Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare disease (5–10 cases per million persons per year) characterised by the massive formation of platelet rich-thrombi in the microcirculation of multiple organs1,2. It affects both sexes, although the incidence is two to three times higher among females3. TTP was first described by Moschowitz in 1924 in a 16-year old girl who presented with fever, anaemia, thrombocytopenia and focal changes in the central nervous system and kidneys4. Several reports of TTP followed this initial description leading to a definition based upon the following pentad of symptoms: fever, mechanical haemolytic anaemia, thrombocytopenia, central nervous system abnormalities and renal impairment5. However, fever, neurological abnormalities and renal impairment are not constant symptoms, especially during the early stage of the disease, thus leading to the currently accepted definition of TTP consisting of the association of mechanical haemolytic anaemia with fragmented erythrocytes and thrombocytopenia (platelet count <100 × 109/L) without alternative causes6.
In parallel to the evolution of clinical criteria, the pathophysiological mechanisms of TTP were elucidated when Joel Moake and his colleagues observed that the plasma of a patient with recurrent TTP contained very high molecular weight [so-called ultralarge (UL)] multimers of von Willebrand factor (VWF), a multimeric adhesion glycoprotein contained in endothelial cells, platelets and plasma7. Once released from the abnormally stimulated endothelial cells, these ultralarge forms of VWF, present in the endothelium but not in plasma in physiological conditions, promote intravascular aggregation of platelets and the consequent microvascular thrombosis and haemolytic anaemia caused by mechanical damage, particularly in blood flow conditions characterised by high fluid shear stress such as in the microcirculation3.
Moake himself hypothesised the deficiency of a cleaving protease as being responsible for the presence of UL VWF7, but it was Furlan et al. and Tsai and Lian in 1996 who managed to isolate from human plasma a metal-dependent protease able to cleave the peptide bond between the tyrosine at position 1605 and the methionine at position 1606 in the central A2 domain of VWF. The same investigators subsequently and independently found that the VWF-cleaving protease was deficient in a retrospective cohort of patients clinically diagnosed as having TTP8,9. The protease, which is responsible for regulating the multimeric structure of VWF, was identified in 2001 by Zheng et al.10 as a new (the thirteenth) member of the ADAMTS (A Disintegrin And Metalloprotease with ThromboSpondin 1 repeats) family of metalloproteases and was called ADAMTS132. While the discovery of ADAMTS13 has renewed interest in TTP, as documented by the exponential increase in the number of publications on this topic in the last few years, it has raised the issue of the diagnostic and prognostic value of ADAMTS13 testing in this condition.
Classification of TTP
There are two different forms of TTP: congenital and acquired2. Congenital TTP, caused by mutations in the ADAMTS13 gene (which is located on chromosome 9q34 and codes for the metalloprotease), is an extremely rare (incidence 1:1,000,000) autosomal recessive condition which manifests often, but not exclusively, at birth or during childhood11–14. The acquired forms can basically be distinguished into two types: immune-mediated forms, due to autoantibodies against ADAMTS1315–17, and those probably secondary to massive endothelial stimulation with consequent release of UL VWF multimers in amounts exceeding the system’s ability to degrade them, despite the presence of normal or only mildly reduced levels of ADAMTS1318. The most common physiological or pathological conditions present in the immune-mediated forms, which are often associated with severe ADAMTS13 deficiency (levels less than 10% of the normal), are pregnancy, infections, autoimmune diseases and the use of drugs such as ticlopidine and clopidogrel. The most frequent concomitant conditions associated with TTP forms presenting with normal or mildly reduced levels of ADAMTS13 (greater than 10%) are metastatic tumours, organ transplantation (particularly allogeneic bone marrow transplantation and solid organ transplants) and the use of drugs such as cyclosporine, mitomycin and a-interferon19. In most cases TTP occurs as a single, sporadic acute episode, but there are chronic recurrent forms (20% – 30% of the cases), which have a genetic basis or are associated with the formation and persistence of autoantibodies.
ADAMTS13 assays
The possibility of using plasma ADAMTS13 values to manage TTP patients stems from the current availability of ADAMTS13 assays to measure ADAMTS13 activity, ADAMTS13 antigen and neutralising or non-neutralising anti-ADAMTS13 autoantibodies.
