Abstract
In this study, we have explored the possibility of the combination of the high reactivity of nano Fe3O4 or Au nanoparticles and daunomycin, one of the most important antitumor drugs in the treatment of acute leukemia clinically, to inhibit MDR of K562/A02 cells. Initially, to determine whether the magnetic nanoparticle Fe3O4 and Au can facilitate the anticancer drug to reverse the resistance of cancer cells, we have explored the cytotoxic effect of daunomycin (DNR) with and without the magnetic nano-Fe3O4 or nano-Au on K562 and K562/A02 cells by MTT assay. Besides, the intracellular DNR concentration and apoptosis of the K562/A02 cells was further investigated by flow cytometry and confocal fluorescence microscopic studies. The MDR1 gene expression of the K562/A02 cells was also studied by RT-PCR method. Our results indicate that 5.0 × 10−7 M nano-Fe3O4 or 2.0 × 10−8 M nano-Au is biocompatible and can apparently raise the intracellular DNR accumulation of the K562/A02 cells and increase the apoptosis of tumor cells. Moreover, our observations illustrate that although these two kinds of nanoparticles themselves could not lower the MDR1 gene expression of the K562/A02 cells, yet they could degrade the MDR1 gene level when combining with anticancer drug DNR. This raises the possibility to combine the nano-Fe3O4 or nano-Au with DNR to reverse the drug resistance of K562/A02 cells, which could offer a new strategy for the promising efficient chemotherapy of the leukemia patients.
Keywords: nano-Fe3O4, nano-Au, multidrug resistance, leukemia K562/A02, daunomycin
Introduction
It is well known that the tumor cells can become resistance to the chemotherapeutics. Multidrug resistance (MDR) has been a major cause leading final failure of the tumor chemotherapy clinically (Bradley et al 1988). MDR is usually characterized by the over-expression of P-gp (Litman et al 1997; Soma et al 2000; Ferrao et al 2001) that acts as an energy-dependent drug efflux pump and leads to a decrease in cytotoxic drug accumulation (Hamada and Tsuruo 1988).
Unlike solid tumor, hematological malignant tumor can’t be cured by surgical treatment. The main strategy to treat it is chemotherapy. Considering the importance of MDR in clinical hematological oncology, various kinds of P-gp-selective antagonisms have been developed to reverse P-gp-mediated MDR in vitro or in clinical trials. Undoubtedly, the reverse drugs can enhance the sensitization of the tumor to different chemotherapeutics in vivo or in vitro and thus resolve MDR to some extent (Hegewisch-Becker 1996; Twentyman et al 1992; Advani et al 1999; Tan et al 2000). Except some drugs such as tetrandrine (Tet), cyclosporin A (CsA) and PSC 833, most P-gp-selective antagonisms can’t be applied in large scale clinically because of their serious side effects on the pharmacokinetics of other accompanying chemotherapeutic drugs. Much effort has been devoted to the reversal of Tet in our laboratory in recent years and it was verified that Tet would be a potential reversal drugs in vivo and in vitro (Xu et al 2006). Furthermore, one new strategy of target administering by drug carrier systems such as liposome (Alf and Jean-Pierre 2006), nanoparticle (Cuvier et al 1992; Brigger et al 2002; Vauthier et al 2003; Jain et al 2005), and others, has been developed to enhance the efficiency of anticancer drug delivery. Nanomaterials are well known to have potential applications in various biomedical fields, such as disease diagnostics and therapeutics (Farokhzad et al 2005). They have attracted more and more attention to reverse MDR recently. It was reported that gold nanoparticles had been utilized for gene delivery (Sanford et al 1993). Moreover, some nanoparticles have been explored to illustrate the mechanisms of biochemical modulation and have potentially promising use in clinical cancer chemotherapy applications (Kirchner et al 2005).
