Abstract
Mutation of the crr-ptsI gene locus revealed that Streptomyces coelicolor uses the phosphotransferase system (PTS) for N-acetylglucosamine uptake. crr, ptsI, and ptsH, which encode the three general PTS phosphotransferases, are induced by N-acetylglucosamine but not by other PTS substrates. Thus, the S. coelicolor PTS is biased for N-acetylglucosamine utilization, a novel feature that distinguishes this PTS from others.
The bacterial phosphotransferase system (PTS) is a multifaceted system that is required for carbohydrate uptake, carbon catabolite repression, and chemotaxis (19). In addition, there are reports that indicate a linkage between C metabolism and other cellular processes, for example, nitrogen fixation, stress response, starvation, and pathogenicity, via the PTS (5, 9, 20, 27). While PTS research is quite advanced in gram-negative and low-GC gram-positive bacteria, knowledge about it is limited in high-GC gram-positive bacteria, to which the antibiotic-producing soil bacterium Streptomyces coelicolor belongs (15, 16). Analysis of ptsH, which encodes the general PTS phosphotransferase HPr, revealed that S. coelicolor uses the PTS to internalize fructose, but no role of the PTS in carbon catabolite repression could be demonstrated (2, 14, 17). In silico analysis of the genome led to the identification of pts genes that encode the general phosphotransferases enzyme I (EI) and enzyme IIACrr as well as three further PTS permeases (NagE1, NagE2, and MalX1) (16). We suggested that N-acetylglucosamine could be a possible substrate for NagE1 or NagE2. This is corroborated by an in vitro characterization of IIACrr, which can serve as a IIA protein of an N-acetylglucosamine-specific PTS (PTSNag) (7). In a recent publication, a PTSNag has been described in Streptomyces olivaceoviridis, in which the homologue of nagE2 has been identified as the structural gene for enzyme IINag (29).
We present a mutational analysis of the crr-ptsI gene locus and demonstrate that EI and IIACrr are part of a PTSNag in S. coelicolor. We provide evidence that the two genes form an operon and that their expression, together with the third general gene of the PTS, ptsH, is induced by N-acetylglucosamine. The data suggest that the PTS of S. coelicolor is biased for N-acetylglucosamine metabolism, a novel feature that will be discussed.
Knockout mutation of crr and ptsI.
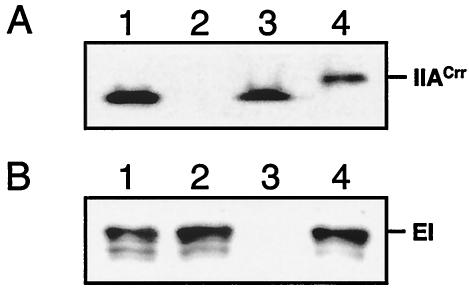
Gene replacement plasmids pFT50 and pFT52 were constructed by several cloning steps to generate a crr and a ptsI mutant (Table 1). Protoplasts of M145 were transformed and mutants were isolated as described previously (4, 8). The resulting strain BAP2 (Δcrr::aacC4) carried a deletion in crr ranging from nucleotides (nt) 146 to 280, in which the apramycin gene cassette (aacC4) was placed. The ptsI gene in strain BAP3 (ptsI::aacC4) was interrupted by the apramycin gene at nt 665. Mutations were verified by PCRs that revealed the presence of aac4 and the correct chromosomal position by the use of oligonucleotides that hybridized in aac4 and on the chromosome just outside the recombination area (data not shown). As can be seen from the Western blots in Fig. 1, no IIACrr (Fig. 1A, lane 2) and no EI (Fig. 1B, lane 3) were detectable in cell extracts of BAP2 and BAP3, respectively. Both mutations had no polar effect on the expression of the adjacent gene, since an immunosignal for EI was present in BAP2 (Fig. 1B, lane 2) extract and vice versa (Fig. 1A, lane 3).