Abstract
Background
The interpretation of “indeterminate” results of the recombinant immunoblot assay (RIBA) is a particularly sensitive issue for Transfusion Services, and donors with such a serological condition require long-term follow-up.
Materials and methods
In the Immunohaematology and Transfusion Medicine Division of Umberto I University Hospital (Rome, Italy), 102,979 donor blood units were screened for hepatitis C virus (HCV) antibodies by enzyme-linked immunosorbent assay (ELISA) over a 5-year period (01.01.2000 – 31.12.2004). Since 24.10.2001, HCV -RNA testing was added. All samples repeatedly reactive by ELISA were then submitted to a HCV confirmatory assay (RIBA).
Results
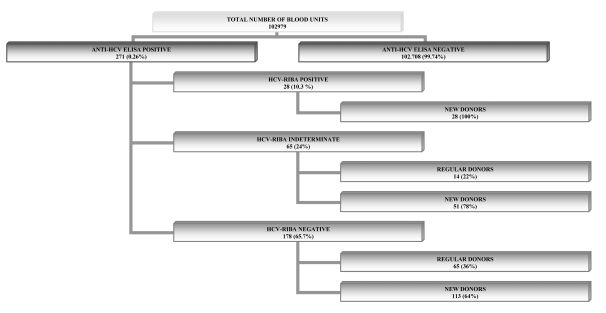
Among the 102,979 donors we found 271 positive to HCV ELISA testing. The results of the RIBA assay for these donors were negative in 178 (65.7%) cases, positive in 28 (10.3%) and indeterminate in 65 (24.0 %).
Of the 65 subjects with an indeterminate pattern, 24 completed a sufficient follow-up (median 25 months; range, 6 – 52), during which some (n=8; 33%) converted to a negative status, some (n=16; 67%) maintained their reactivity pattern, but none became seropositive for HCV.
Conclusions
The HCV-RIBA indeterminate status may indicate either a non-specific reaction (false positive) or a real pre-existing or initial infection and does not, therefore, enable a prediction of outcome. The use of HCV genomic assays (nucleic acid amplification testing), which are more specific than antibody-based assays (ELISA, RIBA), therefore improves HCV blood donor testing by allowing an accurate interpretation of such primary assays.
Keywords: hepatitis C virus (HCV), HCV-RIBA indeterminate, blood donors
Introduction
The problem of the infectious safety of blood transfusions has not been completely solved yet and the risk of contracting an infection, viral or not, after a transfusion still exists1. National epidemiological data do, however, show that the incidence of acute hepatitis C virus (HCV) infections associated with blood transfusions has decreased in recent years. The transfusion of infected blood is now responsible for only a small fraction of cases of HCV infection and the majority of new infections can be attributed to risk factors such us promiscuous sex, beauty treatments, use of drugs and invasive medical procedures2. Blood transfusions have reached high safety standards since the implementation of screening with enzyme-linked immunosorbent assays (ELISA), with their successive improvements3,4. Such screening tests were proven to be both highly reliable and cost-effective, which led to their almost universal utilization as a first-level screening procedure. However, both false positive (HCV-positive according to ELISA, but negative with a second-level recombinant immunoblot assay (RIBA)) and indeterminate results5 (neither negative nor positive results to anti-HCV antibody dosage (HCV-positive with ELISA, indeterminate results with RIBA) may occur. Indeed, although the RIBA is highly specific, its positive predictive value in people at low risk of infection (such as blood donors) is unsatisfactory. In particular, this assay may respond positively to sera that were negative in first level tests 6, and react positively with a non-specific band7,8.
Aim of the study
The aim of this study was to define any changes over time in RIBA-indeterminate cases, determining whether these could evolve to clearly positive, completely negative or otherwise.
Materials and methods
From 01.01.2000 to 31.12.2004, 102,979 donated blood units were assessed for HCV antibodies at the Division of Immunohaematology and Transfusion Medicine, Umberto I University Hospital (Rome, Italy). First-level screening was performed with either Abbott Axsym System HCV version 3.0 assay (Abbott Laboratories, Abbott Park, IL, USA) using the HCr43, c200, c100–3 and NS5 recombinant antigens or Ortho Vitros ECi anti-HCV assay (Ortho-Clinical Diagnostics, Raritan, NJ, USA) using the c22–3, c200 and NS5 recombinant antigens. In samples that repeatedly tested positive with a ≥ 0.70 ratio, the Chiron RIBA HCV SIA version 3.0 (Chiron Corporation, Emeryville, CA, USA) supplementary test was performed. This test is based on the recombinant HCV antigens C33c and NS-5 and the synthetic peptides c100p, 5-1-1p and c22p blotted as single bands on a support membrane, as indicated by the manufacturer. Briefly, serum anti-HCV reactivity against specific viral proteins is assessed through a colorimetric system based on an enzyme-conjugates anti-human IgG antibody and a colorimetric substrate. A human superoxide dismutase (h-SOD) control band enables the identification of anti-h-SOD antibodies that would cause false positive results. The test was interpreted as follows: negative if there was no band or only the h-SOD band; indeterminate if there was a single HCV band or more than one HCV band plus the h-SOD band; and positive if there were two or more HCV bands with no h-SOD band.
