Abstract
Objective
We wanted to compare the human neural stem cell (hNSC) labeling efficacy of different superparamagnetic iron oxide nanoparticles (SPIONs), namely, ferumoxides, monocrystalline iron oxide (MION), cross-linked iron oxide (CLIO)-NH2 and tat-CLIO.
Materials and Methods
The hNSCs (5 × 105 HB1F3 cells/ml) were incubated for 24 hr in cell culture media that contained 25 µg/ml of ferumoxides, MION or CLIO-NH2, and with or without poly-L-lysine (PLL) and tat-CLIO. The cellular iron uptake was analyzed qualitatively with using a light microscope and this was quantified via atomic absorption spectrophotometry. The visibility of the labeled cells was assessed with MR imaging.
Results
The incorporation of SPIONs into the hNSCs did not affect the cellular proliferations and viabilities. The hNSCs labeled with tat-CLIO showed the longest retention, up to 72 hr, and they contained 2.15 ± 0.3 pg iron/cell, which are 59 fold, 430 fold and six fold more incorporated iron than that of the hNSCs labeled with ferumoxides, MION or CLIO-NH2, respectively. However, when PLL was added, the incorporation of ferumoxides, MION or CLIO-NH2 into the hNSCs was comparable to that of tat-CLIO.
Conclusion
For MR imaging, hNSCs can be efficiently labeled with tat-CLIO alone or with a combination of ferumoxides, MION, CLIO-NH2 and the transfection agent PLL.
Keywords: Human neural stem cell, Iron oxide nanoparticles, Magnetic resonance (MR)
Human neural stem cells (hNSCs) can be used to replace dead tissue in patients suffering with Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease, stroke and spinal cord injury (1, 2). There is a growing interest in in vivo visualization of transplanted cells with using noninvasive techniques such as magnetic resonance (MR) imaging. The development of an appropriate MR imaging technique with optimized cell labeling conditions will be useful to monitor the effectiveness of cell implantation, homing and differentiation (3).
Superparamagnetic iron oxide nanoparticles (SPIONs) have been used for cell labeling agents in both preclinical and clinical settings. Ferumoxide (AMI-25), a standard SPION agent, is clinically approved and commercially available (4-6). It is coated with dextran and has a hydrodynamic diameter of approximately 100 nm. Monocrystalline iron oxides (MIONs) are smaller (28 nm) than the standard SPIONs so that they can easily pass through the capillary endothelium. The cross-linked iron oxide (CLIO)-NH2 is a modified form of MIONs, and it had the same biophysical properties as MIONs (7). In terms of the particle size, MIONs or CLIO-NH2might be more useful for performing cellular and molecular MR imaging. However, many cell types, including most stem cells, do not take up appreciable amounts of unmodified iron oxide preparations (8-11). One solution has been to modify the surface of the particles with monoclonal antibody or tat-peptide (12), which may result in increased internalization of the particles. Tat-peptide is purified from the human immunodeficiency virus (HIV) tat-peptide, and it carries both a transmembrane and nuclear localization signal within its sequence; it is capable of translocating exogeneous molecules into cells (13-15). The other approach is to use a transfection agent such as superfect, lipofectamine or poly-L-lysine (PLL) (16-19). Recent studies have demonstrated that ferumoxides or MIONs with using the transfection agent PLL are suitable for labeling human stem cells (6). To the best of our knowledge, no study has been performed that's compared the cell labeling efficacy of surface modified SPIONs with using transfection agents. Thus, the purpose of this study was to compare the hNSC labeling efficacy of different SPIONs, namely, ferumoxides, MION, CLIO-NH2 and tat-CLIO.
