Abstract
The periaqueductal gray (PAG) is involved in many gonadal steroid-sensitive behaviors, including responsiveness to pain. The PAG projects to the rostral ventromedial medulla (RVM), comprising the primary circuit driving pain inhibition. Morphine administered systemically or directly into the PAG produces greater analgesia in male compared to female rats, while manipulation of gonadal hormones alters morphine potency in both sexes. It is unknown if these alterations are due to steroidal actions on PAG neurons projecting to the RVM. The expression of androgen (AR) and estrogen (ERα) receptors in the PAG of female rats and within this descending inhibitory pathway in both sexes is unknown. The present study used immunohistochemical techniques (1) to map the distribution of AR and ERα across the rostrocaudal axis of the PAG; and (2) to determine whether AR and/or ERα were colocalized on PAG neurons projecting to the RVM in male and female rats. AR and ERα immunoreactive neurons (AR-IR, ERα-IR) were densely distributed within the caudal PAG of male rats, with the majority localized in the lateral/ventrolateral PAG. Females had significantly fewer AR-IR neurons, while the quantity of ERα was comparable between the sexes. In both sexes, approximately 25-50% of AR-IR neurons and 20-50% of ERα-IR neurons were retrogradely labeled. This study provides direct evidence of the expression of steroid receptors in the PAG and the descending pathway driving pain inhibition in both male and female rats and may provide a mechanism whereby gonadal steroids modulate pain and morphine potency.
Keywords: gonadal steroid receptor, hormone, immunohistochemistry, sex differences, descending modulatory pathway, pain, antinociception
INTRODUCTION
Anatomical and physiological studies have shown that the midbrain periaqueductal gray (PAG) plays a modulatory role in a variety of behaviors including antinociception (Reynolds, 1969; Behbehani & Fields, 1979; Heinricher et al., 1987; Behbehani, 1995; Budai et al., 1998), reproduction (McCarthy et al., 1991; Ogawa et al., 1991; Murphy & Hoffman, 1998; Daniels et al., 1999), fear and anxiety (Kim et al., 1993), aggression (Bandler et al., 1985; Bandler & Carrive, 1988; Depaulis et al., 1992; Scordalakes & Rissman, 2004) and vocalization (Davis et al., 1993; Zhang et al., 1994). While these behaviors have been shown to be modulated by gonadal steroids, our knowledge of the qualitative and quantitative aspects of gonadal steroid receptors in the PAG is incomplete. The PAG has been shown to contain a large number of both androgen receptor (AR) and estrogen receptor (ERα) immunoreactive neurons (Murphy & Hoffman, 1999; Murphy & Hoffman, 2001), however these studies were conducted exclusively in male rats. While the distribution of ERα in the female PAG has been reported in a few species, including the cat (VanderHorst et al., 1998), the golden hamster (Boers et al., 1999), the guinea pig (Turcotte & Blaustein, 1993) and the rhesus monkey (Vanderhorst et al., 2002; VanderHorst et al., 2004), the quantity and distribution of AR and ERα in the female rat is currently unknown.
The PAG projects heavily to the rostral ventromedial medulla (RVM), which in turn projects to the dorsal horn of the spinal cord. This PAG-RVM-spinal cord circuit is the primary neural pathway that elicits the antinociceptive effects of opiates. Previous studies have reported sex differences in the anatomical organization of the projections from the PAG to the RVM and activation of these neurons by inflammatory pain (Loyd & Murphy, 2006). In addition, there are significant sex differences in the activation of the this pathway by systemic morphine, both in the presence and absence of inflammatory pain (Loyd & Murphy, 2006; Loyd et al., 2007; Loyd et al., 2008). To date, it is not known whether ERα and AR are expressed on PAG neurons projecting to the RVM. Numerous behavioral studies have shown that sex differences in opioid analgesia are modulated by both the organizational and activational effects of gonadal steroids (Kepler et al., 1989; Islam et al., 1993; Krzanowska & Bodnar, 1999; 2000; Stoffel et al., 2003; Cataldo et al., 2005; Stoffel et al., 2005). Male rats castrated at birth experience decreased morphine potency in adulthood, while female rats masculinized at birth experience greater morphine potency in adulthood whether morphine is administered systemically (Cicero et al., 2002) or directly into the PAG (Krzanowska et al., 2002). Similarly, both systemic and central administration of morphine is less effective in gonadectomized adult males and more effective in ovariectomized adult females (Kepler et al., 1989; Ratka & Simpkins, 1991; Krzanowska & Bodnar, 1999; Terner et al., 2002; Stoffel et al., 2003; Stoffel et al., 2005; Terner et al., 2005); effects are reversed with hormone replacement (Ratka & Simpkins, 1991; Kiefel & Bodnar, 1992); Stoffel et al., 2003; (Ji et al., 2007).
While the organizational and activational effects of gonadal steroids are likely to contribute to the sexually dimorphic actions of morphine, it is currently unknown whether gonadal steroid receptors are expressed on PAG neurons projecting to the RVM. In addition, the qualitative and quantitative aspects of AR and ERα expression in the PAG of the female rat are not known. The present studies utilized immunohistochemistry to map (1) the quantity and distribution of AR and ERα immunoreactive neurons across the rostrocaudal axis of the PAG; and (2) to determine if the PAG neurons projecting to the RVM express AR and ERα immunoreactivity. Due to a lack of commercially available antibodies at the time these studies were conducted, ERβ was not analyzed in this study. This study is the first to report AR and ERα immunoreactivity in the PAG and its descending projections to the RVM in both male and female rats.
MATERIALS AND METHODS
Subjects
Six adult male and six weight-matched (250-350g; approximately 70-100 days of age) cycling female Sprague-Dawley rats were used in these experiments (Zivic-Miller; Pittsburgh, PA). Rats were housed in same-sex pairs on a 12:12 hour light:dark cycle. Access to food and water was ad libitum throughout the experiment except during surgery. These studies were performed in compliance with the Institutional Animal Care and Use Committee at Georgia State University. All efforts were made to reduce the number of animals used in these experiments and to minimize any possible suffering by the animal.
Vaginal Cytology
Vaginal lavages were performed daily beginning two weeks prior to experimental manipulations to confirm that the female rats were cycling normally and to keep daily records on the stages of their cycle up to the day of sacrifice. Proestrus was identified as a predominance of nucleated epithelial cells and estrus was identified as a predominance of cornified epithelial cells. Diestrus 1 was differentiated from Diestrus 2 by the presence of leukocytes. Rats that appeared between phases were noted as being in the more advanced stage.