From 24.10.2001, qualitative HCV-RNA testing was performed on all donated aliquots using the Roche Ampliscreen Nucleic Acid Test (NAT) version 2.0 (Roche Molecular System, Branchburg, NJ, USA).
Results
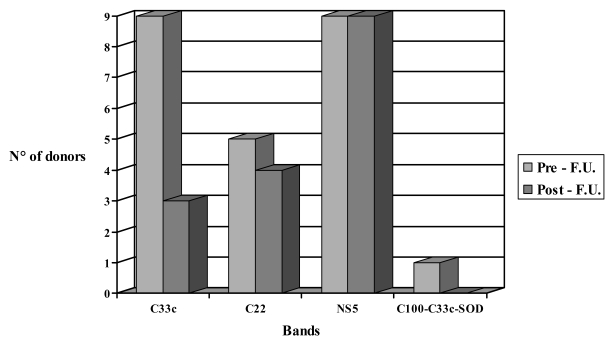
Of the 102,979 donations screened by ELISA, 271 were found to be positive and underwent RIBA testing, which gave the following results: 178 donations (65.7%) resulted negative [65 from regular donors(36%) and 113 from new donors (64%)]; 28 resulted positive (100% from new donors), and 65 indeterminate (24%) [14 from regular donors (22%) and 51 from new donors (78%)] (Figure 1). Out of the 65 indeterminate donations, 24 (37%) came from donors who completed a sufficient follow-up (minimum 6 months; maximum 52 months), with a median duration of 25 months, during which, periodically, ELISA (both methods in use), RIBA and, from when it became available, HCV-RNA testing were performed. In detail, of the 24 donors with a sufficient follow-up, 17 were new donors (71%) and 7 were regular donors (29%). In the study period considered one control was done in 22% of donors, two controls in 58%, three controls in 12% and four controls in 8%. At the time of suspension, 3/17 new donors had reactivity to both screening assays, whereas 3/7 regular donors were reactive with both screening tests. The 21 subjects (87%) who were administered the HCV-RNA test resulted repeatedly negative. With regards to positivity to specific HCV antibodies at the time of diagnosis, 23 subjects (96%) continued to show reactivity to a single band [9 for C33c (39%), 9 for NS-5 (39%), 5 for C22 (22%)]; one subject (4%) showed reactivity to C100, C33c and h-SOD (Figure 2). During the study period, no changes were observed in the RIBA pattern among the 24 donors with an indeterminate status and adequate follow-up. At the end of the follow-up period, out of the nine subjects who had been reactive to C33c, six (67%) converted to having a negative test while three (33%) remained reactive (indeterminate status); all nine subjects (100%) reactive to NS5 remained reactive; four (80%) out of five donors reactive to C22 remained reactive, and in one (20%) the test became negative. The donor who was reactive to C100, C33c and h-SOD converted to a negative status (Figure 2). Out the eight subjects whose RIBA test became negative, two (25%) also became negative with ELISA.
Figure 1.
Results obtained with anti-HCV ELISA and HCV-RIBA on the samples tested
Figura 2.
Evolution of HCV-RIBA inderminate results during follow-up (F.U.)
Discussion
The first observation from these data is that all RIBA-positive subjects were new donors, which emphasizes the efficacy of pre-donation selection, information and follow-up to which regular donors are submitted.
The second point is that the majority (65.7%) of ELISA-positive subjects were repeatedly negative by RIBA throughout the follow-up period, showing that the former results were false positives depending on factors unrelated to HCV infection 9,3. The high percentage of aspecific reactivity is the result of a precise choice of extreme caution in defining the parameters of the grey zone. In fact, the decision to maintain a grey zone, however large (30% of the cutoff), in order to avoid even the weakest reactivity, despite the introduction of NAT assays for the screening of donations, can only contribute to the high number of false positives. None of the subjects reactive to a single antigen became seropositive, thus confirming the fact that regular donors represent a low-risk population, having been selected through self-exclusion, medical follow-up and other means.