MATERIALS AND METHODS
Cell Line and Culture Conditions
HB1F3 is a commercially available hNSC line (1), and this was a gift from a donor (Ajou University, Suwon, Korea). The cells were grown in a humidified 5% CO2 atmosphere at 37℃ in Dulbecco's modified Eagle medium (Sigma, St. Louis, MO) that was supplemented with 5% fetal bovine serum, 100 µg/ml penicillin, 100 U/ml streptomycin (Sigma), 5% horse serum, 50 µg/ml recombinant human epidermal growth factor (PeproTech Inc., Rocky Hill, NJ), and 1 µl/ml recombinant human fibroblast growth factor-basic (PeproTech Inc.).
Iron Oxide Labeling
HB1F3 cells (5 × 105 cell/ml) were separately labeled for 24 hours with using the four different types of SPIONs at 25 µg/ml, i.e., ferumoxides (Feridex IV; Advanced Magnetics, Cambridge, MA), MION-47 (The Center for Molecular Imaging Research, Massachusetts General Hospital, Charlestown, MA), CLIO-NH2 and tat-CLIO (20). The CLIO-NH2 was synthesized by cross-linking MION in a strong base containing epichlorohydrin, and by reacting the obtained product with ammonia. Tat-CLIO was synthesized with using CLIO and tat-peptide, and the tat-peptide was purified from the HIV tat-peptide. The synthesized CLIO-NH2 and tat-CLIO had the same chemical properties as previously reported (7). After 24 hr, the agents were washed out with phosphate-buffered saline (PBS) and the cells were washed three times with PBS. In order to detect the iron in the cells after labeling, the cells were fixed with paraformaldehyde and stored at 4℃.
A transfection agent PLL (Sigma) at 0.25, 0.5, 1 or 2 µg/ml was mixed with ferumoxides, MION-47 or CLIO-NH2 for 60 minutes in a cell culture medium at room temperature on a rotating shaker, and these mixed media were then used to label the HB1F3 cells.
Retention of Iron Oxides in the Cells
In order to investigate retention of iron oxide in HB1F3 cells after in vitro culture, the iron labeled cells were incubated for up to 72 hr in cell culture media. After incubating for 0, 6, 24, 30, 48 or 72 hr, the cultured cells were fixed with 4% paraformaldehyde and stored at 4℃ until they were used for Prussian blue staining (PBS).
Prussian Blue Staining
The fixed HB1F3 cells were washed three times with PBS, incubated for 30 min with 5% potassium ferrocyanide in 5% hydrochloric acid, rewashed and then counterstained with nuclear fast red. Representative labeled cells were examined under a light microscope to determine the intracellular iron oxide distributions.
Cell Proliferation and Viability
The proliferative activities and the viabilities of the iron oxide labeled HB1F3 cells were evaluated by performing long-term (10 days) cell proliferation assays and trypan blue exclusion testing, respectively. The proliferative activities after the magnetic labeling were confirmed by noting the increased amount of cells during the periods of culture time compared with the control. The cell viability value, as compared to a control cell group, was estimated to be 100%.
Measurement of the Iron Contents
The iron contents of the labeled HB1F3 cells were determined by performing atomic absorption spectrophotometry (SpectrAA 800; Varian, Walnut Creek, CA). Briefly, cell suspensions (4 × 106 cells/2 ml PBS) were completely digested in a mixture (2.4 ml) of 35% hydrochloric acid (1.8 ml) and 65% nitric acid (0.6 ml) by heating them for at least three hours at 60℃. They were then diluted to a volume of 10 ml with destabilized water and this was next filtered. The iron concentrations in the samples were calculated with employing a standard curve obtained with using ferrous chloride calibration standards that contained 0, 250, 500 or 1,000 µg/L of iron in the above-mentioned mixture. The iron contents were also confirmed by performing ferrozine-based spectrophotometric assays with using triplicate samples of the acid-digested cell suspensions. The average iron contents per cell were calculated as mean values divided by the number of cells in each sample.
