Abstract
Background
Red cell alloantibodies may disappear over time and cause a delayed haemolytic reaction if their past existence is not known before a transfusion. Only few quantitative data have already been presented on this topic.
Study design and methods
We retrieved the records of alloantibodies detected between 1989 and 2008 in our institution. All warm-reacting alloantibodies were included, with the exception of ABO antibodies, anti-D in women of childbearing age (it was impossible to rule out Rh prophylaxis) and antibodies produced by transfusion-dependent beta-thalassaemia patients (their transfusion history was too unusual).
Results
We found 673 antibodies, produced by 525 patients, which had been tested again after the initial detection. The median follow-up was 319 days. The overall rate of non-persistence was 37%, corresponding to 251 antibodies, produced by 216 patients. Non-persistent antibodies were associated with a longer follow-up (409 vs. 236 days; p=0.012), more tests after detection (2 vs. 1; p<0.001), and a lower maximum score (2+ vs. 3+; p<0.001). Antibody specificity, too, influenced the duration of persistence. Among common antibodies, anti-D was the most long-lived (14% non-persistence); anti-Jka the most short-lived (43% non-persistence). Antibodies detected in the second decade of the study were less persistent (p<0.001). They were also weaker (maximum score: 2+ vs. 3+; p<0.001). This probably reflects the increased sensitivity of the screening tests over the course of time. Age, sex and whether the patient had produced multiple alloantibodies were not significant covariates. A minority of non-persistent antibodies (33/251, 13%) were detected again after a negative result (intermittently-detected antibodies). They had a longer follow-up (885 vs. 341 days; p=0.002), more tests after detection (5 vs. 2; p<0.001), and a higher maximum score (3+ vs. 2+; p=0.001).
Conclusions
Red cell antibodies commonly disappear. To avoid delayed haemolytic reactions, it is necessary to rely on previous records, which should be readily available.
Keywords: antibody persistence, non-persistent antibodies, red cell antibodies, immunohaematology
Introduction
Red cell alloantibodies may become undetectable over the course of time1. This phenomenon is clinically important, as it is at the origin of most cases of delayed haemolytic transfusion reactions2. However, only a few studies have been published on this topic3,4,5, with conflicting results as regards predictor variables. Ramsey and Larson reported on 209 antibodies, followed-up for a median of 10 months3. The overall rate of non-persistence was 37%, with the rates being highest for anti-Jka and -C. A weaker initial score was significantly associated with non-persistence; other patient- or antibody-related characteristics were not, with the doubtful exception of age <20 years. A further study, concerning only 36 antibodies followed-up for more than 5 years, found that 39% were non-persistent4. After 10 years, the rate increased to 45%. The rates of non-persistence were highest for anti-Jka, -S and –C. In a larger study (593 antibodies) by Schonewille et al., 26% of antibodies became undetectable over the course of time (median follow-up: 5–6 months)5. The association with the strength of the antibody was confirmed, but that with antibody specificity was not. Data concerning patients with sickle cell disease only were reported by Rosse et al. (212 antibodies)6. They found a 37% rate of non-persistence, after a follow-up of more than 12 months.
Computer records of immunohaematologic tests exist in this institution since almost two decades. They were used to estimate the rate of disappearance of alloantibodies over time and the influence of a series of covariates, such as age, sex, antibody specificity and others.
Materials and Methods
Our institution is the only transfusion and immunohaematology facility in the province of Ferrara (357,000 inhabitants). It serves several hospitals, including a teaching hospital, for a total of 1,862 beds.
Data collection
Two different databases were used over the years to record the results of immunohaematological tests. Their data were exported and merged into a single Access (Microsoft, Redmond, WA, USA) database, which was then used for further data handling and processing. We retrieved information on all the red cell antibodies detected, with the following exceptions:
- ABO antibodies (anti-A, -A1, -B, -H)
- cold-reacting antibodies (anti-I, -i, -IH, -P1, and selected specimens of anti-M and -N that only reacted in the cold)
- autoantibodies, both warm- and cold-reacting
- anti-D in women of childbearing age (it was generally impossible to rule out Rh prophylaxis)
- antibodies produced by transfusion-dependent beta-thalassaemia major patients, who are common in our region, because of their very unusual transfusion history.
