Abstract
Few interactions have been reported between effectors and components of the type III secretion apparatus, although many interactions have been demonstrated between type III effectors and their cognate chaperones. It is thought that chaperones may play a role in directing effectors to the type III secretion apparatus. The ATPase FliI in the flagellar assembly apparatus plays a pivotal role in interacting with other components of the apparatus and with substrates of the flagellar system. We performed experiments to determine if there were any interactions between the effector Tir and its chaperone CesT and the type III secretion apparatus of enteropathogenic Escherichia coli (EPEC). Specifically, based on analogies with the flagella system, we examined Tir-CesT interactions with the putative ATPase EscN. We showed by affinity chromatography that EscN and Tir bind CesT specifically. Tir is not necessary for CesT and EscN interactions, and EscN binds Tir specifically without its chaperone CesT. Moreover, Tir directly binds EscN, as shown via gel overlay and enzyme-linked immunosorbent assay, and coimmunoprecipitation experiments revealed that Tir interacts with EscN inside EPEC. These data provide evidence for direct interactions between a chaperone, effector, and type III component in the pathogenic type III secretion system and suggest a model for Tir translocation whereby its chaperone, CesT, brings Tir to the type III secretion apparatus by specifically interacting with the type III ATPase EscN.
Numerous substrates of protein transport systems, ranging from the sec export pathway in bacteria to the mitochondrial import system in eukaryotic cells, interact with components of the transport machinery during transit (37, 51). These types of interactions are anticipated for the type III secretion system (TTSS), which facilitates delivery of many bacterial effectors from the cytoplasm of gram-negative bacterial pathogens into host cells. Many protein-protein interactions have been demonstrated for the type III-related flagellar assembly apparatus, in terms of both components and substrates interacting with the flagellar assembly apparatus (6, 19, 23, 29, 42, 44, 46, 54, 61).
The ATPase FliI in the flagellar assembly apparatus appears to play a pivotal role in interacting with other components of the apparatus and substrates of the system. FliI is required for the export of flagellin and other export substrates (41, 54). The substrates of the flagellar assembly apparatus, such as flagellin and other filament-type substrates, interact with both cytosolic components (ATPase FliI, FliH, and FliJ) and membrane-bound components (FlhA and FlhB) of the apparatus (44, 54, 61). FliI is present in the cytoplasm and in association with the inner membrane in Caulobacter crescentus and Salmonella enterica serovar Typhimurium (4, 55). The ATPase activity of S. enterica serovar Typhimurium FliI, localized to the carboxy terminus, is required for flagellar assembly (16, 41). FliI also interacts with a number of components of the flagellar export apparatus and its substrates through its amino terminus (4, 43, 44, 54). FliI interacts not only with substrates of the flagellar assembly apparatus but also with at least one putative chaperone for both hook and filament-type proteins in S. enterica serovar Typhimurium, FliJ (40, 41, 44), suggesting that the ATPase could be the first step in directly facilitating transfer of substrate proteins. Several other flagellar system chaperones have been identified, and they share similarities to TTSS chaperones (reviewed in reference 5). Both are small acidic proteins that bind as homodimers to export substrates, although the type III and flagellar chaperones bind different regions of their substrates.
While few interactions have been demonstrated between effectors and components of the TTSS, demonstrated interactions between type III effectors and their cognate chaperones are plentiful (reviewed in references 3 and 50). While the exact function of type III chaperones is still an area of investigation, many roles have been attributed and proposed for chaperones, including transcriptional regulation (13, 38, 60); prevention of degradation, or a bodyguard function (39); prevention of premature association (39, 49); maintenance of effectors in a secretion-competent conformation (18); escort to the type III apparatus (9); and initiation of secretion and determination of the hierarchy of translocation (7, 9, 32, 33). However, the exact role or roles remain to be determined, and it is possible that a single chaperone has multiple functions in the bacteria and in type III secretion. A considerable and pressing issue in the TTSS field is how chaperones and effectors dock and interact with the type III apparatus.
Enteropathogenic Escherichia coli (EPEC) is a human pathogen responsible for outbreaks of diarrhea in both developing and developed countries (47). During infections, EPEC adheres to intestinal epithelial cells and forms actin-rich pedestals through the binding of the outer membrane protein intimin to its receptor in the host. Using a type III secretion apparatus, EPEC inserts a receptor for intimin, Tir (translocated intimin receptor), into the host cell membrane, where it becomes tyrosine phosphorylated (14, 27). An earlier observation in enterohemorrhagic E. coli found that the EscD homologue Pas, which is thought to be an inner membrane component of the type III apparatus, bound the type III effector Tir in an overlay experiment (30), although there have been no subsequent reports.
CesT is Tir's chaperone. A cesT mutant has significantly reduced levels of Tir in the bacterium and in secreted form and is unable to induce pedestal formation in host cells, but the mutation does not affect the secretion of other EPEC secreted proteins (1, 15). The CesT binding domain is located within the first 100 amino-terminal residues of Tir. Tir can still bind intimin in the presence of CesT, suggesting that Tir is not globally unfolded in the presence of its chaperone (34). CesT was localized to the cytoplasm of EPEC but was not in the membrane or supernatant fractions (15), lending support to the hypothesis that CesT acts inside the bacterium to protect Tir. Interestingly, CesT is important for Tir stability in EPEC, but not when expressed in Yersinia, although it is required for efficient Tir secretion and translocation in both systems (26, 28). This leads to the hypothesis that CesT may play a role in directing Tir to the type III secretion apparatus.
The EPEC TTSS ATPase FliI homologue is EscN. We have shown that EscN is essential for Tir delivery into host cells and for pedestal formation (21). The purpose of this study was to investigate the initial interactions that occur between substrates of the TTSS and the type III apparatus. We examined interactions between Tir and its chaperone CesT and EPEC's type III apparatus, specifically the ATPase EscN.
MATERIALS AND METHODS
Bacterial strains.
EPEC strains (Table 1) were grown in Luria-Bertani (LB) broth supplemented with appropriate antibiotics as standing overnight cultures at 37°C. E. coli strains were grown in LB broth supplemented with appropriate antibiotics as shaking overnight cultures at 37°C.
TABLE 1.
