Abstract
Paraquat (methyl viologen)-resistant mutants of Streptomyces coelicolor A3(2) that grew and sporulated normally in the presence of paraquat were isolated. Based on the positions of the mutant loci in the genetic map, we isolated the pqr (paraquat resistance) gene whose mutation (pqr501) caused a dominant paraquat-resistant phenotype. The pqr locus consists of two genes (pqrA and pqrB) that form a transcription unit. The pqrA gene encodes a protein with a TetR-like DNA-binding motif, and the pqrB gene encodes a putative efflux pump of the major facilitator superfamily. The pqr501 mutation was a base substitution changing arginine-18 to glutamine (R18Q) near the helix-turn-helix motif in PqrA. A pqrA null mutant exhibited similar paraquat resistance, and an increase in the amount of pqrA promoter-driven transcripts of about eightfold was observed for the pqrA501 mutant. These results suggest that PqrA is a negative regulator of its own operon. Deletion of the pqrAB operon caused cells to be very sensitive to paraquat, consistent with the prediction that PqrB may function as a paraquat-efflux pump. Purified PqrA protein specifically bound to the pqrA promoter region, whereas mutant R18Q protein did not, indicating that PqrA is a direct autoregulator of its own operon.
It has been a goal for decades to elucidate the mechanism of generation and removal of reactive oxygen species and to understand how organisms cope with the toxicity and potential damage from these species (4, 39). Redox cycling agents such as paraquat (methyl viologen), lawson, menadione, and plumbagin are powerful propagators of superoxide radicals (O2−) inside the cell. They are reduced to radical forms by a single electron transfer mediated through flavoproteins such as NADPH diaphorase and NADPH cytochrome P-450 reductase, and each radical transfers a single electron to dioxygen, with concurrent generation of superoxide radicals (19). O2− causes damage to cells via multiple routes: it can oxidize low-molecular-weight reductants such as thiols and catechols; inactivate some enzymes; react with NO to yield the strong oxidant peroxinitrite; and oxidize [4Fe-4S] clusters in dehydratases, causing a release of free iron, which then reduces hydroperoxides to hydroxyl or alkoxyl radicals by the Fenton reaction (24). The toxicity of redox cycling agents may result not only from the toxic effect of O2−, but also from depletion of NADPH by a cycling reaction (25). Exploiting this effect, researchers have used various forms of redox cycling agents as antimicrobial agents, herbicides, and anticancer drugs.
The relationship between paraquat toxicity and superoxide radicals is supported by the observations that molecular oxygen is required for toxicity and that the toxicity is abolished by uric acid, a superoxide scavenger, but not by mannitol, known to scavenge hydroxyl radicals (27). However, there are also some reports questioning the direct relationship between paraquat toxicity and the intracellular level of O2− (37). Even though the precise mechanism of toxicity caused by redox cycling agents is not yet fully understood, these reagents are used to mimic and magnify the oxidative stress that cells encounter during normal aerobic respiration, ischemia-reperfusion, or defensive attack by macrophages (19).
Streptomycetes are strongly aerobic and encounter radical oxygen species throughout their complex life cycle. When grown on solid media, they form a mycelium of branching hyphae, develop a mass of aerial hyphae, and eventually form uninucleate spores (5). Streptomyces coelicolor A3(2) is a model organism for this genus. Its complete genome sequence information has been reported recently (2), revealing the presence of 7,825 protein-coding genes on the 8,667,507-bp linear chromosome. A relationship between stress responses and morphological differentiation is indicated by both physiological and genetic data. For example, the aerial mycelium is likely to be in a hyperoxic state, in contrast to the substrate mycelium, which is likely to be hypooxic, and the differentiation of S. coelicolor is hindered in the absence of RsrA (an anti-sigma factor protein) or OxyR, both of which control aspects of the oxidative stress response (16, 31). This idea is also supported by observations made in fungi. In Neurospora crassa, a decrease in the reduced-to-oxidized ratio of NAD and NADP coenzymes accompanies the growth of the aerial mycelium (41). Changes in the cellular concentration of NADH and superoxide dismutase (SOD) activity have also been observed at the initial stage of starvation-induced differentiation in a slime mold (28).
As an initial step to understand the relationship between oxidative stress and cellular differentiation, we previously examined the effect of various redox cycling agents on the growth and differentiation of S. coelicolor A3(2) (11). We found that paraquat inhibited differentiation but not cell survival, unlike menadione or other quinone derivatives. Three paraquat-resistant mutants (U501, U605, and U607) that sporulated normally in the presence of paraquat were isolated, and their loci (pqr, for paraquat resistance) were mapped near the argA gene by genetic crosses. Here, using this information, we describe the isolation and characterization of the genes (pqrA and pqrB) at the pqr locus in the wild type as well as a mutant (U501). The pqrA gene specifies a repressor that binds and negatively regulates its own operon, whereas pqrB encodes a putative efflux pump that most likely contributes to drug resistance.
MATERIALS AND METHODS
Bacterial strains, vectors, and culture conditions.
S. coelicolor A3(2) strains (J1501 and their derivatives) were grown as described by Hopwood et al. (23). For liquid culture, pregerminated spores (about 108 to 109 spores/100 ml of broth) were inoculated in YEME medium containing either 34 or 10.3% sucrose. For plate culture, 107 pregerminated spores or patches of mycelium were inoculated on R2YE or NA medium. The growth phases were determined as described by Cho and Roe (9). Escherichia coli DH5α, methylation-negative E. coli ET12567 (26), and S. coelicolor A3(2) J1501 cells were used as hosts for various recombinant DNAs. The nonmethylating E. coli ET12567, which contains the conjugation driver plasmid, pUZ8002 (gift from D. Figurski), was used for conjugation with J1501. Cosmid clones were from the ordered library of Redenbach et al. (33). Integration vector pDH5 or pSET152 was used to direct recombination through the cloned insert or viral attachment site (φC31 att), respectively (3, 20).