Immunohaematological studies
In the period of study, antibody identification was performed with the gel test (ID, Diamed) or another column agglutination test (BioVue, Ortho). Antibody screening was performed with the same tests mentioned above or, more often, with an indirect antiglobulin tube test, potentiated with a very low ionic strength solution7 or PEG8. The score recorded was that obtained with the identification technique. The scoring system remained constant throughout the study period. It ranged from – to ++++, as follows: –: all red cells at the bottom of the column; +: all red cells within the bottom third of the column, most cells at the bottom; ++: all red cells within the bottom 2/3 of the column, many cells at the bottom; +++: red cells uniformly distributed along the column; ++++: all cells within the top third of the column.
Screening cells were commercially obtained. They were three-cell sets, carefully chosen so as to include Cw, Kpa, and Lua in most cases, and homozygous expression of c, E, Fya, Fyb, Jka, Jkb, M, N, S, and s in all cases. Wra was very rarely present, but we also screened a few hundred patient and donor samples, for a limited period, using an in-house Wra-positive red cell sample.
Data analysis
All antibodies that were tested again after the first detection were included. They were considered persistent if they scored positive in all cases after the first detection and non-persistent if they scored negative at least once after the first detection. A few non-persistent antibodies were detected again after the first negative test: these were considered intermittently-detected antibodies.
The length of follow-up was the interval (days) between the first positive test and the last test (whether positive or negative). The time to non-persistence was the interval between the first detection and the first negative test. In the case of persistent antibodies, it was equal to the length of follow-up (right-censored observations).
Other variables considered were:
- the number of tests after (not including) the first detection
- the number of tests after the first detection up to (including) the first negative result (for persistent antibodies, this was equal to the previous variable)
- the score at first detection
- the maximum score obtained during the follow-up. Titres were available for a few samples only and were not analysed.
Antibodies were also grouped according to the period of detection (divided into approximately two decades from the end of July 1989 to December 1998 and from January 1999 to mid-April 2008) and whether the patient had made multiple alloantibodies.
Statistical analysis
Persistent and non-persistent antibodies were compared, by means of the Mann-Whitney U-test, with regard to age at first detection, length of follow-up, score at first detection, maximum score and number of tests after first detection. Comparisons concerning categorical variables, such as sex, period of detection, and single or multiple alloantibodies, were performed calculating the chi-square statistics. The statistical significance of such multiple comparisons was evaluated by the Holm-Bonferroni method9. The rate of disappearance of antibodies was calculated using the Kaplan-Meier method. Survival curves were also stratified by antibody specificity, maximum score and period of detection.
Many of the above-mentioned variables were entered as covariates into a proportional hazard model (Cox regression), with time to non-persistence as the time variable. The event was non-persistence (the first negative result after the initial detection). Observations regarding persistent antibodies were considered censored.
Statistical analyses were performed using SPSS (v. 16, SPSSInc,Chicago,IL,USA)andOpenStat(v.2.12.07,WGM Consulting, IA, USA).
Results
We retrieved the records of 1859 antibodies, produced by 1502 patients. Of these 1859 antibodies, 673 (from 525 patients;females:332,males:193)weretestedagainafterdetection.
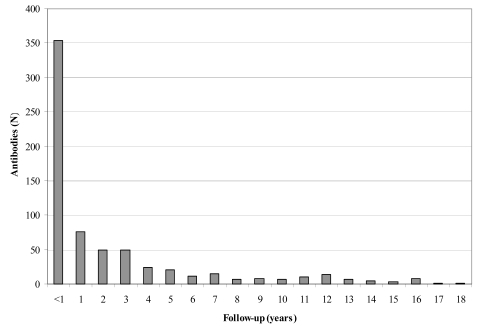
The mean age of the patients at the time of antibody detection was 64±17 years (median: 67; interquartile range (Q1-Q3): 52–75; range: 1–98). On average, the patients' samples were screened for antibodies 2.4 times after first detection (median: 1; Q1-Q3: 1–3; range: 1–34). The frequency distribution of the length of follow-up is shown in Figure 1 (median: 319 days; Q1–Q3: 41–1246). Fifty-seven antibodies (8.5%) were followed-up for 10 years or more. Of those antibodies, 41 (72%) were persistent, including 19 anti-D, 6 anti-C, 6 anti-K, 4 anti-E, 2 anti-c, 2 anti-Fya, 1 anti-e, and 1 anti-Jka; 16 (28%) were non-persistent, including 5 anti-E, 5 anti-K, 2 anti-C, 2 unidentified, 1 antiCw, and 1 anti-e.