Several assays of ADAMTS-13 activity have been developed20–22. They are based on the cleavage of plasma-derived or recombinant VWF multimers by test plasma and the direct or indirect detection of cleaved VWF by ADAMTS13. Direct assays focus on the detection of VWF cleavage products by using agarose or polyacrylamide gel electrophoresis (PAGE), western blotting, and fluorescence resonance energy transfer (FRET) techniques22. The last assay, which uses a truncated synthetic 73-amino-acid VWF peptide as a substrate for the determination of ADAMTS13 activity (FRETS-VWF73 assay)23, is a rapid technique24. Indirect assays depend on measuring the residual substrate (i.e., VWF) or its disappearance. They include the collagen-binding assay, ristocetin-induced aggregation and enzyme-linked immunosorbent assays25. The general principle of ADAMTS13 activity assays is illustrated in figure 1. A multicentre study comparing several of these assays on 30 plasma samples with varying levels of ADAMTS13 activity showed a generally good agreement for the identification of severe ADAMTS13 deficiency, although one false negative and some false positive results were reported by laboratories using the collagen binding assay26. The interlaboratory agreement on samples with mildly reduced or normal activity values was less good.
Figure 1.
General principle of ADAMTS13 activity assays
A number of variables may interfere with the results of these assays. Firstly, all the aforementioned assays measure ADAMTS13 activity upon cleaving VWF in static conditions and thus do not reflect the in vivo physiological blood flow conditions. A flow-based test system, capable of observing in vitro under flow conditions the capacity of plasma ADAMTS13 to cleave UL VWF multimers secreted from stimulated endothelial cells, has been recently proposed but is not quantitative nor clinically validated27. Another important variable is that denaturing agents (e.g., guanidine or urea) are required to make VWF susceptible to cleavage by ADAMTS1325. The use of shorter peptides, such as the FRET-VWF73, instead of full-length VWF in enzyme immunoassay-based methods helps to deal with intra- and inter-laboratory variability only to a limited degree21. Finally, while the stability of ADAMTS13 from normal patients at −70°C has been documented, protease activity in various pathological conditions is not stable in vitro and may be reduced during storage or incubation19.
ELISA to monitor plasma antigen levels of ADAMTS13 have recently become available25,28. Feys et al.28 compared ADAMTS13 antigen and activity in a large set of plasma samples collected from subjects in various physiological states (neonatal period, pregnancy, oral contraceptive intake) or with pathological conditions (liver cirrhosis, inflammatory bowel disease, cardiac surgery) and found that the antigen assay showed less variability than the collagen binding-based activity assay. Thus, the authors concluded that the parallel measurement of both ADAMTS13 activity and antigen levels should be preferred to the measurement of only one parameter.
With regards to the detection of anti-ADAMTS13 autoantibodies, most of them are inhibitory and they can, therefore, be titrated in vitro using classical mixing studies with mixtures of patient’s heat- inactivated plasma and normal plasma8,9. Less frequently, autoantibodies promote the clearance of ADAMTS13 from blood without inhibiting its activity. These non-neutralising antibodies can be detected with more sophisticated methods using recombinant ADAMTS1316.
ADAMTS13 activity testing
Diagnostic value of ADAMTS13 testing in acute TTP
A number of studies have assessed the diagnostic value of ADAMTS13 testing in acute TTP. In the context of two pioneering studies, Furlan et al.8 measured VWF cleaving protease levels in 30 patients with clinically diagnosed acute TTP and found a severe deficiency in 86% (26/30) of them. All TTP patients (37/37) had a severe VWF-cleaving protease deficiency in the other study published by Tsai and Lian9. In a prospective cohort study of 66 acute TTP cases conducted by Veyradier et al.29, 89% (59/66) of the patients had decreased VWF-cleaving protease activity, which was severely reduced in 71% (47/66) of them. Similar results were found by Mori et al. in 18 TTP patients30. By contrast, in the Oklahoma TTP-haemolytic uraemic syndrome (HUS) registry31 only 33% (16/48) of patients with TTP were severely deficient in ADAMTS13 activity. Thirty-one of 46 TTP patients (67%) had a severe VWF cleaving protease deficiency in a study by Coppo et al.32 Finally, Peyvandi et al.33 and Zheng et al.34 found severely reduced protease activity in 48% (48/100) and 80% (16/20) of patients with acute TTP, respectively.