Retrograde Tracer Injections
Animals were deeply anesthetized with a cocktail of ketamine/xylazine/acepromazine (50 mg/kg / 3.3 mg/kg / 3.3 mg/kg; i.p.; Henry Schein, Melville, NY). When a surgical plane of anesthesia was reached each animal was placed in a stereotaxic frame and the skull was adjusted so bregma and lambda were at the same dorsal-ventral plane. Glass micropipettes (10-20 μM) filled with the retrograde tracer Fluorogold (FG; 2% soln. w/v in saline; Fluorochrome LLC; Denver, CO) were lowered into the RVM using the following coordinates (in mm): AP: -2.0 Lambda; ML: 0.0; DV: -8.5). FG was iontophoresed (50/50 duty cycle, 7.5 μA current) into the RVM for 25 minutes to facilitate neuronal uptake. The current was then turned off and the pipettes remained in place for an additional 5 minutes prior to removal to minimize backflow of the tracer along the pipette track. Following tracer injections, wounds were sutured closed, the antibiotic Neosporin was applied to the wound, and the animals were placed in clean cages to recover under a heat lamp. Upon complete recovery from the anesthetic, animals were returned to their original housing facilities.
Perfusion fixation
Ten days following surgery, animals were given a lethal dose of Nembutal (160 mg/kg; i.p.) and transcardially perfused with 200-250 ml of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator to remove blood from the brain. Immediately following removal of blood, 300 ml of 4% paraformaldehyde in 0.1M phosphate buffer containing 2.5% acrolein (Polyscience; Niles, IL) was perfused through the brain as a fixative. A final rinse with 200-250 ml of the sodium chloride/sodium nitrate solution was perfused through the brain to remove any residual acrolein. Immediately following perfusion, the brains were carefully removed, placed in a 30% sucrose solution and stored at 4°C for at least one week prior to sectioning. Sucrose solutions were changed daily to optimize saturation of sucrose into the tissue. To section the brain, the dura and pia mater were carefully removed and the brains were cut into six series of 25 μm coronal sections with a Leica 2000R freezing microtome and stored free-floating in cryoprotectant-antifreeze solution (Watson et al., 1986) at −20°C until immunocytochemical processing. The tissue was sectioned at 25 μm so that 125 μm separates each analyzed level of the PAG thus eliminating any possible bias from counting the same cell twice during data collection.
Immunocytochemistry
A 1:6 series through the rostrocaudal axis of each brain was processed for FG immunoreactivity and AR (n=5 males; n=6 females) or ERα (n=6 males; n=5 females) immunoreactivity as previously described (Murphy & Hoffman, 2001). Briefly, sections were rinsed extensively in potassium phosphate-buffered saline (KPBS) to remove cryoprotectant solution, immediately followed by a 20-minute incubation in 1% sodium borohydride to remove excess aldehydes. The tissue was then incubated in either primary antibody solution rabbit anti-AR (Santa Cruz Biotechnology; Santa Cruz, CA, lot no. L0407; 1:10,000) or rabbit anti-ERα (Santa Cruz Biotechnology; Santa Cruz, CA, lot no. I2607; 1:20,000) in KPBS containing 1.0% Triton-X for one hour at room temperature followed by 48 hours at 4°C. The rabbit anti-AR antiserum was prepared against a peptide mapping at the N-terminus of AR of human origin (MEVQLGLGRVYPRPPSKTYRG) corresponding to amino acids 2-21 (manufacturer’s technical information) and specificity has been confirmed (Creutz & Kritzer, 2004). The rabbit anti-ERα antiserum was prepared against a peptide mapping at the C-terminus of ERα of mouse origin (HSLQTYYIPPEAEGFPNTI) corresponding to amino acids 580-559 (manufacturer’s technical information) and specificity has been confirmed (Quesada et al., 2007).
After rinsing out the primary antibody with KPBS, the tissue was incubated for one hour in biotinylated goat anti-rabbit IgG (Jackson Immunoresearch; West Grove, PA, 1:600), rinsed with KPBS, followed by a one hour incubation in an avidin-biotin peroxidase complex (1:10; ABC Elite Kit, Vector Labs). After rinsing in KPBS and sodium acetate (0.175 M; pH 6.5), AR or ERα immunoreactivity was visualized as a black reaction product using nickel sulfate intensified 3,3’-diaminobenzidine solution containing 0.08% hydrogen peroxide in sodium acetate buffer. After rinsing, AR or ERα labeled sections were then placed in primary antibody solution rabbit anti-FG (Chemicon; Billerica, MA, lot no. 25060005; 1:10,000) in KPBS containing 1.0% Triton-X for one hour at room temperature followed by 48 hours at 4°C. FG was visualized as a brown reaction product using 3,3’-diaminobenzidine containing 0.08% hydrogen peroxide in Trizma buffer (pH 7.2). After 15-30 minutes, three rinses in sodium acetate buffer terminated the reaction and tissue was given a final rinse in KPBS. Sections were then mounted out of saline onto gelatin-subbed slides, air-dried and dehydrated in a series of graded alcohols. Tissue-mounted slides were then cleared in xylene and glass cover-slipped using Permount.
Data Analysis and Presentation
Data were analyzed across six representative levels through the rostrocaudal axis of the PAG (Bregma -6.72, -7.04, -7.74, -8.00, -8.30, -8.80). The number of AR immunoreactive neurons (AR-IR), ERα immunoreactive neurons (ERα-IR), and the number of AR-IR and ERα-IR neurons that were retrogradely labeled (AR/FG+, ERα/FG+) were quantified. The experimenter was blind to the experimental condition. In levels where significant differences were found, a second blinded observer confirmed results. Cell counts were conducted unilaterally as there are no differences in the number of FG+ cells (Loyd & Murphy, 2006) or the number of AR-IR and ERα-IR neurons (Murphy & Hoffman, 2001) for the left versus right side of PAG. Additionally, previous data have shown that there are no sex differences in total area (mm2) of the PAG between weight-matched male and female Sprague-Dawley rats (Loyd & Murphy, 2006).
