation protein 2 (MD-2), or parental HeLa cells were transiently transfected with 0.6 μg TLR5 or TLR7 in 24-well plates. Transfections were done according to the manufacturer’s instructions with Effectene reagent (Qiagen, Valencia, CA, USA). In addition to transfection with individual TLRs, cells were cotransfected with 0.4 μg endothelial leukocyte cell-adhesion molecule (ELAM)-luciferase reporter vector, 0.2 μg pcDNA-CD14, and 0.1 μg CMV-β-galactosidase (β-Gal). Forty-eight hours after transfection, cells were stimulated for 6 h with individual TLR agonists, palmitoyl-3-cysteine-serinelysine-4 (Pam3CSK4; synthetic triacylated lipopeptide, TLR2 agonist, Invivo-Gen, San Diego, CA, USA), LPS (Escherichia coli K12 LCD25, TLR4 agonist, List Biological Laboratories, Campbell, CA, USA), flagellin (from Listeria monocytogenes TLR5 agonist), R-848 (resiquimod, TLR7 agonist, GLSynthesis, Worcester, MA, USA), and varying amounts of heat-killed S. rectivirgula. A luciferase assay was performed using Promega (Madison, WI, USA) reagents according to the manufacturer’s recommendations. Efficiency of transfection was normalized by measuring β-Gal activities in same cell lysates.
Statistical analysis
Results are expressed as mean ± SD. Data were analyzed using One-way ANOVA using GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA). Differences were considered significant at P values of less than 0.05.
RESULTS
Neutrophil chemoattractants are produced in vivo during S. rectivirgula exposures
To determine the kinetics of neutrophil migration into the lungs following S. rectivirgula exposure, mice were intranasally exposed to S. rectivirgula and then analyzed at 6, 12, or 24 h post-exposure (Fig. 1A). Flow cytometry was used to determine the percentage of neutrophils and macrophages in the BALF; we defined neutrophils as Gr-1hi/CD11b+/F4/80-, and macrophages were defined as F4/80+/Gr-1-/CD11b+. As expected, BAL cells isolated from saline-exposed mice consisted predominantly of macrophages (70%) with ∼4% neutrophils. By 6 h post-exposure, there was a significant increase in total BAL cell number (alveolitis), which was comprised predominantly of neutrophils (88%) with a corresponding decrease in macrophages (7%). The alveolitis continues to increase and peaks at 24 h post-exposure; neutrophils were again the predominant cell type recovered from the BALF. By 48 h post-exposure, the BAL cell number has returned to levels similar to saline-exposed controls, and the predominant cell type is macrophages with few neutrophils.
Fig. 1.
Neutrophil chemokines are present in BALF following S. rectivirgula exposure. Mice (five/group) were intranasally exposed to S. rectivirgula (50 μg) or saline and BALF collected from individual mice at 6 and 24 h post-exposure. A multiplex sandwich immunoassay was used to measure production of KC and MCP-1 in the BALF, and an ELISA was used to measure MIP-2 levels. The cells recovered from the BALF were counted, stained with antibody to CD45, CD11b, Gr-1, and F4/80, and run on a BD LSRII flow cytometer to determine the percent of neutrophils and macrophages in individual samples. The data were analyzed using FACSDiva software, and the percent of Gr-1+ or F4/80+ cells was determined using gates set on live cells (using DAPI as an exclusion dye) and CD45+ cells. *, P < 0.05, WT mice exposed to S. rectivirgula compared with saline-exposed mice.
To determine whether neutrophil chemoattractants were produced following S. rectivirgula exposure, BALF was isolated from individual mice and then analyzed using a multiplex sandwich immunoassay for MCP-1 and KC and by ELISA for MIP-2 (Fig. 1, B—D). Our results demonstrate that the neutro-phil chemokines MIP-2 and KC were increased significantly within 6 h of S. rectivirgula exposure and declined to baseline levels by 24 h. MCP-1, a monocyte and T cell chemokine, increased only at 24 h post-exposure, suggesting that its production is dependent on cells recruited into the lung. The increase in MIP-2 and KC correlates with the increase in neutrophils isolated from the BALF. GM-CSF, which promotes neutrophil survival, was not detected at any time-point tested (data not shown).
MIP-2 and KC recruit neutrophils via binding to their cog-nate receptor CXCR2 on the surface of the neutrophil. Flow cytometric analysis of neutrophils recovered from BALF of mice exposed to S. rectivirgula demonstrated that 88 ± 7% of Gr-1+ neutrophils express CXCR2 on the surface (data not shown). In addition, neutrophils isolated from the lungs of mice exposed to S. rectivirgula migrated in a dose-dependent manner to MIP-2 using a standard chemotaxis assay (data not shown). Taken together, these results demonstrate that neutro-phil chemoattractants MIP-2 and KC are rapidly and transiently produced in the lungs of mice following S. rectivirgula exposure and may play an important role in neutrophil recruitment during HP.