Strains used for this study
| Strain | Description | Source or reference |
|---|---|---|
| Δtir | EPEC E2348/69 tir deletion mutant | 20 |
| Δtir/pEscN-HSV | Δtir carrying escN-HSV in pACYC184 | This study |
| ΔescN/pEscN-HSV | ΔescN carrying escN-HSV in pACYC184 | 21 |
| ΔcesT | EPEC E2348/69 cesT deletion mutant | 1 |
| ΔcesT/pEscN-HSV | ΔcesT carrying escN-HSV in pACYC184 | This study |
| B21(DE3) | BL21 (deficient in lon and ompT proteases) containing DE3 (T7 RNA polymerase) | Novagen |
| BL21/pET28a | BL21 carrying a His-tagging pET28a vector | Novagen |
| BL21/pETCesT | BL21 carrying cesT (N-terminal His tag) in pET28a | 22 |
| BL21/pETTir | BL21 carrying tir (N-terminal His) in pET28a | E. Frey, unpublished data |
| BL21/pETInt282 | BL21 carrying eae 658-939 (N-terminal His) in pET28a | 35 |
| BL21/pETTir200-549 | BL21 carrying tir 200-549 (N-terminal His) in pET28a | 22 |
| BL21/pETTir1-391 | BL21 carrying tir 1-391 (N-terminal His) in pET28a | 22 |
| BL21/pETTir201-391 | BL21 carrying tir 201-391 (N-terminal His) in pET28a | 22 |
| BL21/pETTir391-550 | BL21 carrying tir 391-550 (N-terminal His) in pET28a | 22 |
| BL21/pETTir1-200 | BL21 carrying tir 1-200 (N-terminal His) in pET28a | 22 |
EPEC lysate for affinity chromatography.
EPEC from overnight standing cultures was subcultured 1/50 in 200 ml of minimal essential medium for 3 h at 37°C in 5% CO2. The culture was harvested, washed in phosphate-buffered saline (PBS), resuspended in 1.5 ml of sonication buffer (10 mM Tris-HCl [pH 7], 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 1 μM pepstatin, 10 μg of aprotinin per ml), and sonicated three times for 15 s each (amplitude 2.5) (Fisher Sonicator). Unbroken bacteria were removed by centrifugation (16,000 × g for 2 min), and supernatant (EPEC lysate) was stored frozen at −80°C. The protein content of EPEC lysates was determined by use of the bicinchoninic acid protein assay (Sigma).
Protein purification.
BL21 bacteria containing the pET28a vector (for His-tag protein purification; Novagen) carrying the gene for CesT, Tir, or Int-282 were grown with shaking overnight at 37°C in LB supplemented with kanamycin (50 μg/ml). The bacteria were subcultured 1/100 in 250 ml of LB-kanamycin and grown to an optical density at 600 nm of 0.6 to 0.7. One millimolar isopropyl-β-d-thiogalactopyranoside (IPTG; Rose Scientific) (final concentration) was added to induce protein expression, and the bacteria were grown for 3 h. The culture was harvested by centrifugation (6,000 × g, 10 min, 4°C), and the pellet was resuspended in 5 ml of sonication buffer (100 mM sodium phosphate, 100 mM sodium chloride, pH 8) and frozen at −80°C. The culture was thawed and sonicated three times for 20 s each (amplitude 4) (Fisher Sonicator), and the unbroken bacteria and insoluble material were pelleted (16,000 × g, 5 min, 4°C). The supernatant (2.5 ml) was added to 0.5 ml of packed preequilibrated Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) and was incubated for 1 h at 4°C, with rotation. The slurry was then poured into a disposable Poly-Prep chromatography column (Bio-Rad) (0.8 by 4 cm) and washed five times with 8 column volumes of wash buffer (100 mM sodium phosphate, 100 mM sodium chloride [pH 8], 10 mM imidazole). The His-tagged proteins were eluted with increasing concentrations of imidazole (Sigma) (50, 100, 200, and 300 mM in sonication buffer) in 1-column-volume fractions.
Denatured conditions.
BL21/pET28a-His-EscN was cultured and harvested as described above. The bacterial pellet was frozen at −80°C, thawed on ice, and then resuspended in 5 ml of sonication buffer with 8 M urea (Sigma). The culture was sonicated three times for 20 s each (amplitude 4) (Fisher Sonicator) and left on ice for 1 h to solubilize. The unbroken bacteria and insoluble material were pelleted (16,000 × g, 5 min, 4°C). All subsequent steps were performed as described above, with 8 M urea in all buffers.
Affinity chromatography.
BL21 bacteria were cultured, harvested, frozen, and sonicated as described above. The supernatant (2.5 ml) was added to 0.5 ml of packed preequilibrated Ni-NTA agarose (Qiagen) and incubated for 1 h at 4°C, with rotation. The slurry was washed three times with 8 column volumes of wash buffer and one time with 8 column volumes of 1% Triton X-100 in sonication buffer. The slurry was incubated with EPEC lysate (1 mg) for 1 h at 4°C, with rotation. The slurry was then poured into a disposable Poly-Prep chromatography column (Bio-Rad) (0.8 by 4 cm) and washed three times with 8 column volumes of 1% Triton X-100 in sonication buffer and two times with 8 column volumes of wash buffer. The His-tagged proteins were eluted with increasing concentrations of imidazole (Sigma) (50, 100, 200, and 300 mM in sonication buffer) in 1-column-volume fractions. Samples (10 μl) were analyzed by SDS-PAGE followed by Coomassie G-250 (Sigma) or Sypro Ruby (Bio-Rad) staining.
Gel overlay.
Purified Int282, CesT, EscN, and bovine serum albumin (BSA) were prepared in SDS sample buffer with β-mercaptoethanol, boiled for 5 min, resolved by SDS-PAGE (12% gel) at a concentration of 2.5 μg/well, and transferred to an Immobilon-P membrane (Millipore) (polyvinylidene difluoride membrane; pore size, 0.45 μm). Blots were washed three times for 20 min each in 20% isopropanol with 50 mM Tris, pH 8.0, and then three times for 20 min each in Tris, pH 8.0. The proteins were then denatured by incubation two times for 30 min each in 6 M guanidine-HCl with 50 mM Tris, pH 8.0, and then extensively washed in wash buffer (0.05% Tween 20, Tris [pH 8.0]) for >18 h, with numerous changes of the wash buffer. Blots were then blocked for 1 h at room temperature in 5% skim milk in TBST (Tris-buffered saline with 0.1% Tween 20) and were overlaid with 200 μg of purified Tir in 1% skim milk in TBST. Blots were washed six times for 5 min each in TBST and then probed for Tir by using Tir monoclonal antibody 2A5 (diluted 1/1,000 in 1% skim milk in TBST and preabsorbed against fixed EPEC) for 1 h at room temperature. Blots were again washed and probed with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (diluted 1/5,000 in 1% milk in TBST) for 1 h at room temperature, followed by detection with ECL reagent (Amersham).
Immunoprecipitation.
