Abstract
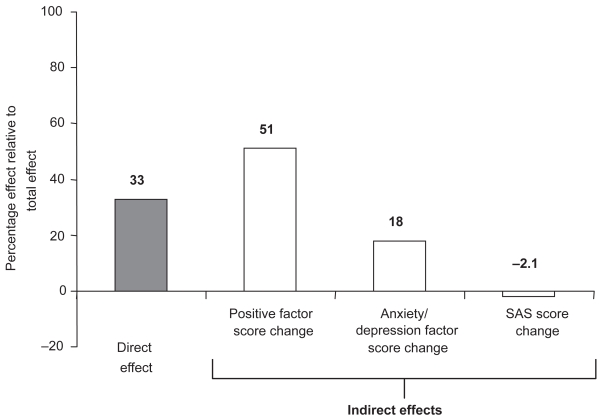
Direct and indirect effects of the new psychotropic paliperidone extended-release (paliperidone ER) tablets on negative symptom improvement in schizophrenia were investigated using path analysis. A post hoc analysis of pooled data from three 6-week, double-blind, placebo-controlled studies of paliperidone ER in patients experiencing acute exacerbation was conducted. Regression analysis explored relationships between baseline/study characteristics and negative symptoms. Change in Positive and Negative Syndrome Scale (PANSS) negative factor score at endpoint was the dependent variable; explanatory variables included demographic and clinical characteristics. Path analysis determined direct and indirect effects of treatment on negative symptom change. Indirect mediators of negative symptom change in the model included changes in positive symptoms, anxiety/depression symptoms and movement disorders. Path analysis indicated that up to 33% of negative symptom improvement was a direct treatment effect. Indirect effects on negative symptoms were mediated through changes in positive symptoms (51%) and anxiety/depression symptoms (18%), whereas changes in movement disorders had a 2.1% inverse effect. Path analysis indicated that paliperidone ER has a direct effect on negative symptoms. Negative symptom improvement also was indirectly mediated via changes in positive and depressive symptoms.
Keywords: antipsychotic, paliperidone ER, path analysis, psychotropic, schizophrenia
Introduction
In the 1970s, schizophrenia researchers began to focus attention on defining and characterizing schizophrenia psychopathology into distinct dimensions. Among the aims of classifying related symptoms into separate categories was to understand better how certain symptom clusters may change over time, how they may influence long-term outcomes and how antipsychotic treatment may affect these different manifestations of illness (Andreasen 1982; Carpenter et al 1985). Schizophrenia symptoms have been conceptualized most broadly as positive (eg, delusions, hallucinatory behavior, grandiosity) or negative (eg, blunted affect, emotional withdrawal, motor retardation) (Andreasen 1982; Kay et al 1987). Recent symptom clusters, derived from factor analysis of the Positive and Negative Syndrome Scale (PANSS), also include anxiety/ depression, disorganized thought and hostility/excitement (Marder et al 1997).
Incorporation of the concept of symptom clusters has clearly advanced the understanding of schizophrenia. However, negative symptoms have proved to be very difficult to define and study. As early as 1985, Carpenter et al (1985) recognized that negative symptoms may have multiple causes, and that not all negative symptoms are intrinsic to schizophrenia. They suggested that negative symptoms could be primary (core features) or secondary consequences of positive and/or mood symptoms, related to extrapyramidal side effects of certain antipsychotic medications or resulting from understimulating social environments, such as those associated with institutionalization (Carpenter et al 1985; Moller 1993).
Because negative symptoms are associated with functional disability and a poor prognosis, alleviation of such symptoms is an important goal of antipsychotic treatment (Davidson and McGlashan 1997; Milev et al 2005). Antipsychotic agents appear to have differential effects on negative symptoms. Indeed, a commonly cited distinction between conventional antipsychotics and atypical agents is the greater efficacy of atypical antipsychotics for negative symptoms (Moller et al 1995; Tollefson and Sanger 1997; King 1998; Tandon 2004). However, it is difficult to discern whether the potential benefits of atypical antipsychotics over conventional agents for negative symptoms are mediated through direct effects on primary symptoms, are indirect effects secondary to improvements in positive or mood symptoms or are due to the lower propensity of atypical agents to cause extrapyramidal symptoms (EPS) (Leucht et al 1999). Several atypical antipsychotic agents may improve negative symptoms both directly (direct effect) and indirectly (indirect effect), as suggested by reports that used path analysis (Moller et al 1995; Tollefson and Sanger 1997; Tandon 2004), which is an extension of multiple regression models (Pedhazur 1982).
Three double-blind, placebo-controlled, 6-week studies have demonstrated that an extended-release formulation of the psychotropic paliperidone (paliperidone extended-release [ER]) significantly reduces negative symptoms in acutely ill patients with schizophrenia (Davidson et al 2007; Kane et al 2007; Marder et al 2007). The objective of the present study was to apply path analysis to a pooled data set from these studies to assess the direct and indirect effects of paliperidone ER on negative symptom improvement.