Data are reported as the mean ± standard error of the mean (SEM) from which percentages were calculated and reported as the percentage of receptor that was localized in retrogradely labeled cells (%AR/FG+; %ERα/FG+) or as the percentage of retrogradely labeled cells that were colocalized with receptor (%FG/AR; %FG/ ERα). A three-way analysis of variance (ANOVA) was used to test for significant main effects of sex (male, female), PAG level (Bregma −6.72 through -8.80), and PAG subdivision (dorsomedial, lateral/ventrolateral). For percentile date, percentages were transformed to standard scores. Fishers’s post hoc tests were used to determine specific group differences when a main effect or interaction was observed. P ≤.05 was considered significant for all analyses. For data presentation, a representative animal from each experimental group was selected and the distribution of (1) AR-IR neurons, (2) ERα-IR neurons, (3) FG+ neurons, (4) AR/FG+ neurons and (5) ERα/FG+ neurons within the PAG were plotted using a Nikon Drawing Tube attached to a Nikon Optiphot microscope. Plots were then scanned onto the computer and adjusted to figure format using Adobe Illustrator 10. Photomicrographs were generated using a Synsys digital camera attached to a Nikon Eclipse E800 microscope. Images were captured with IP Spectrum software and adjusted to figure format by alterations in brightness and contrast levels using Adobe Photoshop 7.0.
RESULTS
Androgen Receptor Distribution in the PAG
AR-IR neurons were distributed across the rostrocaudal axis of the PAG in both male and female rats (Figure 1; red circles). AR-IR neurons were confined to the dorsomedial and lateral/ventrolateral subdivisions of the PAG, with the dorsolateral subdivision of the PAG lacking AR-IR neurons. These results are consistent with previous studies showing AR localization in the PAG of male rats (Murphy & Hoffman, 1999; Murphy & Hoffman, 2001). While the qualitative distribution of AR-IR neurons was similar in both males and females, quantitatively males had a significantly greater number of AR-IR neurons compared to females [F(1, 54)=22.7, p<.00001) (Figure 2; red bars) with a significantly greater number of AR-IR neurons localized in the lateral/ventrolateral PAG compared to the dorsomedial subdivision [F(1, 108)=22.1, p<.0001]. This sex difference was evident across the rostrocaudal axis of the PAG. There was no main effect of level of PAG [F(5,54)=1.2; n.s.] and no significant sex by level interaction [F(5,54)=0.3; n.s.], indicating that the number of AR-IR neurons remained consistent across the rostrocaudal axis of the PAG of both male and female rats.
Figure 1.
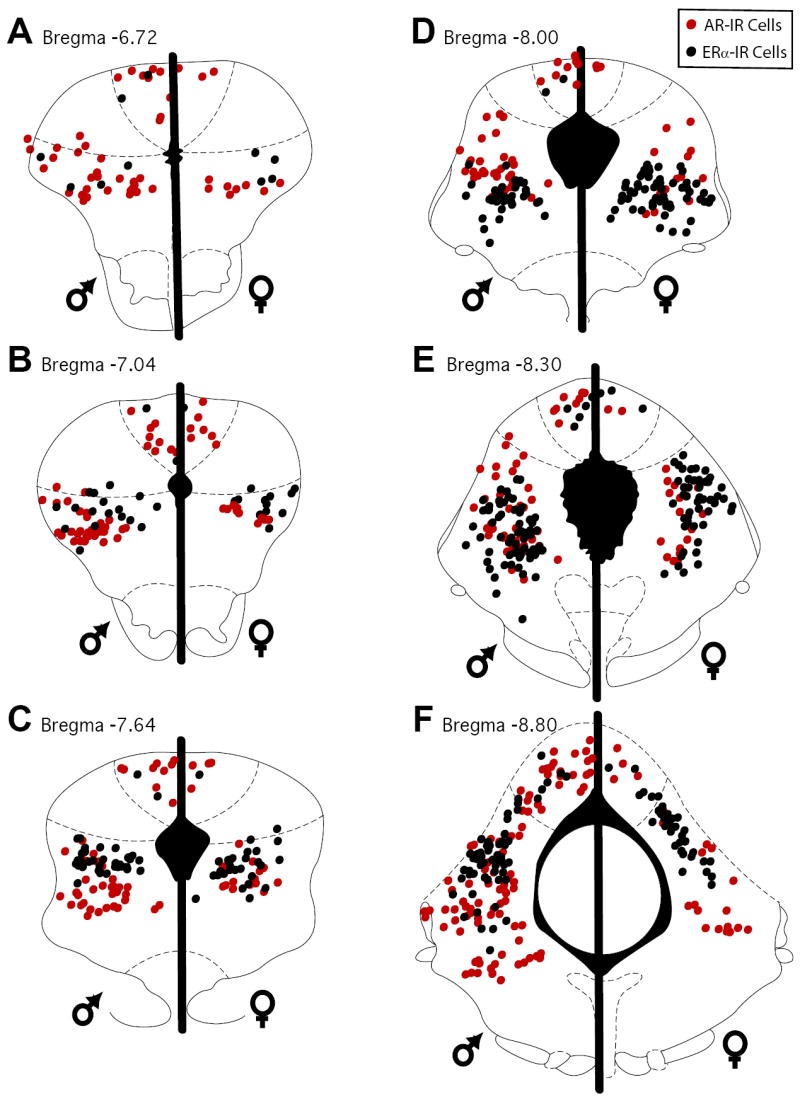
Distribution of cells in the PAG immunoreactive for AR (red circles) and ERα (black circles) in male (left side of plots) and female rats (right side of plots) at six rostrocaudal levels (A-F) of the PAG.
Figure 2.