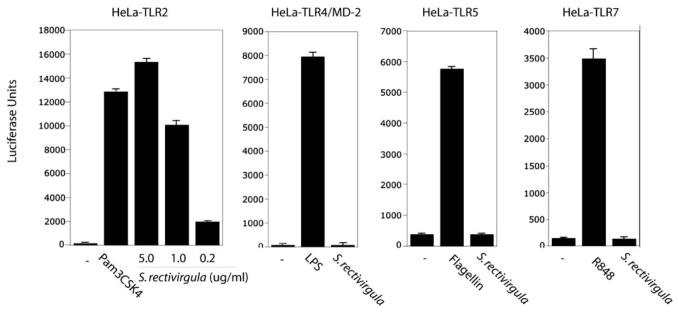
S. rectivirgula stimulates luciferase activity in a cell line transfected with TLR2
Chemokines such as MIP-2 and KC can be induced following stimulation of TLRs by microbial products. To identify the TLRs that may interact with S. rectivirgula, HeLa cells were cotransfected with TLR2, TLR4/MD-2 (MD-2 is a coreceptor for LPS), TLR5, or TLR7 expression vectors and a NF-κB-responsive luciferase reporter vector (ELAM—luciferase reporter vector). The transfected cells were stimulated with media, S. rectivirgula, LPS (TLR4 agonist), flagellin (TLR5 agonist), R848 (TLR7 agonist), or Pam3CSK4 (TLR2 agonist). As shown in Figure 2, the cells transfected with TLR2 were stimulated in a dose-dependent manner by S. rectivirgula. In contrast, S. rectivirgula did not stimulate luciferase activity in HeLa cells expressing TLR4/MD-2, TLR5, or TLR7. These results suggest that chemokine production and subsequently, neutrophil recruitment in mice exposed to S. rectivirgula may be mediated by TLR2.
Fig. 2.
S. rectivirgula stimulates TLR2 but not TLR4, TLR5, or TLR7 in vitro. HeLa cell lines expressing the indicated constructs were transiently transfected with ELAM-luciferase reporter construct, CMV-CD14, CMV-β-Gal (for normalization), and stimulated for 6 h with S. rectivirgula or the indicated TLR agonists used at the following concentrations: LPS (1 ng/ml), Pam3CSK4 (2 μg/ml), flagellin (10 μg/ml), R848 (1 μg/ml), heat-killed S. rectivirgula (5.0, 1.0, or 0.2 ug/ml used for HeLa-TLR2 stimulation; 5.0 μg/ml used for all other cell lines). NF-κB activity was measured by luciferase activity present in cell lysates. Data are expressed as mean ± SD (TLR5 or -7, n=3; TLR2 or -4, n=10).
TLR2 KO mice exhibit neutrophil recruitment into the lung following exposure to S. rectivirgula
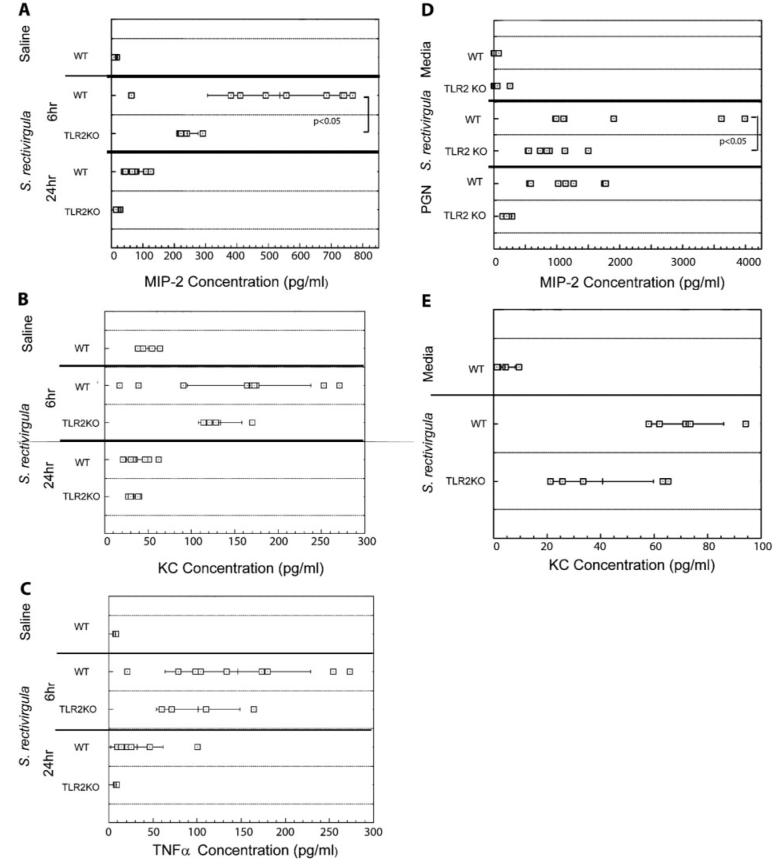
To begin to examine the role of TLR2 in neutrophil recruitment, we exposed TLR2 KO mice to S. rectivirgula and measured the level of neutrophil influx at 6 or 24 h post-exposure. As it is possible that S. rectivirgula may interact with more than one TLR, we exposed the mice to a low dose of S. rectivirgula (30 μg) to increase the chances of identifying a role for TLR2. As shown in Table 1, TLR2 KO mice exposed to S. rectivirgula developed alveolitis at 6 and 24 h post-exposure, similarly to WT mice. The alveolitis that developed in the TLR2 KO mice following exposure was neutrophilic, and there was no significant difference in the total number of neutrophils isolated from the BALF of the TLR2 KO mice compared with WT mice exposed to S. rectivirgula (Table 1). As an indirect measure of neutrophil infiltration, MPO activity was measured in TRL2 KO and WT mice following S. rectivirgula exposure (Fig. 3). WT mice exposed to S. rectivirgula exhibited an increase in MPO activity compared with saline-exposed mice at 6 h post-exposure. However, TLR2 KO mice exposed to S. rectivirgula also exhibited an increase in MPO activity that was reduced but not significantly different from the WT mice. Similar results were seen at 24 h post-exposure (data not shown). These results suggest that TLR2 is dispensable for neutrophil recruitment into the lung following S. rectivirgula exposure.