DNA manipulations.
DNA restriction and modifying enzymes were used as recommended by the manufacturer (Poscochem, Boehringer-Mannheim, or NEB). Standard recombinant DNA techniques were used as described by Sambrook et al. (35). DNA fragments were purified from agarose gels with the GeneClean Kit II (BIO101) or the freeze-squeeze method.
Cloning of the mutant pqr gene by use of ordered cosmids.
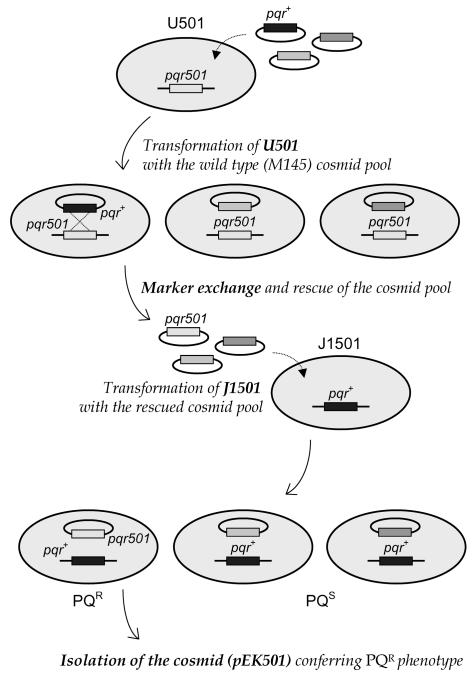
Since the pqr locus of paraquat-resistant mutant U501 was mapped near argA (11), we transformed mutant U501 with each of 40 cosmid clones (from G2 to C54) covering AseI fragments (G, C, and I) near the argA locus of the S. coelicolor genome (33). Each cosmid was first passaged through E. coli ET12567 to avoid methyl-specific restriction in S. coelicolor (26). Over 2,000 transformants all retained the mutant phenotype, suggesting that the mutation might be dominant. We therefore applied a second transformation step, as summarized in Fig. 1. Expecting some marker exchange between the genes on the cosmids and the chromosome, we obtained cosmids from the pool of U501 transformants. The wild-type J1501 cells were then transformed with the rescued cosmid pool. One cosmid clone (pEK501-71) that allowed J1501 to sporulate normally in the presence of paraquat was selected. Various subfragments from pEK501-71 were cloned in pDH5 and tested for paraquat resistance after transformation of J1501 cells.
FIG. 1.
Cloning strategy for the dominant pqr501 mutant gene, using ordered cosmids. The mutant allele conferring the paraquat-resistant sporulation phenotype in strain U501 was isolated on the basis of the dominant nature of the pqr501 mutation, as summarized in the figure. The cosmid pool containing the pqr501 gene was recovered and introduced by marker exchange into the wild-type J1501 cells. Transformants that sporulated normally in the presence of paraquat were selected, and the cosmid containing the mutant allele was isolated.
Cloning of the wild-type pqrAB gene by screening of a phage library.
The 2.2-kb EcoRV-BglII fragment from the mutant pqr gene was used as a probe to screen a λEMBL3 genomic library of S. coelicolor A3(2) M145. Among 20 primary phage candidates, λP123 contained the 2.2-kb EcoRV-BglII fragment that hybridized with the probe DNA. This 2,217-bp EcoRV-BglII fragment and an overlapping 2,436-bp NcoI fragment from λP123, encompassing the whole pqrAB operon, were sequenced and used for further analyses.
Nucleotide sequence analysis.
Nucleotide sequence comparison was done by using the BLAST (1) and Clustal W (40) programs. Omiga 2.0 (Oxford Molecular) was used for general analyses and manipulations of sequence data. Prediction and analysis of open reading frames (ORFs) were carried out by using Frame 2.3 (http://watson.nih.go.jp/∼jun/cgi-bin/frameplot.pl).
RNA analysis by S1 nuclease mapping.
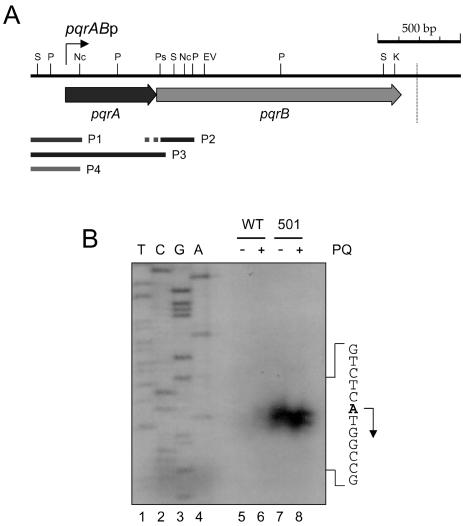
Isolation of RNA and S1 nuclease mapping was carried out as described elsewhere (7). For low-resolution S1 mapping, the probes P2 (156-bp PstI-PvuII-digested fragment with 103 bp of unrelated vector sequences) and P3 (798 bp generated by PCR) were used, as shown in Fig. 5A. PCR amplification was carried out with the downstream primer C1 (5′-GG CTG CAG GGT GCG GTT CAT G-3′; 5′ end at +568 relative to the transcription start site) and the upstream primer B/H (5′-AG CTC GGA TCC AAG CTT CAC G-3′; 5′ end at −230 relative to the transcription start site) (contains BamHI and HindIII sites [underlined], with nucleotide changes shown in bold). For high-resolution S1 mapping, a shorter probe (292 bp; P1 in Fig. 5A) was prepared by PCR using the downstream primer C3 (5′-GT GGA CTT CCG GGT CAG CAG GG-3′; 5′ end at +62 relative to the transcription start site) and the upstream primer B/H.
FIG. 5.