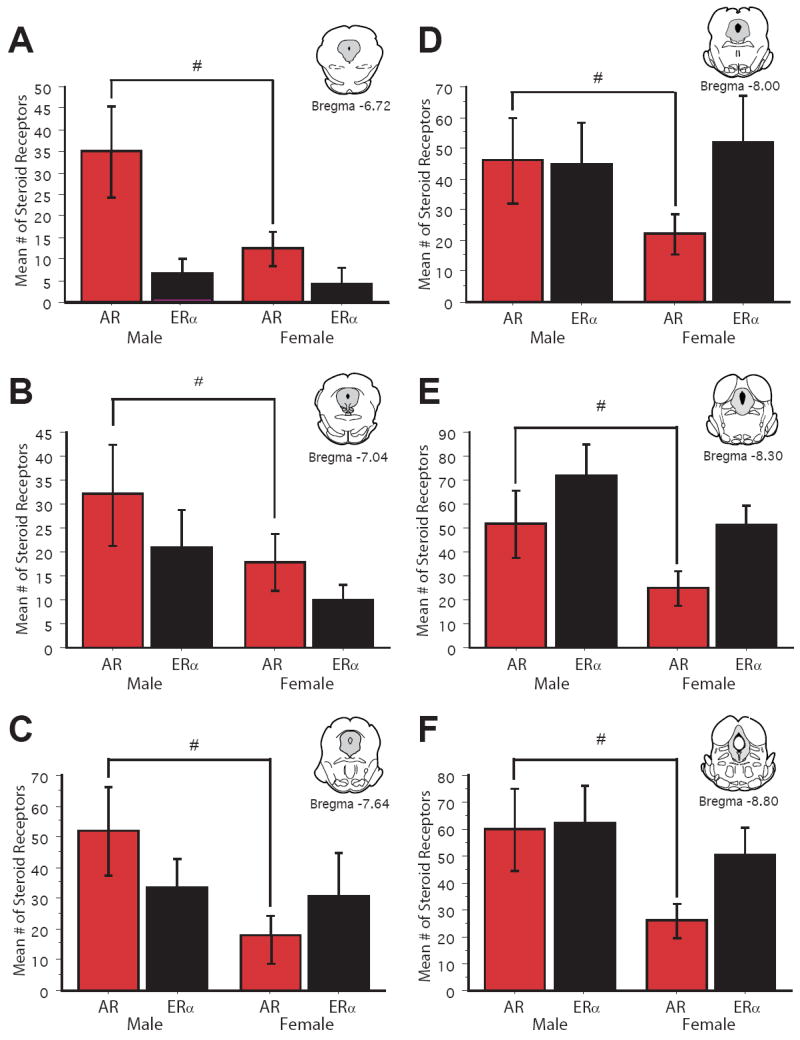
Bar graphs display the mean number (± S.E.M.) of AR immunoreactive cells (green bars) and ERα immunoreactive cells (purple bars) across six rostrocaudal levels of the PAG. Cell counts were combined for the dorsomedial and lateral/ventrolateral subdivisions of PAG. # denotes a significant sex difference in mean # of steroid receptors.
Estrogen (α) Receptor Distribution in the PAG
ERα-IR neurons were densely distributed throughout the rostrocaudal axis of the PAG in both male and female rats (Figure 1; black circles). Similar to the distribution of AR-IR neurons, ERα-IR neurons were confined to the dorsomedial and lateral/ventrolateral subdivisions of the PAG, with the majority of ERα-IR neurons localized in the lateral/ventrolateral PAG [F(1, 108)=105.8, p<.0001]. Overall, there was no sex difference in the number of ERα-IR neurons in the PAG [F(1, 54)=1.2, n.s.] (Figure 2; black bars). A significant increase in the number of ERα-IR neurons [F(5, 54)=9.02, p<.0001] was noted along the rostrocaudal axis of the PAG.
Androgen Receptor Distribution in PAG Neurons Projecting to the RVM
All iontophoretic injections of the retrograde tracer Fluorogold (FG) into the RVM were located on the midline and dorsal to the pyramidal tract, at the level of the caudal pole of the facial nucleus (lambda −2.0mm). Analysis was limited to injection sites that occurred between the facial nucleus and the olivary complex across approximately 2mm rostrocaudally (Bregma -9.30 to -11.60). In our studies using anterograde tracing from the PAG to the RVM, we have noted that this region of RVM contains the highest density of anterogradely labeled fibers and is remarkably consistent throughout this 2mm window of RVM (unpublished observations). Injections outside of the RVM were not included for analysis. Only male and female rats with comparable injection sites were used for analysis. Injection of FG into the RVM produced dense retrograde labeling throughout the rostrocaudal axis of the PAG consistent with our previous studies (Loyd et al, 2006, Loyd et al., 2007; Loyd et al., 2008). Females had a significantly greater number of PAG cells retrogradely labeled from the RVM compared to males [F(1,70)=14.4, p<.0003].
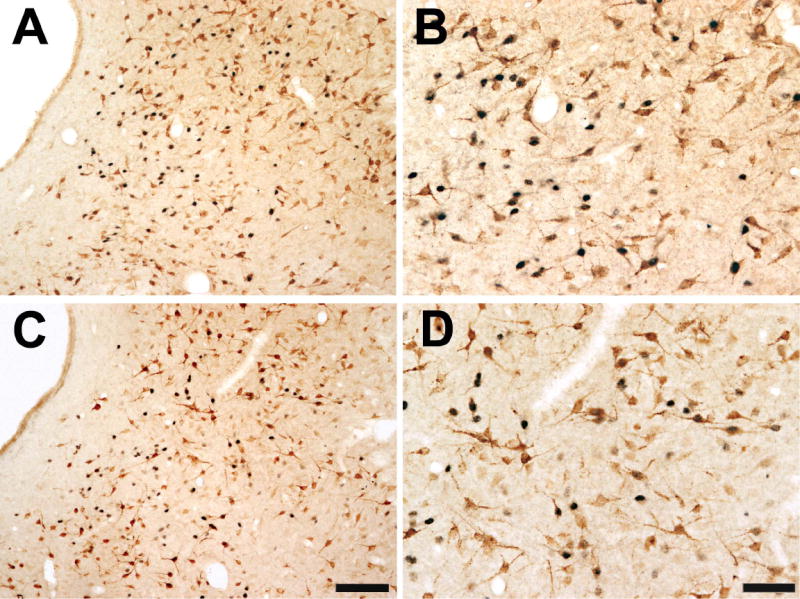
Figure 3 shows an example of AR and FG immunoreactivity within the lateral PAG of a representative male (A-B) and female (C-D) rat. AR-IR neurons there were retrogradely labeled were densely localized throughout the rostrocaudal axis of the PAG in both male and female rats (Figure 4; red stars), with males expressing more dual labeled cells [F(1,54)=19.5; p<.0001]. The percentage of retrogradely labeled cells that expressed AR was comparable between the sexes [F(1,54)=13.51; n.s.] (Figure 5, %FG/AR), and significantly increased moving caudally through the PAG [F(5,54)=7.29; p<.0001]. Since female rats had a greater number of PAG neurons projecting to the RVM compared to males, the percentage of AR that was localized in retrogradely labeled cells was also determined (Figure 5; %AR/FG+) and was found to be comparable between the sexes [F(1, 54)=1.4; n.s.].