Figure 1.
Frequency distribution of length of follow-up (from the first positive test to the last test). 319 (47%) antibodies were followed-up for 1 year or more; 57 (8.5%) for 10 years or more
Two hundred and fifty-one antibodies (37%) in 216 patients (41%; female/male: 129/87) became undetectable over the course of time (Tables I and II). After the last positive sample, they were tested again 2.2 times, on average (median: 1; Q1–Q3: 1–2; range: 1–15).
Table I.
Number of non-persistent antibodies.
| Antibody | N | % * | Non- persistent | % ** |
|---|---|---|---|---|
| E | 136 | 20.2 | 53 | 39.0 |
| K | 118 | 17.5 | 40 | 33.9 |
| D | 108 | 16.0 | 15 | 13.9 |
| ? | 57 | 8.5 | 44 | 77.2 |
| C | 38 | 5.6 | 9 | 23.7 |
| c | 36 | 5.3 | 8 | 22.2 |
| Jka | 30 | 4.5 | 13 | 43.3 |
| M | 25 | 3.7 | 7 | 28.0 |
| Cw | 24 | 3.6 | 13 | 54.2 |
| Fya | 19 | 2.8 | 4 | 21.1 |
| Lea | 14 | 2.1 | 8 | 57.1 |
| Leb | 10 | 1.5 | 6 | 60.0 |
| S | 10 | 1.5 | 5 | 50.0 |
| Kpa | 9 | 1.3 | 3 | 33.3 |
| Wra | 8 | 1.2 | 5 | 62.5 |
| Lua | 7 | 1.0 | 4 | 57.1 |
| e | 6 | 0.9 | 3 | 50.0 |
| Jkb | 6 | 0.9 | 3 | 50.0 |
| Cob | 2 | 0.3 | 1 | 50.0 |
| N | 2 | 0.3 | 2 | 100.0 |
| cE | 1 | 0.1 | 1 | 100.0 |
| Cha | 1 | 0.1 | 1 | 100.0 |
| Dia | 1 | 0.1 | 1 | 100.0 |
| f | 1 | 0.1 | 0 | 0.0 |
| Fyb | 1 | 0.1 | 0 | 0.0 |
| Jsa | 1 | 0.1 | 1 | 100.0 |
| Lub | 1 | 0.1 | 0 | 0.0 |
| Xga | 1 | 0.1 | 1 | 100.0 |
| Total | 673 | 100 | 251 | 37.3 |
The antibodies are listed in order of frequency.
‘?’ indicates that the specificity was not identified with certainty (auto- and pan-antibodies were excluded). Anti-D from women of childbearing age were also excluded because it was impossible to rule out Rh prophylaxis.
Percentage with respect to the total number of antibodies
Percentage of non-persistent antibodies, with respect to the number of antibodies with the same specificity.
Table II.
Number of non-persistent antibodies, grouped according to the genetic system.
| Genetic System | N | % | Non- persistent | % |
|---|---|---|---|---|
| Rh | 350 | 52.0 | 102 | 29.1 |
| Kell | 128 | 19.0 | 44 | 34.4 |
| Unidentified | 57 | 8.5 | 44 | 77.2 |
| MNSs | 37 | 5.5 | 14 | 37.8 |
| Kidd | 36 | 5.3 | 16 | 44.4 |
| Lewis | 24 | 3.6 | 14 | 58.3 |
| Duffy | 20 | 3.0 | 4 | 20.0 |
| Lutheran | 8 | 1.2 | 4 | 50.0 |
| Others | 13 | 1.9 | 9 | 69.2 |
| Total | 673 | 100 | 251 | 37.3 |
Genetic systems are listed in order of frequency. 'Others' is a miscellaneous group.
The frequency of non-persistence varied widely among the different antibody specificities. Excluding the specificities for which there were fewer than six samples, the lowest frequency was 14% (anti-D) and the highest 77% (unidentified antibodies).
Eighty-two of the 251 non-persistent antibodies (33%) were undetectable in more than one consecutive specimen.