The structure of the pqrAB operon. (A) Restriction map around the pqrAB operon. The coding regions of pqrA and pqrB are indicated by arrows. Abbreviations for restriction enzymes are as described for Fig. 2A and as follows: P, PvuII; S, SalI. The probes used for this study are shown by solid lines; P1 (292 bp) was used for high-resolution S1 mapping, P2 (259 bp, with the 103-bp unrelated sequences dotted) and P3 (798 bp) were used for low-resolution S1 mapping, and P4 (290 bp) was used for gel mobility shift assays. The transcription start site is marked by a bent arrow (pqrABp). The vertical dotted line downstream of the pqrB gene marks the position of a predicted intrinsic transcription termination signal. (B) High-resolution S1 mapping of the 5′ end of the pqrAB transcript. J1501 (WT) (lanes 5 and 6) and U501 (lanes 7 and 8) cells were grown in YEME medium containing 34% sucrose for 30 h and subjected to 200 μM paraquat treatment (lanes 6 and 8) or no further treatment (lanes 5 and 7) for 1 h before cell harvesting. The 292-bp probe P1 was generated by PCR. The same downstream primer was used to generate the DNA sequencing ladders (lanes 1 to 4). The transcription start site for pqrA is indicated by an arrow from the A residue (bold) on the sense sequence.
Construction of pqrA and pqrAB null mutants.
To create the pqrA and pqrAB null mutants, Redirect technology was used as described previously (15). Disruption cassettes containing oriT and an apramycin gene were generated by PCR using pIJ773 as a template and appropriate primer pairs. For pqrA deletion, the A-up (GAG ACA AAA TTG TCT CAT GTG GGT TAT TGT TGT CTC ATG ATT CCG GGG ATC CGT CGA CC) and A-down (GGG CCG GCT GCA GGG TGC GGT TCA TGG TTC CTC TCT CCG TGT AGG CTG GAG CTG CTT C) primers were used, both of which have 39-nucleotide extensions (underlined) around the start and stop codons (in bold) of the pqrA coding region. A-down includes the putative ribosome-binding site for pqrB (italics) to ensure its translation in the pqrA deletion background. For pqrAB deletion, the A-up and B-down (TGA GCG GTC CGC CGT ATC GCG TTG GCG CGC CCC GGG TTA TGT AGG CTG GAG CTG CTT C) primers were used; B-down contains a 39-nucleotide sequence (underlined) extending downstream of the pqrB stop codon (bold). Both PCR products were gel purified and introduced into E. coli BW25113 containing the pqrAB cosmid L24 (provided by H. Kieser, John Innes Center) and a second plasmid, pIJ790, with λRED functions for linear recombination. The PCR-targeted recombinant cosmid was passaged through E. coli ET12567 containing pUZ8002 and then introduced into S. coelicolor J1501 by conjugation as described previously (6). Double-crossover mutants were identified as being apramycin resistant and kanamycin sensitive. Representative recombinants, JA603 (ΔpqrA) and JS707 (ΔpqrAB), were chosen for further analysis after verification of their gene structures by Southern blot analyses.
Overproduction and purification of PqrA proteins.
PCR was done with J1501 genomic DNA as a template and with primer C1 and a mutagenic primer containing an NdeI site at the translational start codon. The resulting PCR product was initially cloned in pUC18 at the HincII site, and the insert was cut out by NdeI and BamHI. The NdeI-BamHI fragment was cloned into pET-15b (Novagen) to yield pET506. A fresh colony of E. coli BL21(DE3)pLysS harboring pET506 was grown and induced as described previously (6). Cells were resuspended in a buffer composed of 5 mM imidazole, 500 mM NaCl, and 20 mM Tris-HCl (pH 7.9) and were disrupted by sonication. The recombinant His-tagged PqrA protein was recovered from the soluble fractions. The lysate was centrifuged at 16,000 × g for 10 min, and the supernatant was subjected to Ni-nitrilotriacetic acid column chromatography as recommended by the manufacturer (Novagen). The eluate was dialyzed against TGED buffer (50 mM NaCl, 50 mM Tris-HCl [pH 7.9], 5% glycerol, 0.1 mM EDTA, 0.1 mM dithiothreitol) containing 50% glycerol and was used for a gel mobility shift assay. The same primers and procedures were applied to obtain mutant PqrA protein [PqrA(R18Q)], except for the template (U501 genomic DNA).
Western blot analysis.
Mycelial cells were harvested and suspended in TGED buffer containing 1 mM phenylmethylsulfonyl fluoride. They were disrupted by sonication and clarified by centrifugation at 4°C. The protein concentration in cell extracts was determined by use of a protein assay kit (Bio-Rad). Anti-PqrA antiserum was prepared against purified PqrA protein, and Western blot analysis was performed as described elsewhere (8).
Gel mobility shift assay.
The gel mobility shift assay was carried out as described previously (10, 18). The 290-bp pqrAB promoter region from −218 to +72 nucleotides relative to the transcription start site was prepared by digesting the 798-bp PCR product with HindIII and NcoI (P4 in Fig. 5A). The labeled probe (about 30,000 cpm for <0.1 pmol per each reaction) was incubated with 10 pmol of purified PqrA in 20 μl of binding buffer (4 mM Tris-HCl [pH 8.0], 1 mM EDTA, 4 mM dithiothreitol, 5 mM MgCl2, 20 mM KCl, 0.3 μg of bovine serum albumin per ml, 10% glycerol) containing 1 μg of poly(dI-dC) at 25°C for 30 min. The DNA-protein mixture was electrophoresed on a 5% native polyacrylamide gel in 0.5× Tris-borate-EDTA buffer (35) and was analyzed by autoradiography.
Nucleotide sequence accession number.
The nucleotide sequences of the pqrAB operon and its flanking region were submitted to the DDBJ/EMBL/GenBank databases under accession number AF160959.
RESULTS
Cloning of the mutant and wild-type pqr genes.