Figure 3.
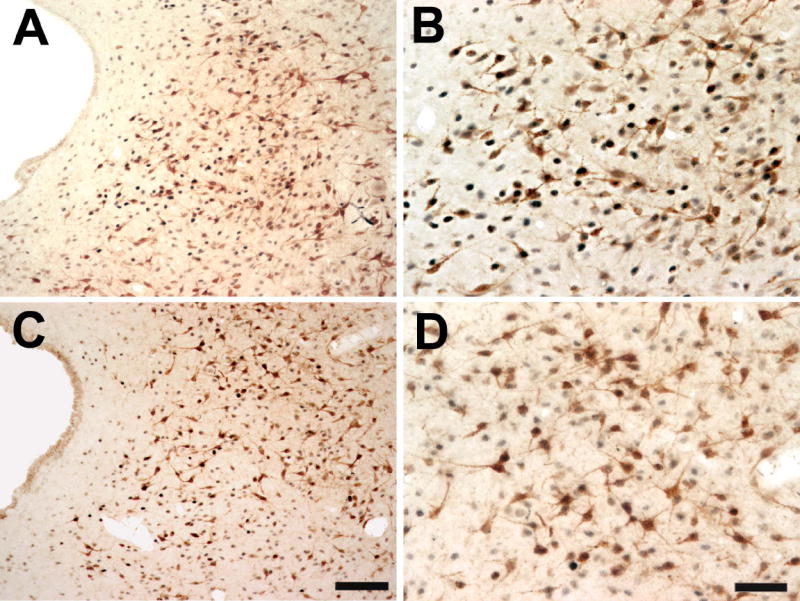
Color photomicrograph showing a low (A,C) and high (B,D) power example of single- and double-labeled AR and FG immunoreactive cells in the lateral PAG (bregma -8.00) of a male (A-B) and female rat (C-D). Scale bar = 100 μm for low power images; scale bar = 50 μm for high power images.
Figure 4.
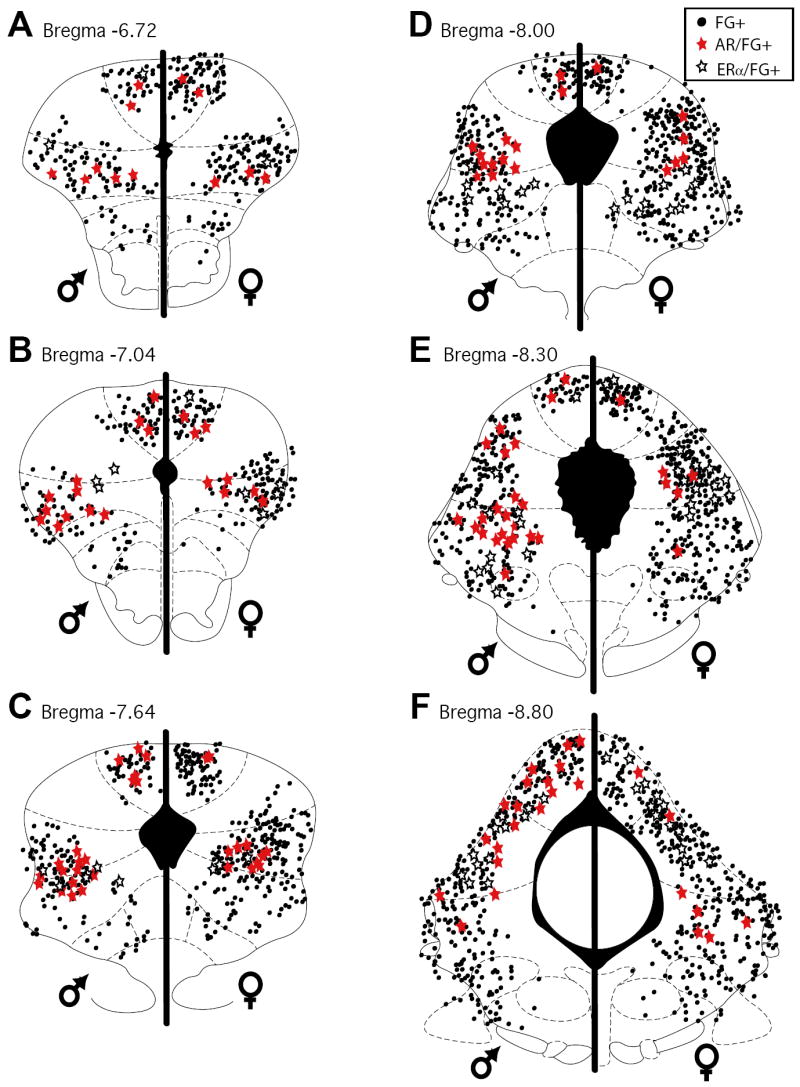
Distribution of cells in the PAG retrogradely labeled from the rostral ventromedial medulla (black circles) and immunoreactive for AR (red stars) and ERα (open stars) in male (left side of plots) and female rats (right side of plots) at six rostrocaudal levels of the PAG.
Figure 5.