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | Recipient for cloning experiments | 1 |
| ET12567 | Host for preparation of nonmethylated DNA | 10 |
| Streptomyces coelicolor | ||
| M145 A3(2) | SCP1− SCP2−; prototroph | 8 |
| BAP2 | M145 Δcrr::accC4 Aprr | This study |
| BAP3 | M145 ptsI::accC4 Aprr | This study |
| Plasmids | ||
| pBluescript SK(+) | Cloning vector, ColE1 replicon; Apr | Stratagene |
| pSU2718 | Cloning vector, P15A replicon; Cmr | 11 |
| pHLW1 | Plasmid with accC4 apramycin gene; Aprr Apr | U. Wehmeier |
| pWHM3 | E. coli-Streptomyces shuttle vector; Apr Tsrr | 28 |
| pUWL-SK+ | E. coli-Streptomyces shuttle vector; Apr Tsrr | 30 |
| pMN406 | Plasmid containing mycgfp2+; Hygr | This study |
| pFT32 | 2,881-bp EcoRV-PvuII crr region from cosmid SC1A8A cloned into SmaI of pSU2718; Cmr | This study |
| pFT33 | accC4 gene from pHLW1 cut with PvuII, inserted into TthIII1 site of pFT32; Cmr Aprr | This study |
| pFT35 | Overexpression vector for ptsI of S. coelicolor | 14 |
| pFT50 | 4.8-kb SbfI-NheI crr::accC4 fragment from pFT33 cloned into PvuII site of pWHM3; Apr Aprr Tsrr | This study |
| pFT51 | 1.8-kb EcoRI-HindIII ptsI fragment from pFT35 cloned into EcoRI-HindIII of pWHM3; Apr Tsrr | This study |
| pFT52 | accC4 gene from pHLW1 cut with BamHI inserted into ptsI of pFT51 cut with BglII; Apr Tsrr; Aprr | This study |
| pFT73 | mycgfp2+ cloned into BamHI-PstI of pUWL-SK+; Apr Tsrr | |
| pFT102 | pFT73 with 765-bp (nt −638 to nt 127 of crr) KpnI-PstI fragment; Apr Tsrr | This study |
| pFT103 | pFT73 with 383-bp (nt −256 to nt 127 of crr) KpnI-PstI fragment; Apr Tsrr | This study |
| pFT104 | pFT73 with 184-bp (nt −57 to nt 127 of crr) KpnI-PstI fragment; Apr Tsrr | This study |
| pFT105 | pFT73 with 305-bp (nt −228 to nt 77 of ptsI) KpnI-PstI fragment; Apr Tsrr | This study |
Ap, ampicillin, used at 100 mg/liter; Tet, tetracycline, used at 12 mg/liter; Tsr, thiostreptone; Apr, apramycin, and Cm, chloramphenicol, both used at 25 mg/liter.
FIG. 1.
Western blots of sodium dodecyl sulfate-10% polyacrylamide gels show the absence of IIACrr and EI in BAP2 and BAP3, respectively. Blotting was performed as described with polyclonal antibodies raised against His-tagged EI (Eurogentec) and His-tagged IIACrr (7). Ten micrograms of cellular protein prepared from cells grown on tryptic soy broth without dextrose or 10 ng of purified His-tagged protein was subjected to gel electrophoresis. (A) IIACrr-immunoreactive signal in cell extracts of M145 (lane 1), BAP2 (lane 2), BAP3 (lane 3), and His-tagged IIACrr (lane 4). (B) EI-immunoreactive signal in cell extracts of M145 (lane 1), BAP2 (lane 2), BAP3 (lane 3), and His-tagged EI (lane 4).
Phenotypes.