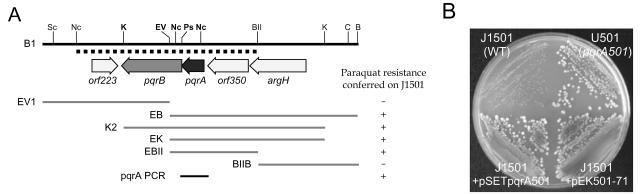
The pqr loci of the three paraquat-resistant mutants (U501, U605, and U607) were all mapped near argA on the S. coelicolor chromosome (11). Following the scheme summarized in Fig. 1, and based on initial indications that the mutation might be dominant, we cloned the mutant pqr (pqr501) gene from U501 by using ordered cosmids encompassing sufficient regions around argA. We screened and isolated a cosmid (pEK501-71) that contained pqr501 by marker exchange as described in Materials and Methods. When cosmid pEK501-71 was introduced into J1501, the transformants showed a mutant phenotype, sporulating normally in the presence of paraquat (Fig. 2B). Several restriction fragments of the insert in the pEK501-71 cosmid were subcloned into pDH5 and introduced into J1501 as shown in Fig. 2A. Thiostrepton-resistant transformants were selected and tested for paraquat resistance. The smallest restriction fragment that conferred resistance was the EcoRV-BglII fragment of about 2.2 kb (EBII in Fig. 2A). This fragment and flanking DNA to a total of about 4.5 kb were sequenced. The fragment EBII contained two complete (pqrA and orf350) and two truncated (argH and pqrB) coding regions. Sequence comparison with the S. coelicolor genome database (http://www.sanger.ac.uk/Projects/S_coelicolor/) revealed that the pqrAB gene resides within cosmid L24 (accession number AL157956), which also contains the argA gene, consistent with our previous genetic mapping result. The coding regions of pqrA and pqrB correspond to the annotated ORFs SCL24.04c and SCL24.03c, respectively.
FIG. 2.
Identification of the pqrA gene conferring paraquat resistance. (A) Physical mapping of the gene conferring the paraquat resistance phenotype. A restriction map of the 7-kb EcoRI-BamHI (B1) fragment from the pEK501-71 cosmid is presented. Five ORFs are shown as arrows. Various subfragments (EV1, EB, K2, EK, EBII, and BIIB) from fragment B1 were cloned into pDH5, introduced into the wild-type (J1501) cells, and examined for the ability to confer paraquat resistance to J1501 cells on NA plates containing 100 μM paraquat. The dotted line beneath the physical map denotes the 4,577-bp NcoI-BglII fragment whose nucleotide sequence was determined. The following abbreviations were used for the restriction enzymes: B, BamHI; BII, BglII; C, ClaI; EV, EcoRV; K, KpnI; Nc, NcoI; Ps, PstI; Sc, SacII. (B) Verification of the paraquat resistance phenotype conferred by the pqrA501 gene. The entire coding region of the pqrA501 gene from the pEK501-71 cosmid was amplified by PCR (pqrA PCR) and cloned into pSET152 to make pSETpqrA501. Pregerminated spores from the parental wild-type J1501, the resistant mutant U501, J1501 harboring pEK501-71, and J1501 harboring pSETpqrA501 were streaked on NA containing 100 μM paraquat and incubated at 30°C for 4 days.
The pqrA stop codon overlapped the pqrB initiation codon (A TGA), suggesting that the two genes are cotranscribed. We cloned the PCR-amplified pqrA501 gene into the pSET152 vector to make pSETpqrA501, which can integrate into the chromosome via the φC31 att site. When pSETpqrA501 was introduced into J1501, it conferred resistance to paraquat (Fig. 2B). These results clearly demonstrate that the pqrA501 gene is a dominant allele that caused the paraquat-resistant phenotype in U501.
pqrA and pqrB encode a repressor-like DNA-binding protein and an efflux pump protein, respectively.
The pqrA gene encodes a protein of 183 amino acids (aa) (20,052 Da). PqrA contains a predicted DNA-binding motif (12) resembling those found in TetR-like DNA-binding proteins and is most closely similar to NfxB of Pseudomonas aeruginosa (30.3% identity, 51.9% similarity) and also similar to YvkB of Bacillus subtilis (17.7% identity, 39.2% similarity). NfxB negatively regulates the mexC-mexD-oprJ operon encoding a drug efflux system (13, 29, 32). NfxB also binds to the upstream region of its own gene, resulting in autorepression (38). The function of YvkB in B. subtilis is not yet known.
The pqrB gene encodes a predicted membrane protein of 510 aa (52,144 Da) which is highly homologous to the major facilitator superfamily (MFS) proteins of multidrug resistance efflux pumps found in many bacteria (34). Some of the related proteins in gram-positive bacteria are involved in drug resistance, probably by serving as proton-dependent drug efflux systems. Interestingly, pqrB is homologous to yvkA of B. subtilis, the start codon of which also overlaps with yvkB, a pqrA homologue. The similarity between the S. coelicolor pqrAB and B. subtilis yvkBA genes implies a conserved function for these putative regulator-efflux pump systems in both organisms and a common regulatory mechanism that may involve similar upstream cis elements.
A pqrA null mutant is as resistant to paraquat as the pqrA501 mutant, whereas a pqrAB null mutant is hypersensitive to paraquat.