Figure 2 summarises the rates of ADAMTS13 deficiencies in reported case series of TTP. A severe protease deficiency is present in the majority but not in all patients diagnosed with acute TTP (range, 33% – 100%), thus challenging the previous observations35 that severely deficient activity of VWF-cleaving protease is a specific diagnostic marker to discriminate TTP from other microangiopathies. On the other hand, some authors have found severe ADAMTS13 deficiency in microangiopathies other than TTP36–38. It is possible that these discrepancies reflect differences in case definitions for TTP or assay methodologies39.
Figure 2.
Rates (%) of ADAMTS13 (partial or severe) in patients with clinically-diagnosed acute TTP
Short-term prognostic value of ADAMTS13 testing in acute TTP
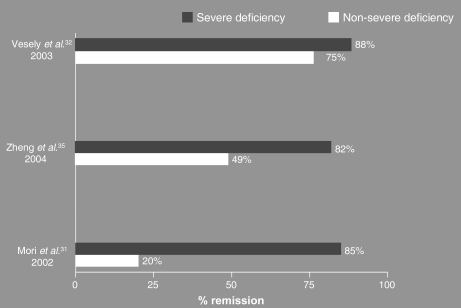
Since the development of ADAMTS13 assays8,9, several investigators have evaluated whether or not protease activity testing is useful to predict short-term outcomes (remission and mortality rate). With regards to the prognostic value of ADAMTS13 activity testing in predicting remission in acute TTP, data from the Oklahoma TTP-HUS Registry showed a slightly higher remission rate in TTP patients with severe ADAMTS13 deficiency than in those without a severe deficiency (88% versus 75%)31. Zheng et al.34 obtained substantially similar results (82% remission rate in TTP patients with severe ADAMTS13 deficiency versus 49% in patients with non-severe deficiency). A more marked difference in the remission rate between patients with severe or non-severe VWF-cleaving protease deficiency (85% versus 20%) was found by Mori et al.30 (Figure 3a).
Figure 3a.
Remission rates (%) in patients with acute TTP with severe vs non-severe ADAMTS13 deficiency
The impact of ADAMTS13 activity levels on mortality rate in patients with acute TTP has also been studied. In the Oklahoma TTP-HUS Registry31, the mortality rate was lower in patients with severe deficiency of ADAMTS13 activity than in those with non-severe deficiency (16% versus 45%). Similar results were reported by Zheng et al.34, Mori et al.30, and Raife et al.40 (Figure 3b). By contrast, Coppo et al.32 found that patients with severe ADAMTS13 deficiency had a higher mortality rate (13% in TTP patients with severe ADAMTS13 deficiency versus 0% in those with non–severe deficiency).
Figure 3b.
Mortality rate (%) in patients with acute TTP with severe vs non-severe ADAMTS13 deficiency
Overall, these data suggest that, during acute TTP, a severe deficiency of ADAMTS13 is associated with a greater likelihood of favourable short-term outcomes (remission and survival rates). Conversely, TTP cases with detectable ADAMTS13 activity are associated with a high mortality rate. It cannot be excluded, though, that the latter finding is simply due to the fact that patients with detectable ADMATS13 develop TTP in association with severe diseases or conditions such as metastatic cancer and organ allotransplantation.
Anti-ADAMTS13 testing
Diagnostic value of anti-ADAMTS13 testing
As for ADAMTS13 activity, also the prevalence of ADAMTS13 neutralising inhibitors in acute TTP patients at presentation varies greatly in the different studies, ranging from 38% to 95%29–31,33,34,41, with an apparently higher prevalence (67%–87%) in patients with severe ADAMTS13 deficiency33,42. This variability could reflect the low reproducibility of anti-ADAMTS13 antibody assays between different studies. Furthermore, it is possible that the assessment of inhibitory ADAMTS13 antibodies in vitro may be an incomplete evaluation of ADAMTS13 activity impairment in vivo, where inhibitory and non-inhibitory anti-ADAMTS13 antibodies may act in concert.
Short-term prognostic value of anti-ADAMTS13 testing
Most of the aforementioned studies also analysed the short-term prognostic value of anti-ADAMTS13 testing (Figure 4a and 4b). In the study by Zheng et al.34, all patients (8/8) with undetectable inhibitory anti-ADAMTS13 achieved a complete remission, while one death was detected among the four patients with detectable inhibitory anti-ADAMTS13. Similarly, Mori et al.30 reported that complete remission was reached in all eight patients with undetectable ADAMTS13 inhibitors, while among six patients with anti-ADAMTS13 antibodies, four (67%) obtained a response and two (33%) died.
Figure 4a.