Magnetic nano-Fe3O4 could be used in the biomedicine areas because of their biocompatibility, simple preparation, and other unique properties (Zhang et al 2006; Li et al 2007). Fe3O4 nanoparticles were found readily to interact with proteins (Chen et al 2002). It is reported that the trichloro-s-triazine (TsT) can be used as an easy linker to couple magnetic Fe3O4 nanoparticles with hydrophilic monomethoxy-poly (ethylene glycol) (mPEG), and the mPEG – dopamine-coated nanoparticles were stable in buffer at various pH values greater than 7 or at the temperatures ranging from 25 °C to 70 °C. On the basis of these observations, it is possible to couple various hydrophilic macromolecules, including nucleotides and peptides, to iron oxide nanoparticles, which could be used for highly sensitive DNA sequence detection (Xie et al 2006) and will contribute much to biomedical science.
Our recent reports have demonstrated that some anticancer drugs could be readily modified on some biocompatible nanomaterials, which could readily afford the sustained drug delivery for the target cancer cell lines and reduce the relevant toxicity towards normal cells and tissues (Li et al 2007; Lv et al 2008). Thus, in this study, we have explored the combination of the high reactivity of nano-Fe3O4 or Au nanoparticles with daunomycin, one of the most important antitumor drugs in the treatment of acute leukemia clinically, to inhibit MDR of K562/A02 cells. The main point of this strategy is to increase the intracellular concentration of the targeted drug by the synergistic effect of nanoparticles. The reverse effects of nano-Fe3O4 and nano-Au towards K562 and K562/A02 cells have been investigated in this report.
Materials and methods
Cell lines and cell culture
K562 cells, derived from human leukemic cells of a chronic myeloid leukemia patient in blastic crisis, had been constantly preserved in our laboratory. K562/A02 cells (provided by Prof. C. Yang, Institute of Hematology, Chinese Academy of Medical Sciences and Peking Union Medical College, China) were incubated in the medium containing 1 μg/ml ADM for maintaining the resistant characteristics. K562/A02 cells were kept in medium without ADM for 2 weeks before using these cells. Both these two kinds of cell lines were cultured in a flask in RPMI 1640 medium (GIBCO, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS; GIBCO), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C, 5% CO2. K562/A02 cells were maintained in a complete RPMI 1640 medium containing 1 μg/ml adriamycin, and cultured for two weeks in drug-free medium prior to their use in each experiment.
Reagents
Daunomycin (DNR) was purchased from Farmitalia (Italian). MTT was purchased from Sigma (St. Louis, MO, USA). PCR reagents kit was purchased from TaKaLa (China). TRI-zol reagent was purchased from Gibco/BRL, USA. MDR1 and β-actin primer were synthesized by Shanghai Biotechnology Company (China) and Invitrogen (Carlsbad, CA, USA). All reagents used in this study are analytical grade.
Preparation of drug-loaded nanoparticles
Nano-Fe3O4 and nano-Au were prepared by the electro-chemical deposition under oxidizing conditions (EDOC). Before applied in the present experiment, the magnetite nanoparticles were well-distributed in RPMI 1640 medium freshly added with 10% heat-inactivated FBS by using ultrasound treatment in order to obtain nano-Fe3O4 and nano-Au colloidal suspension. ADM conjugated with nano-Fe3O4 (nano-Fe3O4-ADM) or ADM conjugated with nano-Au (nano-Au-ADM) were prepared by mechanical absorption polymerization referring to previously reported (Gao et al 2004; Bennis et al 1994). We also investigated the temperature effect and chose 37 °C or 4 °C for the polymerization process. Briefly, different concentrations (V/V), nano-Fe3O4 and nano-Au were respectively added under mechanical stirring to 200 μl of an aqueous medium with certain concentration of ADM that in the final nanoparticle and cell suspension was 50 μmol/l (pH 7.4). At different temperatures (37 °C, 4 °C), the overall polymerization process lasted for 24 h.