Methods
Data for this post hoc analysis were pooled from 3 multicenter, double-blind, randomized, placebo-controlled, parallel-group, 6-week studies of paliperidone ER. Preliminary efficacy and safety findings of these studies have been reported elsewhere (Davidson et al 2007; Kane et al 2007; Marder et al 2007). All 3 studies had similar populations and identical study designs, with the same entry criteria, efficacy variables, rating guidelines, rater training procedures and time points. Geographical regions/countries included Western Europe (France, Greece, The Netherlands, and Spain), Eastern Europe (Bulgaria, Croatia, Estonia, Poland, Russia, Slovakia, Ukraine, and Romania), North America (United States and Canada), Asia (Hong Kong, Malaysia, Republic of Korea, Singapore, and Taiwan), India, Israel, Mexico, and South Africa. Independent ethics committees or institutional review boards approved the final study protocol before study initiation. Each subject provided written consent according to local requirements after the nature of the study had been fully described and was willing and able to complete self-administered questionnaires and be compliant with medication. The studies were conducted in accordance with current Good Clinical Practice guidelines and the Declaration of Helsinki and its subsequent revisions.
Subjects
Included subjects were male or female, ≥18 years of age, with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of schizophrenia for ≥1 year. Subjects had to have been experiencing active symptoms at the time of enrollment, with a PANSS total score of 70–120 points at screening and baseline, and to have agreed to voluntary hospitalization for at least 14 days. Female subjects of childbearing age had to have a negative pregnancy test result and be using an acceptable form of contraception.
Subjects were excluded if they had poor general health or chronic health conditions; a history of severe pre-existing gastrointestinal narrowing; any laboratory results not within the reference range (or deemed by the investigator to be clinically significant); an Axis I diagnosis other than schizophrenia; a DSM-IV diagnosis of substance dependence within 6 months before screening or if deemed at significant risk of suicide or violent behavior. Subjects were also excluded if they had allergy or hypersensitivity to phenytoin, carbamazepine, barbiturates, lamotrigine, risperidone, paliperidone, or olanzapine; previous lack of response to risperidone or a history of neuroleptic malignant syndrome. Other exclusions included treatment with any of the following: monoamine oxidase inhibitors (within 4 weeks before screening), other antidepressants (unless on a stable dose for ≥3 months before baseline), beta-blockers (for any use other than hypertension), depot antipsychotic (within 120 days before screening), paliperidone palmitate (within 10 months of screening), mood stabilizers (within 2 weeks before baseline), any experimental device or drug (within 90 days before screening), or electroconvulsive therapy (within 3 months before screening).
Treatment
Subjects included in this post hoc analysis were those randomly assigned to receive paliperidone ER (3, 6, 9, 12, or 15 mg) or placebo for 6 weeks once daily in the morning. No dose adjustment was permitted, except for a titration during the first week for subjects receiving paliperidone ER 15 mg.
Measures
In order to ensure inter-rater reliability, clinicians received training and qualification on key efficacy and safety variables. The primary efficacy measures of interest in these studies were collected via the PANSS (Kay et al 1987). PANSS negative symptoms were described by the negative factor score (sum of blunted affect, emotional withdrawal, poor rapport, passive social withdrawal, lack of spontaneity, motor retardation, and active social avoidance), positive symptoms were described by the positive factor score (sum of delusions, hallucinatory behavior, grandiosity, suspiciousness, stereotyped thinking, somatic concern, unusual thought content, lack of judgment, and insight) and anxiety/depression symptoms were described by the anxiety/depression factor score (sum of anxiety, guilty feelings, tension and depression) (Marder et al 1997). The PANSS was administered at baseline and study days 4, 8, 15, 22, 29, 36, and 43.
Other measures included the Clinical Global Impressions–Severity (CGI-S) scale (Guy 1976) and the Personal and Social Performance Scale (PSP), a clinician-rated instrument that indicates an overall rating of personal and social functioning (Morosini et al 2000). The PSP measures 4 domains of functioning: socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behaviors. Ratings of 71–100 indicate only mild difficulties; ratings of 31–70 indicate varying degrees of disability and ratings of 0–30 indicate functioning so poor that the patient requires intensive support or supervision (Morosini et al 2000). The PSP was administered at baseline and endpoint.
Safety and tolerability were assessed via adverse event (AE) reporting and evaluation of EPS. AEs that occurred between the first and last study-related procedure were tabulated; these included AEs reported by subjects voluntarily, as well as those collected via interviewing of subjects in a nondirected manner. A treatment-emergent AE was defined as an AE that was either new in onset or increased in severity following the initiation of study treatment. Subjects were monitored for the incidence of EPS-related AEs, which included tremor, dystonia, hyperkinesia, parkinsonism, and dyskinesia. Severity of EPS was evaluated using the Simpson-Angus Scale (SAS) (Simpson and Angus 1970), the Abnormal Involuntary Movement Scale (AIMS) (Guy 1972), and the Barnes Akathisia Rating Scale (BARS) (Barnes 1989). The SAS rates 10 items, including gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, head dropping, glabella tap, tremor, and salivation, on a scale ranging from 0 (normal) to 4 (extreme). EPS assessments were performed at baseline and study weeks 1–6.