EPEC from overnight standing cultures was subcultured 1/50 in 10 ml of minimal essential medium for 3 h at 37°C in 5% CO2. The culture was harvested, washed in PBS, divided into two parts, and pelleted (6,000 × g, 5 min). The bacteria were resuspended in 1 ml of lysis buffer (1% Triton X-100, 10 mM Tris-HCl [pH 7], 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 1 μM pepstatin, 10 μg of aprotinin per ml). Bacteria were sonicated three times for 15 s each (amplitude 2.5) (Fisher Sonicator). Unbroken bacteria were removed by centrifugation (16,000 × g for 2 min). The supernatant was incubated for 2 hours at 4°C with 2 μl of Tir monoclonal antibody clone 2A8 (5 mg/ml). BSA-blocked protein G-Sepharose beads (25 μl packed) (Amersham Pharmacia Biotech) were added to the supernatant-antibody mix and incubated overnight at 4°C. Beads were pelleted by gentle centrifugation (2,000 × g, 1 min). The supernatant (post-immunoprecipitation [IP]) was removed, and SDS sample buffer with β-mercaptoethanol was added. The beads were washed three times with 1 ml of lysis buffer, the last wash was removed with a 25-gauge needle, and the samples were resuspended in 50 μl of SDS sample buffer with β-mercaptoethanol. Samples (15 μl) were analyzed by SDS-PAGE.
Immunoblotting.
Samples were subjected to SDS-PAGE and transferred to nitrocellulose (pure nitrocellulose; 0.45-μm pore size) (Bio-Rad). Blots were blocked overnight at 4°C in 5% (wt/vol) skim milk-TBST and then incubated with primary antibody diluted in 1% skim milk-TBST for 1 h at room temperature. Blots were washed in TBST and then incubated in secondary antibody diluted in 1% skim milk-TBST for 1 h at room temperature followed by detection with ECL reagent (Amersham Pharmacia Biotech). Primary antibodies were mouse anti-Tir monoclonal antibody clone 2A5 (1/200) and mouse anti-herpes simplex virus (HSV) (Novagen) (1/1,000). The secondary antibody was horseradish peroxidase-conjugated goat anti-mouse antibody (Sigma) (1/5,000).
ELISA and competitive ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (22). In brief, Immulon-2 96-well plates (Dynatech Laboratories, Inc.) were coated with 0.5 μg of His-purified EscN, CesT, or BSA in 100 μl of PBS for 1 h at 37°C, blocked for 1 h in 5% skim milk in 0.1% Tween 20-PBS, and overlaid with increasing amounts of His-purified Tir (0, 50, 100, 200, 500, 750, and 1,000 nM) for 1 h at 37°C. Tir was detected by use of mouse anti-Tir monoclonal antibody clone 2A5 (used at 1/500) and a peroxidase-conjugated goat anti-mouse secondary antibody (Sigma) (used at 1/5,000). The Immulon-2 plates were washed three times between reagent additions and five times before development. The ELISA was developed by adding 100 μl of OPD solution (30 mg of o-phenylenediamine dihyrochloride [OPD] in 30 ml of 0.1 M citric acid, pH 4.5). The reaction was quenched with 100 μl of 3 N sulfuric acid and read at 492 nm in a TECAN Spectra-Fluor Plus plate reader. For competitive ELISAs, Immulon-2 96-well plates were coated with 0.5 μg of EscN, CesT, or BSA in 100 μl of PBS for 1 h at 37°C and blocked for 1 h in 5% skim milk in 0.1% Tween 20-PBS. Wells were overlaid with 100 nM His-purified Tir and increasing concentrations of His-purified CesT (0, 50, 100, 200, 400, 800, and 1,000 nM) for 1 h at 37°C. Tir was detected as described above.
RESULTS
CesT binds both Tir and EscN.
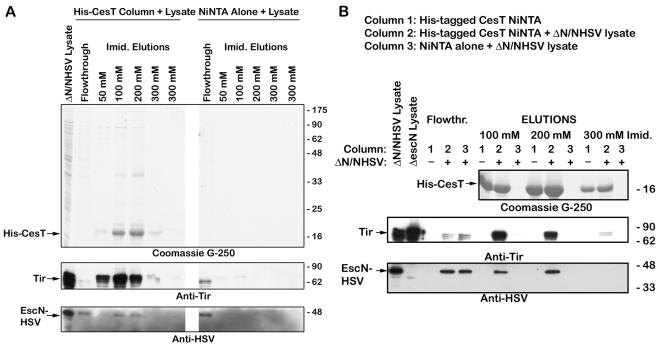
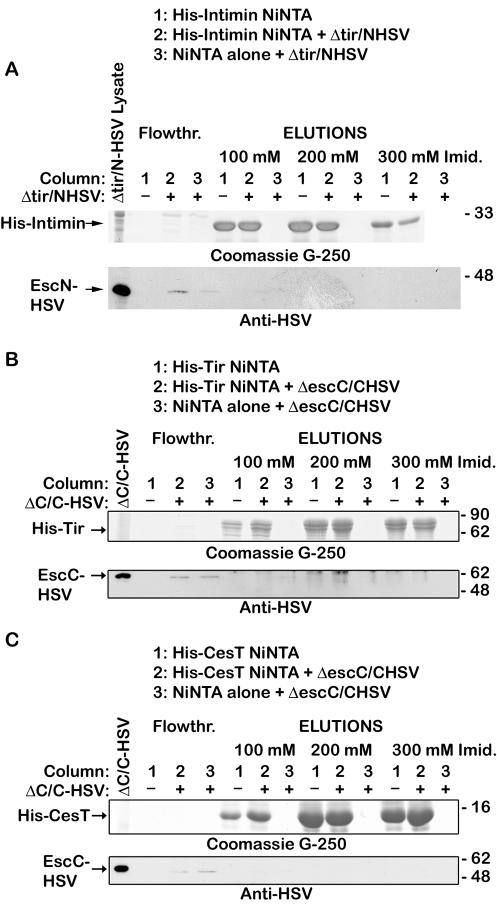
Previous experiments have demonstrated that Tir interacts with CesT (1, 15), and taken together with the data on the ATPase FliI in the flagellar assembly apparatus (40, 41, 44, 54), this suggests that CesT could play a role in delivering Tir to the TTSS via the ATPase EscN. The interactions between these proteins were examined by use of affinity chromatography. His-tagged CesT was bound to a Ni-NTA column, and EPEC lysates were poured over the column, washed, and then eluted with increasing concentrations of imidazole. HSV-tagged EscN and Tir coeluted with His-tagged CesT (Fig. 1). While EscN and Tir were in the flowthrough fractions of a Ni-NTA-only control column, they were not detected in the elution fractions (Fig. 1). Tir and EscN-HSV were also recovered from the wash fractions (data not shown) from the Ni-NTA column alone, although they were diluted in the wash buffer to barely detectable levels. As a control to ensure that HSV-tagged EscN was not binding to a CesT (or Tir) column in a nonspecific manner, affinity chromatography experiments were performed using His-tagged intimin-280, an EPEC outer membrane protein that should not interact with EscN, as they are not related and are not found in the same fractions of the bacteria (21). While HSV-tagged EscN was present in the flowthrough fractions, EscN did not coelute with His-intimin-280 (Fig. 2). Additionally, HSV-tagged EscC, EPEC outer membrane secretin, did not coelute with His-tagged Tir or His-tagged CesT (Fig. 2). Thus, EscN and Tir bind CesT specifically, and we hypothesize that CesT can bind Tir and EscN simultaneously on an affinity column.