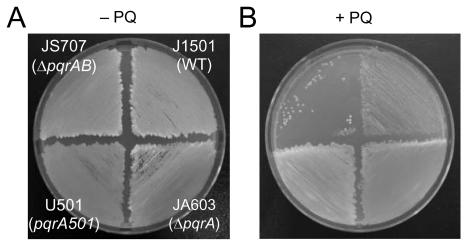
To assess the roles that pqrA and pqrB play in paraquat resistance, we constructed pqrA and pqrAB null mutants of S. coelicolor J1501 cells, using a newly developed, PCR-targeted mutagenesis system called Redirect (15). As described in Materials and Methods, apramycin-resistant and kanamycin-sensitive colonies were isolated as apparent double-crossover exconjugants whose expected genomic structures were verified by Southern blot analyses (data not shown). As shown in Fig. 3, a pqrA null mutant (JA603) exhibited almost the same resistance to paraquat as the pqrA501 mutant (U501), implying that pqrA501 is a loss-of-function mutant whose dominant nature might be attributed to a dominant-negative suppression of the wild-type allele. A pqrAB null mutant (JS707), however, exhibited hypersensitivity to paraquat, as measured by growth impairment in the presence of 200 μM paraquat (Fig. 3B). These results suggest that pqrA is antagonistic to the paraquat-resistant phenotype, probably acting as a negative regulator for the pqrB gene, which is required for paraquat resistance.
FIG. 3.
Paraquat sensitivity of pqrA and pqrAB null mutants. Equal amounts of pregerminated spores from the wild type (J1501) (WT) and from pqrA501 (U501), ΔpqrA (JA603), and ΔpqrAB (JS707) mutants were streaked on NA plates without (A) or with (B) 200 μM paraquat (PQ). Plates were incubated at 30°C for 4 days before pictures were taken.
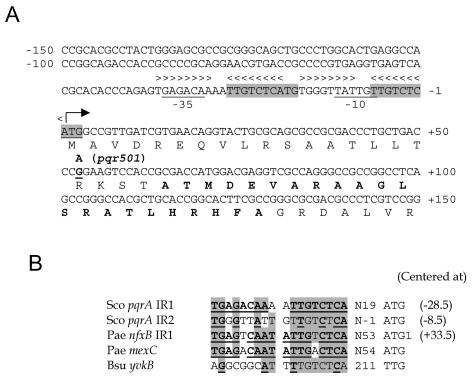
The comparison between the pqr genes from the wild type (M145) and pEK501-71 revealed that the pqr501 mutation is a transition from G to A, resulting in an amino acid change from arginine (R) to glutamine (Q) at position 18 (R18Q) in the pqrA coding region (Fig. 4). We verified, by PCR amplification and sequencing of the pqrA alleles from both the original mutant U501 and the parental strain J1501, that the sequence data from the M145 strain and the marker-exchanged cosmid, pEK501-71, were the same as those from the original strains (data not shown). As demonstrated in Fig. 4, Arg-18 lies just ahead of the putative helix-turn-helix motif of 22 aa starting from Ala-22 (12).
FIG. 4.
Amino acid sequence comparison of PqrA and related proteins. PqrA protein (ScoPqrA) was compared with NfxB from P. aeruginosa (PaeNfxB) and a hypothetical protein from B. subtilis (BsuYvkB). The TetR-type helix-turn-helix motifs of 22 aa are marked in bold. The R-to-Q substitution in the pqrA501 mutant is marked in bold and underlined. The residues that are mutated in the nfxB13E (R42G) and K385 (H87R) alleles from P. aeruginosa are shaded. Fully conserved residues are marked by asterisks. Similar matches are marked by colons and dots according to the definitions in the Clustal W program (40).
Determination of the transcription start site.
The transcription start site was determined by both low- and high-resolution S1 nuclease mapping. To evaluate the cotranscription of pqrA and pqrB, we performed low-resolution S1 mapping using a 798-bp probe, P3, generated by PCR (Fig. 5A). This probe encompasses the entire pqrA gene and an N-terminal portion (18 bp) of pqrB (Fig. 5A). We observed only one protected product, of about 570 nucleotides, suggesting that there is no separate promoter for pqrB (data not shown). The amount of protected band was very small for J1501 in comparison with that for the U501 mutant, suggesting that pqrAB expression is derepressed in the U501 mutant. We determined the transcription start site for pqrA, by high-resolution S1 mapping, to be the A residue that is the first base of the predicted initiation codon, ATG (Fig. 5B).
Inspection of the sequence upstream of the coding region revealed a putative promoter element (GAGACA-N18-TATTGT) (Fig. 6A) that shows some marginal resemblance to the promoters recognized by the principle sigma factor σHrdB in Streptomyces spp. At least two pairs of inverted repeat sequences were found upstream of the pqrA gene that resemble NfxB binding sites in nfxB and mexC genes in P. aeruginosa (Fig. 6B) (38). Although the positions of the inverted repeat sequences in pqrA (IR1 and IR2) relative to the transcription start site differ from those recognized by NfxB in P. aeruginosa, we postulate that PqrA may act as an autoregulatory repressor like NfxB.
FIG. 6.
Nucleotide sequence of the promoter region of the pqrA gene. (A) The nucleotide sequences around the promoter region of the pqrAB genes are presented. The transcription start site is marked by a bent arrow. The putative promoter elements and the initiation codon are underlined. Two sets of inverted repeat sequences, from −37 to −20 and from −17 to + 1, are marked with arrowheads. One direct repeat of nucleotides from −27 to + 3 is shaded. The amino acids for the TetR-type helix-turn-helix motif are marked in bold. The point of one nucleotide change, from G to A, at +53 in the pqrA501 allele is marked. (B) Comparison of inverted repeat sequences. The two inverted repeat sequences (IR1 and IR2) of pqrA (Sco pqrA) were aligned with related sequences. The binding sites of NfxB in the nfxB and mexC genes of P. aeruginosa (Pae) are presented (38), along with a similar sequence from the yvkBA operon of B. subtilis (Bsu). Nucleotides common to at least four sequences are shaded. Palindromic residues are marked in bold and underlined for each sequence. The center positions relative to the transcription start site are shown in parentheses.
PqrA functions as an autoregulator.