Analysis
The overall intent-to-treat (ITT) population was defined as all randomly assigned subjects who took at least one dose of paliperidone ER or placebo and had one postbaseline efficacy assessment. The path analysis included patients in the overall ITT population who had no missing data at both baseline and endpoint on the PANSS negative, positive, and anxiety/depression factor scores and on the SAS scores (path ITT population).
Outcome variables were analyzed at the 6-week endpoint, which utilized the last-observation-carried-forward approach. Analysis of covariance (ANCOVA) models compared change scores at endpoint for each paliperidone ER group with placebo. The ANCOVA model included treatment, study and analysis center within study as factors and the baseline score for the analyzed variable as a covariate. No adjustments for multiplicity were performed. Least squares estimates and p values for pairwise differences between paliperidone ER and placebo groups were based on this model. Statistical testing was performed at the 0.05 level using 2-tailed tests.
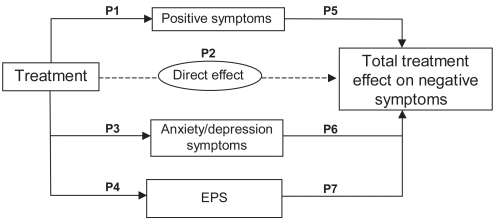
Path analysis was used to separate the total effects of paliperidone ER treatment on negative symptoms into direct and indirect effects. For the analysis, change in the PANSS negative factor score at endpoint was the dependent variable. Independent variables were chosen based on prior research (Moller et al 1995; Tollefson and Sanger 1997; Tandon 2004) and clinical judgment and included those that are thought to be mediators of negative symptom change: change in the PANSS positive and anxiety/depression factor scores and change in the SAS score for EPS at endpoint. Path coefficients determined the direct and indirect effects of paliperidone on the negative factor score (Figure 1). Structural equation modeling was used to examine the direct and indirect effects of paliperidone on negative symptoms. The regression models were as follows:
Figure 1.
Path model illustrating the relationships between the direct and indirect effects of treatment from positive symptoms, anxiety/depression symptoms, and EPS symptoms on negative symptoms. P1 Through P7 are path coefficients.
Abbreviation: EPS, extrapyramidal symptoms.
In these equations, ΔPositive is the change in PANSS positive factor score; ΔNegative is the change in PANSS negative factor score; ΔDepressive is the change in the anxiety/depression factor score; and ΔEPS is the change in the SAS score at endpoint. Baseline in each formula indicates the baseline score of the respective variable.
The total effect of treatment was determined by:
P1 through P7 in the above equation are the path coefficients and P2 is the direct effect of treatment. The direct effect of negative symptoms was defined as the treatment effect remaining after controlling for changes in positive symptoms, anxiety/depression symptoms and EPS. Path analysis assumes that a number of requirements have been met regarding the data as well as the theoretical model itself. Some important assumptions are that the data should follow a multivariate normal distribution; that there is absence of multicollinearity in the predictors; that error terms are uncorrelated and that a linear additive relationship exists between independent and dependent variables.
An initial multiple regression model was used to explore the relationship between negative symptoms and other selected demographic/clinical characteristics (ie, age, sex, race, change in PSP, duration of study drug exposure, duration since last psychotic episode) in the path ITT population.
Results
Approximately 15% of screened patients were not randomly assigned (2% due to missing data, and 98% due to screen failure). The overall ITT population included 1306 subjects and the path ITT population comprised 1274 subjects. Characteristics of the overall and path ITT populations were similar. A total of 937 patients in the path ITT population received paliperidone ER (3 mg, n = 120; 6 mg, n = 224; 9 mg, n = 243; 12 mg, n = 238; 15 mg, n = 112) and 337 patients received placebo. Baseline demographic and clinical characteristics were similar across treatment groups (Table 1). The majority of subjects were male (62%), mean age range was 36.2–39.5 years across the groups and most subjects (62%) were Caucasian. The most common diagnosis, occurring in 81% of patients, was paranoid schizophrenia. Mean baseline PANSS total score, ranging across groups from 91.6–94.4, reflected an acutely ill population, as did mean scores for negative, positive and anxiety/depression factor scores. Mean SAS scores were also similar and low across treatment groups at baseline (Table 1).
Table 1.