FIG. 1.
Coelution of Tir and EscN with His-tagged CesT. An EPEC escN/pEscN-HSV lysate was prepared and applied to a His-tagged CesT-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The flowthrough was collected, and the columns were washed and proteins were eluted with increasing concentrations of imidazole. The same treatment was given to a His-tagged CesT-Ni-NTA column without EPEC lysate (column 1). Samples were resolved by SDS-PAGE (12% gel), and proteins were detected with Coomassie G-250 or transferred to nitrocellulose membranes and probed with anti-Tir or anti-HSV antiserum. (A) Samples from columns 1 and 2 were loaded. (B) Samples from columns 1, 2, and 3 were loaded beside one another in triplets.
FIG. 2.
EscN does not bind intimin-280. (A) An EPEC tir/pEscN-HSV lysate was prepared and applied to a His-tagged intimin-280-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The same treatment was given to a His-tagged intimin-280-Ni-NTA column without EPEC lysate (column 1). (B) An EPEC escC/pEscC-HSV lysate was prepared and applied to a His-tagged Tir-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The same treatment was given to a His-tagged Tir-Ni-NTA column without EPEC lysate (column 1). (C) An EPEC escC/pEscC-HSV lysate was prepared and applied to a His-tagged CesT-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The same treatment was given to a His-tagged CesT-Ni-NTA column without EPEC lysate (column 1). The flowthrough was collected, and the columns were washed and proteins were eluted with increasing concentrations of imidazole. Samples were resolved by SDS-PAGE (12% gel), and proteins were detected with Coomassie G-250 (top) or transferred to nitrocellulose membranes and probed with anti-HSV antiserum (bottom).
CesT binds EscN without Tir.
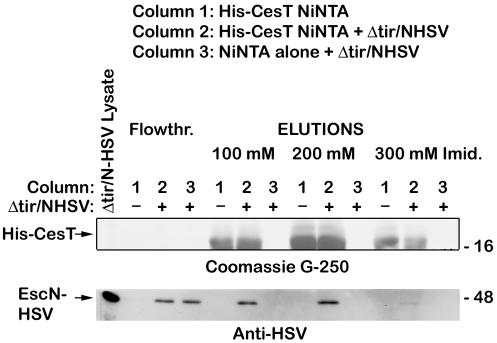
To examine if Tir was necessary for CesT to interact with EscN, affinity chromatography experiments were performed on a His-tagged CesT Ni-NTA column with a Δtir EPEC lysate (Fig. 3). Under these conditions, HSV-tagged EscN still coeluted with His-tagged CesT, but not with the Ni-NTA column alone, indicating that Tir is not necessary for CesT and EscN interactions.
FIG. 3.
CesT binds EscN without Tir. An EPEC tir/pEscN-HSV lysate was prepared and applied to a His-tagged CesT-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The flowthrough was collected, and the columns were washed and proteins were eluted with increasing concentrations of imidazole. The same treatment was given to a His-tagged CesT-Ni-NTA column without EPEC lysate (column 1). The columns were washed, and proteins were eluted with increasing concentrations of imidazole. Samples were resolved by SDS-PAGE (12% gel), and proteins were detected with Coomassie G-250 (top), or transferred to nitrocellulose membranes and probed with anti-HSV antiserum (bottom).
Tir binds EscN without CesT.
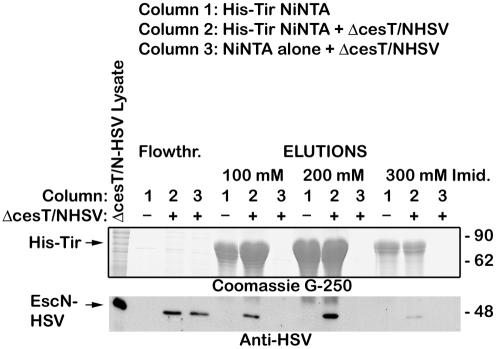
We next examined if Tir could interact with EscN without the presence of CesT by constructing a His-tagged Tir Ni-NTA column with a ΔcesT EPEC lysate (thus, no CesT is present under these experimental conditions). HSV-tagged EscN coeluted with Tir, but not with Ni-NTA beads alone, indicating that EscN binds Tir specifically without its chaperone CesT (Fig. 4).
FIG. 4.
Tir binds EscN without CesT. An EPEC cesT/pEscN-HSV lysate was prepared and applied to a His-tagged Tir-Ni-NTA column (column 2) or an empty Ni-NTA column (column 3). The flowthrough was collected, and the columns were washed and proteins were eluted with increasing concentrations of imidazole. The same treatment was given to a His-tagged Tir-Ni-NTA column without EPEC lysate (column 1). The columns were washed, and proteins were eluted with increasing concentrations of imidazole. Samples were resolved by SDS-PAGE (12% gel), and proteins were detected with Coomassie G-250 (top) or transferred to nitrocellulose membranes and probed with anti-HSV antiserum (bottom).
Direct binding of Tir and EscN.
The interactions observed between Tir and EscN in the affinity chromatography experiments could be due to an unknown protein in the EPEC lysate acting as a mediator. Thus, direct binding was demonstrated by performing a gel overlay experiment in which purified EscN was resolved by SDS-PAGE, transferred to Immobilon-P membranes, blocked, overlaid with purified Tir, and detected by use of anti-Tir antisera. Intimin-280 and CesT were also blotted as positive controls, and BSA was used as a negative control for Tir binding (Fig. 5). When blots were not overlaid with Tir, the anti-Tir antibody did not cross-react with EscN, CesT, intimin-280, or BSA (data not shown). Purified Tir bound EscN, CesT, and intimin-280, but not BSA (Fig. 5, lower panel) or the molecular weight markers (data not shown). Note that the light marks in the BSA lane in the bottom overlay panel are smaller than the molecular weight of BSA and thus are not Tir binding to BSA. Note that it appears that Tir bound intimin-280 most strongly, but conclusions cannot be made about the relative affinities of interaction in this experiment due to potential differences in how well the resolved proteins were renatured following transfer. However, these results indicate that Tir binds EscN directly under these in vitro conditions.