Cytotoxicity assays
K562 and K562/A02 cells (2 × 104 cells per well) in log phase were incubated into 96-well flat-bottomed plates (Costar, Charlotte, NC) respectively. Different concentrations of DNR, nano-Fe3O4 and nano-Au were added into these cells alone and cultured for 48 h to measure their cytotoxicity. On the basis of these results, different concentrations of DNR, nano-Fe3O4 and nano-Au were combined to find the best combination that the compound will kill the tumor cells most. After incubation for 48 h, MTT solution (20 μl at 5 mg/ml) was added to each well and incubated for 4 h, followed by reading the optical density value (A) at 540 nm using a plate reader (Model 550, BIO-RAD, Japan) to measure the amount of cell proliferation and/or killing. The inhibition ratio (IR) of cells was determined as follows: (1-A of tests cells/A of blank control) ×100%. Each assay was repeated at least three times.
DNR accumulation in K562/A02 cells and K562/A02 cells apoptosis
Briefly, 1 × 106 cells were incubated with DNR (10 mg/L) in the presence or absence of nano-Fe3O4 (5.0 × 10−7 M) or nano-Au (2.0 × 10−8 M). After incubation for 48 h, cells were harvested and washed twice in ice-cold PBS and the amount of DNR accumulated in K562/A02 cells was determined by measuring the cell-associated fluorescence emission (480 nm) using flow cytometry (FACS Calibar, Becton – Dickinson, USA) according to a previously descried method (Suzuki et al 1997). Briefly, 100 pL cell suspensions were treated with 5 pL Annexin V-FITC for 30 min in the dark, and the sample were analyzed with flow cytometry. The apoptosis of several subgroup K562/A02 cells was measured by flow cytometry again to further identify the cytotoxicity of DNR with nano-Fe3O4 or nano-Au.
Confocal fluorescence microscopic studies
50 mg/L DNR and 5.0 × 10−7 M nano-Fe3O4 or 2.0 × 10−8 M nano-Au magnetic solution were injected into a cell culture for incubation. In a control experiment, only DNR was injected into a cell culture for incubation. The culture was detected using a confocal fluorescence microscope (Leica TCS SP2) after incubating for 30 min. The freshly prepared cell culture was dropped on a rigorously cleaned glass plate immediately before the measurement. The excitation wavelength of the fluorescence was 480 nm.
RT-PCR study
The several subgroup K562/A02 cells (above) were centrifuged by 1000 rpm for 5 min and collected. Total cellular RNA was extracted using the TRI-zol reagent as described by the manufacturer. One microgram of RNA was used for cDNA synthesis employing avian myeloblastosis virus (AMV) reverse transcriptase and oligo dT as the primer. cDNA was amplified with specific primers for MDR1. β-actin was used as an internal control to confirm successful cDNA synthesis. For MDR1 and β-actin, the amplify conditions as follows: 94 °C 30 sec, 56 °C 30 sec, 72 °C 1 min, 30 cycles. Primer sequences were as follows: MDR1 (437 bp) sense: 5′-TGG TTT GAT GTG CAC GAT GTT GGG-3′, antisense: 5′-AGA TCA GCA GGA AAG CAG CAC CTA-3′, β-actin (661 bp) sense: 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′, antisense: 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′ (Tan et al 2003). PCR products were separated on 1.5% agarose gels. The band intensities on gels photographs were directly quantitated by ImagerMaster VDS image analysis (Pharmacia Biotech, USA), and the target/control ratios were calculated.