Baseline demographic and clinical characteristics (path analysis intent-to-treat population; n = 1274)
| Parameter | Paliperidone ER daily dose
|
||||||
|---|---|---|---|---|---|---|---|
| 3 mg (n = 120) | 6 mg (n = 224) | 9 mg (n = 243) | 12 mg (n = 238) | 15 mg (n = 112) | Combined (n = 937) | Placebo (n = 337) | |
| Age, mean years (±SD) | 36.2 (10.9) | 39.5 (10.6) | 37.4 (11.2) | 38.4 (11.0) | 37.7 (9.9) | 38.0 (10.8) | 39.2 (11.0) |
| Male gender, n (%) | 76 (63) | 129 (58) | 149 (61) | 144 (61) | 72 (64) | 570 (61) | 217 (64) |
| Age at diagnosis, mean years (±SD) | 25.8 (8.3) | 25.9 (8.6) | 26.6 (8.5) | 25.6 (9.0) | 25.2 (7.8) | 25.9 (8.5) | 26.6 (9.7) |
| Race, n (%) | |||||||
| Caucasian | 58 (48) | 148 (66) | 169 (70) | 156 (66) | 50 (45) | 581 (62) | 210 (62) |
| Black | 25 (21) | 58 (26) | 21 (9) | 63 (27) | 26 (23) | 193 (21) | 73 (22) |
| Asian | 30 (25) | 0 (0) | 28 (12) | 0 (0) | 29 (26) | 87 (9) | 27 (8) |
| Other | 7 (6) | 18 (8) | 25 (10) | 19 (8) | 7 (6) | 76 (8) | 27 (8) |
| Type of schizophrenia, n (%) | |||||||
| Paranoid | 89 (74) | 196 (88) | 193 (79) | 197 (83) | 84 (75) | 759 (81) | 271 (80) |
| Disorganized | 7 (6) | 3 (1) | 13 (5) | 9 (4) | 6 (5) | 38 (4) | 13 (4) |
| Catatonic | 1 (1) | 1 (<1) | 1 (<1) | 1 (<1) | 1 (1) | 5 (1) | 0 (0) |
| Undifferentiated | 22 (18) | 18 (8) | 31 (13) | 26 (11) | 18 (16) | 115 (12) | 46 (14) |
| Residual | 1 (1) | 6 (3) | 5 (2) | 5 (2) | 3 (3) | 20 (1) | 7 (2) |
| PANSS total score, mean (±SD) | 91.6 (12.3) | 93.4 (1.1) | 93.6 (12.6) | 94.4 (11.1) | 92.4 (12.4) | 93.4 (11.8) | 93.9 (11.6) |
Abbreviations: Paliperidone ER, paliperidone extended-release; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation.
Effect of paliperidone ER treatment on negative, positive and anxiety/ depression symptoms and EPS (path ITT population)
All doses of paliperidone ER resulted in significant improvements in negative symptom scores at endpoint compared with placebo (p < 0.001). Significant improvements for all doses of paliperidone ER were also noted for the positive factor and anxiety/depression factor scores (p < 0.001). Changes in mean SAS scores at endpoint were significantly different from placebo (p < 0.001) in the ER paliperidone 9- and 12-mg groups and in the combined paliperidone ER group (Table 2). Changes at endpoint in other efficacy variables are provided in Table 2.
Table 2.
Change at endpoint in path analysis variables and other clinical measures (path analysis ITT population; n = 1274)
| Parameter | Paliperidone ER daily dose
|
||||||
|---|---|---|---|---|---|---|---|
| 3 mg (n = 120) | 6 mg (n = 224) | 9 mg (n = 243) | 12 mg (n = 238) | 15 mg (n = 112) | Combined (n = 937) | Placebo (n = 337) | |
| Path analysis variables | |||||||
| PANSS negative factor | |||||||
| Baseline, mean (±SD) | 22.3 (5.7) | 23.2 (5.4) | 23.2 (5.1) | 23.6 (5.4) | 23.0 (5.6) | 23.2 (5.4) | 23.1 (5.2) |
| Endpoint (±SD) | 18.5 (6.0) | 18.8 (6.1) | 19.6 (6.1) | 19.1 (6.0) | 18.8 (6.4) | 19.0 (6.1) | 21.6 (6.9) |
| LS mean change (±SE)a | −4.2 (0.6)b | −4.0 (0.4)b | −3.8 (0.4)b | −4.1 (0.4)b | −4.4 (0.6)b | −4.0 (0.2)b | −1.4 (0.3) |
| PANSS positive factor | |||||||
| Baseline (±SD) | 27.6 (4.8) | 27.9 (4.9) | 27.8 (5.2) | 27.6 (4.8) | 27.6 (5.1) | 27.7 (4.9) | 27.8 (4.5) |
| Endpoint (±SD) | 22.5 (7.3) | 21.8 (7.0) | 21.7 (7.7) | 20.4 (6.6) | 20.8 (6.4) | 21.4 (7.1) | 25.4 (7.6) |
| LS mean change (±SE)a | −4.8 (0.7)b | −5.5 (0.5)b | −5.6 (0.4)b | −6.9 (0.5)b | −7.0 (0.7)b | −6.0 (0.2)b | −2.2 (0.4) |