Sex, single or multiple alloantibodies, and period of detection
Frequency distributions according to sex, single or multiple alloantibodies and period of detection are listed in Table III. Female patients produced 64% and 61% of persistent and non-persistent antibodies, respectively (chi-square test, not statistically significant difference). Four hundred and seven patients made a single alloantibody, 92 patients made two alloantibodies, 22 patients made three, and 4 patients made four. Multiple alloantibodies accounted for 41% of the persistent antibodies and 37% of the nonpersistent antibodies (chi-square test, not significantly different). Non-persistent antibodies accounted for 45% of the alloantibodies in the first period of detection, but 63% in the second period. This difference is statistically significant (chi-square test, p<0.001). Sixty-nine out of 108 anti-D were identified in the first decade (chi-square test, p=0.001).
Table III.
Comparison between persistent and nonpersistent antibodies: categorical variables.
| Persistent | Non- persistent | p | ||
|---|---|---|---|---|
| Sex | F | 272 | 152 | NS |
| M | 150 | 99 | ||
| Multiple alloantibodies | No | 249 | 158 | NS |
| Yes | 173 | 93 | ||
| Period of detection* | 1 | 234 | 93 | <0.001 |
| 2 | 188 | 158 |
Frequency distribution of some categorical variables. The chi-square statistics was calculated to test for significance.
NS: statistically not significant.
The period of detection was divided into two decades (1: from 1989 to 1998; 2: from 1999 to 2008).
In the first decade, 4/69 anti-D were non-persistent; in the second, 11/39 (chi-square test, p=0.001). Unidentified antibodies were found more often in the second decade of study (38/57; chi-square test, p=0.016).
Age at first detection, length of follow-up, and number of tests after first detection
There was no difference (Mann-Whitney U-test) between persistent and non-persistent antibodies as regards age at first detection (Table IV). Non-persistent antibodies were followed-up for longer (median: 409 days; persistent: median: 236; Mann-Whitney U-test, p=0.012) and were tested more times after the first detection (median: 2 tests; persistent: median: 1; Mann-Whitney U-test, p<0.001) (Table IV).
Table IV.
Comparison between persistent and non-persistent antibodies: ordinal and scalar variables
| Persistent | Non-persistent | p | |
|---|---|---|---|
| Age (y) | 63±17 | 64±18 | NS |
| Follow-up (d) | 236 (25–1363) | 409 (104–1166) | 0.012 |
| N. of tests after first detection | 1 (1–2) | 2 (1–4) | <0.001 |
| Initial score | 3 (2–3) | 2 (1–3) | <0.001 |
| Maximum score | 3 (2–4) | 2 (2–3) | <0.001 |
Initial and maximum scores are ordinal variables. Age of the patient at the time of detection, length of follow-up, number of tests after first detection are scalar variables. Data are reported as means±1SD, or as medians with the interquartile range in parentheses, as appropriate. The statistical significance of the differences was evaluated using the Mann-Whitney U-test.
Initial score and maximum score
Non-persistent antibodies were significantly weaker than persistent antibodies, both at the time of first detection (initial score: persistent: 3+; non-persistent: 2+; Mann-Whitney U-test, p<0.001) and as regards the maximum score reached during the follow-up (persistent: 3+; nonpersistent: 2+; Mann-Whitney U-test, p<0.001) (Table IV).
Initial and maximum scores of antibodies detected during the first decade (initial score: median: 3+; Q1–Q3: 2–3+; maximumscore:median:3+;Q1–Q3:2–4+)weresignificantly higher than during the second decade (initial score: median: 2+;Q1–Q3:1–3+;maximumscore:median:2+;Q1–Q3:2–3+) (Mann-Whitney U-test, p<0.001 in both cases).
Variables affecting the time to non-persistence
The following covariates were entered into a proportional hazard model: age at first detection, sex, antibody specificity, initial and maximum scores, period of detection (first or second decade), and single or multiple alloantibodies.
Cox regression analysis showed that antibody specificity, maximum score and period of detection significantly influenced the time to non-persistence (Tables V and VI). The same analysis was repeated, substituting the genetic system for the antibody specificity. The results were very similar, with genetic system (Table VII), maximum score and period of detection being significant covariates.
Table V.