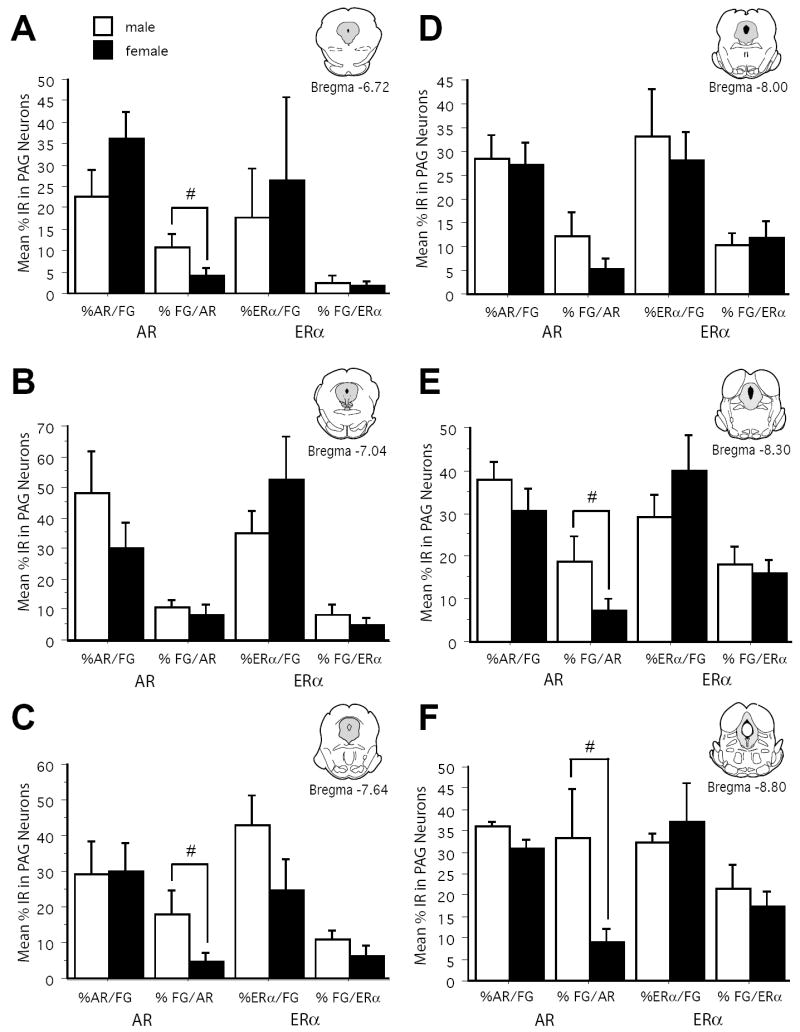
Bar graphs display the mean (± S.E.M.) %AR/FG+, %FG/AR+, %ERα/FG+, and %FG/ERα+ immunoreactive neurons for the dorsomedial combined with lateral/ventrolateral regions of PAG across six rostrocaudal levels of the PAG.
Estrogen (α) Receptor Distribution in PAG Neurons Projecting to the RVM
An example of ERα and FG immunoreactivity within the lateral PAG of a representative male (A-B) and female (C-D) rat is shown in Figure 6. ERα-IR neurons that were retrogradely labeled were densely localized throughout the rostrocaudal axis of the PAG in both male and female rats (Figure 4; open stars) [F(1,54)=1.1; n.s.], with the majority of localized in the lateral/ventrolateral subdivision [F(1, 54)=7.5; p<.0001]. Across all levels and regions of the PAG, the percentage of retrogradely labeled cells that expressed ERα was comparable between the sexes [F(1,54)=0.176; n.s.] (Figure 5, %FG/ERα). Similarly, the percentage of ERα that was localized in retrogradely labeled cells was comparable between the sexes [F(1, 54)=0.292; n.s.] (Figure 5; %ERα/FG+). Additionally, a significantly greater percentage of retrogradely labeled cells that expressed ERα were observed in the caudal PAG [F(5,54)=15.1; p<.0001].
Figure 6.
Color photomicrograph showing a low (A,C) and high (B,D) power example of single- and double-labeled ERα and FG immunoreactive cells in the lateral PAG (bregma -8.00) of a male (A-B) and female rat (C-D). Scale bar = 100 μm for low power images; scale bar = 50 μm for high power images.
DISCUSSION
The PAG has been implicated in a variety of hormone-sensitive behaviors (Bandler & Shipley, 1994; Keay & Bandler, 2001; 2002); however, gonadal steroid receptor expression in the PAG had not been reported in both male and female rats. Here we report that the expression of AR is sexually dimorphic along the entire rostrocaudal axis of the PAG, with males having a significantly greater number of immunoreactive neurons. No sex differences were noted in the qualitative or quantitative aspects of ERα expression in the PAG. In the present study, ERβ was not examined due to a lack of a reliable antibody at the time these studies were conducted; therefore, the possibility remains that the expression of ERβ in the PAG is sexually dimorphic. Similarly, in this study we were unable to determine the effects of estrous on ERα expression in the PAG due to low number of animals. On the day of sacrifice, ten days following tracer injections, all female rats were in either the estrus (n=4) or proestrus (n=2) phase of their cycle; no animals were in the diestrus phase.
The sex difference in the expression of AR in the PAG may play a role in sex differences in pain and analgesia. There are numerous behavioral studies indicating a role of gonadal steroids in modulating morphine potency. Gonadectomy reduces morphine potency in male rats (Kepler et al., 1989) and increases morphine potency in females (Terner et al., 2002; Terner et al., 2005), while hormone replacement reverses these effects (Stoffel et al., 2003; Stoffel et al., 2005). Masculinizing female rat pups with testosterone increases morphine potency to male-like levels (Cicero et al., 2002). In addition, testosterone has been shown to oppose the effects of estradiol on neuronal excitability (Edwards et al., 1999) and decrease pain sensitivity in both male and female rats (Aloisi et al., 2004). A greater expression of AR in the PAG of males may provide an anatomical substrate for the sexually dimorphic modulation of pain by gonadal steroids.
The distribution of AR-IR and ERα-IR neurons was remarkably similar; both receptor types were preferentially localized within the dorsomedial and lateral/ventrolateral subdivisions of the PAG and both increased in density along the rostrocaudal axis of the PAG. These results are similar to the distribution of AR-IR and ERα-IR neurons previously reported in the PAG of the male rat (Murphy & Hoffman, 1999; Murphy & Hoffman, 2001). In addition, the distribution of ERα-IR neurons in the female rat PAG is similar to that previously reported in the cat (VanderHorst et al., 1998), the golden hamster (Boers et al., 1999), the guinea pig (Turcotte & Blaustein, 1993) and the rhesus monkey (Vanderhorst et al., 2002; VanderHorst et al., 2004).
Steroid Receptor Colocalization within the Endogenous Descending Pathway Driving Pain Inhibition
The dense projections from the PAG to the RVM provide an essential neural circuit for the antinociceptive effects of opiates. Many behavioral studies have reported an effect of steroid hormones on morphine potency; however, this study is the first to report the expression of AR and ERα within the endogenous descending pathway driving pain inhibition. Using immunohistochemical analysis, we report that AR and ERα were expressed on PAG neurons projecting to the RVM in both the dorsomedial and lateral/ventrolateral subdivisions of the PAG. Male rats had a greater number AR-IR neurons that were retrogradely labeled, however, there was so sex difference in either the percentage of retrogradely labeled cells that expressed AR or the percentage of AR that was located within retrogradely labeled cells. Similarly, the percentage of ERα that was localized in retrogradely labeled cells was comparable between the sexes and was significantly greater in the caudal PAG with the majority localized in the lateral/ventrolateral subdivision.