TABLE 1.
TLR2 KO Mice Develop a Neutrophilic Alveolitis following S. rectivirgula Exposure
| Alveolitis (cells×106)a |
Neutrophils (cells×106)b |
|||
|---|---|---|---|---|
| WT | TLR2 KO | WT | TLR2 KO | |
| Saline exposure | 0.21 ± 0.10 | N.D. | 0.001 ± 0.0009 | N.D. |
| S. rectivirgula 6 h exposure | 11.1 ± 1.5 | 9.5 ± 3 | 11.0 ± 1.4 | 9.0 ± 0.3 |
| S. rectivirgula 24 h exposure | 2.9 ± 2.6 | 1.2 ± 0.726 | 1.5 ± 1.0 | 0.7 ± 0.8 |
WT (n=9/group) or TLR2 KO (n=4/group) mice were exposed to saline or S. rectivirgula (30μg) one time and sacrificed at 6 or 24 h after exposure.
BAL was performed, and the recovered cells were counted using trypan blue dye exclusion. N.D., Not determined.
Total neutrophil cell count was derived by staining cells recovered from the BALF with antibody to CD45, Gr-1, CD11b, and F4/80; neutrophils were identified as Gr1+/CD45+/CD11b+/F480-. The cells were run on a BD LSRII flow cytometer, and the data were analyzed using FACSDiva software. There was no significant difference between WT and TLR2 KO mice in alveolitis or neutrophil cell count at any time-point tested.
Fig. 3.

TLR2 is not required for neutrophil recruitment following S. rectivirgula stimulation. TLR2 KO (n=4 mice/group) or WT mice (n=9 mice/group) were intranasally exposed to S. rectivirgula (30 μg) one time and neutrophil recruitment analyzed at 6 h post-exposure. One lung lobe from individual mice was homogenized, and MPO activity was measured as described in Materials and Methods. There was no statistically significant difference between TLR2 KO and WT mice exposed to S. rectivirgula at any of the time-points measured.
TLR2 is not required for cytokine production following S. rectivirgula exposure
To determine whether TLR2 was necessary for cytokine production following S. rectivirgula exposure, ELISAs were performed on BALF from individual mice at 6 and 24 h post-exposure (Fig. 4, A—C). The results demonstrated that there was no decrease in KC or TNF-α production at 6 h post-exposure in the TLR2 KO mice compared with the WT mice. However, there was a significant decrease in MIP-2 production in the TLR2 KO mice compared with the WT mice exposed to S. rectivirgula. These results suggest that TLR2 may play a role in MIP-2 production; however, it is not necessary for KC or TNF-α production following S. rectivirgula exposure in vivo. Additional in vitro studies were performed to determine whether TLR2 plays a role in production of the neutrophil chemokines MIP-2 or KC (Fig. 4, D and E). Adherent cells were prepared from spleens of WT or TLR 2KO mice and stimulated overnight with S. rectivirgula. Culture supernatants were harvested and analyzed by ELISA for MIP-2 and KC production. The results demonstrate that cells from WT mice stimulated with S. rectivirgula produced large amounts of MIP-2, whereas there was a significant decrease in MIP-2 production by TLR2-deficient cells stimulated with S. rectivirgula. In contrast, there was no significant difference in KC production between WT and TLR2 KO cells following S. rectivirgula stimulation. These results suggest that TLR2 plays a role in MIP-2 production but is dispensable for KC and TNF-α production and that additional PRRs might contribute to the production of these cytokines following S. rectivirgula stimulation.
Fig. 4.
TLR2 is not necessary for cytokine production following S. rectivirgula exposure. (A—C) TLR2 KO or WT mice were intranasally exposed to S. rectivirgula (30 μg) one time, and cytokine production was analyzed at 6 or 24 h post-exposure. TNF-α, MIP-2, and KC were measured in unconcentrated BALF recovered from individual mice by ELISA and are expressed as pg/ml. (D and E) Splenic adherent cells were purified from WT or TLR2 KO mice and stimulated overnight with heat-killed S. rectivirgula, PGN (a TLR2 agonist), or media alone. Culture supernatants were collected and analyzed by ELISA for MIP-2 or KC.