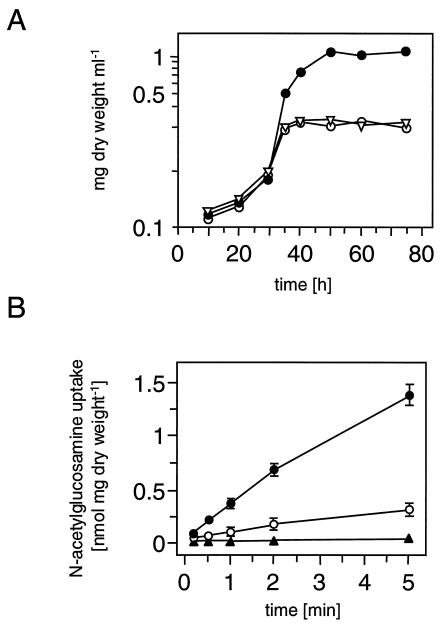
BAP2 and BAP3 were examined regarding their growth phenotype on mineral medium (MM) agar plates (14). Both mutants could not grow on N-acetylglucosamine, whereas utilization of galactose, glucosamine, glucose, glutamate, glycerol, lactose, maltose, mannitol, mannose, ribose, sorbitol, sorbose, sucrose, and xylose was not affected. The ptsI mutant was additionally impaired in fermentation of fructose, which is in agreement with previous publications (14, 16, 17). A growth curve in MM supplemented with 0.1% Casamino Acids and either 50 mM glycerol, glucose, or N-acetylglucosamine was recorded as described to corroborate the N-acetylglucosamine-negative phenotype (14). Within the first 35 h, the strains showed similar increases in biomass (Fig. 2A). BAP2 and BAP3 then entered stationary phase when N-acetylglucosamine was present in the medium while the wild type continued growing for 20 h. The mutants and the wild type showed similar growth curves when glucose or glycerol served as the source of carbon (data not shown).
FIG. 2.
N-Acetylglucosamine utilization and N-acetylglucosamine transport. (A) Growth curve of M145 (•), BAP2 (○), and BAP3 (▿) on MM supplemented with 0.1% Casamino Acids and 50 mM N-acetylglucosamine. (B) Time course of N-acetylglucosamine transport. Mycelia of M145, BAP2, and BAP3 were grown in MM supplemented with 0.1% Casamino Acids and 50 mM glycerol (○) or 50 mM glycerol plus 50 mM N-acetylglucosamine (•). The triangle indicates transport activity of BAP2 and BAP3 grown on glycerol with or without N-acetylglucosamine. Standard deviations indicated by error bars represent the means of three independent experiments.
Transport of N-[14C]acetyl-d-glucosamine (6.2 mCi mmol−1) at a final concentration of 20 μM was performed (23). BAP2 and BAP3 showed no detectable transport (<10 pmol of N-acetylglucosamine min−1 mg [dry weight]−1), while the wild type incorporated N-acetylglucosamine at a rate of 87 ± 5 U when grown on glycerol and 344 ± 31 U when grown on glycerol plus N-acetylglucosamine. Hence, under these conditions transport of N-acetylglucosamine was inducible by a factor of four in the wild type and was impaired in both mutants. PTS assays were carried out to investigate whether EI and IIACrr are directly required for PTSNag activity (17). Phosphoenolpyruvate-dependent phosphorylation of N-acetylglucosamine in cell extracts of the wild type increased about fivefold (from 7.3 ± 0.3 to 40.5 ± 2.1 nmol of N-acetylglucosamine-phosphate min−1 mg of protein−1) when grown in the presence of N-acetylglucosamine (MM plus 0.1% Casamino Acids, 50 mM glycerol, ± 50 mM N-acetylglucosamine). This activity was absent in cell extracts of BAP2 and BAP3 but could be restored upon addition of His-tagged EI or His-tagged IIACrr, respectively.
Carbon source-dependent synthesis.