Results
Growth rates of K562 and K562/A02 cells by MTT assay
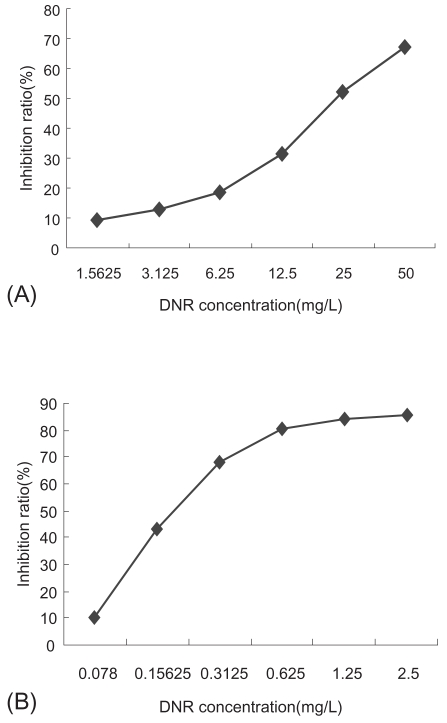
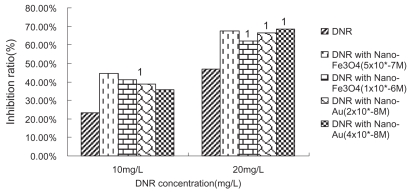
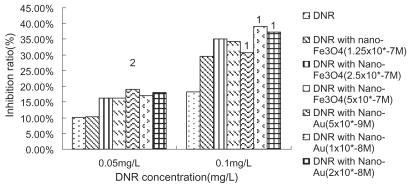
We have examined the cytotoxic activity of DNR, nano-Fe3O4 or nano-Au alone to K562 cells and its Pgp-overexpression MDR counterparts-K562/A02. K562/A02 was significantly resistant to DNR: 50% inhibiting concentration (IC50) values after incubation for 48 h was 23.23 mg/L, which is 76-fold higher than K562 cells, while the IC50 values after incubation for 48 h of K562 cells was 0.307 mg/L. The growth inhibition curves of two cell lines to DNR were shown in Figure 1. It was noted that nano-Fe3O4 or nano-Au themselves alone could hardly inhibit K562/A02 and K562 proliferation when their doses were between 2.0 × 10−9 M to 1.0 × 10−6 M (P > 0.05), which also confirmed their good biocompatibility. In another experiment, we chose DNR in concentration of 10 mg/L, 20 mg/L, and 0.05 mg/L, 0.1 mg/L (smaller than IC50 to K562/A02 and K562, respectively) combined with different concentration of nano-Fe3O4 or nano-Au to find the optimum combination condition which affected most tumor cells. It was observed that nano-Fe3O4 or nano-Au combining with DNR could inhibit two cell lines proliferation significantly (P < 0.001). 5.0 × 10−7 M nano-Fe3O4 or 2.0 × 10−8 M nano-Au did the best in K562/A02, while 2.5 × 10−7 M nano-Fe3O4 or 1.0 × 10−8 M nano-Au did the best in K562 (as shown in Figures 2, 3).
Figure 1.
Effect of the different concentrations of daunomycin (DNR) on growth inhibitiom rates of K562/A02 cells (A) and K562 cells (B) by MTT assay.
Figure 2.
Growth inhibiting rate of daunomycin (DNR) with or without nanoparticle treated-K562/A02 cells (48 h).
Notes: P < 0.05, compared to DNR without nanoparticle treated-K562/A02 cells (single factor analysis of variance); 1P < 0.01, compared with DNR without nanoparticle treated-K562/A02 cells (single factor analysis of variance).
Figure 3.
Growth inhibiting rate of daunomycin (DNR) with or without nanoparticle treated-K562 cells (48 h).
Notes: P < 0.05, compared with DNR without nanoparticle treated-K562 cells (single factor analysis of variance); 1P < 0.01, compared with DNR without nanoparticle treated-K562 cells (single factor analysis of variance); 2P > 0.05, compared with DNR without nanoparticle treated-K562 cells (single factor analysis of variance).