MR Imaging
Phantom cell suspensions (312, 625, 1,250, 2,500, 5,000 and 10,000 cells/µl) were prepared in 2% agarose gel. MR imaging of the phantoms was performed under a standard knee coil and with using a 1.5 T MR imager (Signa Horizon; GE Medical Systems, Milwaukee, WI) to obtain the T2-axial images. The sequence parameters were a repetition time = 5,000 ms, an effective echo time = 90 ms, a field of view = 120 × 120 mm, a flip angle = 90°, a matrix = 256 × 160, a slice thickness = 2.0 mm a slice separation = 0 mm and the number of excitations = 3.0.
The T2 and T2* values were measured by using the conventional spin-echo (TR/TE = 2000 ms/10, 15, 20, 25, 30, 40, 50, 60 and 70 ms) and gradient-echo sequences (TR/TE = 1000 ms/4, 11, 18, 25, 32 and 39 ms) with one echo for each sequence, while varying the TE. The T2 and T2* values were calculated by fitting the decreased signal intensities with the increasing TEs into a mono-exponential function. The T1 was determined by using the inversion-recovery fast spin-echo sequences (TR/TE/TI = 2200/18/50, 100, 200, 500, 800, 1200, 2100 ms) while varying the TI and keeping the TR and TE constant. The image intensities with the various TIs were proportional to |[1-(1-k)exp(-TI/T1)]Mo | and T1 was measured using a least squares fit of the image intensities in this equation (25).
Statistical Analysis
All the data is presented as means ± standard deviations. The data was statistically processed using the Mann-Whitney test. For all the tests, p values of < 0.05 were considered to indicate statistical significance. All the calculations were performed using commercially available statistical software (GraphPad Prism, version 4; GraphPad, San Diego, CA).
RESULTS
Labeling
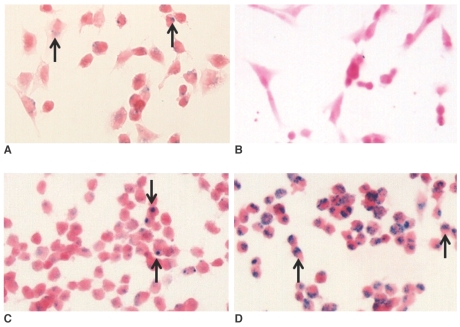
The HB1F3 cells exposed to the ferumoxides, MION-47, CLIO-NH2 or tat-CLIO showed intracellular uptake of the iron oxide (Fig. 1). However, no intracellular uptake of the iron oxide was detected in cells incubated with MION-47.
Fig. 1.
Photomicrographs of the hNSCs treated for 24 hr with ferumoxides (A), MION-47 (B), CLIO-NH2 (C) or tat-CLIO (D) at 25 µg/ml. The intracellular uptake of iron oxide nanoparticles (arrows) is seen in cells exposed to ferumoxides (A), CLIO-NH2 (C) or tat-CLIO (D). However, no intracellular uptake of iron oxide was found for the cells incubated with MION-47 (B). (Prussian blue stain, objective magnification: × 40)
Retention
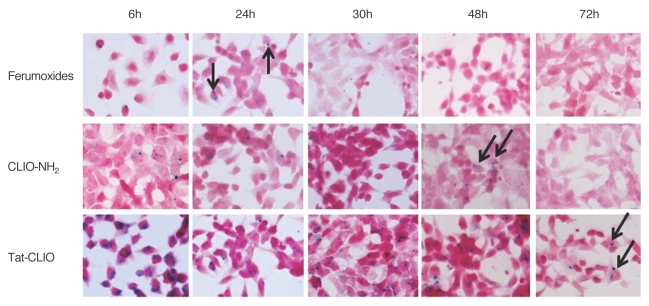
The number of iron-containing cells decreased as the incubation time increased for all the SPION-labeled cells (Fig. 2). The cells loaded with tat-CLIO showed a greater numbers of blue stained cells at all time points compared to the ferumoxides and the CLIO-NH2 labeled cells. The ferumoxide-exposed cells and the CLIO-NH2 exposed cells showed iron labeling until 24-48 hr, whereas iron-containing cells were visible after 72 hr for the tat-CLIO exposed cells. However, more than 90% of the HB1F3 cells labeled with tat-CLIO were unlabeled after 48 hr of incubation.