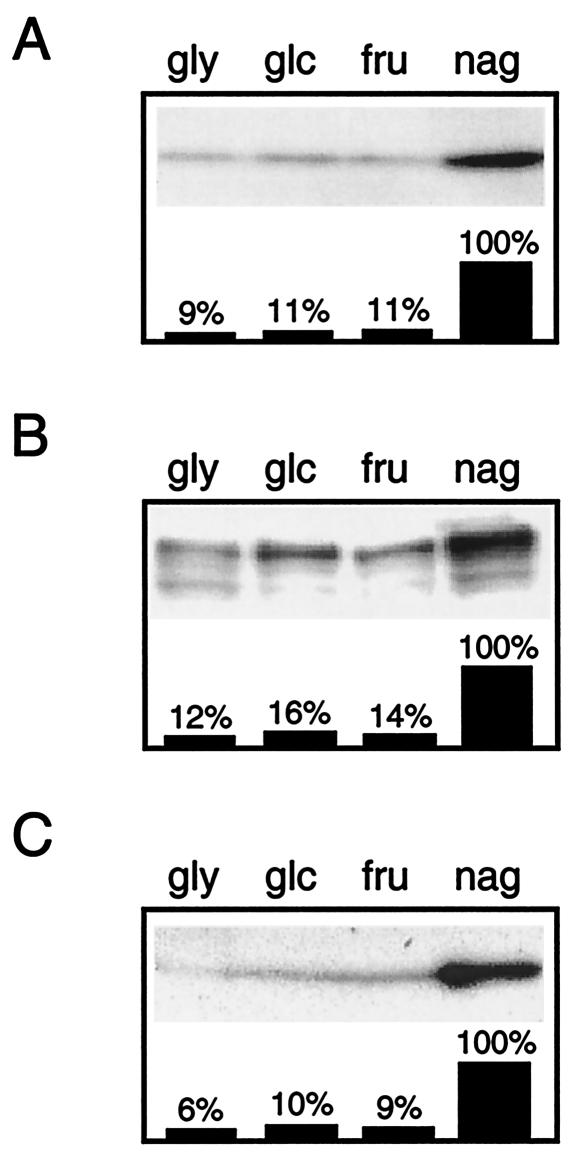
Since IIACrr and EI as well as the previously characterized HPr protein can be considered pleiotropically acting PTS proteins, we raised the question whether their synthesis is stimulated by growth on PTS substrates in comparison to growth on non-PTS carbon sources like glycerol and glucose. Protein levels of IIACrr, EI, and HPr were monitored by Western blotting (Fig. 3). All three proteins showed 8- to 10-fold-higher amounts in mycelia grown on N-acetylglucosamine than in mycelia grown on fructose, glycerol, or glucose. Interestingly, growth on the other PTS sugar, fructose, did not lead to an enhanced synthesis of the PTS proteins.
FIG. 3.
Protein levels of IIACrr, EI, and HPr. Western blots of sodium dodecyl sulfate-10% polyacrylamide gels show the immunoreactive signals corresponding to IIACrr (A), EI (B), and HPr (C). Crude cell extracts were prepared from mycelia grown on MM supplemented with 0.1% Casamino Acids and 50 mM glycerol (gly), glucose (glc), fructose (fru), or N-acetylglucosamine (nag). In each lane 5 μg of cellular protein extract was subjected to gel electrophoresis. The bars show the signal quantification of IIACrr, EI, and HPr protein levels obtained by densitometric analysis of the gray scales with the TINA software program (version 2.08; Raytest). The signal value detected in N-acetylglucosamine-grown cells was set to 100%. Similar results were obtained in three independent experiments.
Transcription of crr and ptsI.
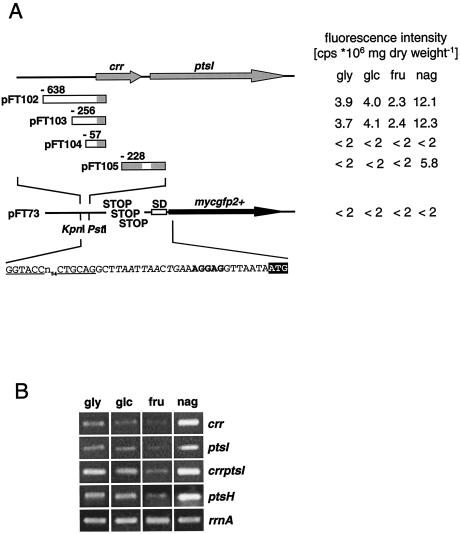
Next, we studied transcription of the crr-ptsI locus. Promoter activities were examined by taking advantage of the reporter gene mycgfp2+, a GC-rich variant of an improved gfp gene (24). The mycgfp2+ gene was amplified from pMN406 by using oligonucleotides GFP1 (5′-TGCCACGGATCTGCAGGCTTAATTAACTGAAAGG-3′) and GFP2 (5′-CGACGGATCCGATAAAATAAAAAAGGGG-3′) introducing PstI and BamHI restriction sites (underlined) and stop codons in all reading frames (italics) prior to the ribosomal binding site and ligated in the Streptomyces-Escherichia coli shuttle plasmid pUWL-SK digested with the same endonucleases, giving plasmid pFT73 (Fig. 4A) (30). A set of transcriptional fusions of putative promoter fragments was cloned into pFT73, yielding plasmids pFT102 to pFT105 (Table 1). They were transformed into the wild type. Each strain was grown on glycerol, fructose, glucose, and N-acetylglucosamine and subjected to green fluorescent protein fluorescence quantification. The results revealed the presence of an N-acetylglucosamine-dependent promoter upstream of crr between nt −256 and −57. This promoter was also active when the strains were grown in the presence of glycerol, glucose, and fructose. A second promoter was found to precede ptsI. This promoter was also inducible by N-acetylglucosamine.