Tumor cells apoptosis
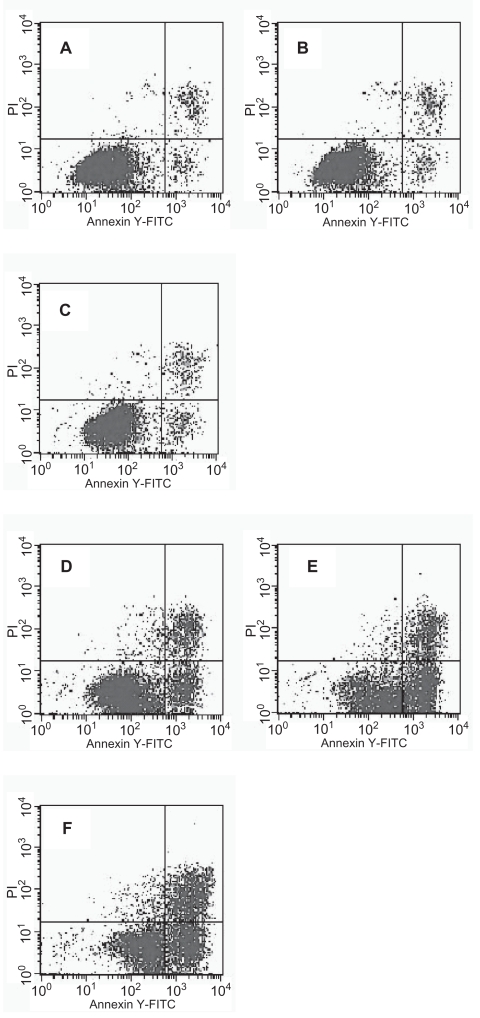
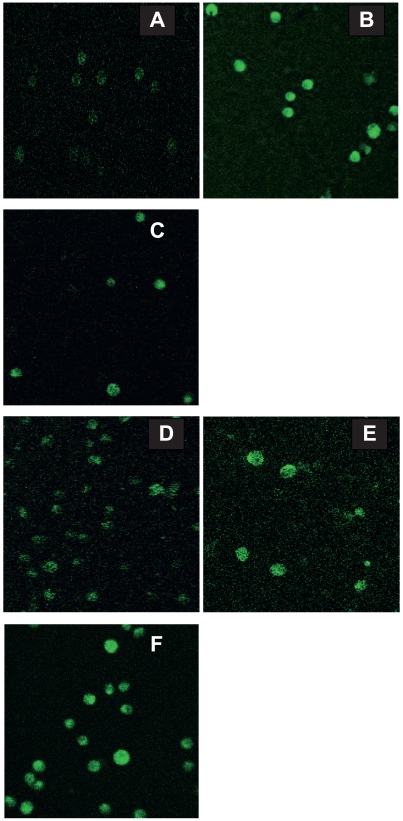
Based on these observations, it appeared that DNR combining with nano-Fe3O4 or nano-Au could efficiently increase the apoptosis ratio of K562/A02 cells. The flow cytometry results show that nano-Fe3O4 or nano-Au themselves can hardly induce the apoptosis of K562/A02 cells. 12.57% apoptosis of K562/A02 cells was observed under DNR alone, while 32.76% and 29.38% apoptosis of K562/A02 cells were observed when the K562/A02 cells were treated with DNR in combination with nano-Fe3O4 or nano-Au (Figure 4).
Figure 4.
The apoptosis of K562/A02 cells under daunomycin (DNR) with and without nanoparticles. K562/A02 cells (A–D) were incubated with Nano-Fe3O4 (5.0 × 10−7 M) (B), Nano-Au (2.0 × 10−8 M) (C), DNR (10 mg/L) (D) and nothing (A), respectively, and K562/A02 cells (E–F) were incubated with DNR (10 mg/L) in the presence of Nano-Fe3O4 (5.0 × 10−7 M) (E) or Nano-Au (2.0 × 10−8 M) (F) at 37 °C for 48 h.
The intracellular free DNR concentration
The intracellular DNR concentration of the K562/A02 cells was explored by flow cytometry. Compared with that treated with DNR alone, the intracellular DNR concentration of the K562/A02 cells were found to increase by 71% and 51%, respectively when the K562/A02 cells were treated with DNR in combination with nano-Fe3O4 and nano-Au, respectively. Similar observations were also illustrated through confocal fluorescent microscope (Figure 5). Considering that the anticancer drug daunorubicin is a fluorescent molecule while the relevant nanoparticles have no fluorescence, our observations of the laser confocal fluorescence microscopy further confirm the synergistic effect of these nanoparticles on the cellular uptake of daunorubicin.
Figure 5.
Effect of nanoparticle on the intracellular accumulation of daunomycin (DNR) in K562/A02 cells (×1000). K562/A02 (A–C) and K562 (D–F) cells were incubated with DNR (50 mg/L) in the absence (A, D) or the presence of Nano-Fe3O4 (5.0×10−7 M) (B, E) or Nano-Au (2.0 × 10−8 M) (C, F) at 37 °C for 30 min. The cells were observed and photographed under a confocal fluorescent microscope.