| PANSS anxiety/depression factor | |||||||
| Baseline (±SD) | 10.9 (3.6) | 11.5 (2.9) | 11.2 (3.0) | 11.8 (3.3) | 11.0 (3.2) | 11.4 (3.2) | 11.8 (3.3) |
| Endpoint (±SD) | 9.1 (4.0) | 9.4 (3.5) | 8.9 (3.5) | 9.1 (3.6) | 8.5 (3.6) | 9.0 (3.6) | 10.9 (4.3) |
| LS mean change (±SE)a | −2.0 (0.4)b | −2.0 (0.2)b | −2.2 (0.2)b | −2.5 (0.2)b | −2.8 (0.4)b | −2.3 (0.1)b | −0.8 (0.2) |
| SAS scale | |||||||
| Baseline | 0.20 (0.34) | 0.10 (0.18) | 0.11 (0.21) | 0.10 (0.23) | 0.17 (0.33) | 0.12 (0.25) | 0.14 (0.27) |
| Endpoint | 0.13 (0.29) | 0.06 (0.13) | 0.15 (0.28) | 0.13 (0.25) | 0.15 (0.29) | 0.12 (0.25) | 0.09 (0.24) |
| LS mean change (±SE)a | −0.05 (0.02) | −0.05 (0.01) | 0.03 (0.01)b | 0.02 (0.01)b | 0.00 (0.02) | −0.01 (0.01)c | −0.04 (0.01) |
| Other variables | |||||||
| CGI-S | |||||||
| Baseline (±SD) | 4.7 (0.7) | 4.7 (0.7) | 4.7 (0.7) | 4.8 (0.7) | 4.7 (0.7) | 4.7 (0.7) | 4.7 (0.7) |
| Endpoint (±SD) | 3.9 (1.1) | 3.8 (1.1) | 3.9 (1.1) | 3.7 (1.1) | 3.6 (1.0) | 3.8 (1.1) | 4.4 (1.2) |
| LS mean change (±SE)a | −0.7 (0.1)b | −0.8 (0.1)b | −0.8 (0.1)b | −1.0 (0.1)b | −1.1 (0.1)b | −0.9 (0.0)b | −0.3 (0.1) |
| PSP total score | |||||||
| Baseline (±SD) | 48.3 (14.0) | 46.8 (13.7) | 48.9 (15.2) | 46.0 (13.4) | 47.5 (14.2) | 47.4 (14.1) | 47.6 (13.9) |
| Endpoint (±SD) | 56.9 (17.3) | 56.0 (16.2) | 56.8 (16.6) | 55.4 (15.4) | 59.9 (14.8) | 56.7 (16.1) | 48.6 (17.1) |
| LS mean change (±SE)a | 8.0 (1.5)b | 8.4 (1.0)b | 7.6 (1.0)b | 8.2 (1.0)b | 11.8 (1.6)b | 8.5 (0.5)b | 0.4 (0.8) |
ANCOVA model with treatment, study and analysis center within study as factors; the baseline score for the analyzed variable as a covariate.
p ≤ 0.001 vs placebo;
p < 0.005 vs placebo.
Note: Adjustments for multiple comparisons were not performed.
Abbreviations: CGI-S, Clinical Global Impression-Severity; ER, extended-release; ITT, intent-to-treat; LS, least squares; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance Scale; SAS, Simpson-Angus Rating Scale; SE, standard error.
Path analysis (path ITT population)
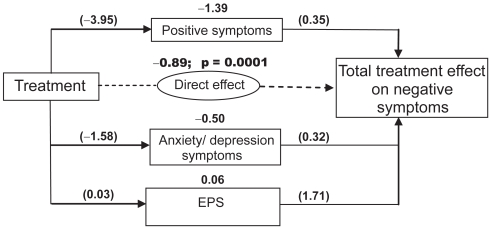
Path analysis indicated that the total (direct and indirect) effects of paliperidone ER treatment resulted in greater improvement in negative symptoms than did placebo treatment. The total treatment effect was reflected in a 2.7-point greater improvement in the negative factor score in the combined treatment group than in the placebo group (Table 3). Paliperidone ER was shown to have both direct and indirect effects on negative symptoms (Figure 2). In the combined paliperidone ER group, the direct effect of treatment was indicated by a 0.89-unit greater improvement in negative symptoms compared with placebo (p = 0.0001); the direct effect of treatment on the negative symptoms factor score was estimated to be 33% (Table 3 and Figure 3). Among the domains studied, the largest effects of paliperidone treatment were mediated indirectly, through changes in positive symptoms (51%) and anxiety/depression symptoms (18%). Essentially no contribution was made by change in SAS scores (−2.1%; Table 3 and Figure 3). Each individual dose of paliperidone ER had a significantly greater (p < 0.05) direct effect than placebo on negative symptoms, which ranged from 21% for the 12-mg dose to 52% for the 3-mg dose. Although the total effect of treatment was generally consistent over doses, there was a differential effect on different symptom domains (Table 3).
Table 3.