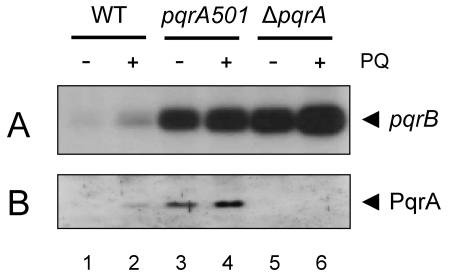
We measured the level of pqrAB mRNA from the wild type (J1501) and pqrA501 and ΔpqrA mutants by S1 nuclease mapping with the 259-bp probe P2 (Fig. 5A). As shown in Fig. 7A, the basal transcript level was elevated about sixfold in the pqrA501 mutant and about eightfold in the ΔpqrA mutant compared with that of the wild type. The level of PqrA protein was also elevated about fivefold in the pqrA501 mutant, when monitored by Western blot analysis. No PqrA protein was detected in the ΔpqrA mutant, as expected (Fig. 7B). These results imply that PqrA functions as a negative regulator for its own operon, and the mutant protein (R18Q) has lost this ability, thus derepressing the operon in the pqrA501 mutant. When treated with paraquat (200 μM), the amount of pqrAB transcript as well as PqrA protein increased about twofold in the wild type. In the two mutants, the induction was very marginal, about 20%.
FIG. 7.
Gene expression of pqrAB in pqrA501 and ΔpqrA mutants. Expression of the pqrAB genes in the wild type (J1501) and in pqrA501 and ΔpqrA mutants was examined by S1 nuclease mapping with probe P2 to detect pqrB transcripts (A) and by Western blot analysis with antibody against PqrA (B) as described in Materials and Methods. Cells were grown in YEME medium containing 10.3% sucrose to mid-exponential-growth phase (18 h) and were treated with 200 μM paraquat (PQ) (lanes 2, 4, and 6) for 1 h before preparing RNA or cell extracts.
PqrA specifically binds to its promoter region.
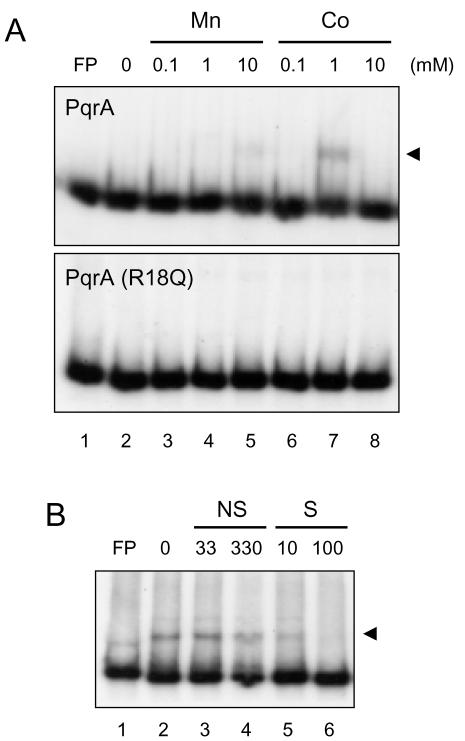
To examine the interaction of PqrA with the pqrAB promoter region, we performed gel mobility shift assays with cell extracts prepared from the wild type and the pqrA501 and ΔpqrA mutants. With all the extracts tested, the 290-bp DNA probe (−218 to +72 nt; P4 in Fig. 5A) formed a strong complex, suggesting that cell extracts are not good to use for detecting PqrA-specific binding (data not shown). We purified both wild-type and PqrA501 mutant proteins and performed gel shift assays. As shown in Fig. 8, a specific binding complex was observed between PqrA and probe P4 in the presence of 1 mM cobalt cation (Co2+). Manganese (Mn2+) at 10 mM slightly enhanced binding as well. The effect of Co2+ on PqrA binding in vitro is consistent with the observation that pqrAB transcripts decrease when cells are treated with Co2+ (data not shown). Under all the conditions we tested, we were not able to observe any significant binding of purified PqrA501 (R18Q) to the DNA probe. These results clearly demonstrate that PqrA acts as a direct autoregulator of its own operon and that PqrA501 lost the ability to bind to the pqrA promoter and caused derepression.
FIG. 8.
Binding assay with purified PqrA proteins. (A) Gel mobility shift assay using 10 pmol of either purified wild-type PqrA (top) or the PqrA(R18Q) variant (bottom) and the labeled 290-bp pqrAB promoter fragment (probe P4) in the presence of either MnCl2 or CoCl2, from 0.1 to 10 mM as indicated. FP, free probe only. Arrowheads indicate binding complexes. (B) Specific binding of purified PqrA to the pqrAB promoter fragment. The binding buffer contained 1 mM Co2+. Ten picomoles of purified PqrA was incubated with either specific (S) or nonspecific (NS) competitors and then with the labeled probe. Nonspecific competitors [HaeIII digests of pGEM-3Zf(+)] (lanes 3 and 4) and specific competitors (unlabeled probe DNA) (lanes 5 and 6) were added in molar excess as indicated.
DISCUSSION
Taking advantage of the ordered cosmid library, we applied a map-based cloning strategy to isolate the dominant pqr501 mutant allele responsible for normal sporulation in the presence of paraquat. In S. coelicolor, it has been possible to clone novel genes by transforming the recessive mutant cells with a minimal set of genomic cosmids encompassing the mutated loci (17). To isolate the dominant mutant gene, we applied a novel strategy exploiting a marker exchange between the genes on cosmids and a mutant chromosome. The marker exchange proceeds through integration of cosmid via homologous recombination and excision of cosmid with the mutant allele via another homologous recombination. Some U501 transformants showed stronger resistance phenotypes in the course of our screening procedure (data not shown). This might be attributed to the rare event of homogenotization, resulting in the loss of a wild-type pqr allele and establishing two copies of the pqr501 allele. This procedure is an attractive approach to isolate the mutant gene that is dominant to the wild type, as an alternative to the construction of shotgun libraries for each mutant strain.