Relationship between patient- and antibody related variables and time to non-persistence.
| Covariate | Hazard Ratio | 95%CI | p |
|---|---|---|---|
| Age | 1.005 | 0.996–1.014 | NS |
| Sex | 0.982 | 0.741–1.302 | NS |
| Initial score | 1.281 | 0.941–1.742 | NS |
| Maximum score | 0.629 | 0.539–0.736 | <0.001 |
| Period of detection | 2.196 | 1.575–3.061 | <0.001 |
| Multiple alloantibody | 1.034 | 0.768–1.391 | NS |
In the Cox regression analysis, time to non-persistence was the time variable and non-persistence (the first negative test after the first detection) was the event. All covariates were entered together into the proportional hazard model. Non-significant covariates were then excluded and the hazard ratios calculated again for the significant ones. Antibody specificities are reported separately in Table VI. A hazard ratio less than 1 means that any increase in the covariate decreases the chance of non-persistence. The opposite is true if the hazard ratio is higher than 1. 95%CI indicates the confidence interval. When the 95%CI includes 1, the effect of the covariate is not statistically significant.
Table VI.
Relationship between antibody specificity and time to non-persistence.
| Antibody | Hazard Ratio | 95%CI | p |
|---|---|---|---|
| ? | 7.080 | 3.745–13.385 | <0.001 |
| Cw | 4.609 | 2.068–10.271 | <0.001 |
| E | 2.562 | 1.409–4.658 | 0.002 |
| e | 4.238 | 1.216–14.766 | 0.023 |
| Jka | 2.561 | 1.153–5.691 | 0.021 |
| Jkb | 5.319 | 1.515–18.678 | 0.009 |
| K | 2.754 | 1.505–5.042 | 0.001 |
| Lea | 3.379 | 1.333–8.566 | 0.010 |
| Leb | 4.295 | 1.650–11.178 | 0.003 |
| Lua | 3.598 | 1.146–11.302 | 0.028 |
| Wra | 10.496 | 3.730–29.537 | <0.001 |
The Cox regression analysis concerned antibody specificities for which there were at least six samples each. Hazard ratios are calculated with reference to anti-D. For brevity, only the statistically significant hazard ratios are reported. All antibodies listed have hazard ratios higher than that of anti-D.
Table VII.
Relationship between genetic system and time to non-persistence
| Genetic System | Hazard Ratio | 95% CI | p |
|---|---|---|---|
| Duffy | 0.842 | 0.308–2.299 | NS |
| Kell | 1.326 | 0.920–1.913 | NS |
| Kidd | 1.386 | 0.787–2.442 | NS |
| Lewis | 1.839 | 1.017–3.323 | 0.044 |
| Lutheran | 1.625 | 0.584–4.518 | NS |
| MNSs | 1.025 | 0.568–1.850 | NS |
| Other | 3.923 | 1.949–7.897 | <0.001 |
| Unidentified | 3.494 | 2.342–5.213 | <0.001 |
In the Cox regression analysis, genetic system was entered instead of antibody specificity. Hazard ratios are calculated with reference to Rh antibodies. All statistically significant covariates have greater hazard ratios than that of Rh antibodies.
However, when both antibody specificity and genetic system were entered into the same model, antibody specificity emerged as the only significant covariate.
Rate of disappearance of antibodies
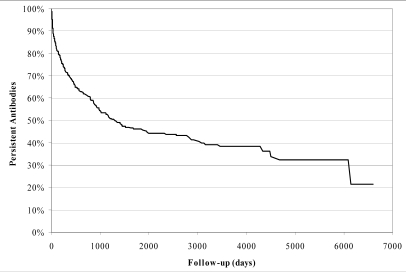
The Kaplan-Meier estimate of the "survival" curve of all antibodies (N=673) is shown in Figure 2.
Figure 2.
Kaplan-Meier estimate of the "survival" curve of all antibodies (N=673). 50% of antibodies disappear within 1300 days (3 years and 7 months, approximately)
The curve predicts that 50% of antibodies disappear within 1300 days (approximately 3 years and 7 months).