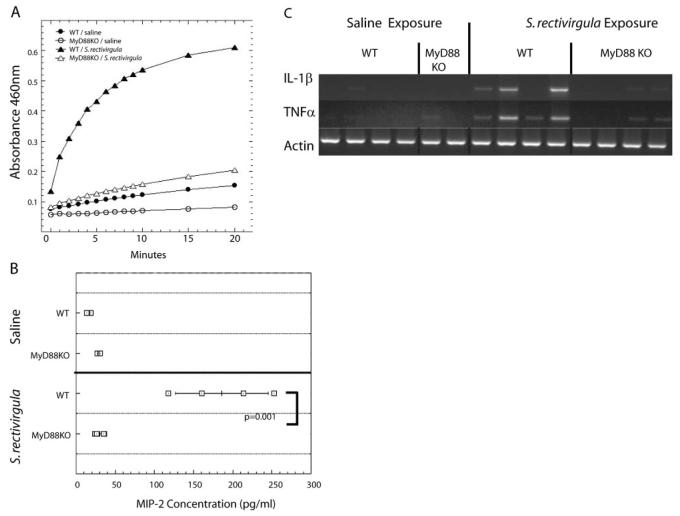
MyD88 is necessary for neutrophil recruitment following S. rectivirgula exposure
To identify individual PRRs that play a role in neutrophil recruitment following S. rectivirgula exposure, it was necessary to identify which family of PRRs is involved. Unlike with most TLR family members, other PRRs, such as neuronal apoptosis inhibitory protein, MHC class II transcription activator, incompatibility locus protein from Podospora anserina, and telomerase-associated protein-LRR (NLR) family members, nucleotide-binding oligomerization domain 1 (NOD1) and NOD2, do not use MyD88 [29]. Therefore, to determine whether MyD88-dependent TLRs or other PRR families are required for cytokine production and neutrophil recruitment in HP, we exposed MyD88 KO mice to S. rectivirgula. MyD88 KO mice and WT littermates were exposed once/day for 2 days to S. rectivirgula and analyzed 24 h after the last exposure. Mice were exposed to S. rectivirgula two times to exaggerate any low-level activation of a MyD88-independent pathway. We chose to examine neutrophil recruitment 24 h following the last S. rectivirgula exposure, as it is the peak of neutrophil influx into the lungs. If a deficiency in MyD88 only delayed the kinetics of neutrophil recruitment, we should be able to detect it by this time-point. As shown in Table 2, WT mice exposed to S. rectivirgula developed alveolitis compared with MyD88 KO mice, which did not exhibit alveolitis; the number of cells recovered from the BALF of S. rectivirgula exposed MyD88 KO mice was similar to saline controls. The alveolitis that developed in WT littermates was composed mainly of neutrophils compared with MyD88 KO mice that did not have an increase in the number of neutrophils in the BALF following S. rectivirgula exposure (Table 2). As an indirect measure of neutrophil infiltration, MPO activity was measured in MyD88 KO and WT littermate controls following S. rectivirgula exposure (Fig. 5A). The results demonstrated that WT mice exposed to S. rectivirgula exhibited an increase in MPO activity following S. rectivirgula exposure, whereas there was no increase in MPO activity in lungs from MyD88 KO mice exposed to S. rectivirgula. To determine whether there was a low level of neutrophil recruitment into MyD88 KO mice immediately following S. rectivirgula exposure, which may be gone by 24 h post-exposure, pilot studies were performed at 6 h post-exposure. Results from these studies revealed that at 6 h post-exposure, MyD88 KO mice had levels of alveolitis similar to saline and significantly decreased from WT mice exposed to S. rectivirgula (WT/saline=0.1×106±0.02 cells; WT/S. rectivirgula=3.4×106±2.3 cells; MyD88 KO/S. rectivirgula=0.12×106±0.05 cells; n=4/group). The results from these studies suggest that the adaptor protein MyD88 is necessary for neutrophil recruitment into the lung following S. rectivirgula exposure.
TABLE 2.
MyD88 KO Mice Do Not Develop Alveolitis or a Neutrophil Influx following S. rectivirgula Exposure
| Alveolitis (cells×106)a |
Neutrophils (cells×106)b |
|||
|---|---|---|---|---|
| WT | MyD88 KO | WT | MyD88 KO | |
| Saline exposure | 0.45 ± 0.096 | 0.43 ± 0.36 | 0.001 ± 0.0008 | N.D. |
| S. rectivirgula exposure | 5.6 ± 2.68 | 0.41 ± 0.31 | 4.4 ± 2.1 | 0.09 ± 0.07 |
Mice (n=4/group) were exposed to saline or S. rectivirgula (60 μg) once/day for 2 days and BAL performed 24 h after the last exposure.
BAL was performed, and the recovered cells were counted using trypan blue dye exclusion.
Total neutrophil cell count was derived by staining cells recovered from the BALF with antibody to CD45, Gr-1, CD11b, and F4/80; neutrophils were identified as Gr1+/CD45+/CD11b+/F480-. The cells were run on a BD LSRII flow cytometer, and the data were analyzed using FACSDiva software. There was no significant difference between WT and TLR2 KO mice in alveolitis or neutrophil cell count at any time-point tested.
Fig. 5.
MyD88 KO is required for neutrophil recruitment into the airways following S. rectivirgula exposure. WT and MyD88 KO (n=4 mice/group) were exposed to saline or S. rectivirgula (60 μg) once/day for 2 days and analyzed for neutrophil recruitment 24 h after the last exposure. (A) One lung lobe from individual mice was homogenized, and MPO activity was measured as described in Materials and Methods. (B) MIP-2 was measured in BALF recovered from individual mice by ELISA. (C) RT-PCR was performed on RNA isolated from one lung lobe from individual WT or MyD88 KO mice exposed to saline or S. rectivirgula using primers specific for TNF-α and IL-1β. The housekeeping gene β-actin was used as an internal control. *, P < 0.05, MyD88 KO exposed to S. rectivirgula compared with WT mice exposed to S. rectivirgula.