Fig. 2.
Retention of SPIONs in hNSCs. Iron oxide nanoparticles (arrows) are seen within the ferumoxides labeled cells and the CLIO-NH2 labeled cells for up to 24 and 48 hr, respectively and inside the tat-CLIO labeled cells for up to 72 hr. (Prussian blue stain, objective magnification: × 40)
Viability and Proliferation
The average viability of the control cells, as determined by trypan blue exclusion assay, was 100 ± 4.5%. The viable percentage of the ferumoxide-labeled, MION-47 labeled, CLIO-NH2 labeled or tat-CLIO-labeled HB1F3 cells versus the unlabeled control cells was 105 ± 3.9, 101 ± 5.1, 101 ± 2.5 and 95 ± 6.5, respectively. The cells exposed to these SPIONs showed no differences of viability versus the unlabeled cells, and there was no effect on the proliferative capability of the cells labeled with all four SPIONs during 10 days of culture.
Increased Intracellular Iron Uptake Induced by Poly-L-Lysine
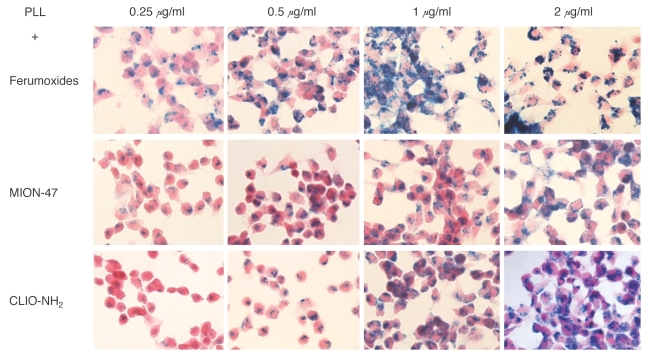
Prussian blue staining of the SPION-exposed cells with PLL revealed a dose-dependent increase of the iron oxide uptake into the cells (Fig. 3). Almost 100% of the cells were labeled with iron oxide when the HB1F3 cells were incubated in SPION media that contained 2 µg/ml PLL. So, the concentration 2 µg/ml of PLL was selected to transfect iron oxide into the cells. The viabilities and proliferations of the SPION exposed cells with using PLL were similar to those of the unlabeled control cells.
Fig. 3.
Photomicrographs of hNSCs treated for 24 hr with three different SPIONs (ferumoxides, MION-47 or CLIO-NH2) at 25 µg/ml in the presence of different doses of PLL. As the PLL concentration increased in the media from 0.25 µg/ml to 2 µg/ml, more iron oxide nanoparticles are seen inside the labeled cells. (Prussian blue stain, objective magnification: × 40)
Iron Contents with and without Poly-L-Lysine
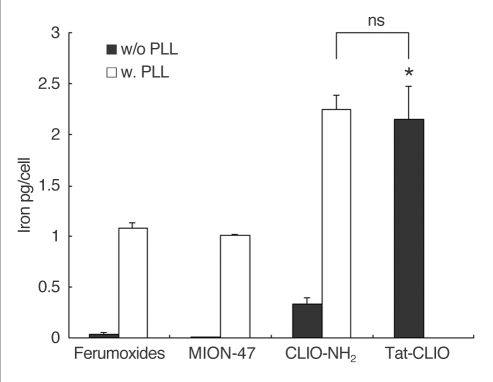
Atomic absorption spectrophotometry revealed the highest iron incorporation in the tat-CLIO-exposed cells (2 ± 0.3 pg/cell) and the lowest in the MION-47-exposed cells (0.005 pg/cell) (Fig. 4). The ferumoxides and CLIO-NH2 showed 0.04 ± 0.01 and 0.34 ± 0.07 pg of iron/cell, respectively. The tat-CLIO-labeled cells showed 59-fold, 430-fold and 6-fold higher uptakes than did the ferumoxide-labeled, MION-47 labeled and CLIO-NH2 labeled cells, respectively.