RT-PCR
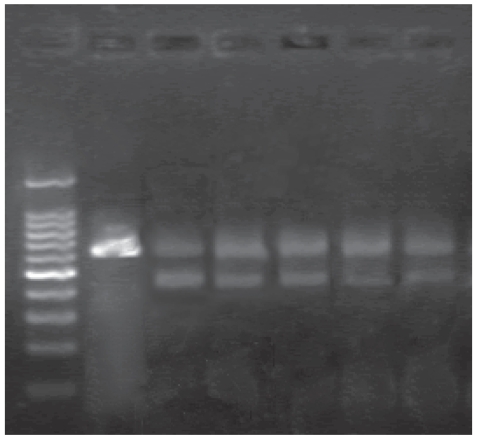
Additionally, our RT-PCR studies showed that these two kinds of nanoparticles themselves could not lower the MDR1 gene expression of the K562/A02 cells, but they could down regulate the MDR1 gene level when combined with DNR. K562 cells were negative in MDR1 gene expression. Computer-assisted image analysis showed that the ratios of MDR1/β-actin band intensities were as follows: Nano-Fe3O4: 0.793 ±0.03, Nano-Au: 0.770 ± 0.033, Nano-Fe3O4 with DNR: 0.619 ± 0.034 (P < 0.01) and Nano-Au with DNR: 0.635 ± 0.041 (P < 0.01), where the descent rate were 23.3% and 21.6%, respectively (as shown in Table 3 and Figure 6).
Figure 6.
The MDR1 mRNA level of K562/A02 cells with nanoparticles (5.0 × 10−7 M Nano-Fe3O4 or 2.0 × 10−8 M Nano-Au) in the absence or presence of DNR (10 mg/L) Lane:1, marker; 2, K562 (negative control); 3, K562/A02 (positive control); 4, Nano-Fe3O4; 5, Nano-Au; 6, DNR with Nano-Fe3O4; 7, DNR with Nano-Au.
Discussion
As described above, we have demonstrated that nanoparticles themselves can not reverse MDR-mediated resistance in K562/A02 cells, but they did if combined with certain chemotherapeutics such as DNR. However, it is reported that nano-Fe3O4 with better histocompatibililty to human body was suitable to prepare some materials imbedded in human body (Dresco et al 1999; Du et al 2005). Our observations indicate that nanoparticles themselves can not inhibit the growth of K562/A02 and K562 cells or induce their apoptosis. In our studies, we choose DNR with 10 mg/L or 20 mg/L and 0.05 mg/L or 0.1 mg/L to K562/A02 and K562 cells, respectively, which are smaller than IC50 of DNR, to combine different volume fraction of nanoparticles ranging from 2.0 × 10−9 M to 1.0 × 10−6 M so that they can interact with each other with water-bath at 50 °C for 30 min, following by standing to stay overnight. Our observations indicate that the presence of nano-Fe3O4 could enhance the cytotoxic effect of DNR to K562/A02 and K562 cells, having significant difference with DNR alone group (P < 0.05). To K562/A02 cells, the effective combination, composed by 5.0 × 10−7 M nano-Fe3O4 or 2.0 × 10−8 M nano-Au and 10 mg/L DNR, could efficiently induce more K562/A02 cells apoptosis. These results may be attributed to that these nanoparticles could readily interact with DNR and form so-called conjugated nanocomposites, which could facilitate the cellular uptake of DNR and thus enhance the cytotoxic effect of K562/A02 cells. MTT and confocal fluorescent microscope studies also indicate that these functionalized nanoparticle could efficiently strengthen DNR lethal effect to target cancer cells and increase the intracellular accumulation of DNR. Meanwhile, we also find the non-specific cytotoxocity of these nanoparticles was similar for both the parent tumor cell line and its MDR counterpart (Table 1) which suggests that the relevant nanoparticles conjugated with DNR may exert its MDR-reversal activity via down-regulation of MDR gene transcription of K562/A02 cells.