MyD88 is necessary for cytokine production following S. rectivirgula exposure
To determine whether the decrease in neutrophil recruitment in MyD88 KO mice following S. rectivirgula exposure correlated with a decrease in MIP-2 production, an ELISA was performed using BALF from individual mice (Fig. 5B). WT mice exposed to S. rectivirgula had an increase in MIP-2 in the BALF; however, there was no increase in MIP-2 in BALF from S. rectivirgula-exposed MyD88 KO mice. At 24 h post-exposure, the level of cytokines such as TNF-α and IL-1β, which may play an indirect role in neutrophil recruitment, is difficult to detect in the BALF using ELISA. Therefore, we analyzed the production of these cytokines in the lung tissue by the more sensitive RT-PCR (Fig. 5C). The results demonstrate that WT mice exposed to S. rectivirgula have an increase in mRNA for TNF-α and IL-1β compared with saline control mice. However, there was no increase in mRNA for these cytokines in the MyD88 KO mice exposed to S. rectivirgula. We could not detect KC mRNA at this time-point in our samples (data not shown). Additional in vitro studies have shown that macrophages isolated from WT mice produce MIP-2 and KC in response to in vitro S. rectivirgula stimulation; in contrast, macrophages from MyD88 KO mice produced significantly less MIP-2 and KC following S. rectivirgula stimulation (Fig. 6). Taken together, these results suggest that MyD88 is necessary for neutrophil recruitment following S. rectivirgula exposure, and the decrease in neutrophil recruitment in MyD88-deficient mice is a result of decreased cytokine/chemokine production. It is likely that the combined lack of MIP-2 as well as TNF-α and IL-1β contributes to the decrease in neutrophil recruitment, as TNF-α and IL-1β are important for adhesion molecule expression. In addition, these results suggest that MyD88-dependent TLRs likely play a role in stimulating neutrophil recruitment following S. rectivirgula exposure.
Fig. 6.

MyD88 is necessary for MIP-2 and KC production following S. rectivirgula stimulation in vitro. Adherent cell populations were prepared from MyD88 KO or WT spleens. Cells were stimulated with S. rectivirgula (10 μg) or media alone, and supernatants were collected at 24 h. The amounts of MIP-2 and KC present in the supernatants were measured by ELISA and are expressed as pg/ml.
DISCUSSION
The results from our studies reveal the importance of MyD88 in neutrophil recruitment following S. rectivirgula exposure.
The number of cells in the BALF was increased by 6 h post-S. rectivirgula exposure, continued to increase through 24 h, and then returned to normal levels by 48 h post-S. rectivirgula exposure. The influx was predominantly composed of neutrophils; ∼88% of the BALF was neutrophils throughout the 6-, 12-, and 24-h time-points. However, whenever the BAL cell counts returned to normal levels, the cellular composition was similar to saline controls consisting predominantly of macrophages with few neutrophils detected. The increase in neutrophil recruitment into the lung was accompanied by an increase in chemokines and cytokines that play a direct or indirect role in neutrophil recruitment. The neutrophil chemokines MIP-2 and KC were increased in the BALF by 6 h post-exposure and returned to baseline levels by 24 h post-exposure. Additionally, TNF-α, which indirectly plays a role in neutrophil recruitment via stimulation of adhesion molecule expression and chemokine production, was also increased in the BALF at 6 h post-exposure. Conversely, the monocyte chemoattractant MCP-1 did not increase until 24 h post-S. rectivirgula exposure. As neutrophils are a source of MCP-1, the early influx of neutrophils may be important for the production of chemokines that help to recruit other cell types into the lung. The chemokines MIP-2 and KC act by binding to their cognate receptor CXCR2 expressed on neutrophils and other cell types. In our studies, CXCR2 was expressed on 75–98% of neutrophils isolated from the BALF of mice that had been exposed to S. rectivirgula. Although there was some variability in the percentage of neutrophils expressing CXCR2, this may be a result of down-regulation of the receptor by a number of factors such as TNF-α, fMLP, a tripeptide found often in bacteria, MCP-1, and prolonged KC stimulation [30]. In addition to neutrophils, CXCR2 has been found on some macrophages, endothelial, epithelial, and skeletal muscle cells [31–34]. Our studies revealed that CXCR2 was also expressed by Ly6G- cells; as these cells were CD45+/CD11b+, they may have been monocytes or dendritic cells. Therefore, MIP-2 and KC may also play important roles in recruitment of cells other than neutrophils into the lung.