Fig. 4.
Quantitative iron determination by an atomic absorption spectrophotometer. The graph shows the highest iron incorporation in the tat-CLIO labeled cells (2.15 ± 0.3 pg/cell) and the lowest in the MION-47 labeled cells (0.005 pg/cell) in absence of PLL (black bars). With PLL (white bars), the iron content in the ferumoxides labeled, MION-47 labeled or CLIO-NH2 labeled cells increased to 1.08 ± 0.07 pg/cell, 1.01 ± 0.02 pg/cell and 2.24 ± 0.17 pg/cell, respectively, which are 27 fold, 202 fold and 7-fold greater uptakes, respectively, compared with the cells without using PLL. The cells labeled with CLIO-NH2 in the presence of PLL show a similar intracellular iron content as the tat-CLIO-labeled cells (p > 0.1).
Note.-w/o = without, w = with, ns = statistically non-significant
With using PLL, the iron content in the ferumoxide-labeled, MION-47-labeled and CLIO-NH2-labeled cells increased to 1.1 ± 0.07 pg/cell, 1.0 ± 0.02 pg/cell and 2.2 ± 0.17 pg/cell, which are 27 fold, 202 fold and 7-fold uptakes, respectively, compared to the comparably treated cells without PLL. The cells treated with CLIO-NH2 and PLL showed a similar intracellular iron content as the tat-CLIO-labeled cells (p > 0.1) (Fig. 4).
MR Imaging
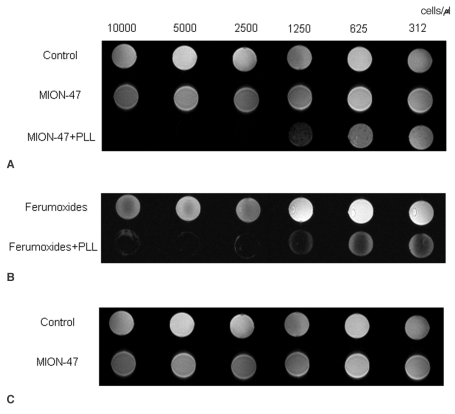
Phantoms with more than 1,250 cells/µl of tat-CLIO-labeled cells were visualized on the T2-weighted MR images (Fig. 5). For the ferumoxides and CLIO-NH2, the phantoms with more than 2,500 cells/µl and 10,000 cells/µl had a visibly decreased signal when not using PLL. With using PLL, a decreased signal was found in phantoms with 625-1,250 cells/µl with the ferumoxide labeled, MION-47 labeled and CLIO-NH2 labeled cells.
Fig. 5.
Gradient-echo T2-weighted MR images of the phantoms with different cell numbers (i.e., 312, 625, 1,250, 2,500, 5,000 and 10,000 cells/µl). MR scans were performed at different time points with (A) the control, the MION-47 treated cells and the MION-47+PLL treated cells, (B) the ferumoxides treated cells and the ferumoxides with PLL treated cells, and (C) the CLIO-NH2 treated cells, the CLIO-NH2 with PLL treated cells and the tat-CLIO treated cells. In the absence of PLL, the phantoms with more than 1,250 cells/µl of the tat-CLIO-labeled cells show a decreased signal on the T2-weighted MR images. For the ferumoxides treated cells and the CLIO-NH2 treated cells, the phantoms with more than 2,500 cells/µl show a visibly decreased signal without PLL. With PLL, a decreased signal is found in the phantoms with the ferumoxides treated cells, in the phantoms with the MION-47 treated cells and in the phantoms with the CLIO-NH2 treated cells, with 625-1,250 cells/µl respectively. 'Control' represents the MR image of the phantom with the non-labeled control cells.