Remission rates (%) in patients with acute TTP with or without anti-ADAMTS13
Figure 4b.
Mortality rate (%) in patients with acute TTP with or without anti-ADAMTS13
In the study conducted by Coppo et al.41, deaths (4/21, 19%) were observed only in patients with detectable inhibitor, while all cases with undetectable inhibitor (12/12) evolved favourably obtaining durable complete remissions. Finally, in a study including both patients with an initial episode of TTP and relapsed patients, Böhm et al.42 found that all TTP cases with absent anti-ADAMTS13 (7/7) achieved a complete response to treatment, while two of the 18 patients with detectable anti-ADAMTS13 died.
Overall, these data indicate that inhibitors are associated with a worse prognosis. The sample sizes of these studies was invariably small, so that all results and conclusions should be interpreted with great caution. While some authors reported a positive correlation between high inhibitor titres and severity of clinical presentation, treatment refractoriness and the rate of deaths30,41,43, suggesting that the strength of ADAMTS13 inhibitors may be associated with treatment responsiveness and outcome, others failed to demonstrate such correlations32,43.
ADAMTS13 and anti-ADAMTS13 testing for the prediction of TTP recurrence
Given the higher rate of relapse (20–30% of cases) in patients surviving an acute initial episode of TTP, many investigators have focused on the search for predictors of TTP recurrence44. With this aim, a number of studies assessed the value of ADAMTS13 and anti-ADAMTS13 in predicting the likelihood of recurrence in TTP patients during both the acute phase and first remission.
So far no single prospective study has been of adequate size, but pooled data from Veyradier et al.29, Vesely et al.31, and Zheng et al.34 indicate that ADAMTS13 deficiency caused by inhibitors is associated with a relapsing course of TTP (15/35, 43%), while patients without inhibitors during acute TTP rarely relapse (1/19, 5%). Similarly, severe deficiency of ADAMTS13 activity during the acute phase of TTP identifies a subgroup of patients with a higher likelihood of relapse (37% in patients with severe ADAMTS13 deficiency versus 6% in patients with non-severe ADAMTS13 deficiency).
In a prospective cohort study, Ferrari et al.45 investigated 32 patients who had low plasma levels of ADAMTS13 activity at the time of the first acute episode of TTP and subsequently achieved remission. Interestingly, the presence of high levels of inhibitory anti-ADAMTS13 IgG at presentation was associated with the persistence of undetectable ADAMTS13 activity in remission, which was in turn predictive of relapses within 18 months. These results were in accordance with those published by Peyvandi et al. in a retrospective cohort study of 109 TTP patients46. Survivors of an acute TTP episode with severely reduced ADAMTS13 levels and/or with anti-ADAMTS13 antibodies during remission had an approximately three-fold greater likelihood of having another episode of TTP than patients with higher protease activity and no antibody46.
Collectively, these data suggest that severe ADAMTS13 deficiency and the presence of anti-ADAMTS13 antibodies during acute TTP or in first remission are associated with a higher risk of recurrence.
Conclusions
In spite of the recent progress in our understanding of the pathophysiology of TTP, a number of issues remain unsolved. First of all, the possibility of managing TTP patients guided by the results of ADAMTS13 tests is jeopardised by the unsatisfactory and limited availability of standardised ADAMTS13 assays suitable for clinical laboratories. From this point of view, the use of the fluorogenic ADAMTS13 testing (FRETS-VWF73 assay) appears to be particularly promising23. However, despite these advances in protease testing, increasing evidence has raised questions about the sensitivity of decreased ADAMTS13 activity for the diagnosis of TTP and the specificity of ADAMTS13 deficiency as a means of discriminating TTP from other microangiopathies, thus suggesting that as yet unidentified environmental or genetic factors contribute to the aetiology of TTP.
Another controversial issue regards the prognostic value of ADAMTS13 testing. Although literature data suggest that the detection of an anti-ADAMTS13 inhibitor is associated with increased treatment refractoriness and a higher mortality rate, the number of patients evaluated is too limited to draw firm conclusions. Additional studies are also required to elucidate the relationship between the inhibitor titre and short-term outcomes. Similarly, the finding that severe ADAMTS13 deficiency and the presence of anti-ADAMTS13 antibodies during acute TTP or first remission are associated with an increased risk of relapses needs to be confirmed by further prospective studies on larger populations of TTP patients.