The results from our studies with MyD88 KO mice demonstrated that MyD88 was necessary for neutrophil recruitment following exposure to S. rectivirgula. MyD88 KO mice exposed to S. rectivirgula did not develop alveolitis and did not exhibit a neutrophil influx into the lungs, as measured by flow cytometry and the MPO assay compared with the WT mice exposed to S. rectivirgula. As there is no intrinsic defect in neutrophil migration in MyD88 KO mice, the lack of neutrophil recruitment is likely a result of a lack of cytokines and/or chemokines that are important in neutrophil recruitment [35]. Following S. rectivirgula exposure, there was no increase in MIP-2 in the BALF of MyD88 KO mice compared with WT mice, suggesting that MyD88 is necessary for MIP-2 production. The cytokines TNF-α and IL-1β also play an indirect role in neutrophil recruitment, and our results demonstrated that MyD88 KO mice did not increase expression of mRNA for TNF-α or IL-1β following S. rectivirgula exposure. These results suggest that MyD88 is necessary for production of proinflammatory cytokine production following S. rectivirgula exposure in vivo, although we cannot rule out that S. rectivirgula interacts with some of the intracellular receptors such as the NOD-like receptors (NLRs) that recognize bacterial molecules present in the cytosol. Members of the NLRs play a critical role in activation of caspase-1 necessary for processing of IL-1β and IL-18 [36]. As the IL-1Rβ uses the MyD88 adaptor protein, IL-1R signaling would also be abrogated in these mice. As production of cytokines and chemokines decreases by 24 h after the last exposure to S. rectivirgula in vivo, we examined the role of MyD88 in chemokine production using in vitro S. rectivirgula stimulation of adherent cells. Direct stimulation of splenic adherent cells from WT mice with S. rectivirgula results in the production of MIP-2 and KC; however, S. rectivirgula stimulation of cells from MyD88 KO mice resulted in a significant decrease in MIP-2 and KC production. These results suggest that MyD88 plays a direct role in induction of MIP-2 and KC by S. rectivirgula stimulation, and the lack of neutrophil recruitment in vivo is a result of decreased production of cytokines and chemokines necessary for neutrophil recruitment. Our previous studies demonstrated that neutrophils were a source of IFN-γ following S. rectivirgula exposure. Therefore, a decrease in neutrophil recruitment in the MyD88 KO mice would be expected to result in decreased IFN-γ gene expression, as IFN-γ is necessary for granuloma formation, and fibrosis in HP factors that modulate neutrophil recruitment may impact on the severity of the disease. Studies are currently in progress to identify the cytokines and signaling pathway(s) that stimulate neutrophil IFN-γ production following S. rectivirgula exposure.
As MyD88 is necessary for neutrophil recruitment following S. rectivirgula exposure, it is likely that PRRs upstream of MyD88 interact with S. rectivirgula. Our in vitro studies using cells transfected with various TLRs demonstrated that S. rectivirgula is capable of stimulating TLR2 in a dose-dependent manner; there was no stimulation of TLR4, -5, or -7. As S. rectivirgula is a gram-positive bacteria containing the ligands for TLR2, these results suggest that S. rectivirgula may be interacting with TLR2 in vivo. Additionally, PGN has been shown to stimulate MIP-2 production in a TLR2-dependent manner, suggesting that TLR2 may be important in chemokine production necessary for neutrophil recruitment [37]. However, the results from the TLR2 KO mice exposed to S. rectivirgula revealed that TLR2 was dispensable for neutrophil recruitment at 6 or 24 h post-exposure. The influx of neutrophils as measured by flow cytometry and MPO assays showed no significant differences in the percentage of neutrophils and macrophages in the airways or interstitial lung tissue between WT and TLR2 KO mice. Additionally, KC and TNF-α levels in the BALF were not decreased significantly in the TLR2 KO mice compared with the WT mice, suggesting that TLR2 is not necessary for their induction in vivo. In contrast, there was a significant decrease in the amount of MIP-2 produced in TLR2 KO mice compared with WT at the 6-h time-point, suggesting that TLR2 may play a role in MIP-2 production. The involvement of TLR2 in S. rectivirgula-induced MIP-2 production was confirmed using in vitro studies. Stimulation of cells from TLR2 KO mice with S. rectivirgula resulted in significantly less MIP-2 production than WT cells stimulated with S. rectivirgula. Despite the decrease in MIP-2 production in the TLR2 KO mice following S. rectivirgula exposure, neutrophil recruitment is similar to WT mice, demonstrating that other cytokines/chemokines compensate for the lack of MIP-2. These results are similar to other studies using gram-positive organisms. Miller et.al. [38] found that MyD88 was necessary for neutrophil recruitment following infection with S. aureus, whereas TLR2 was not required. Although TLR2 did not play a role in neutrophil recruitment, TLR2 KO mice did exhibit increased susceptibility to S. aureus infection, albeit not to the level of MyD88 KO mice. Our studies only examined a role for TLR2 in neutrophil recruitment, and it is possible that TLR2 may be playing another role in the disease process. Studies have shown that activation of TLR2 causes changes in neutrophil respiratory burst, IL-8 expression, and an overall increase in neutrophil activation. Using an animal model of Aspergillosis, signaling through TLR2 led to an increase in neutrophil activity, as assessed by oxidative function, granule content release, and increased cytokine production [39, 40]. Other studies revealed that signaling through TLR2 leads to an increased responsiveness of neutrophils to the effects of IL-8 [41]. Our studies did not measure lifespan or activation status of the infiltrating cells, so although there is no difference in the number of neutrophils recruited, there could be a difference in disease outcome in TLR2 KO mice following S. rectivirgula exposure.