FIG. 4.
Transcriptional analyses. (A) Quantification of green fluorescent protein fluorescence was carried out with mycelia grown in 30 ml of MM supplemented with 0.1% Casamino Acids and 50 mM glycerol (gly), fructose (fru), glucose (glc), or N-acetylglucosamine (nag) under vigorous shaking for 48 h at 28°C. Cells were harvested and washed twice in buffer A (1× phosphate-buffered saline and 0.05% Tween 80), resuspended in the same buffer, and adjusted to 2 mg (dry weight) per ml. Cells (0.5 ml) were mixed with 1.5 ml of buffer A in acrylic cuvettes (Sarstedt) and stirred to prevent sedimentation. Probes were analyzed by fluorescence spectroscopy with a Spex Fluorolog 1680. Emission spectra of mycgfp2+ fluorescence were taken from 500 to 524 nm with an excitation wavelength of 485 nm and a slit width of 2 mm. cps, counts per second. (B) RT-PCR. A 1% agarose gel shows specific RT-PCR amplification products of crr, ptsI, and ptsH after the 27th cycle of the PCR. Total RNA was prepared from cultures grown on MM supplemented with 0.1% Casamino Acids and 50 mM glycerol (gly), glucose (glc), fructose (fru), or N-acetylglucosamine (nag). Oligonucleotides for RT-PCR experiments were combined as follows: RTcrr1 (22-mer) and RTcrr2 (21-mer) (nt 4 to 446 of crr), RTptsI1 (23-mer) and RTptsI2 (21-mer) (nt 277 to 746 of ptsI), RTcrrptsI1 (24-mer) and RTcrrptsI2 (24-mer) (nt 233 of crr to nt 119 of ptsI), RTptsH1 (29-mer) and RTptsH2 (25-mer) (nt 1 to nt 268 of ptsH), and 16SrRNA1 (21-mer) and 16SrRNA2 (21-mer) (nt 787 to nt 1313 of rrnA) for detection of the 16S rRNA, which served as the invariant standard.
Reverse transcription-PCR (RT-PCR) experiments were performed to corroborate these findings (Fig. 4B). RNA of S. coelicolor grown on MM in the presence of 0.1% Casamino Acids plus a 50 mM concentration of either glycerol, fructose, glucose, or N-acetylglucosamine was prepared as described previously (14). The One-Step RT-PCR kit (Qiagen) was applied according to the manufacturer's instructions with gene-specific oligonucleotides. Analysis of the mRNA of ptsH, crr, and ptsI and of a potential crr-ptsI operon transcript showed that they all were increased in RNA preparations from wild-type mycelia grown in the presence of N-acetylglucosamine, while RNA from fructose-, glucose-, or glycerol-grown mycelia exhibited lower amounts of transcript. Hence, the gene expression data confirmed the observed stimulation by N-acetylglucosamine of HPr, EI, and IIACrr synthesis and revealed that crr and ptsI constitute an operon.