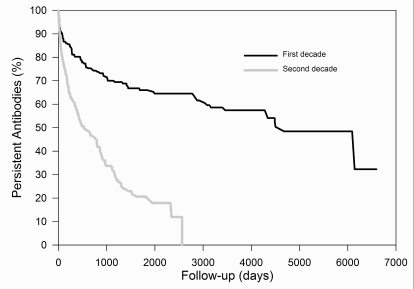
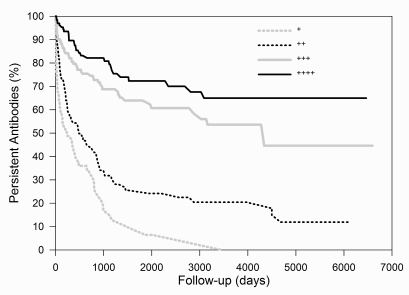
Figure 3 and 4 show the rate of disappearance, stratified by period of detection (first period: N=327; second period: N=346) and by maximum score (+: N=91; ++: N=197; +++: N=226; ++++: N=153), respectively.
Figure 3.
Survival curves of antibodies, stratified by period of detection.
- : first decade; - : second decade. Antibodies detected during the second decade had a significantly faster rate of disappearance (Cox regression analysis, p < 0.001)
Figure 4.
Survival curves of antibodies, stratified by maximum score.
- : ++++; - : +++; ··· : ++; ··· : +. The rate of disappearance increases as the maximum score decreases (Cox regression analysis, p<0.001)
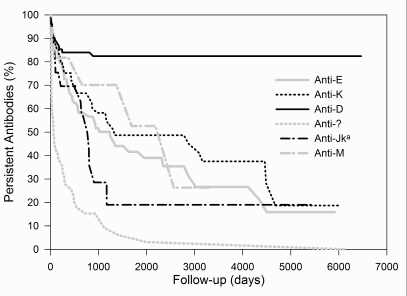
Fifty percent of antibodies detected during the first period disappear within 4500 days. The corresponding value for the second period is 526 days. The "half-life" of antibodies with a maximum score of + to ++++ is 236, 487, 4303, and >6465 days, respectively. Separate curves for a few representative antibodies (anti-D, -E, -K, unidentified, -Jka and -M) are presented in Figure 5.
Figure 5.
Survival curves of antibodies, stratified by antibody specificity.
Only a few representative specificities are shown: - : anti-D; - : anti-E; ··· : anti-K; ··· : unidentified; - ·: anti-Jka; - ·: anti-M
Their curves reach 50% of disappearance at >6465, 1234, 1319, 66, 767, and 2222 days, respectively.
Intermittently-detected antibodies
A few non-persistent antibodies were detected again after a negative result (N=33). These comprised eight anti-K, seven anti-E, six unidentified, two anti-Cw and anti-Lea, and one each of anti-D, -e, -Kpa, -Jka, -Lea, -Lua, -M, and -Wra.
They were compared with the rest of the non-persistent antibodies (Tables VIII and IX).
Table VIII.
Comparison between intermittently-detected antibodies and the other non-persistent antibodies: categorical variables
| Intermittently- detected | Other non-persistent | p | ||
|---|---|---|---|---|
| Sex | Female | 15 | 137 | NS |
| Male | 18 | 81 | ||
| Multiple alloantibodies | No | 21 | 137 | NS |
| Yes | 12 | 81 | ||
| Period of detection | 1 | 15 | 78 | NS |
| 2 | 18 | 140 |
The chi-square statistics was calculated to test for significance.
Table IX.
Comparison between intermittently-detected antibodies and the other non-persistent antibodies: ordinal and scalar variables.
| Intermittently- detected | Other non-persistent | p | |
|---|---|---|---|
| Age (years) | 66±17 | 64±18 | NS |
| Follow-up (days) | 885 (414–2144) | 341 (87–1023) | 0.002 |
| N. of tests after first detection | 5 (3–6) | 2 (1–3) | <0.001 |
| Initial score | 2 (1–3) | 2 (1–3) | NS |
| Maximum score | 3 (2–3) | 2 (1–3) | 0.001 |
The statistical significance of the differences was evaluated using the Mann-Whitney U-test.
Intermittently-detected antibodies were followed-up for longer (median: 885 days; other non-persistent antibodies: median: 341; Mann-Whitney U-test, p=0.002) and had more tests after the initial detection (median: 5; other nonpersistent antibodies: median: 2; Mann-Whitney U-test, p<0.001). They were also stronger than the rest (maximum score: median: 3+; other non-persistent antibodies: median: 2+; Mann-Whitney U-test, p=0.001).