Estimated path coefficients and corresponding percentage effect on PANSS Negative Symptom Factor Score (path analysis ITT population; n = 1274)
| Paliperidone ER vs placebo
|
||||||
|---|---|---|---|---|---|---|
| 3 mg (n = 120) | 6 mg (n = 224) | 9 mg (n = 243) | 12 mg (n = 238) | 15 mg (n = 112) | Combined (n = 937) | |
| Direct effect (%) | −1.405 (52) | −1.173 (41) | −0.760 (33) | −0.612 (21) | −0.805 (28) | −0.891 (33) |
| Indirect, change in positive symptoms (%) | −0.934 (35) | −1.259 (44) | −1.107 (49) | −1.870 (64) | −1.620 (56) | −1.387 (51) |
| Indirect, change in anxiety/depression symptoms (%) | −0.351 (13) | −0.446 (15) | −0.527 (23) | −0.527 (18) | −0.500 (17) | −0.504 (18) |
| Indirect, change in EPS (%) | −0.004 (0.14) | −0.003 (0.11) | 0.115 (−5) | 0.085 (−2.9) | 0.046 (−1.6) | 0.058 (−2.1) |
| Total effect of treatment on change in negative symptoms | −2.694 | −2.881 | −2.279 | −2.923 | −2.888 | −2.723 |
| r2 estimate | 0.350 | 0.388 | 0.333 | 0.422 | 0.345 | 0.420 |
| GFI | 0.8897 | 0.8850 | 0.8746 | 0.8849 | 0.8843 | 0.8895 |
Notes: The r2 estimate represents the proportion of the total variation of changes in negative symptoms that is explained by the treatment effect. The GFI is analogous to a squared multiple correlation; it indicates the proportion of the observed covariance explained by the model covariance. The GFI varies from 0 to 1, with 1 being a perfect fit.
Abbreviations: EPS, extrapyramidal symptoms; ER, extended-release; GFI, Goodness of Fit Index; PANSS, Positive and Negative Syndrome Scale; ITT, intent-to-treat.
Figure 2.
Estimated path coefficients for the comparisons of effects of all paliperidone ER doses combined vs placebo.
The numbers in parentheses are the coefficients of the intermediate paths; the coefficient of the indirect path can be obtained by multiplying the coefficients of the intermediate paths. Sample interpretation, for positive symptoms: the first intermediate path shows that paliperidone ER treatment (all doses combined) was associated with a P1: −3.95-unit greater change in positive symptoms than placebo, after controlling for the baseline score; the second intermediate path shows that there was a P5: 0.35-unit effect of positive symptoms on negative symptoms, after controlling for the other symptoms. The product of the intermediate path coefficients ([−3.95]*[0.35] = −1.39) shows that paliperidone ER treatment (all doses combined) was associated with a 1.39-unit greater improvement in negative symptoms via positive symptom improvement vs placebo.
Abbreviations: EPS, extrapyramidal symptoms; ER, extended-release.
Figure 3.
Percentage of the total treatment effect due to direct or indirect effects contributing to change in the negative factor score at endpoint, all paliperidone ER doses combined.
Abbreviations: ER, extended-release; SAS, Simpson-Angus Scale.
Correlations between negative symptoms and demographic and clinical variables (path ITT population)
A multiple regression model explored the relationship between negative symptoms and demographic/clinical characteristics. A significant relationship was found between improvement in negative symptoms at endpoint and duration of exposure to paliperidone ER (r = −0.175; p < 0.001), as well as improvements in the PSP total score at endpoint (r= −0.521; p < 0.001).
Tolerability and safety
Preliminary safety and tolerability data for each individual study have been presented elsewhere (Davidson et al 2007; Kane et al 2007; Marder et al 2007). Pooled data indicated that paliperidone ER was well tolerated. Common treatment-emergent AEs (occurring in ≥10% of subjects in either the paliperidone ER combined group or placebo treatment arms) included headache (14% vs 12%) and insomnia (12% vs 15%; Table 4). Most of the frequently reported AEs showed no apparent dose relationship among paliperidone ER–treated subjects, with the exception of akathisia, which occurred more often in groups receiving 9-, 12-, or 15-mg doses (Table 4). The discontinuation rate due to treatment-emergent AEs was similar with paliperidone ER (3 mg: 3%, n = 2; 6 mg: 6%, n = 15; 9 mg: 4%, n = 10; 12 mg: 5%, n = 13; 15 mg: 4%, n = 4; all doses combined: 5%, n = 45) and placebo (5%; n = 18).
Table 4.
Incidence of common TEAS occurring in ≥10% of subjects receiving paliperidone ER or placebo (path analysis ITT population; n = 1274)
| Adverse event | Paliperidone ER daily dose
|
||||||
|---|---|---|---|---|---|---|---|
| 3 mg (n = 120) n (%) | 6 mg (n = 224) n (%) | 9 mg (n = 243) n (%) | 12 mg (n = 238) n (%) | 15 mg (n = 112) n (%) | Combined (n = 937) n (%) | Placebo (n = 337) n (%) | |
| Headache | 14 (12) | 25 (11) | 34 (14) | 34 (14) | 20 (18) | 127 (14) | 41(12) |
| Insomnia | 14 (12) | 27 (12) | 35 (14) | 26 (11) | 14 (13) | 116 (12) | 50 (15) |
| Akathisia | 5 (4) | 7 (3) | 19 (8) | 23 (10) | 11 (10) | 65 (7) | 14 (4) |
| Anxiety | 12 (10) | 16 (7) | 14 (6) | 11 (5) | 9 (8) | 62 (7) | 27 (8) |
Abbreviations: ER, extended-release; ITT, intent-to-treat; TEAS, treatment-emergent adverse events.