References
- 1.Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. J Am Soc Nephrol. 2003;14:1072–81. doi: 10.1097/01.asn.0000060805.04118.4c. [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE, Moake JL, Miyata T, et al. Recent advances in thrombotic thrombocytopenic purpura. Hematology (Am Soc Hematol Educ Program) 2004:407–23. doi: 10.1182/asheducation-2004.1.407. [DOI] [PubMed] [Google Scholar]
- 3.Mannucci PM, Lavoretano S, Peyvandi S. The thrombotic microangiopathies. Blood Transf. 2005;3:120–35. [Google Scholar]
- 4.Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc NY Pathol Soc. 1924;24:21–4. [Google Scholar]
- 5.Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine (Baltimore) 1966;45:139–59. [Google Scholar]
- 6.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 7.Moake JL, Rudy CK, Troll JH, et al. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–5. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 8.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 9.Tsai HM, Lian EC. Antibodies to von Willebrand factor cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Chung D, Takayama TK, et al. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–63. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HM. Molecular mechanisms in thrombotic thrombovytopenic purpura. Semin Thromb Hemost. 2004;30:549–56. doi: 10.1055/s-2004-835675. [DOI] [PubMed] [Google Scholar]
- 12.Schulman I, Pierce M, Lukens A, et al. Studies on thrombopoeisis. A factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency. Blood. 1960;16:943–57. [PubMed] [Google Scholar]
- 13.Upshaw JD. Congenital deficiency of a factor in normal plasma that reverses microangiopathic hemolysis and thrombocytopenia. N Engl J Med. 1978;298:1350–2. doi: 10.1056/NEJM197806152982407. [DOI] [PubMed] [Google Scholar]
- 14.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 15.Klaus C, Plaimauer B, Studt JD, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103:4514–9. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 16.Scheiflinger F, Knöbl P, Trattner B, et al. Nonneutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura. Blood. 2003;102:3241–3. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- 17.Rieger M, Mannucci PM, Hovinga JA, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106:1262–7. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 18.van der Plas RM, Schiphorst ME, Huizinga EG, et al. Von Willebrand factor proteolysis is deficient in classic but not in bone marrow transplantation-associated, thrombotic thrombocytopenic purpura. Blood. 1999;93:3798–802. [PubMed] [Google Scholar]
- 19.Tsai HM. Current concepts in thrombotic thrombocytopenic purpura. Annu Rev Med. 2006;57:419–36. doi: 10.1146/annurev.med.57.061804.084505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan M, Lammle B. Assays of von Willebrand factor-cleaving protease: a test for diagnosis of familial and acquired thrombotic thrombocytopenic purpura. Semin Thromb Hemost. 2002;28:167–72. doi: 10.1055/s-2002-27819. [DOI] [PubMed] [Google Scholar]
- 21.Veyradier A, Girma JP. Assays of ADAMTS-13 activity. Semin Hematol. 2004;41:41–7. doi: 10.1053/j.seminhematol.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Shelat S, Ai J, Zheng XL. Molecular biology of ADAMTS13 and diagnostic utility of ADAMTS13 proteolytic activity and inhibitor assays. Semin Thromb Hemost. 2005;31:659–72. doi: 10.1055/s-2005-925472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokame K, Nobe Y, Kokubo Y, et al. FRETS-VWF73, a fluorogenic substrate for ADAMTS13 assay. Br J Haemost. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 24.Groot E, Hulstein JJ, Rison CN, et al. FRETS-VWF73: a rapid and predictive tool for thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:698–9. doi: 10.1111/j.1538-7836.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyata T, Kokame K, Banno F. Measurement of ADAMTS13 activity and inhibitors. Curr Opin Hematol. 2005;12:384–9. doi: 10.1097/01.moh.0000169286.74464.3a. [DOI] [PubMed] [Google Scholar]
- 26.Studt JD, Bohm M, Budde U, et al. Measurement of von Willebrand factor-cleaving protease (ADAMTS-13) activity in plasma: a multicenter comparison of different assay methods. J Thromb Haemost. 2003;1:1882–7. doi: 10.1046/j.1538-7836.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 27.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 28.Feys HB, Canciani MT, Peyvandi F, et al. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. Br J Haematol. 2007;138:534–40. doi: 10.1111/j.1365-2141.2007.06688.x. [DOI] [PubMed] [Google Scholar]