Discussion
In the absence of an antigenic stimulus, antibody production should decrease and eventually cease. Thus, it is the long-term persistence of an antibody that should be considered surprising10.
This phenomenon has been repeatedly documented11,12, in patients who were never transfused and whose last antigenic stimulus dated back as far as 44 years previously11. In the present study, 41 antibodies persisted for 10 years or more, and 11 for 15 years or more (up to 18 years). A detailed obstetric and transfusion history of these 11 patients is not available, unfortunately, but four of them were males and all females but one were at the limit or beyond childbearing age when the antibody was initially detected. Moreover, none of these patients suffered a haemolytic transfusion reaction. Therefore, any continuous or repeated stimulus is very unlikely.
The primary aim of the present study was to collect data on non-persistent antibodies. We found an overall frequency of 37%, which is exactly identical to that seen in two other studies3,6. Others found a lower percentage (26%), but their median follow-up was shorter than in the present study (5–6 months vs. 10 months)5. Conversely, after more than 10 years, the percentage increased to 45%4.
Age, sex, and whether the patient had made multiple alloantibodies did not influence the rate of disappearance (Table V) and were not distributed differently between persistent and non-persistent antibodies (Tables III and IV). This is in agreement with what was generally seen in previous studies3,5, although Ramsey and Larson found that patients less than 20 years old tended to lose antibodies more frequently3. In the present study, only seven patients were less than 20 years old and only two of them made non-persistent antibodies.
The strength of the antibody, as indicated by its score, is one of the best predictors of persistence. Both initial and maximum scores were significantly greater for persistent antibodies (Table IV). However, only the maximum score was a significant covariate in the Cox regression analysis (Table V). Not unexpectedly, initial and maximum scores were correlated and the two variables had the same value for 518/673 (77%) antibodies. Thus, in most cases the two scores convey the same information. When they do not, it means that there has been a successive stimulation or that the immune response was detected at an early stage. Both situations should be linked to longer persistence. The association with the strength of the antibody has been described previously3.
A curious observation is that the antibodies detected in the second decade of study were more short-lived than those detected in the first decade (Tables IV and V, and Figure 3). We were prompted to verify this by a similar observation in another study5. Probably, this is just a reflection of the improved sensitivity of screening tests over the years, which allowed the detection of weaker antibodies. In fact, initial and maximum scores of antibodies detected in the second decade were significantly lower. Others, too, noted that antibodies detected by a more sensitive technique (gel test, as opposed to a tube albumin antiglobulin test) were less persistent5.
The follow-up of non-persistent antibodies was longer and these antibodies were tested more often after the initial detection (Table IV). This suggests that all antibodies would probably become undetectable, provided their follow-up is sufficiently prolonged.
The frequency of non-persistence varied widely between the different antibody specificities. Among the antibodies observed in sufficient numbers, the frequency of non-persistence was lowest for anti-D (14%). An identical value was found in two other studies3,5, while a third one found a somewhat higher frequency (26%)6. Schonewille et al. noted that most anti-D samples were already present at the beginning of their study5. These antibodies were also associated with a longer persistence. We found a similar trend, too. Unidentified antibodies were the most short-lived. This is not surprising, because most of them were probably common specificities, but too weak to be reliably identified. They were found more often in the second decade of study.
Anti-Jka, -K, and -E were the most frequent specificities associated with delayed haemolytic reactions13.Anti-E and anti-K are not particularly short-lived. However, they are among the most common antibodies and this probably explains why they are easily encountered in cases of delayed haemolytic reactions. In contrast, Kidd antibodies are known for being transient3,13, and we confirmed this.
Antigens such as Cw, Kpa, Lua, and especially Wra, are not always represented in screening panels. We tried to ensure that our cells included the first three antigens and Cw was almost always present. Therefore, the nonpersistence rate of anti-Cw (54%) is not explained by a lack of the corresponding antigen.
In general, there seems not to be any association between the known immunogenicity of the antigen1 and the rate of persistence of the antibody.
Antibody specificity was a very significant covariate in the proportional hazard model. This is at variance with the findings of Schonewille et al., who reported no influence on the time to non-persistence5. This is surprising, considering the similarity of their other findings with ours. We can offer no explanation for this.