In Bacillus subtilis, ptsH and ptsI form an operon that is constitutively expressed and that is localized downstream of ptsG, the gene for the glucose-specific PTS permease (6, 26). In E. coli, ptsH, ptsI, and crr form a tricistronic operon, which is regulated in a complex fashion by the globally acting transcription factors catabolite activator protein and Mlc (18). The expression varies about three- to fourfold and is stimulated either by cyclic AMP or by the presence of PTS substrates (3, 12, 21). Hence, the N-acetylglucosamine-specific induction of the genes of the S. coelicolor PTS is a novel feature that distinguishes this PTS from others.
What can be the physiological rationale for an N-acetylglucosamine-biased PTS? Streptomycetes are an integral part of the indigenous soil microflora. Chitin (a β-1,4-linked polymer of N-acetylglucosamine) and its breakdown products are commonly found in the soil. As chitin cannot directly enter the cell, it has to be degraded to chito-oligosaccharides and N-acetylglucosamine (22, 25). It is obvious that the genes for chitin and N-acetylglucosamine metabolism are coordinately regulated. We found a common cis element, a 12-bp palindrome that is present in one copy 122 nt upstream of crr, in two copies in the promoter region of ptsH, and in front of chitinase genes (16, 17). Transcriptional analyses of chitinase genes revealed that the cis element is involved in substrate induction and glucose repression (13, 22). Thus, it will be worthwhile to identify the trans-acting element(s) to uncover the regulation of chi and pts genes and to determine whether both pathways are subject to a common regulatory mechanism.
Acknowledgments
We thank Anke Engels and Udo Wehmeier for helpful discussions and gifts of plasmids.
The work was supported by grant SFB473 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidmann, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, N.Y.
- 2.Butler, M. J., J. Deutscher, P. W. Postma, T. J. Wilson, A. Galinier, and M. J. Bibb. 1999. Analysis of a ptsH homologue from Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 177:279-288. [DOI] [PubMed] [Google Scholar]
- 3.De Reuse, H., and A. Danchin. 1988. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol. 170:3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink, D., D. Falke, W. Wohlleben, and A. Engels. 1999. Nitrogen metabolism in Streptomyces coelicolor A3(2): modification of glutamine synthetase I by an adenylyltransferase. Microbiology 145:2313-2322. [DOI] [PubMed] [Google Scholar]
- 5.Gaurivaud, P., J. L. Danet, F. Laigret, M. Garnier, and J. M. Bove. 2000. Fructose utilization and phytopathogenicity of Spiroplasma citri. Mol. Plant-Microbe Interact. 13:1145-1155. [DOI] [PubMed] [Google Scholar]
- 6.Gonzy-Treboul, G., and M. Steinmetz. 1987. Phosphoenolpyruvate:sugar phosphotransferase system of Bacillus subtilis: cloning of the region containing the ptsH and ptsI genes and evidence for a crr-like gene. J. Bacteriol. 169:2287-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamionka, A., S. Parche, H. Nothaft, J. Siepelmeyer, K. Jahreis, and F. Titgemeyer. 2002. The phosphotransferase system of Streptomyces coelicolor: IIACrr exhibits properties that resemble transport and inducer exclusion function of enzyme IIAGlucose of Escherichia coli. Eur. J. Biochem. 269:2143-2150. [DOI] [PubMed] [Google Scholar]
- 8.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 9.Lodge, J., and G. R. Jacobson. 1988. Starvation-induced stimulation of sugar uptake in Streptococcus mutans is due to an effect on the activities of preexisting proteins of the phosphotransferase system. Infect. Immun. 56:2594-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. H. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61-68. [DOI] [PubMed] [Google Scholar]
- 11.Martinez, E., B. Bartolome, and F. de la Cruz. 1988. pACYC184-derived cloning vectors containing the multiple cloning site and lacZ alpha reporter gene of pUC8/9 and pUC18/19 plasmids. Gene 68:159-162. [DOI] [PubMed] [Google Scholar]