FIG. 5.
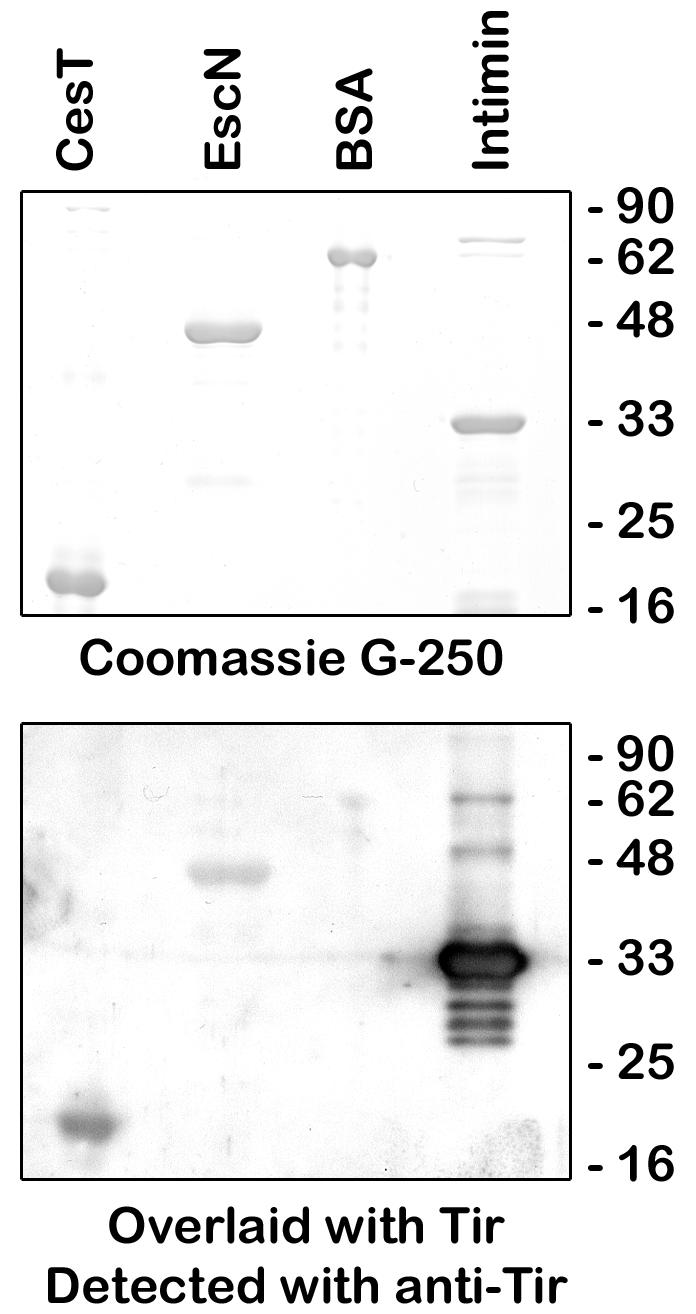
Direct binding of Tir and EscN. Samples (2.5 μg) of purified CesT, EscN, intimin-280, and BSA were resolved by SDS-PAGE (12% gel) and stained with Coomassie G-250 (top) or transferred to Immobilon-P, overlaid with 200 μg of purified Tir, and detected with anti-Tir antiserum (bottom).
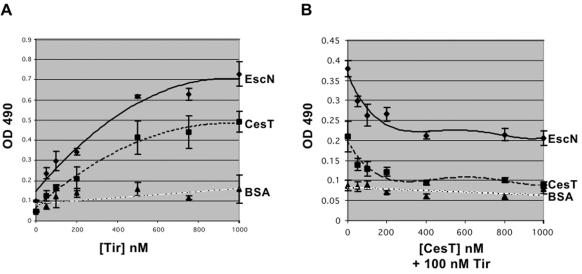
To determine if this binding was concentration dependent, an ELISA was performed. Immulon-2 plates were coated with 0.5 μg of EscN, CesT (as a positive control), and BSA (as a negative control) and overlaid with increasing amounts of His-purified Tir. Tir bound both EscN and CesT in a concentration-dependent manner, saturating at 500 nM Tir, but did not bind BSA (Fig. 6A). A competitive ELISA was performed by overlaying coated wells with a constant amount of Tir and an increasing amount of CesT to examine if CesT affected Tir-EscN binding. As a control, Tir binding to CesT was examined and decreased to background levels by adding increasing amounts of soluble CesT (Fig. 6B). Fifty percent inhibition of binding occurred at 50 nM CesT. Tir binding to EscN was also inhibited, but notably not to background levels (only 60% inhibited), by adding increasing amounts of soluble CesT (Fig. 6B). This suggests that CesT does not completely compete out the binding of Tir and EscN. Collectively, these results emphasize that Tir can directly bind EscN in vitro in a specific manner.
FIG. 6.
Tir binds EscN and CesT but not BSA in ELISA. Immulon-2 plates were coated with 0.5 μg of EscN, CesT (as a positive control), and BSA (as a negative control). (A) Coated wells were overlaid with increasing amounts of His-purified Tir to determine if binding was concentration dependent. (B) Competitive ELISA was performed by overlaying coated wells with a constant amount of Tir and increasing amounts of CesT to examine if CesT affected Tir-EscN binding. In both cases, Tir was detected with an anti-Tir monoclonal antibody and a peroxidase-conjugated secondary antibody. The results are shown as means ± standard deviations and are representative of three separate experiments performed in triplicate for each ELISA.
Interaction of Tir and EscN inside EPEC.