The third point is that several of the subjects with indeterminate results had probably contracted HCV infection prior to screening (C22 band or C33c band)10, especially those new donors unknown to our transfusion service. For those regular donors whose reactivity did not correspond to a change in test results and who were known to be negative on initial testing, the indeterminate status in the absence of viraemia probably represents an aborted infection11, while for regular donors with different reactivity to the screening test, it is easier to think of non-specific reactivity 7,8. Isolated C100 reactivity is thought to be less significant than C33c and C22 as marker of a former infection and is often considered non-specific12. Isolated NS-5 reactivity may have different implications. This marker is known to be diagnostically relevant for HCV infection screening in high-risk subjects, because it may accompany viraemia13. In blood donors, such reactivity may have a different significance. In our series, eight out of nine subjects (89%) with isolated reactivity to NS-5 repeatedly tested negative with the HCV-RNA and did not seroconvert throughout the follow-up period. Our data do, therefore, support other authors’ conclusions6,12 that NS-5 reactivity in blood donors is mostly non-specific. Considering that subjects reactive only for NS-5 represent almost half the donors with an indeterminate status (27 out of 65 in the whole cohort, 9 out of 24 of those who completed follow-up), since the introduction of viral RNA amplification testing, the finding of this band in first-level donor selection tests is counterproductive rather than beneficial, causing numerous transfusable blood units to be discarded and expensive testing to be conducted in those donors with an indeterminate HCV status.
Conclusions
Serological screening for HCV in blood donors has been designed in order to improve transfusion safety. It is, therefore, preferable to use extremely sensitive methods that can detect even the slightest reactivity. The RIBA can help to discriminate whether a positive result can be attributed to a previous infection or a falsely reactive screening test in subjects with a low or absent viral load (HCV RNA negative). However, the interpretation of RIBA indeterminate results leaves doubts, as demonstrated by our survey in which the follow-up showed that 16 of 24 donors maintained an unchanged pattern over time.
Nowadays, however, given the improvement in diagnostic tests and the implementation of nucleic acid amplification technology, with a direct evaluation of infectivity, indeterminate results can be interpreted better, allowing prompt definition of the real status of HCV infection in those numerous potential donors with an indeterminate status. This is, of course, of considerable importance for counselling.
References
- 1.Tripodi G, Imberciadori G. Transfusion-transmissible infections: diagnosis, prevention and residual risk. Blood Transfusion. 2005;3:19–31. [Google Scholar]
- 2.Mele A, Tosti ME, Spada E, et al. Epidemiology of acute viral hepatitis: twenty years of surveillance through SEIEVA in Italy and review of the literature. RAPPORTI ISTISAN 06/12. Roma. 2006 [Google Scholar]
- 3.Colin C, Lanoir D, Touzet S, et al. Sensitivity and specificity of third generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Hamid M, El-Daly M, El-Kafrawy S, et al. Comparison of second and third generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002;40:1656–9. doi: 10.1128/JCM.40.5.1656-1659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Poel CL, Cuypers HTM, Reesink HW, et al. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991;337:317–9. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]
- 6.Kitchen AD, Tucker NV. The specificity of anti-HCV supplementary assays. Vox Sang. 1995;69:100–3. doi: 10.1111/j.1423-0410.1995.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 7.Kiely P, Wood E. Can we improve the management of blood donors with nonspecific reactivity in viral screening and confirmatory assays? Transfus Med Rev. 2005;19:58–65. doi: 10.1016/j.tmrv.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Prati D, Capelli C, Contino G, et al. Score on a four-antigen recombinant immunoblot assay (RIBA-2) and hepatitis C virus RNA detection in RIBA-2-indeterminate blood donors. Transfusion. 1994;34:454–5. doi: 10.1046/j.1537-2995.1994.34594249064.x. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen L, Buffington J, Shapiro CN, et al. Multiple false reactions in viral antibody assays after influenza vaccination. Am J Epidemiol. 1995;141:1089–96. doi: 10.1093/oxfordjournals.aje.a117374. [DOI] [PubMed] [Google Scholar]
- 10.Kondili LA, Chionne P, Costantino A, et al. Infection rate and spontaneous seroreversion of anti-hepatitis C virus during the natural course of hepatitis C virus infection in the general population. Gut. 2002;50:693–6. doi: 10.1136/gut.50.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeff LB, Hollinger FB, Alter HG, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–63. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 12.Lemaire JM, Courouce AM, Defer C, et al. HCV RNA in blood donors with isolated reactivities by third-generation RIBA. Transfusion. 2000;40:867–70. doi: 10.1046/j.1537-2995.2000.40070867.x. [DOI] [PubMed] [Google Scholar]
- 13.Cavazza S, Lagging M. Indeterminate third-generation hepatitis C recombinant immunoblot assay and HCV RNA analysis: isolated reactivity against NS5 associated with HCV viraemia in clinical patients but not blood donors. Scand J Infect Dis. 2005;37:488–92. doi: 10.1080/00365540510037821. [DOI] [PubMed] [Google Scholar]