The incidence of EPS-related AEs, which were examined in detail, was higher in paliperidone ER-treated subjects (all doses combined, 20%) compared with those receiving placebo (11%). This difference was due primarily to higher rates of such EPS-related AEs as dystonia, hyperkinesia, parkinsonism, and dyskinesia in subjects who received higher paliperidone ER doses. EPS-related AEs occurred in 15 subjects (13%) receiving 3 mg of paliperidone ER; 24 (11%) subjects receiving 6 mg; 60 (25%) subjects receiving 9 mg; 62 (26%) subjects receiving 12 mg and 27 (24%) subjects receiving 15 mg. A total of 38 (11%) subjects receiving placebo had EPS-related AEs. Regardless of treatment, the percentages of subjects reporting the onset of any EPS-related AE tended to decrease over time. The median severity score for specific types of EPS was rated as 0 on the SAS (parkinsonism), AIMS (dyskinesia), and BARS (akathisia) scales for all patients receiving placebo or paliperidone ER at baseline and remained unchanged at endpoint.
Discussion
This exploratory analysis was performed to generate a hypothesis regarding a direct impact of paliperidone ER on negative symptoms. Paliperidone ER treatment, compared with placebo, was associated with a significant improvement in negative symptoms, representing a 22% reduction from baseline. In the context of this acutely ill population, in whom a reduction in negative symptoms may be particularly difficult to detect, this degree of improvement may be meaningful. A clinically meaningful response in negative symptoms has not yet been determined (Kirkpatrick et al 2006). Although improvement in negative symptoms was indirectly mediated through changes in positive and anxiety/ depression symptoms, this analysis supports the hypothesis that paliperidone ER may also have a direct effect on the negative symptoms of schizophrenia in an acutely ill patient population. Changes in EPS had little effect on changes in negative symptoms.
These results are generally consistent with results of previous path analyses that examined direct and indirect effects of risperidone vs haloperidol (Moller et al 1995), olanzapine vs haloperidol and placebo (Tollefson and Sanger 1997) and quetiapine vs placebo (Tandon 2004). All three of these studies demonstrated a direct effect of treatment on negative symptoms, although the mediators of indirect effects differed. Different populations, study designs, and assessments (particularly the different symptom rating scales and clusters used in the various path analytic models) (Tollefson and Sanger 1997; Tandon 2004) may account for any disparities in study findings.
Duration of exposure to paliperidone ER and improvement in functioning were significantly correlated with improvement in negative symptoms. Our data for duration of drug exposure are consistent with previous research highlighting that continued antipsychotic treatment is associated with optimal outcomes in multiple symptom domains and decreases in the likelihood of relapse and rehospitalization (Ayuso-Gutierrez and del Rio Vega 1997; Docherty et al 2002; Valenstein et al 2002; Weiden et al 2004; Gharabawi et al 2007).
The finding that functioning and negative symptoms are linked is also consistent with previous work (Milev et al 2005) and emphasizes the damaging effects that negative symptoms have on the ability of patients with schizophrenia to participate fully in society.
Pooled safety data indicated that paliperidone ER was generally well tolerated. Discontinuations related to treatment-emergent AEs were similarly low for patients receiving paliperidone ER or placebo. Although the incidence of EPS-related AEs was higher in paliperidone ER-treated patients, primarily those receiving higher doses, the severity of EPS was very low throughout the study.
Several study limitations must be noted. Path analysis requires the usual assumptions of regression with a linear relationship among variables, no or minimal multicollinearity in the predictors, multivariate normal data and absence of measurement error. The goodness-of-fit index ranged from 0.88 to 0.89, less than the acceptable fit range of >0.9. Univariate and multivariate normality test results indicated that there are some moderate violations of this assumption; however, for this study, the sample size was large enough to support the asymptotic multivariate normal distribution for obtaining robust estimates. A Pearson Correlation Matrix was calculated to test the multicollinearity in the predictors. All the correlations were <0.2 with p values >0.05. Path analysis is particularly sensitive to model specification; failure to include relevant causal variables or the inclusion of extraneous variables can substantially affect results. In addition, path analysis can only consider the secondary effects of factors included in the model. Although the factors chosen for use in the present analysis are considered the major contributors to negative symptoms in schizophrenia, the possibility that other unidentified factors contributed to the results cannot be completely eliminated. The r2 estimates ranged from 0.33 to 0.44. Furthermore, the measure for movement disorders used in our path model was the SAS, which rates symptoms of parkinsonism and may not be a comprehensive measure of the various types of motor disorders. However, parkinsonism, rather than dyskinesia or akathisia, is the type of movement disorder most likely to confound the assessment of negative symptoms (Prosser et al 1987). Improvements in negative symptoms in paliperidone ER vs placebo groups were observed in the paliperidone-treated group, which reported a 20% rate of EPS-related AEs (EPS-related AEs were reported by 11% of placebo-treated subjects). Nonetheless, future analysis should further explore the effect of different types of movement disorders on negative symptoms. It should be noted that this was a post hoc analysis of phase 3 paliperidone studies (Davidson et al 2007; Kane et al 2007; Marder et al 2007); these studies were not designed to assess direct or indirect treatment effects on negative symptoms. Further, to assess better the impact of treatment on negative symptoms, prospective studies with a longer duration and patient populations with minimal positive symptoms and persistent negative symptoms should be conducted, as recommended by the National Institute of Mental Health Measurement and Treatment Research to Improve Cognition in Schizophrenia consensus statement on negative symptoms (Kirkpatrick et al 2006). Finally, there was no evaluation of the reliability of ratings after initial training, a limitation typical to this type of study.