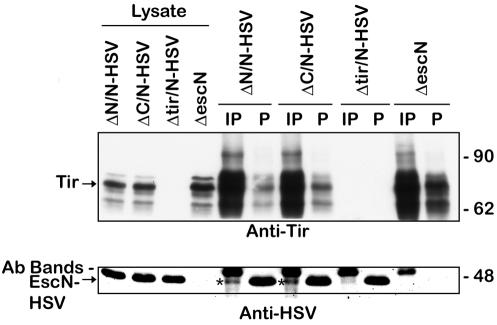
Tir-EscN interactions were examined inside EPEC by performing coimmunoprecipitation reactions with bacterial lysates and anti-Tir antibody. Tir was immunoprecipitated from all EPEC strains that contained Tir (Fig. 7, upper panel, IP lanes). A small amount of EscN-HSV was coimmunoprecipitated from the EPEC strains that contained both Tir and EscN (ΔN/N-HSV and ΔC/N-HSV), but not from the tir or escN mutant. The band just above EscN on the Western blots is the cross-reacting antibody band from the immunoprecipitation. Notably, while EscN coimmunoprecipitates with Tir, the majority of EscN-HSV remains in the postimmunoprecipitation supernatant (lane P). Coimmunoprecipitating Tir and EscN in a ΔescC background to prevent Tir from being secreted did not increase the amount of coimmunoprecipitated EscN-HSV. Overall, this confirms the above in vitro interaction between Tir and EscN, although a significant portion of EscN was not coimmunoprecipitated under these conditions.
FIG. 7.
Interaction of Tir and EscN inside EPEC. Bacteria were lysed and immunoprecipitated with anti-Tir antisera. Immunoprecipitate (IP) and postimmunoprecipitate (P) supernatant samples were resolved by SDS-PAGE (8% gel), transferred to nitrocellulose, and probed with anti-Tir (top) or anti-HSV (bottom) antiserum. Coimmunoprecipitated EscN-HSV is indicated with an asterisk.
DISCUSSION
The energy requirements and chaperone functions in the type III secretion and translocation process are not well understood. In this study, we have demonstrated trimolecular interactions between Tir, its chaperone CesT, and the type III putative ATPase EscN. Previous work has demonstrated that CesT interacts with and stabilizes Tir inside of EPEC (1, 15). By affinity chromatography, the present work has demonstrated not only that these three proteins interact but that the binding of CesT to EscN is not dependent on Tir and the binding of Tir to EscN is not dependent on CesT. Limitations in affinity chromatography include the potential for nonspecific binding, the excess of protein used, the interactions being indirect, and the fact that interactions are observed under in vitro conditions. To account for nonspecific binding and the amount of protein, we monitored EscN-HSV binding to the Ni-NTA column resin as well as EscN-HSV binding to another protein (intimin-280; data not shown). EscN-HSV did not coelute under either of these conditions. Alos, another HSV-tagged protein (EscC) did not coelute with His-tagged Tir or CesT (data not shown). Moreover, direct binding of Tir and EscN was demonstrated by gel overlay and ELISA. Interactions between Tir and EscN inside EPEC were demonstrated by coimmunoprecipitation. Only a small amount of the EscN-HSV was coimmunoprecipitated with Tir, suggesting that the interaction between the two proteins is not strong or that these lysis conditions were not altogether conducive for monitoring the interaction. It can be hypothesized that the strength of interaction between Tir and EscN is either weak or reversible; otherwise, Tir would not leave EPEC. Additionally, it is probable that EscN interacts with a number of other proteins, including other type III apparatus components and other effectors and chaperones, and thus is not all available to interact (and thus be coimmunoprecipitated) with Tir. Overall, these results are evidence in support of the hypothesis that CesT plays a role in directing Tir to the type III secretion apparatus, but further investigations are required to test and prove this hypothesis.
If CesT “shepherds” Tir to EscN for translocation through the type III secretion machinery, the results indicating that Tir is secreted at low levels in a cesT mutant (15) and interacts with EscN without CesT (Fig. 4 to 7) are contradictory to the above hypothesis. However, these results, taken together with the facts that Tir is very unstable inside EPEC in a cesT mutant (1, 15) and that CesT interacts directly with EscN without Tir (Fig. 3), suggest that CesT functions in stabilizing Tir and escorting it to EscN and the TTSS. When CesT is not present, Tir is less stable, but as evidenced by the small amount secreted, it can direct itself to EscN and the type III secretion machinery. Moreover, a recent study of secretion and translocation of Tir deletions fused to adenylate cyclase suggested that the amino-terminal 26 residues of Tir function as a CesT-independent signal for translocation and that CesT's role in stability may mediate rapid delivery of Tir into host cells (11). CesT has also been shown to interact and chaperone another EPEC type III effector called Map, and CesT cannot bind Map and Tir simultaneously (12). It will be interesting to determine if these EscN interactions are present with this other EPEC effector.
Data for other TTSSs have suggested that type III chaperones could function to deliver effectors to the type III apparatus, but this is the first evidence for direct interactions between a chaperone, an effector, and a type III component in the pathogenic TTSS. For example, glutathione S-transferase fusions to either the amino or carboxy termini of the Yersinia chaperone SycE can bind YopE similarly to wild-type SycE but fail to secrete YopE, suggesting that the chaperone not only prevents degradation but functions to deliver the effector to the type III apparatus (10). Interestingly, the EPEC type III chaperones CesD and CesD2 are found in the inner membrane and cytoplasmic fractions of bacteria (48, 58), further strengthening a role in effector delivery.
The type III ATPase has been shown to be important for needle formation, since both Shigella and Salmonella ATPase mutants have normal base structures but no needles (56, 57). It is possible that the type III ATPase, by itself or with other components, forms the lower cytoplasmic part of the TTSS. Yersinia YscN has been shown to form a complex with three other cytoplasmic and/or inner membrane-associated Ysc proteins (25). Shigella ATPase homologue Spa47 has 33% identity to the β-subunit of F1-ATPase. When modeled on the F1-ATPase structure, Spa47, as a homohexamer, fits at the inner membrane base of the type III needle complex structure, with a central channel aligned with the one found in the needle complex (8). Further, we have shown that EscN is localized to the cytoplasm and associated with the inner membrane in EPEC (21). While the present paper was in the review process, a paper was published showing that the type III ATPase in Pseudomonas syringae is a peripheral membrane protein that clusters at the inner membrane and that its ATPase activity is stimulated when it oligomerizes (52).
The cytosolic and inner membrane components of the pathogenic TTSS are homologous to those of the flagellar assembly system (2). As discussed in the introduction, the ATPase FliI in the flagellar system plays a pivotal role in interacting with flagellin and other substrates and chaperones as well as other components of the flagellar assembly apparatus (4, 40, 41, 43-45, 54, 61). Moreover, FliI's ATPase activity increases in the presence of flagellin or other export substrates, indicating that energy is required for the export process (54). This study suggests that the EscN type III ATPase is functionally analogous to FliI.
A model for type III translocation can be suggested based on our findings and inferences from the flagellar assembly pathway (36, 44) whereby type III effectors interacting with their cognate chaperones are delivered to the type III ATPase, which interacts with the membrane-spanning type III apparatus complex (possibly with a protein such as EscV or EscU). While it is too preliminary to extend these results further, it is possible that the docking of the effector-ATPase complex to the type III apparatus may trigger ATP hydrolysis and translocation of the effector, but this entire model remains to be tested.