Role in Pain and Analgesia
The present results report a dense colocalization of gonadal steroid receptors on PAG neurons projecting to the RVM, which may provide the anatomical substrate for the reported sex differences in morphine potency. Between 27-50% of PAG neurons projecting to the RVM contain mu opioid receptor (MOR); these MOR+ cells are localized primarily within the caudal lateral/ventrolateral PAG (Commons et al., 2000; Wang & Wessendorf, 2002), in the same subdivision of the PAG that we report a dense distribution of both steroid hormone receptors. Estradiol has been shown to both uncouple MORs from G protein-gated inwardly rectifying potassium channels causing a reduction in hyperpolarization by MOR agonists (Kelly et al., 2003) and induce mu opioid receptor (MOR) internalization (Eckersell et al., 1998). Furthermore, MOR internalization requires the presence of ERα (Micevych et al., 2003) suggesting that colocalization of MOR and ERα in the descending inhibitory circuit may provide a mechanism through which gonadal hormones differentially affect morphine potency in male and female rats.
Although not determined in the present study, it is possible that both AR and ERα are colocalized within the same PAG cells, as is the case in other brain areas (Wood & Newman, 1995); (Greco et al., 1998). A population of neurons expressing both AR and ERα in the PAG may provide a potential mechanism for the diverse effects of gonadal steroid hormones. For example, there are numerous reports of sex differences in pain sensitivity; however, there is no clear consensus on the direction of the sex difference (Mogil et al., 2000; Gaumond et al., 2002; Aloisi et al., 2004; LaCroix-Fralish et al., 2005). In addition, pain sensitivity varies across the rat estrous cycle (Gintzler, 1980) and the human menstrual cycle (Cogan & Spinnato, 1986; Hellstrom & Anderberg, 2003). Sex differences in circulating gonadal steroids acting via a differential expression of AR and ERα within the same PAG neuron may provide a mechanism for the diverse effects of gonadal steroids on pain sensitivity.
Other Functional Considerations
The PAG has also been implicated in the regulation of the autonomic system controlling blood pressure, heart rate, and regional blood flow, all of which have been shown have shown to be modulated by gonadal hormones (Alper & Schmitz, 1996); (Morgan & Pfaff, 2001). In parallel, the PAG initiates defensive and aggressive behaviors, such as the ‘fight or flight’ response (Bandler et al., 1985; Bandler & Carrive, 1988; Depaulis et al., 1992; Scordalakes & Rissman, 2004), and evidence suggests that gonadal hormones increase these behaviors in both male and female rats (Albert et al., 1990; Albert et al., 1991; Johansson et al., 2000). Additionally, the PAG has also been implicated in initiating sex behavior, in that stimulation of the PAG facilitates lordosis in female rats (Sakuma & Pfaff, 1979a; 1979b; McCarthy et al., 1991), while lesions of the PAG suppress this behavior (Sakuma & Pfaff, 1979b; Lonstein & Stern, 1998). Here we report that the PAG, an anatomical substrate essential for the integration of sensory input and autonomic output, contains a large population of gonadal steroid receptor-expressing neurons, which appear to be involved in modulating both autonomic and sensory responses involved in producing steroid-sensitive behaviors.
Summary
The present study demonstrates that there are sex differences in the qualitative and quantitative aspects of the gonadal steroid receptors in the PAG. These reported differences in AR and ERα immunoreactivity in the PAG have an important impact on steroid-sensitive behaviors modulated by the PAG, such as reproduction, aggression, and autonomic regulation. We additionally report that the primary neural circuit for the antinociceptive effects of opioids expresses steroid receptors and may provide a direct mechanism for sex differences in morphine analgesia.
Acknowledgments
The authors would like to acknowledge the technical assistance of Leslie Bush on data collection. This work was supported by NIH grants DA16272 and P50 AR49555 awarded to Anne Z. Murphy, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert DJ, Jonik RH, Walsh ML. Hormone-dependent aggression in the female rat: testosterone plus estradiol implants prevent the decline in aggression following ovariectomy. Physiol Behav. 1991;49:673–677. doi: 10.1016/0031-9384(91)90300-d. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Watson NV, Gorzalka BB, Walsh ML. Hormone-dependent aggression in male rats is proportional to serum testosterone concentration but sexual behavior is not. Physiol Behav. 1990;48:409–416. doi: 10.1016/0031-9384(90)90336-3. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci Lett. 2004;361:262–264. doi: 10.1016/j.neulet.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Alper RH, Schmitz TM. Estrogen increases the bradycardia elicited by central administration of the serotonin1A agonist 8-OH-DPAT in conscious rats. Brain Res. 1996;716:224–228. doi: 10.1016/0006-8993(96)00069-8. [DOI] [PubMed] [Google Scholar]
- Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav Brain Res. 1985;15:107–119. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? TINS. 1994;19:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Boers J, Gerrits PO, Meijer E, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the mesencephalon, pons and medulla oblongata of the female golden hamster. Neurosci Lett. 1999;267:17–20. doi: 10.1016/s0304-3940(99)00318-3. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Budai D, Harasawa I, Fields HL. Midbrain periaqueductal gray (PAG) inhibits nociceptive inputs to sacral dorsal horn nociceptive neurons through alpha2-adrenergic receptors. J Neurophysiol. 1998;80:2244–2254. doi: 10.1152/jn.1998.80.5.2244. [DOI] [PubMed] [Google Scholar]
- Cataldo G, Bernal S, Markowitz A, Ogawa S, Ragnauth A, Pfaff DW, Bodnar RJ. Organizational manipulation of gonadal hormones and systemic morphine analgesia in female rats: effects of adult ovariectomy and estradiol replacement. Brain Res. 2005;1059:13–19. doi: 10.1016/j.brainres.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- Cogan R, Spinnato JA. Pain and discomfort thresholds in late pregnancy. Pain. 1986;27:63–68. doi: 10.1016/0304-3959(86)90223-X. [DOI] [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW. Presynaptic and postsynaptic relations of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary-projecting periaqueductal gray neurons. J Comp Neurol. 2000;419:532–542. doi: 10.1002/(sici)1096-9861(20000417)419:4<532::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- Daniels D, Miselis RR, Flanagan-Cato LM. Central Neuronal Circuit Innervating the Lordosis-Producing Muscles Defined by Transneuronal Transport of Pseudorabies Virus. J Neurosci. 