Paraquat resistance might result from several causes, including (i) an increase in export, (ii) a decrease in import, (iii) an increase in removal of toxic effects, or (iv) a decrease in generation of toxic effects. When paraquat-resistant mutants were initially isolated, the levels of several antioxidant defense enzymes were monitored. We observed that the levels of superoxide dismutases (NiSOD and Fe/ZnSOD) and catalases (CatA, CatB, and CatC) did not change in pqr mutants (data not shown), suggesting that the resistance may not be due to enhanced removal of toxic reactive oxygen species generated by paraquat. The isolation and characterization of the pqr gene revealed that PqrA is a TetR family repressor that negatively regulates the pqrAB operon encoding PqrA itself and PqrB, a putative drug efflux protein. Therefore, it is most likely that the paraquat resistance phenotype of U501 resulted from overproduction of PqrB, which can export paraquat, due to the failure to repress the operon in the mutant. The paraquat-resistant and -sensitive phenotypes of ΔpqrA and ΔpqrAB mutants, respectively, support this prediction. This proposal is consistent with the action mode postulated for PqrB homologues in other organisms, such as MvrA in E. coli and SmvA in Salmonella enterica serovar Typhimurium, that are required for paraquat resistance (22, 27).
We found that the R18Q mutation in pqr501 causes almost complete loss of DNA-binding activity, and it is dominant to the wild type. Arg-18 resides three residues ahead of the helix-turn-helix motif. PqrA may bind to its target sequence as an oligomer, most likely as a dimer, as observed for other TetR family regulators (21, 36). If Pqr501 is impaired in DNA-binding ability but still able to form oligomers with wild-type proteins, then the dominant nature of the mutant can be explained. NfxB in P. aeruginosa, the closest homologue of PqrA, negatively regulates its own gene as well as the mexC-mexD-oprJ operon (32). Two known nfxB mutations (H87R and R42G) that fail to repress target genes and cause multiple antibiotic resistance are recessive to the wild-type allele (30, 32). Although the in vitro binding results are not available, both mutations are thought to reduce the DNA-binding activity of NfxB. Considering that Arg-42 in NfxB resides within the helix-turn-helix motif, it is conceivable that R42G mutation affected the DNA-binding activity. The contribution of His-87, which is located further downstream from the helix-turn-helix motif, is less clear. More mutant alleles of pqrA need to be analyzed in order to elucidate the precise action mechanism of PqrA.
In P. aeruginosa, the nfxB gene resides immediately upstream of the mexC-mexD-oprJ operon in the opposite orientation, sharing the two inverted repeats where NfxB binds (38). As a gram-negative bacterium, P. aeruginosa possesses a resistance-nodulation-division family efflux transporter that exports various drugs. It consists of an outer membrane channel (OprJ), a cytoplasmic membrane efflux transporter (MexD), and an accessory linker protein (MexC) that is associated with OprJ and MexD in the periplasm (29). As a gram-positive bacterium, S. coelicolor possesses MFS efflux systems. An inspection of the genome sequence revealed that there are at least 28 putative MFS efflux systems in S. coelicolor, as judged by their sequence characteristics (14). As one of the MFS membrane proteins, PqrB could be one component involved in drug efflux, extruding paraquat. In S. coelicolor, the repressor PqrA is synthesized from the same transcription unit as PqrB. The presence of inverted repeat sequences that resemble the NfxB binding site implies that PqrA may also recognize this sequence. B. subtilis, another gram-positive bacterium, possesses yvkBA genes that are highly homologous to pqrAB, and there is a similar cis element upstream of the yvkB gene. These observations led us to postulate that the drug efflux systems of P. aeruginosa, B. subtilis, and S. coelicolor share common features in function as well as in regulation. The role that the pqrAB system plays in growth, stress responses, and differentiation needs to be elucidated further. The regulation mechanism for this operon includes autoregulation by PqrA, a small induction by paraquat, and repression by certain metals such as Co2+. Whether the activity of PqrA itself is sensitive to paraquat remains to be determined.
Acknowledgments
We are indebted to H. Kieser for kind provision of strains and ordered cosmids.
This work was supported by a research grant from Korea Science and Engineering Foundation (KOSEF) to J.-H. Roe (R01-1999-00068) and by funding from the BBSRC/KOSEF United Kingdom-Korea Joint Programme in Actinomycete Research. B.-E. Ahn was the recipient of a predoctoral fellowship from the BK21 Program of the Ministry of Education and Human Resources. Y.-H. Cho was supported by a Sogang University Research Grant (2002).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentley, S. D., et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 3.Bierman, M., R. Logan, K. O' Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 4.Candenas, E. 1989. Biochemistry of oxygen toxicity. Annu. Rev. Biochem. 58:79-110. [DOI] [PubMed] [Google Scholar]
- 5.Chater, K. F. 1998. Taking a genetic scalpel to the Streptomyces colony. Microbiology 144:1465-1478. [DOI] [PubMed] [Google Scholar]
- 6.Cho, Y.-H., E.-J. Lee, B.-E. Ahn, and J.-H. Roe. 2001. SigB, an RNA polymerase sigma factor required for osmoprotection and proper differentiation of Streptomyces coelicolor. Mol. Microbiol. 42:205-214. [DOI] [PubMed] [Google Scholar]
- 7.Cho, Y.-H., J.-S. Hahn, and J.-H. Roe. 2000. Analysis of the dual promoters and the negative cis-element, HRE, controlling H2O2-responsive expression of Streptomyces coelicolor catA. J. Microbiol. 38:239-244. [Google Scholar]