Overall, the current data on the EPEC TTSS and studies on the flagellar assembly apparatus evoke similarities to SecB/SecA targeting in the sec export pathway (reviewed in references 5 and 37; discussed in reference 10). SecB is a homotetrameric chaperone that binds sec-dependent export substrates and facilitates their targeting to the translocase (SecYEG) because of its affinity for the SecA ATPase (17, 24). The substrate-SecB complex binds the ATPase SecA with much greater affinity than SecB alone, and binding strongly stimulates SecA's ATPase activity (24, 31). The SecB chaperone functions both as an antifolding factor and a secretion pilot. Moreover, it was recently observed by electron and atomic force microscopy that the SecA ATPase forms ring-like pore structures with diameters of 8 nm and holes of 2 nm in the presence of phospholipids, suggesting that ATPases may indeed form the core of the bacterial protein secretion channels (59). Very recently, the crystal structure of Mycobacterium tuberculosis SecA was solved and the structure predicts that SecA can interact with the SecYEG pore (53). The result of the present study that a type III chaperone and its effector interact with the type III ATPase lends support for the extension of these ideas to TTSS.
In summary, we have demonstrated a new role for the type III chaperone CesT through the trimolecular interactions observed with its cognate effector Tir and the type III apparatus ATPase EscN. To our knowledge, this is the first evidence for direct interactions between a chaperone, an effector, and a type III component in the pathogenic TTSS, and we suggest a model for Tir translocation whereby its chaperone, CesT, brings Tir to the type III apparatus by specifically interacting with the type III ATPase EscN. This work strengthens the evidence supporting similar modes of action among homologous and nonhomologous bacterial secretion systems.
Acknowledgments
A.G. is supported by doctoral research awards from Medical Research Council of Canada, Imperial Order of the Daughters of the Empire, and Michael Smith Foundation for Health Research. B.B.F. is a Howard Hughes Medical Institute (HHMI) International Research Scholar, a Canadian Institutes for Health Research (CIHR) Distinguished Investigator, and the Peter Wall Distinguished Professor. Operating grants from HHMI, CIHR, and the Canadian Bacterial Disease Network to B.B.F supported this work.
We are grateful to Samantha Gruenheid and Nikhil Thomas for helpful discussions and for critically reading the manuscript.
REFERENCES
- 1.Abe, A., M. de Grado, R. A. Pfuetzner, C. Sanchez-Sanmartin, R. Devinney, J. L. Puente, N. C. Strynadka, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol. Microbiol. 33:1162-1175. [DOI] [PubMed] [Google Scholar]
- 2.Aizawa, S.-I. 2001. Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett. 202:157-164. [DOI] [PubMed] [Google Scholar]
- 3.Aldridge, P., and K. T. Hughes. 2001. How and when are substrates selected for type III secretion? Trends Microbiol. 9:209-214. [DOI] [PubMed] [Google Scholar]
- 4.Auvray, F., A. J. Ozin, L. Claret, and C. Hughes. 2002. Intrinsic membrane targeting of the flagellar export ATPase FliI: interaction with acidic phospholipids and FliH. J. Mol. Biol. 318:941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennet, J. C. Q., and C. Hughes. 2000. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 8:202-204. [DOI] [PubMed] [Google Scholar]
- 6.Bennet, J. C. Q., J. Thomas, G. M. Fraser, and C. Hughes. 2001. Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol. Microbiol. 39:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birtalan, S. C., R. M. Phillips, and P. Ghosh. 2001. Structure of the Yersinia type III secretory system chaperone SycE. Nat. Struct. Biol. 8:974-978. [DOI] [PubMed] [Google Scholar]
- 8.Blocker, A., K. Komoriya, and S.-I. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 6:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd, A. P., I. Lambermont, and G. R. Cornelis. 2000. Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J. Bacteriol. 182:4811-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, L. W., and O. Schneewind. 1999. Yersinia enterocolitica type III secretion: on the role of SycE in targeting YopE into HeLa cells. J. Biol. Chem. 274:22102-22108. [DOI] [PubMed] [Google Scholar]
- 11.Crawford, J. A., and J. B. Kaper. 2002. The N-terminus of enteropathogenic Escherichia coli (EPEC) Tir mediates transport across bacterial and eukaryotic cell membranes. Mol. Microbiol. 46:855-868. [DOI] [PubMed] [Google Scholar]
- 12.Creasey, E. A., R. M. Delahay, A. A. Bishop, R. K. Shaw, B. Kenny, S. Knutton, and G. Frankel. 2003. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Mol. Microbiol. 47:209-221. [DOI] [PubMed] [Google Scholar]
- 13.Darwin, K. H., and V. L. Miller. 2001. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 20:1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. [DOI] [PubMed] [Google Scholar]
- 15.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 16.Fan, F., and R. M. Macnab. 1996. Enzymatic characterization of FliI. An ATPase involved in flagellar assembly in Salmonella typhimurium. J. Biol. Chem. 271:31981-31988. [DOI] [PubMed] [Google Scholar]
- 17.Fekkes, P., C. van der Does, and A. J. Driessen. 1997. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 16:6105-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman, M. F., S. Muller, E. Wuest, and G. R. Cornelis. 2002. SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 46:1183-1197. [DOI] [PubMed] [Google Scholar]
- 19.Fraser, G. M., J. C. Q. Bennet, and C. Hughes. 1999. Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol. Microbiol. 32:569-580. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier, A., M. de Grado, and B. B. Finlay. 2000. Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect. Immun. 68:4344-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauthier, A., J. L. Puente, and B. B. Finlay. 2003. Enteropathogenic Escherichia coli's type III secretion system secretin requires components of the type III apparatus for assembly and localization. Infect. Immun. 71:3310-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goosney, D. L., R. DeVinney, R. A. Pfuetzner, E. A. Frey, N. C. Strynadka, and B. B. Finlay. 2000. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with alpha-actinin. Curr. Biol. 10:735-738. [DOI] [PubMed] [Google Scholar]
- 23.Grunenfelder, B., S. Gehrig, and U. Jenal. 2003. Role of cytoplasmic C terminus of the FliF motor protein in flagellar assembly and rotation. J. Bacteriol. 185:1624-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartl, F. U., S. Lecker, E. Schiebel, J. P. Hendrick, and W. Wickner. 1990. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63:269-279. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, M. W., and G. V. Plano. 2000. Interactions between type III apparatus components from Yersinia pestis detected using the yeast two-hybrid system. FEMS Microbiol. Lett. 186:85-90. [DOI] [PubMed] [Google Scholar]