1999;19:2823–2833. doi: 10.1523/JNEUROSCI.19-07-02823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP, Bandler R. Pulmonary and upper airway afferent influences on the motor pattern of vocalization evoked by excitation of the midbrain periaqueductal gray of the cat. Brain Res. 1993;607:61–80. doi: 10.1016/0006-8993(93)91490-j. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90:307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HE, Burnham WM, MacLusky NJ. Testosterone and its metabolites affect afterdischarge thresholds and the development of amygdala kindled seizures. Brain Res. 1999;838:151–157. doi: 10.1016/s0006-8993(99)01620-0. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Gintzler AR. Endorphin-mediated increases in pain threshold during pregnancy. Science. 1980;210:193–195. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards D, Michael R, Clancy A. Androgen receptors and estrogen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinology. 1998;67:18–28. doi: 10.1159/000054294. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Cheng ZF, Fields HL. Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J Neurosci. 1987;7:271–278. doi: 10.1523/JNEUROSCI.07-01-00271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Percept Mot Skills. 2003;96:201–211. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- Islam AK, Cooper ML, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon morphine antinociception in rats. Physiol Behav. 1993;54:45–53. doi: 10.1016/0031-9384(93)90042-e. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. J Pain. 2007;8:494–502. doi: 10.1016/j.jpain.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Johansson P, Lindqvist A, Nyberg F, Fahlke C. Anabolic androgenic steroids affects alcohol intake, defensive behaviors and brain opioid peptides in the rat. Pharmacol Biochem Behav. 2000;67:271–279. doi: 10.1016/s0091-3057(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: observations and speculation. Exp Physiol. 2002;87:275–279. doi: 10.1113/eph8702355. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34:119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- Kiefel JM, Bodnar RJ. Roles of gender and gonadectomy in pilocarpine and clonidine analgesia in rats. Pharmacol Biochem Behav. 1992;41:153–158. doi: 10.1016/0091-3057(92)90075-q. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicted from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Analysis of sex and gonadectomy differences in B-endorphin antinociception elicted from the ventrolateral periaqueductal gray in rats. Eur J Pharm. 2000;392:157–161. doi: 10.1016/s0014-2999(00)00110-2. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71–80. doi: 10.1016/j.pain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 1998;804:21–35. doi: 10.1016/s0006-8993(98)00642-8. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147:456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ. Sexually Dimorphic Activation of the Periaqueductal Gray - Rostral Ventromedial Medullary Circuit during the Development of Tolerance to Morphine in the Rat. European Journal of Neuroscience. 2008;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Pfaff DW, Schwartz-Giblin S. Midbrain central gray GABAA receptor activation enhances, and blockade reduces, sexual behavior in the female rat. Exp Brain Res. 1991;86:108–116. doi: 10.1007/BF00231045. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24:375–389. doi: 10.1016/s0149-7634(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Murphy A, Hoffman G. Distribution of gonadal steroid receptor containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A unique circuit for male sex behavior. Soc Neurosci City. 24:81–82. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of androgen and estrogen receptor containing neurons in the male rat periaqueductal gray. Horm Beh. 1999;36:98–108. doi: 10.1006/hbeh.1999.1528. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kow L-M, McCarthy MM, Pfaff DW, Schwartz-Giblin S. Midbrain PAG control of female reproductive behavior: In vitro electrophysiological characterization of actions of lordosis-relevant substances. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 211–238. [Google Scholar]
- Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratka A, Simpkins JW. Effects of estradiol and progesterone on the sensitivity to pain and on morphine-induced antinociception in female rats. Horm Beh. 1991;25:217–228. doi: 10.1016/0018-506x(91)90052-j. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff D. Facilitation of female reprodcutve behavior from mesencephalic central gray in the rat. Am J Physiol. 1979a;237:R278–284. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff D. Mesencephalic mechanisms for integration of female reproductive behavior in the rat. Am J Physiol. 1979b;237:R285–290. doi: 10.1152/ajpregu.1979.237.5.R285. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Folk JE, Rice KC, Craft RM. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain. 2005;6:261–274. doi: 10.1016/j.jpain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner JM, Barrett AC, Grossell E, Picker MJ. Influence of gonadectomy on the antinociceptive effects of opioids in male and female rats. Psychopharmacology (Berl) 2002;163:183–193. doi: 10.1007/s00213-002-1143-x. [DOI] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- Turcotte JC, Blaustein JD. Immunocytochemical localization of midbrain estrogen receptor- and progestin receptor-containing cells in female guinea pigs. J Comp Neurol. 1993;328:76–87. doi: 10.1002/cne.903280106. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Schasfoort FC, Meijer E, van Leeuwen FW, Holstege G. Estrogen receptor-alpha-immunoreactive neurons in the periaqueductal gray of the adult ovariectomized female cat. Neurosci Lett. 1998;240:13–16. doi: 10.1016/s0304-3940(97)00900-2. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ., 3rd Estrogen receptor-alpha immunoreactive neurons in the ventrolateral periaqueductal gray receive monosynaptic input from the lumbosacral cord in the rhesus monkey. J Comp Neurol. 2002;443:27–42. doi: 10.1002/cne.10098. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ., 3rd Projections from estrogen receptor-alpha immunoreactive neurons in the periaqueductal gray to the lateral medulla oblongata in the rhesus monkey. Neuroscience. 2004;125:243–253. doi: 10.1016/j.neuroscience.2003.12.044. [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW. Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience. 2002;109:619–634. doi: 10.1016/s0306-4522(01)00328-1. [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain longterm peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wood R, Newman S. Androgen and estrogen receptors coexist within individual neurons in the brain of the syrian hamster. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Davis PJ, Bandler R, Carrive P. Brain stem integration of vocalization: role of the midbrain periaqueductal gray. J Neurophysiol. 1994;72:1337–1356. doi: 10.1152/jn.1994.72.3.1337. [DOI] [PubMed] [Google Scholar]