- 8.Cho, Y.-H., E.-J. Lee, and J.-H. Roe. 2000. A developmentally regulated catalase required for proper differentiation and osmoprotection in Streptomyces coelicolor. Mol. Microbiol. 35:150-160. [DOI] [PubMed] [Google Scholar]
- 9.Cho, Y.-H., and J.-H. Roe. 1997. Isolation and expression of the catA gene encoding the major vegetative catalase in Streptomyces coelicolor Müller. J. Bacteriol. 179:4049-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung, H.-J., J.-H. Choi, E.-J. Kim, Y.-H. Cho, and J.-H. Roe. 1999. Negative regulation of the gene for Fe-containing superoxide dismutase by an Ni-responsive factor in Streptomyces coelicolor. J. Bacteriol. 181:7381-7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, H.-J., E.-J. Kim, E. Park, and J.-H. Roe. 1995. Isolation and genetic mapping of paraquat-resistant sporulating mutants of Streptomyces coelicolor. J. Microbiol. 33:215-221. [Google Scholar]
- 12.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottesman, M. M., and I. Pastan. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385-427. [DOI] [PubMed] [Google Scholar]
- 14.Griffith, J. K., M. E. Baker, D. A. Rouch, M. G. Page, R. A. Skurray, I. T. Paulsen, K. F. Chater, S. A. Baldwin, and P. J. Henderson. 1992. Membrane transport proteins: implications of sequence comparisons. Curr. Opin. Cell Biol. 4:684-695. [DOI] [PubMed] [Google Scholar]
- 15.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 100:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn, J.-S., S.-Y. Oh, and J.-H. Roe. 2002. Role of OxyR as a peroxide-sensing positive regulator in Streptomyces coelicolor A3(2). J. Bacteriol. 184:5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn, J.-S., S.-Y. Oh, K. F. Chater, and J.-H. Roe. 2000. Isolation of the regulator gene responsible for overproduction of catalase A in H2O2-resistant mutant of Streptomyces coelicolor. J. Microbiol. 38:18-23. [Google Scholar]
- 18.Hahn, J.-S., S.-Y. Oh, K. F. Chater, Y.-H. Cho, and J.-H. Roe. 2000. H2O2-sensitive Fur-like repressor CatR regulating the major catalase gene in Streptomyces coelicolor. J. Biol. Chem. 275:38254-38260. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell, B., and J. M. C. Gutteridge. 1989. Free radicals in biology and medicine, 2nd ed. Clarendon Press, Oxford, England.
- 20.Hillemann, D., A. Pühler, and W. Wohlleben. 1991. Gene disruption and gene replacement in Streptomyces via single stranded DNA transformation of integration vectors. Nucleic Acids Res. 19:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinrichs, W., C. Kisker, M. Duvel, A. Muller, K. Tovar, W. Hillen, and W. Saenger. 1994. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science 264:418-442. [DOI] [PubMed] [Google Scholar]
- 22.Hongo, E., M. Morimyo, K. Mita, I. Machida, H. Hama-Inaba, H. Tsuji, S. Ichimura, and Y. Noda. 1994. The methyl viologen-resistance-encoding gene smvA of Salmonella typhimurium. Gene 148:173-174. [DOI] [PubMed] [Google Scholar]
- 23.Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, J. M. Ward, and H. Schrempf. 1985. Genetic manipulation of Streptomyces: a laboratory manual. The John Innes Foundation, Norwich, Conn.
- 24.Liochev, S. I., and I. Fridovich. 1999. Superoxide and iron: partners in crime. IUBMB Life 48:157-161. [DOI] [PubMed] [Google Scholar]
- 25.Liochev, S. I., A. Hausladen, W. F. Beyer, Jr., and I. Fridovich. 1994. NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc. Natl. Acad. Sci. USA 91:1328-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacNeil, D. J. 1988. Characterization of a unique methyl-specific restriction system in Streptomyces avermitilis. J. Bacteriol. 170:5607-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morimyo, M. 1988. Isolation and characterization of methyl viologen-sensitive mutants of Escherichia coli K-12. J. Bacteriol. 170:2136-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nations, C., V. F. Allison, H. C. Aldrich, and R. G. Allen. 1989. Biological oxidation and the mobilization of mitochondrial calcium during the differentiation of Physarum polycephalum. J. Cell. Physiol. 140:311-316. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-387. [DOI] [PubMed] [Google Scholar]
- 30.Okazaki, T., and K. Hirai. 1992. Cloning and nucleotide sequence of the Pseudomonas aeruginosa nfxB gene, conferring resistance to new quinolones. FEMS Microbiol. Lett. 97:197-202. [DOI] [PubMed] [Google Scholar]
- 31.Paget, M. S., J.-B. Bae, M.-Y. Hahn, W. Li, C. Kleanthous, J.-H. Roe, and M. J. Buttner. 2001. Mutational analysis of RsrA, a zinc-binding anti-sigma factor with a thiol-disulphide redox switch. Mol. Microbiol. 39:1036-1047. [DOI] [PubMed] [Google Scholar]
- 32.Poole, K., N. Gotoh, H. Tsujimoto, Q. Zhao, A. Wada, T. Yamasaki, S. Neshat, J. Yamagishi, X. Z. Li, and T. Nishino. 1996. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol. Microbiol. 21:713-724. [DOI] [PubMed] [Google Scholar]
- 33.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 34.Saier, M. H., Jr., I. T. Paulsen, M. K. Sliwinski, S. S. Pao, R. A. Skurray, and H. Nikaido. 1998. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 12:265-274. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Schumacher, M. A., M. C. Miller, S. Grkovic, M. H. Brown, R. A. Skurray, and R. G. Brennan. 2002. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 21:1210-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott, M. D., and J. W. Eaton. 1996. Superoxide is not the proximate cause of paraquat toxicity. Redox Rep. 2:113-119. [DOI] [PubMed] [Google Scholar]
- 38.Shiba, T., K. Ishiguro, N. Takemoto, H. Koibuchi, and K. Sugimoto. 1995. Purification and characterization of the Pseudomonas aeruginosa NfxB protein, the negative regulator of the nfxB gene. J. Bacteriol. 177:5872-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toledo, I., A. A. Noronha-Dutra, and W. Hansberg. 1991. Loss of NAD(P)-reducing power and glutathione disulfide excretion at the start of induction of aerial growth in Neurospora crassa. J. Bacteriol. 173:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]