Table 1.
The MDR1 mRNA level of K562/A02 cells with nanoparticle in the absence or presence of DNR (at 48 h) (n = 3)
| Groups | Positive control | Nano-Fe3O4 | Nano-Au | DNR with Nano-Fe3O4 | DNR with Nano-Au |
|---|---|---|---|---|---|
| MDR1/β-actin | 0.809 ± 0.033 | 0.793 ± 0.037 | 0.770 ± 0.040 | 0.619 ± 0.042* | 0.635 ± 0.050* |
| descent rate (%) | 1.90 ± 0.069 | 4.57 ± 0.072 | 23.33 ± 0.069 | 21.60 ± 0.035 |
Notes: P < 0.01, compared with K562/A02 cells (single factor analysis of variance).
In summary, we have demonstrated that the functional nanoparticles could help DNR entering K562/A02 and K562 cells and increase the intracellular accumulation of DNR.
Although the relative mechanisms need to be explored further, the conjugated nanocomposites could have promising application to inhibit the drug resistant and facilitate the target drug delivery in the relevant cancer chemotherapy.
Acknowledgments
This paper was supported by a grant from the National Nature Science Foundation of China (No. 39970832, 20675014).
Footnotes
Disclosure
Bao-An Chen and Yong-Yuan Dai contributed equally to this work. There are no conflicts of interest to report.
References
- Advani R, Saba HI, Tallman MS, et al. Treatment of refractory and relapsed acute myelogenous leukemia with combination chemotherapy plus the multidrug resistance modulator PSC 833 (Valspodar) Blood. 1999;93:787–95. [PubMed] [Google Scholar]
- Lamprecht A, Benoit JP. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Release. 2006;112:208–13. doi: 10.1016/j.jconrel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Bennis S, Chapey C, Couvreur P, et al. Enhanced cytotoxicity of doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres against multidrug-resistant tumour cells in culture. Eur J Cancer. 1994;30A:89–93. doi: 10.1016/s0959-8049(05)80025-5. [DOI] [PubMed] [Google Scholar]
- Bradley G, Juranka PF, Ling V. Mechanisms of multidrug resistance. Biochimica et Biophysica Acta. 1999;948:87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Drug Delivery. 2002;54:631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Liao M. Preparation and characterization of YADH-bound magnetic nanoparticles. J Mol Catal B Enzym. 2002;16:283–91. [Google Scholar]
- Cuvier C, Roblot-Treupel L, Millot JM, et al. Doxorubicin-loaded nanospheres bypass tumor cell multidrug resistance. Biochem Phamacol. 1992;44:509–17. doi: 10.1016/0006-2952(92)90443-m. [DOI] [PubMed] [Google Scholar]
- Dresco PA, Zaitsev VS, Gambino RJ, et al. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir. 1999;15:1945–51. [Google Scholar]
- Du D, Ju H, Zhang X, et al. Electrochemical immunoassay of membrane P-glycoprotein by immobilization cells on gold nanoparticles modified on a methoxysilyl-terminated butyrylchitosan matrix. Biochemistry. 2005;44:11539–45. doi: 10.1021/bi0507332. [DOI] [PubMed] [Google Scholar]
- Farokhzad OC, Khademhosseini A, Jon S, et al. Microfluidic system for studying the interaction of nanoparticles and microparticles with cells. Anal Chem. 2005;77:5453–9. doi: 10.1021/ac050312q. [DOI] [PubMed] [Google Scholar]
- Ferrao P, Sincock P, Cole S, et al. Intracellular Pgp contributes to functional drug efflux and resistance in acute myeloid leukemia. Leuk Res. 2001;25:395–405. doi: 10.1016/s0145-2126(00)00156-9. [DOI] [PubMed] [Google Scholar]
- Gao H, Wang J, Shen X, et al. Preparation of magnetic polybutyl-cyanoacrylate nanospheres encapsulated with aclacinomycin A and its effect on gastric tumor. World J Gastroenterol. 2004;10:2010–13. doi: 10.3748/wjg.v10.i14.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Tsuruo T. Characterization of the ATPase activity of the Mr 170000 to 180000 membrane glycoprotein (Pglycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988;48:4926–32. [PubMed] [Google Scholar]