- 29.Veyradier A, Obert B, Houllier A, et al. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98:1765–72. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 30.Mori Y, Wada H, Gabazza EC, et al. Predicting response to plasma exchange in patients with thrombotic thrombocytopenic purpura with measurement of vWF-cleaving protease activity. Transfusion. 2002;42:572–80. doi: 10.1046/j.1537-2995.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 31.Vesely SK, George JN, Lammle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–8. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 32.Coppo P, Bengoufa D, Veyradier A, et al. Reseau d’Etude des Microangiopathies Thrombotiques de l’Adulte. Severe ADAMTS13 deficiency in adult idiopathic thrombotic microangiopathies defines a subset of patients characterized by various autoimmune manifestations, lower platelet count, and mild renal involvement. Medicine (Baltimore) 2004;83:233–44. doi: 10.1097/01.md.0000133622.03370.07. [DOI] [PubMed] [Google Scholar]
- 33.Peyvandi F, Ferrari S, Lavoretano S, et al. von Willebrand factor cleaving protease (ADAMTS-13) and ADAMTS-13 neutralizing autoantibodies in 100 patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2004;127:433–9. doi: 10.1111/j.1365-2141.2004.05217.x. [DOI] [PubMed] [Google Scholar]
- 34.Zheng XL, Kaufman RM, Goodnough LT, et al. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–9. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi V, Robles R, Alberio L, et al. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002;100:710–3. doi: 10.1182/blood-2002-02-0344. [DOI] [PubMed] [Google Scholar]
- 36.Hovinga JA, Studt JD, Alberio L, et al. von Willebrand factor-cleaving protease (ADAMTS-13) activity determination in the diagnosis of thrombotic microangiopathies: the Swiss experience. Semin Hematol. 2004;41:75–82. doi: 10.1053/j.seminhematol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Mal H, Veyradier A, Brugiere O, et al. Thrombotic microangiopathy with acquired deficiency in ADAMTS 13 activity in lung transplant recipients. Transplantation. 2006;81:1628–32. doi: 10.1097/01.tp.0000226066.82066.fa. [DOI] [PubMed] [Google Scholar]
- 38.Blot E, Decaudin D, Veyradier A, et al. Cancer-related thrombotic microangiopathy secondary to von Willebrand factor-cleaving protease deficiency. Thromb Res. 2002;106:127–30. doi: 10.1016/s0049-3848(02)00095-6. [DOI] [PubMed] [Google Scholar]
- 39.Tsai HM. Is severe deficiency of ADAMTS-13 specific for thrombotic thrombocytopenic purpura? Yes. J Thromb Haemost. 2003;1:625–31. doi: 10.1046/j.1538-7836.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 40.Raife T, Atkinson B, Montgomery R, et al. Severe deficiency of VWF-cleaving protease (ADAMTS13) activity defines a distinct population of thrombotic microangiopathy patients. Transfusion. 2004;44:146–50. doi: 10.1111/j.1537-2995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 41.Coppo P, Wolf M, Veyradier A, et al. Reseau d’Etude des Microangiopathies Thrombotiques de l’Adulte. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2005;132:66–74. doi: 10.1111/j.1365-2141.2005.05837.x. [DOI] [PubMed] [Google Scholar]
- 42.Böhm M, Betz C, Miesbach W, et al. The course of ADAMTS-13 activity and inhibitor titre in the treatment of thrombotic thrombocytopenic purpura with plasma exchange and vincristine. Br J Haematol. 2005;129:644–52. doi: 10.1111/j.1365-2141.2005.05512.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsai HM. High titers of inhibitors of von Willebrand factor-cleaving metalloproteinase in a fatal case of acute thrombotic thrombocytopenic purpura. Am J Hematol. 2000;65:251–5. doi: 10.1002/1096-8652(200011)65:3<251::aid-ajh13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Willis MS, Bandarenko N. Relapse of thrombotic thrombocytopenic purpura: is it a continuum of disease? Semin Thromb Hemost. 2005;31:700–8. doi: 10.1055/s-2005-925476. [DOI] [PubMed] [Google Scholar]
- 45.Ferrari S, Scheiflinger F, Rieger M, et al. French Clinical and Biological Network on Adult Thrombotic Microangiopathies. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 2007;109:2815–22. doi: 10.1182/blood-2006-02-006064. [DOI] [PubMed] [Google Scholar]
- 46.Peyvandi F, Lavoretano S, Palla R, et al. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;2:232–9. doi: 10.3324/haematol.11739. [DOI] [PubMed] [Google Scholar]