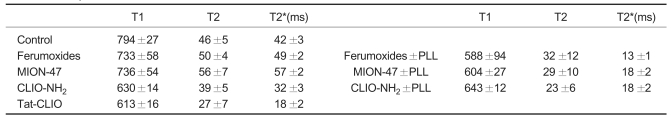
Table 1 shows the T1, T2, and T2* values that were measured in the phantoms with 1,250 cells/µl of the different SPIONs. A stronger susceptibility effect was found in the cells labeled with tat-CLIO (a T2* value of 18 ± 2 msec) than that of the cells labeled with ferumoxides (49 ± 2 msec), MION-47 (57 ± 2 msec) or CLIO-NH2 (32 ± 3 msec). However, in the presence of PLL, the strongest susceptibility was obtained for the ferumoxide labeled cells (13 ± 1 msec). In all cases, mono-exponential signal intensity decreases were found that allowed very unambiguous determination of the T2 and T2* values.
Table 1.
The T1-, T2- and T2*- Values Measured in the Phantoms with 1,250 cells/µl of Different Superparamagnetic Iron Oxide Nanoparticles
Note.-Data are the means with standard deviations. ms = msec, MION = monocrystalline iron oxide, CLIO= cross-linked iron oxide, PLL = poly-L-lysine
DISCUSSION
Of the four different SPIONs, i.e., ferumoxides, MION-47, CLIO-NH2 and tat-CLIO, we found that tat-CLIO, a SPION with its surface modified with transmembrane translocation signals, was the most efficient agent for introducing iron oxide into hNSCs when these agents were added to cell culture media alone. However, ferumoxides, which are a clinically approved contrast media, were as efficient as tat-CLIO for introducing iron oxide into the hNSCs when PLL was used as a supportive transfection agent during the cell incubation. Labeling cells by means of simple incubation with ferumoxides is more practical than using the complicated surface modification method.
Our results are consistent with other previous studies; the previous reports showed that transfection agents improve the labeling efficiency of ferumoxides or MION to stem cells and to other mammalian cells (6, 16-22). In a study by Jendelova et al. (5), ferumoxides were incorporated into mouse embryonic stem cells and rat mesenchymal stem cells, without the aid of any transfection agent, when the cells were incubated at 112.4 mg/ml for three passages or for five days, respectively. Yet in our study, only 17% tagging of the hNSCs was obtained at a lower concentration (25 µg/ml) of ferumoxides and after a shorter incubation time (24 hrs). Although some studies have used ferumoxides or MION alone to label cells, they were not efficiently taken up by cells, except for the phagocytic cells, or the cells were put in higher concentrations of iron. We found that the MION-47 treated hNSCs were not labeled either, and that the number of CLIO-NH2 labeled cells was less than the tat-CLIO labeled cells, like a previous report (13). For the carboxydextran-coated SPIONs, a larger particle size resulted in improved cellular uptake (65 nm, 4.4 µg ± 0.08 Fe per 100,000 cells; 17 nm, 2.1 µg ± 0.06 Fe per 100,000 cells; p < 0.05) (18).
The transfection agent PLL has a relatively small toxic/efficacious ratio, with PLL concentrations as low as 10 mg/ml in media causing significant cell death (16). Ferumoxides complexed to PLL effectively label cells, but residual Fe-PLL complexes may remain on the surface of the cells or they cause cells to clump together in the final cell preparation prior to infusion. A recent report indicated that labeling mesenchymal stem cells with ferumoxides-PLL complexes inhibited the chondrogenic differentiation capacity of mesenchymal stem cells (23). In contrast, labeling cells with ferumoxides-protamine sulfate complexes did not alter the viability and functional capacity of a variety of cell types (24). In our study, SPIONS, including tat-CLIO, showed no adverse effect on the cell proliferations and viabilities. However, we did not check the effect on cell differentiation and so further studies on these issues are necessary.