As neutrophil recruitment following S. rectivirgula exposure depends on MyD88, the results from the TLR2 KO mice reveal that there must be some other MyD88-dependent PRR involved in neutrophil recruitment. S. rectivirgula is a gram-positive organism and to our knowledge, does not possess LPS, suggesting that TLR4 (LPS ligand) would not be involved in neutrophil recruitment. Accordingly, S. rectivirgula stimulation of cells cotransfected with TLR4/MD-2 and a reporter gene did not result in an increase in luciferase activity. However, additional studies using TLR4 KO mice will demonstrate conclusively whether TLR4 is necessary for neutrophil recruitment and/or chemokine production following S. rectivirgula exposure. Another MyD88-dependent TLR that may be stimulated by S. rectivirgula is TLR9, an intracellular TLR found in endosomes that interacts with unmethylated CpG DNA found in bacteria and viruses [42]. Several studies have demonstrated that TLR9 plays a role in cytokine production following infection with live bacteria, including intracellular and extracellular pathogens [43, 44].
Alternatively, our results may suggest that a combination of TLRs is responsible for neutrophil recruitment following S. rectivirgula exposure. S. rectivirgula is a complex organism that potentially contains many different PAMPs that can activate multiple MyD88-dependent TLRs. Studies with Mycobacterium tuberculosis or Trypanosoma cruzi infections using TLR2/TLR9 double-KO mice reveal that these two TLRs cooperate in the control of infection [45, 46]. TLR2/TLR9 double-KO mice have an enhanced susceptibility to these infections with decreased production of cytokines compared with infected TLR2-single KO, TLR9-single KO, or WT mice alone. As a result of the complexity of the organism and the redundancy in the TLR system, it is likely that multiple MyD88-dependent TLRs act in a cooperative manner to induce neutrophil recruitment following S. rectivirgula exposure. The results of our studies have demonstrated that MyD88 is necessary for neutrophil recruitment following S. rectivirgula exposure, and therefore, TLRs that are dependent on MyD88 are involved in initiating the cytokine and chemokine cascade that results in neutrophil recruitment. Our previous studies have suggested that neutrophils play a pivotal role in IFN-γ production, which is required for granuloma formation [12]. Identifying the factors that lead to neutrophil recruitment into the lung may lead to the development of therapeutics to treat the disease. In addition, although a large number of individuals are exposed to the environmental antigens that cause HP, only a small proportion of these individuals develops disease. The host cofactor(s) that play a role in determining whether an individual is susceptible to disease are unknown. Several of the TLRs (TLR2, TLR4) have polymorphisms that change their responsiveness to their ligands [47, 48]. For example, two gene polymorphisms in human TLR2 (Arg753 Gln and Arg677 Trp mutation) have decreased responsiveness to bacterial lipoproteins, which are TLR2 ligands [49]. The Arg677 Trp mutation has been reported to be associated with lepromatous leprosy in a Korean population, whereas the Arg753 Gln mutation was associated with tuberculosis [50]. There have been no studies to determine whether TLR or MyD88 polymorphisms correlate with S. rectivirgula-induced HP; however, identification of the TLRs involved in recognition of S. rectivirgula will lead to future studies to determine whether TLR polymorphisms contribute to disease susceptibility.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants HL084172 (E. A. F.) and AI053137 (A-K.Y.), the Children’s Foundation Transgenic Mice Program, and the University of Tennessee Bacterial Pathogenesis Center. The authors thank S. Akira (Osaka University, Japan) for providing the MyD88 KO mice.
REFERENCES
- 1.Sharma OP, Fujimura N. Hypersensitivity pneumonitis: a non-infectious granulomatosis. Semin. Respir. Infect. 1995;10:96–106. [PubMed] [Google Scholar]
- 2.Sharma SK, Pande JN, Verma K, Guleria JS. Bronchoalveolar lavage fluid (BALF) analysis in interstitial lung diseases—a 7-year experience. Indian J. Chest Dis. Allied Sci. 1989;31:187–196. [PubMed] [Google Scholar]
- 3.Salvaggio JE, DeShazo RD. Pathogenesis of hypersensitivity pneumonitis. Chest. 1986;89:190S–193S. doi: 10.1378/chest.89.3_supplement.190s. [DOI] [PubMed] [Google Scholar]
- 4.Gurney JW. Hypersensitivity pneumonitis. Radiol. Clin. North Am. 1992;30:1219–1230. [PubMed] [Google Scholar]
- 5.Ando M, Suga M. Hypersensitivity pneumonitis. Curr. Opin. Pulm. Med. 1997;3:391–395. doi: 10.1097/00063198-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Agostini C, Trentin L, Facco M, Semenzato G. New aspects of hypersensitivity pneumonitis. Curr. Opin. Pulm. Med. 2004;10:378–382. doi: 10.1097/01.mcp.0000133067.71469.b2. [DOI] [PubMed] [Google Scholar]
- 7.Patel AM, Ryu JH, Reed CE. Hypersensitivity pneumonitis: current concepts and future questions. J. Allergy Clin. Immunol. 2001;108:661–670. doi: 10.1067/mai.2001.119570. [DOI] [PubMed] [Google Scholar]
- 8.Denis M, Cormier Y, Fournier M, Tardif J, Laviolette M. Tumor necrosis factor plays an essential role in determining hypersensitivity pneumonitis in a mouse model. Am. J. Respir. Cell Mol. Biol. 1991;5:477–483. doi: 10.1165/ajrcmb/5.5.477. [DOI] [PubMed] [Google Scholar]