- Hegewisch-Becker S. MDR1 reversal: criteria for clinical trails designed to overcome the multidrug resistance phenotype. Leukemia. 1996;10(Suppl 3):S32–8. [PubMed] [Google Scholar]
- Jain TK, Morales MA, Sahoo SK, et al. Iron oxide nanoparticles for sustained delivery anticancer agents. Molecular Pharmaceutics. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005;5:331–8. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Wang C, et al. The enhancement effect of gold nanoparticles to facilitate the drug delivery and biomarker of drug-resistant cancer cells. Chem Med Chem. 2007;2:374–8. doi: 10.1002/cmdc.200600264. [DOI] [PubMed] [Google Scholar]
- Li J, Wu C, Dai Y, et al. Doxorubicin-CdS nanoparticles: A potential anticancer agent for enhancing the drug uptake of cancer cells. J Nanosci Nanotechnol. 2007;7:435–9. [PubMed] [Google Scholar]
- Litman T, Nielsen D, Skovsgaard T, et al. Structure-activity relationships of P-glycoprotein interacting drugs: kinetic characterization of their effects on ATPase activity. Biochimica et Biophysica Acta. 1997;1361:159–68. doi: 10.1016/s0925-4439(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Lv G, He F, Wang X, et al. Novel nanocomposite of nano Fe3O4 and polylactide nanofibers for application in drug uptake and induction of cell death of leukemia cancer cells. Langmuir. 2008;24:2151–6. doi: 10.1021/la702845s. [DOI] [PubMed] [Google Scholar]
- Sanford JC, Smith FD, Russell JA. Optimizing the biolistic process for different biological applications. Methods Enzymol. 1993;217:483–509. doi: 10.1016/0076-6879(93)17086-k. [DOI] [PubMed] [Google Scholar]
- Soma CE, Dubernet C, Bentoliba D, et al. Reversion of multidrug resistance by co-encapsulation of doxorubicin and cyclosporin A in poly alkylcyanoacrylate nanoparticles. Biomaterials. 2000;21:1. doi: 10.1016/s0142-9612(99)00125-8. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Inoue K, Hongoh A, et al. Modulation of doxorubicin resistance in a doxorubicin-resistant human leukemia cell by an immunoliposome targeting transferring receptor. Br J Cancer. 1997;76:83–9. doi: 10.1038/bjc.1997.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12:450–8. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Tan Y, Li G, Zhao C, et al. Expression of sorcin predicts poor outcome in acute myeloid leukemia. Leukemia Res. 2003;27:125–31. doi: 10.1016/s0145-2126(02)00083-8. [DOI] [PubMed] [Google Scholar]
- Twentyman PR. Cyclosporins as drug resistance modifiers. Biochem Pharmacol. 1992;43:109–17. doi: 10.1016/0006-2952(92)90668-9. [DOI] [PubMed] [Google Scholar]
- Vauthier C, Dubernet C, Chauvierre C, et al. Drug delivery to resistant tumors: the potential of poly (alkyl cyanoacrylate) nanoparticles. J Control Release. 2003;93:151–60. doi: 10.1016/j.jconrel.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Xie J, Xu C, Xu Z, et al. Linking hydrophilic macromolecules to monodisperse magnetite (Fe3O4) nanoparticles via trichloro-s-triazine. Chem Mater. 2006;18:5401–3. doi: 10.1021/cm061793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Shen H, Ao Z, et al. Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leukemia Res. 2006;30:407–13. doi: 10.1016/j.leukres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang X, Wu C, et al. Synergistic enhancement effect of magnetic nanoparticles on anticancer drug accumulation in cancer cells. Nanotechnology. 2006;17:3622–6. doi: 10.1088/0957-4484/17/14/043. [DOI] [PubMed] [Google Scholar]