- 26.Kenny, B. 2002. Enteropathogenic Escherichia coli (EPEC)—a crafty subversive little bug. Microbiology 148:1967-1978. [DOI] [PubMed] [Google Scholar]
- 27.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 28.Kenny, B., and J. Warawa. 2001. Enteropathogenic Escherichia coli (EPEC) Tir receptor molecule does not undergo full modification when introduced into host cells by EPEC-independent mechanisms. Infect. Immun. 69:1444-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kihara, M., G. U. Miller, and R. M. Macnab. 2000. Deletion analysis of the flagellar switch protein FliG of Salmonella. J. Bacteriol. 182:3022-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kresse, A. U., K. Schulze, C. Deibel, F. Ebel, M. Rohde, T. Chakraborty, and C. A. Guzman. 1998. Pas, a novel protein required for protein secretion and attaching and effacing activities of enterohemorrhagic Escherichia coli. J. Bacteriol. 180:4370-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lill, R., W. Dowhan, and W. Wickner. 1990. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 60:271-280. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd, S. A., A. Forsberg, H. Wolf-Watz, and M. S. Francis. 2001. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 9:367-371. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd, S. A., M. Norman, R. Rosqvist, and H. Wolf-Watz. 2001. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39:520-531. [DOI] [PubMed] [Google Scholar]
- 34.Luo, Y., M. G. Bertero, E. A. Frey, R. A. Pfuetzner, M. R. Wenk, L. Creagh, S. L. Marcus, D. Lim, F. Sicheri, C. Kay, C. Haynes, B. B. Finlay, and N. C. Strynadka. 2001. Structural and biochemical characterization of the type III secretion chaperones CesT and SigE. Nat. Struct. Biol. 8:1031-1036. [DOI] [PubMed] [Google Scholar]
- 35.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 36.Macnab, R. M. 2000. Type III protein pathway exports Salmonella flagella. ASM News 66:738-745. [Google Scholar]
- 37.Manting, E. H., and A. J. Driessen. 2000. Escherichia coli translocase: the unravelling of a molecular machine. Mol. Microbiol. 37:226-238. [DOI] [PubMed] [Google Scholar]
- 38.Mavris, M., A. L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543-1553. [DOI] [PubMed] [Google Scholar]
- 39.Menard, R., P. Sansonetti, and C. Parsot. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of Shigella flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 40.Minamino, T., R. Chu, S. Yamaguchi, and R. M. Macnab. 2000. Role of FliJ in flagellar protein export in Salmonella. J. Bacteriol. 182:4207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minamino, T., and R. M. Macnab. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minamino, T., and R. M. Macnab. 2000. Domain structure of Salmonella FlhB, a flagellar export component responsible for substrate specificity switching. J. Bacteriol. 182:4906-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minamino, T., and R. M. Macnab. 2000. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol. Microbiol. 37:1494-1503. [DOI] [PubMed] [Google Scholar]
- 44.Minamino, T., and R. M. Macnab. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052-1064. [DOI] [PubMed] [Google Scholar]
- 45.Minamino, T., J. R. H. Tame, K. Namba, and R. M. Macnab. 2001. Proteolytic analysis of the FliH/FliI complex, the ATPase component of the type III flagellar export apparatus of Salmonella. J. Mol. Biol. 312:1027-1036. [DOI] [PubMed] [Google Scholar]
- 46.Minamino, T., S. Yamaguchi, and R. M. Macnab. 2000. Interaction between FliE and FlgB, a proximal rod component of the flagellar basal body of Salmonella. J. Bacteriol. 182:3029-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neves, B. C., R. Mundy, L. Petrovska, G. Dougan, S. Knutton, and G. Frankel. 2003. CesD2 of enteropathogenic Escherichia coli is a second chaperone for the type III secretion translocator protein EspD. Infect. Immun. 71:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neyt, C., and G. Cornelis. 1999. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol. 31:143-156. [DOI] [PubMed] [Google Scholar]
- 50.Page, A. L., and C. Parsot. 2002. Chaperones of the type III secretion pathway: jacks of all trades. Mol. Microbiol. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 51.Pfanner, N., and A. Geissler. 2001. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell. Biol. 2:339-349. [DOI] [PubMed] [Google Scholar]
- 52.Pozidis, C., A. Chalkiadaki, A. Gomez-Serrano, H. Stahlberg, I. Brown, A. P. Tampakaki, A. Lustig, G. Sianidis, A. S. Politou, A. Engel, N. J. Panapoulos, J. Mansfield, A. P. Pugsley, S. Karamanou, and A. Economou. 2003. Type III protein translocase: HrcN is a peripheral membrane ATPase that is activated by oligomerization. J. Biol. Chem. 278:25816-25824. [DOI] [PubMed] [Google Scholar]
- 53.Sharma, V., A. Arockiasamy, D. R. Ronning, C. G. Savva, A. Holzenburg, M. Braunstein, W. R. Jacobs, Jr., and J. C. Sacchettini. 2003. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl. Acad. Sci. USA 100:2243-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva-Herzog, E., and G. Dreyfus. 1999. Interaction of FliI, a component of the flagellar export apparatus, with flagellin and hook protein. Biochim. Biophys. Acta 1431:374-383. [DOI] [PubMed] [Google Scholar]
- 55.Stephens, C., C. Mohr, C. Boyd, J. Maddock, J. Gober, and L. Shapiro. 1997. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J. Bacteriol. 179:5355-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sukhan, A., T. Kubori, J. Wilson, and J. E. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainwright, L. A., and J. B. Kaper. 1998. EspB and EspD require a specific chaperone for proper secretion from enteropathogenic Escherichia coli. Mol. Microbiol. 27:1247-1260. [DOI] [PubMed] [Google Scholar]
- 59.Wang, H.-W., Y. Chen, H. Yang, X. Chen, M.-X. Duan, P. C. Tai, and S.-F. Sui. 2003. Ring-like pore structures of SecA: Implication for bacterial protein-conducting channels. Proc. Natl. Acad. Sci. USA 100:4221-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wulff-Strobel, C. R., A. W. Williams, and S. C. Straley. 2002. LcrQ and SycH function together at the Ysc type III secretion system in Yersinia pestis to impose a hierarchy of secretion. Mol. Microbiol. 43:411-423. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, K., B. Gonzalez-Pedrajo, and R. M. Macnab. 2002. Interactions among membrane and soluble components of the flagellar export apparatus of Salmonella. Biochemistry 41:9516-9524. [DOI] [PubMed] [Google Scholar]