- 9.Denis M, Cormier Y, Tardif J, Ghadirian E, Laviolette M. Hypersensitivity pneumonitis: whole Micropolyspora faeni or antigens thereof stimulate the release of proinflammatory cytokines from macrophages. Am. J. Respir. Cell Mol. Biol. 1991;5:198–203. doi: 10.1165/ajrcmb/5.2.198. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsson G, Hunninghake GW. Interferon-γ is necessary for the expression of hypersensitivity pneumonitis. J. Clin. Invest. 1997;99:2386–2390. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nance S, Cross R, Fitzpatrick E. Chemokine production during hypersensitivity pneumonitis. Eur. J. Immunol. 2004;34:677–685. doi: 10.1002/eji.200324634. [DOI] [PubMed] [Google Scholar]
- 12.Nance S, Cross R, Yi AK, Fitzpatrick EA. IFN-γ production by innate immune cells is sufficient for development of hypersensitivity pneumonitis. Eur. J. Immunol. 2005;35:1928–1938. doi: 10.1002/eji.200425762. [DOI] [PubMed] [Google Scholar]
- 13.Clark-Lewis I, Kim KS, Rajarathnam K, Gong JH, Dewald B, Moser B, Baggiolini M, Sykes BD. Structure-activity relationships of chemokines. J. Leukoc. Biol. 1995;57:703–711. doi: 10.1002/jlb.57.5.703. [DOI] [PubMed] [Google Scholar]
- 14.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hedrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and IL-8/CXCL8. J. Biol. Chem. 2006;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- 15.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 18.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Akira S. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 2004;34:73–82. doi: 10.1016/j.jdermsci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 21.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J. Biol. Chem. 2004;279:49315–49322. doi: 10.1074/jbc.M402111200. [DOI] [PubMed] [Google Scholar]
- 22.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 23.Lien E, Sellati TJ, Yoshimura A, Flo TH, Rawadi G, Finberg RW, Carroll JD, Espevik T, Ingalls RR, Radolf JD, Golenbock DT. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 24.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 25.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Röschmann K, Jung G, Wiesmüller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 2007 doi: 10.1189/jlb.0807586., Epub ahead of print.
- 26.Robichon C, Vidal-Ingigliardi D, Pugsley AP. Depletion of apolipoprotein N-acyltransferase causes mislocalization of outer membrane lipoproteins in Escherichia coli. J. Biol. Chem. 2005;280:974–983. doi: 10.1074/jbc.M411059200. [DOI] [PubMed] [Google Scholar]
- 27.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 28.Koike K, Moore EE, Moore FA, Read RA, Carl VS, Banerjee A. Gut ischemia/reperfusion produces lung injury independent of endotoxin. Crit. Care Med. 1994;22:1438–1444. doi: 10.1097/00003246-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Kufer TA, Fritz JH, Philpott DJ. NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol. 2005;13:381–388. doi: 10.1016/j.tim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Asagoe K, Yamamoto K, Takahashi A, Suzuki K, Maeda A, Nohgawa M, Harakawa N, Takano K, Mukaida N, Matsushima K, Okuma M, Sasada M. Down-regulation of CXCR2 expression on human polymorphonuclear leukocytes by TNF-α. J. Immunol. 1998;160:4518–4525. [PubMed] [Google Scholar]
- 31.Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-regulated expression of the CXCR2 ligand KC/GRO-α in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am. J. Pathol. 2006;168:1385–1395. doi: 10.2353/ajpath.2006.040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkas L, Hahn MC, Schmoczer M, Jentsch N, Kratzel K, Pfeifer M, Schulz C. Expression of CXC chemokine receptors 1 and 2 in human bronchial epithelial cells. Chest. 2005;128:3724–3734. doi: 10.1378/chest.128.5.3724. [DOI] [PubMed] [Google Scholar]
- 33.Frydelund-Larsen L, Penkowa M, Akerstrom T, Zankari A, Nielsen S, Pedersen BK. Muscle: exercise induces interleukin-8 receptor (CXCR2) expression in human skeletal muscle. Exp. Physiol. 2007;92:233–240. doi: 10.1113/expphysiol.2006.034769. [DOI] [PubMed] [Google Scholar]
- 34.Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, Mao L, Wistuba I, Strieter RM, Kurie JM. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 35.Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning CJ, Wagner H, Holzmann B. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 2002;169:2823–2827. doi: 10.4049/jimmunol.169.6.2823. [DOI] [PubMed] [Google Scholar]
- 36.Kanneganti T-D, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 40.Bellocchio S, Moretti S, Perruccio K, Fallarino F, Bozza S, Montagnoli C, Mosci P, Lipford GB, Pitzurra L, Romani L. TLRs govern neutrophil activity in aspergillosis. J. Immunol. 2004;173:7406–7415. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 41.Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868. [PubMed] [Google Scholar]
- 42.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 43.Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B. Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J. Immunol. 2005;175:3964–3970. doi: 10.4049/jimmunol.175.6.3964. [DOI] [PubMed] [Google Scholar]
- 44.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- 45.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 46.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Corbett SA, Calvano SE, Lowry SF. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J. Infect. Dis. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 48.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 49.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect. Immun. 2000;68:6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogus AC, Yoldas B, Ozdemir T, Uguz A, Olcen S, Keser I, Coskun M, Cilli A, Yegin O. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 2004;23:219–223. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]