Intermittently-detected antibodies are a minor subpopulation of non-persistent antibodies, characterised by a strength intermediate between the strengths of persistent and non-persistent antibodies, the longest median follow-up and many tests after the initial detection. This suggests that the production of the antibody may not be interrupted, but only too low to be detected constantly. Intermittent detection of antibodies is frequently observed in transfusion-dependent beta-thalassaemia patients (unpublished observations). Cyclical variations of up to 30% in antibody production of anti-D in volunteers have been described14. A further possible reason for a fluctuation in the antibody levels is an acute haemorrhage, followed by a massive transfusion.
In conclusion, red cell alloantibodies tend to disappear over the course of time, especially when they are weak. The increased sensitivity of our current screening tests does not protect us against this risk. In fact, the risk is becoming greater because we are now able to identify the antibodies earlier and to avoid a further stimulation, which would increase the strength of the antibodies and render them more easily detectable. There is no immediate solution to this problem, except to rely on previous records10, which should be readily available when necessary. Patients should be informed of their immunisation status and provided with suitable immunohaematological reports, particularly when they are going to be transfused elsewhere.
Acknowledgments
The author thanks the staff of the Immunohaematology Laboratory of the Blood Transfusion Service of Ferrara, particularly Dr. Carla Turbiani and Dr. Daniela Resca, who directed the Laboratory over the years, and Dr. Eleonora Bedendo, who helped in the writing of a previous draft of this paper.
References
- 1.Klein HG, Anstee DJ. Mollison's blood transfusion in clinical medicine. 11. Oxford, Great Britain: Blackwell Science; 2005. [Google Scholar]
- 2.Engelfriet CP, Reesink HW. International Forum: prevention and diagnosis of haemolytic transfusion reactions. Vox Sang. 2006;91:353–68. doi: 10.1111/j.1423-0410.2006.00812_1.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey G, Larson P. Loss of red cell antibodies over time. Transfusion. 1988;28:162–5. doi: 10.1046/j.1537-2995.1988.28288179022.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey G, Smietana SJ. Long-term follow-up testing of red cell alloantibodies. Transfusion. 1994;34:122–4. doi: 10.1046/j.1537-2995.1994.34294143938.x. [DOI] [PubMed] [Google Scholar]
- 5.Schonewille H, Haak HL, van Zijl AM. RBC antibody persistence. Transfusion. 2000;40:1127–31. doi: 10.1046/j.1537-2995.2000.40091127.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–7. [PubMed] [Google Scholar]
- 7.Reverberi R, Ferrari L, Balugani S. Fattori che influenzano la sensibilità dei test per gli anticorpi anti-eritrocitari: confronto tra bassa e bassissima forza ionica e tra protamina ed anti-IgG. La Trasfusione del Sangue. 1996;41:10–6. [Google Scholar]
- 8.Verenini M, Reverberi R. Uso del PEG in immunoematologia: ottimizzazione della tecnica. La Trasfusione del Sangue. 1997;42:220–4. [Google Scholar]
- 9.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–8. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelfriet CP, Overbeeke MAM. The persistence of blood group antibodies. Transfusion. 1994;34:98–9. doi: 10.1046/j.1537-2995.1994.34294143960.x. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison HE, McLennan W. Long persistence of Rhesus antibodies. Vox Sang. 1966;11:517–8. doi: 10.1111/j.1423-0410.1966.tb04249.x. [DOI] [PubMed] [Google Scholar]
- 12.Cox MT, McMican A, Blumberg N. Case report of an anti-Jkb persisting for sixteen years (letter) Transfusion. 1983;23:362. doi: 10.1046/j.1537-2995.1983.23483276883.x. [DOI] [PubMed] [Google Scholar]
- 13.Taswell HF, Pineda AA, Moore SB. Haemolytic transfusion reactions: frequency and clinical and laboratory aspects. In: Bell CA, editor. A seminar on immune-mediated cell destruction. Chicago, IL: AABB; 1981. [Google Scholar]
- 14.Rubinstein P. Cyclical variations in anti-Rh titer detected by automatic quantitative hemagglutination. Vox Sang. 1972;23:508–22. doi: 10.1111/j.1423-0410.1972.tb03844.x. [DOI] [PubMed] [Google Scholar]