- 12.Mattoo, R. L., and E. B. Waygood. 1983. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can. J. Biochem. Cell Biol. 61:29-37. [DOI] [PubMed] [Google Scholar]
- 13.Ni, X., and J. Westpheling. 1997. Direct repeat sequences in the Streptomyces chitinase-63 promoter direct both glucose repression and chitin induction. Proc. Natl. Acad. Sci. USA 94:13116-13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nothaft, H., S. Parche, A. Kamionka, and F. Titgemeyer. 2003. In vivo analysis of HPr reveals a fructose-specific phosphotransferase system that confers high affinity uptake in Streptomyces coelicolor. J. Bacteriol. 185:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parche, S., A. Burkovski, G. A. Sprenger, B. Weil, R. Kramer, and F. Titgemeyer. 2001. Corynebacterium glutamicum: a dissection of the PTS. J. Mol. Microbiol. Biotechnol. 3:423-428. [PubMed] [Google Scholar]
- 16.Parche, S., H. Nothaft, A. Kamionka, and F. Titgemeyer. 2000. Sugar uptake and utilisation in Streptomyces coelicolor: a PTS view to the genome. Antonie Leeuwenhoek 78:243-251. [DOI] [PubMed] [Google Scholar]
- 17.Parche, S., R. Schmid, and F. Titgemeyer. 1999. The phosphotransferase system (PTS) of Streptomyces coelicolor: identification and biochemical analysis of a histidine phosphocarrier protein HPr encoded by the gene ptsH. Eur. J. Biochem. 265:308-317. [DOI] [PubMed] [Google Scholar]
- 18.Plumbridge, J. 2002. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5:187-193. [DOI] [PubMed] [Google Scholar]
- 19.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabus, R., J. Reizer, I. Paulsen, and M. H. Saier, Jr. 1999. Enzyme I(Ntr) from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J. Biol. Chem. 274:26185-26191. [DOI] [PubMed] [Google Scholar]
- 21.Saffen, D. W., K. A. Presper, T. L. Doering, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J. Biol. Chem. 262:16241-16253. [PubMed] [Google Scholar]
- 22.Saito, A., T. Fujii, T. Yoneyama, M. Redenbach, T. Ohno, T. Watanabe, and K. Miyashita. 1999. High-multiplicity of chitinase genes in Streptomyces coelicolor A3(2). Biosci. Biotechnol. Biochem. 63:710-718. [DOI] [PubMed] [Google Scholar]
- 23.Schlösser, A., and H. Schrempf. 1996. A lipid-anchored binding protein is a component of an ATP-dependent cellobiose/cellotriose-transport system from the cellulose degrader Streptomyces reticuli. Eur. J. Biochem. 242:332-338. [DOI] [PubMed] [Google Scholar]
- 24.Scholz, O., A. Thiel, W. Hillen, and M. Niederweis. 2000. Quantitative analysis of gene expression with an improved green fluorescent protein. Eur. J. Biochem. 267:1565-1570. [DOI] [PubMed] [Google Scholar]
- 25.Schrempf, H. 2001. Recognition and degradation of chitin by streptomycetes. Antonie Leeuwenhoek 79:285-289. [DOI] [PubMed] [Google Scholar]
- 26.Stülke, J., I. Martin-Verstraete, M. Zagorec, M. Rose, A. Klier, and G. Rapoport. 1997. Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator. Mol. Microbiol. 25:65-78. [DOI] [PubMed] [Google Scholar]
- 27.Ueguchi, C., N. Misonou, and T. Mizuno. 2001. Negative control of rpoS expression by phosphoenolpyruvate: carbohydrate phosphotransferase system in Escherichia coli. J. Bacteriol. 183:520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vara, J., M. Lewandowska-Skarbek, Y. G. Wang, S. Donadio, and C. R. Hutchinson. 1989. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J. Bacteriol. 171:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, F., X. Xiao, A. Saito, and H. Schrempf. 2002. Streptomyces olivaceoviridis possesses a phosphotransferase system that mediates specific, phosphoenolpyruvate-dependent uptake of N-acetylglucosamine. Mol. Genet. Genomics 268:344-351. [DOI] [PubMed] [Google Scholar]
- 30.Wehmeier, U. F. 1995. New multifunctional Escherichia coli-Streptomyces shuttle vectors allowing blue-white screening on XGal plates. Gene 165:149-150. [DOI] [PubMed] [Google Scholar]