In conclusion, our study indicates that hNSCs can be safely and efficiently labeled for MR imaging with using either tat-CLIO alone or a combination of ferumoxides, MION-47 and CLIO-NH2 and the transfection agent PLL. These labeling methods could be used to noninvasively track hNSCs.
Footnotes
This research was supported by the Korean Health 21 R & D Project, Ministry of Health & Welfare (Project No. A040004), by the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (Grant no. 0420080-1), and by the National R & D Program of the Korean Ministry of Science and Technology (Project No. SC-3111).
References
- 1.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 3.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 5.Jendelová P, Herynek V, Urdziková L, Glogarová K, Kroupová J, Andersson B, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 6.Daldrup-Link HE, Rudelius MR, Piontek G, Metz S, Brauer R, Debus G, et al. Migration of iron oxide-labeled human hematopoietic progenitor cells in a mouse model: in vivo monitoring with 1.5-T MR imaging equipment. Radiology. 2005;234:197–205. doi: 10.1148/radiol.2341031236. [DOI] [PubMed] [Google Scholar]
- 7.Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked Iron Oxides (CLIO): a new platform for the development of targeted MR contrast agents. Acad Radiol. 2002;9:S304–S306. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 8.Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR., Jr Efficient transfer of genetic material into mammalian cells using starburst polyamidoamine dendrimers. Proc Natl Acad Sci USA. 1996;93:4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang MX, Redemann CT, Szoka FC., Jr In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 10.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Human Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 11.DeLong R, Stephenson K, Loftus T, Fisher M, Alahari S, Nolting A, et al. Characterization of complexes of oligonucleotides with polyamidoamine starburst dendrimers and effects on intracellular delivery. J Pharm Sci. 1997;86:762–764. doi: 10.1021/js960409f. [DOI] [PubMed] [Google Scholar]
- 12.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugate. Bioconjug Chem. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 13.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 14.Zhao M, Kircher MF, Josephson L, Weissleder R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjug Chem. 2002;13:840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]
- 15.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 16.Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, et al. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3:24–32. doi: 10.1162/15353500200403190. [DOI] [PubMed] [Google Scholar]
- 17.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838–846. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 18.Matuszewski L, Persigehl T, Wall A, Schwindt W, Tombach B, Fobker M, et al. Cell tagging with clinically approved iron oxides: feasibility and effect of lipofection, particle size, and surface coating on labeling efficiency. Radiology. 2005;235:155–161. doi: 10.1148/radiol.2351040094. [DOI] [PubMed] [Google Scholar]
- 19.Montet-Abou K, Montet X, Weissleder R, Josephson L. Transfection agent induced nanoparticle cell loading. Mol Imaging. 2005;4:165–171. doi: 10.1162/15353500200505100. [DOI] [PubMed] [Google Scholar]
- 20.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–268. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R, Cheng HC, Bogdanova A, Bogdanova A., Jr Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging. 1997;7:258–263. doi: 10.1002/jmri.1880070140. [DOI] [PubMed] [Google Scholar]
- 22.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 23.Bulte JW, Kraitchman DL, Mackay AM, Pittenger MF. Chondrogenic differentiation of mesenchymal stem cells is inhibited after magnetic labeling of with ferumoxides. Blood. 2004;104:3410–3412. doi: 10.1182/blood-2004-06-2117. [DOI] [PubMed] [Google Scholar]
- 24.Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 25.Zhu DC, Penn RD. Full-brain T1 mapping through inversion recovery fast spin echo imaging with time-efficient slice ordering. Magn Reson Med. 2005;54:725–731. doi: 10.1002/mrm.20602. [DOI] [PubMed] [Google Scholar]