In summary, within the limitations of the path analysis method used here, these data suggest that paliperidone ER improves the negative symptoms of schizophrenia through a direct effect as well as an indirect effect on positive and mood symptoms. Given the substantial impact that negative symptoms have on patient functioning, it will be of great interest to explore how paliperidone ER may affect negative symptoms and outcomes during long-term treatment in a prospectively defined study.
Acknowledgments
The authors wish to acknowledge Dr Mariana Ovnic and Helix Medical Communications (funding supported by Ortho-McNeil Janssen Scientific Affairs, LLC) for assisting with the development and submission of this paper.
Footnotes
Disclosures
All the authors are employees of Ortho-McNeil Janssen.
References
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Ayuso-Gutierrez JL, del Rio Vega JM. Factors influencing relapse in the long-term course of schizophrenia. Schizophr Res. 1997;28:199–206. doi: 10.1016/s0920-9964(97)00131-x. [DOI] [PubMed] [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, et al. Treatment of negative symptoms. Schizophr Bull. 1985;11:440–52. doi: 10.1093/schbul/11.3.440. [DOI] [PubMed] [Google Scholar]
- Davidson L, McGlashan TH. The varied outcomes of schizophrenia. Can J Psychiatry. 1997;42:34–43. doi: 10.1177/070674379704200105. [DOI] [PubMed] [Google Scholar]
- Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93:117–30. doi: 10.1016/j.schres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Docherty JP, Grogg AL, Kozman C, et al. Antipsychotic maintenance in schizophrenia: partial compliance and clinical outcome. American College of Neuropsychopharmacology 41st Annual Meeting; San Juan, Puerto Rico. December 8–12.2002. [Google Scholar]
- Gharabawi G, Bossie C, Turkoz I, et al. The impact of insight on functioning in patients with schizophrenia or schizoaffective disorder receiving risperidone long-acting injectable. J Nerv Mental Dis. 2007;195:976–82. doi: 10.1097/NMD.0b013e31815c1982. [DOI] [PubMed] [Google Scholar]
- Guy WA. ECDEU assessment manual for psychopharmacology. Washington, DC: US Department of Health, Education and Welfare; 1972. Abnormal Involuntary Movement Scale (AIMS) pp. 533–7. [Google Scholar]
- Guy WA. Clinical global impressions. In: ECDEU assessment manual for psychopharmacology. Washington, DC: US Department of Health, Education and Welfare; 1976. pp. 218–222. [Google Scholar]
- Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90:147–61. doi: 10.1016/j.schres.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- King DJ. Drug treatment of the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 1998;8:33–42. doi: 10.1016/s0924-977x(97)00041-2. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Jr, et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D, et al. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–46. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62:1363–70. doi: 10.1016/j.biopsych.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Moller HJ. Neuroleptic treatment of negative symptoms in schizophrenic patients. Efficacy problems and methodological difficulties. Eur Neuropsychopharmacol. 1993;3:1–11. doi: 10.1016/0924-977x(93)90289-x. [DOI] [PubMed] [Google Scholar]
- Moller HJ, Muller H, Borison RL, et al. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re-evaluation of the North American risperidone study. Eur Arch Psychiatry Clin Neurosci. 1995;245:45–9. doi: 10.1007/BF02191543. [DOI] [PubMed] [Google Scholar]
- Morosini PL, Magliano L, Brambilla L, Ugolini S, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101:323–9. [PubMed] [Google Scholar]
- Pedhazur EJ. Path analysis. Multiple regression in behavioral research. New York: Holt; 1982. [Google Scholar]
- Prosser ES, Csernansky JG, Kaplan J, et al. Depression, parkinsonian symptoms, and negative symptoms in schizophrenics treated with neuroleptics. J Nerv Mental Dis. 1987;175:100–5. doi: 10.1097/00005053-198702000-00006. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Tandon R. Quetiapine has a direct effect on the negative symptoms of schizophrenia. Hum Psychopharmacol. 2004;19:559–63. doi: 10.1002/hup.642. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry. 1997;154:466–74. doi: 10.1176/ajp.154.4.466. [DOI] [PubMed] [Google Scholar]
- Valenstein M, Copeland LA, Blow FC, et al. Pharmacy data identify poorly adherent patients with schizophrenia at increased risk for admission. Med Care. 2002;40:630–9. doi: 10.1097/00005650-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55:886–91. doi: 10.1176/appi.ps.55.8.886. [DOI] [PubMed] [Google Scholar]