Abstract
The oral bacterium Streptococcus salivarius takes up lactose via a transporter called LacS that shares 95% identity with the LacS from Streptococcus thermophilus, a phylogenetically closely related organism. S. thermophilus releases galactose into the medium during growth on lactose. Expulsion of galactose is mediated via LacS and stimulated by phosphorylation of the transporter by HPr(His∼P), a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase transport system (PTS). Unlike S. thermophilus, S. salivarius grew on lactose without expelling galactose and took up galactose and lactose concomitantly when it is grown in a medium containing both sugars. Analysis of the C-terminal end of S. salivarius LacS revealed a IIA-like domain (IIALacS) almost identical to the IIA domain of S. thermophilus LacS. Experiments performed with purified proteins showed that S. salivarius IIALacS was reversibly phosphorylated on a histidine residue at position 552 not only by HPr(His∼P) but also by HPr(Ser-P)(His∼P), a doubly phosphorylated form of HPr present in large amounts in rapidly growing S. salivarius cells. Two other major S. salivarius PTS proteins, IIABLMan and IIABHMan, were unable to phosphorylate IIALacS. The effect of LacS phosphorylation on growth was studied with strain G71, an S. salivarius enzyme I-negative mutant that cannot synthesize HPr(His∼P) or HPr(Ser-P)(His∼P). These results indicated that (i) the wild-type and mutant strains had identical generation times on lactose, (ii) neither strain expelled galactose during growth on lactose, (iii) both strains metabolized lactose and galactose concomitantly when grown in a medium containing both sugars, and (iv) the growth of the mutant was slightly reduced on galactose.
Streptococcus salivarius is the predominant bacterial species among the pioneer microorganisms that colonize the mouth (19). Acquisition of and competition for nutrients, particularly sugars, which serve as the major energy source for oral streptococci, constitute vital ecological determinants for the survival of oral bacteria. S. salivarius is able to metabolize a broad variety of sugars that can be grouped into two categories, non-PTS sugars, which are taken up by transport systems energized by proton motive force or ATP, and PTS sugars, which are transported by the phosphoenolpyruvate:sugar phosphotransferase system (PTS) (4, 35, 38). The PTS uses phosphoenolpyruvate (PEP) in a group translocation process to phosphorylate incoming mono- and disaccharides via a phosphoryl-transfer cascade involving the non-sugar-specific proteins, Enzyme I (EI) and HPr, and a family of sugar-specific enzyme II complexes (EII) (27). In gram-positive bacteria, the PTS controls sugar metabolism by regulating transporter activities and gene transcription via the protein HPr (6, 29). This protein can be phosphorylated by EI at the expense of PEP on a histidine at position 15, generating HPr(His∼P), and by a ATP-dependent protein kinase/phosphorylase, called HPrK/P, on a serine at position 46, generating HPr(Ser-P) (6, 8, 29). Both HPr(His∼P) and HPr(Ser-P) possess regulatory functions. HPr(His∼P) accomplishes its regulatory functions by reversibly phosphorylating its targets, and HPr(Ser-P) accomplishes its regulatory functions by protein-protein interactions (7, 13, 31, 42). In addition to the aforementioned phosphorylated forms of HPr, rapidly growing streptococcal cells contain substantial amounts of the doubly phosphorylated form HPr(Ser-P)(His∼P), whose functions remain unclear (33, 36, 37).
Lactose (milk sugar) is a disaccharide composed of glucose and galactose and is an important energy source for oral streptococci. It is taken up by S. salivarius via a non-PTS transport system (14) composed of a single membrane protein, lactose permease (LacS), that possesses an amino acid sequence that shares 95% identity with the sequence of Streptococcus thermophilus LacS (40). S. thermophilus and S. salivarius are closely related and belong, together with Streptococcus vestibularis, to the same phylogenetic cluster, forming the salivarius group of oral streptococci (16). Most S. thermophilus strains are unable to grow on galactose and release galactose into the medium during growth on lactose (15). The release of galactose has even been observed with Gal+ mutant strains (34). Expulsion of galactose by S. thermophilus is mediated via LacS during lactose-galactose exchange, a process that is strengthened after phosphorylation of the transporter at a histidine residue that is part of a IIA domain at the C-terminal end of the protein (11, 17, 23, 24, 41). The phosphorylation, which chiefly occurs at the end of the logarithmic growth phase, is catalyzed by HPr(His∼P), whose intracellular concentrations increase at the end of the exponential growth phase (11, 12).
Unlike S. thermophilus, S. salivarius readily metabolizes galactose and lactose, and growth on lactose is not accompanied by an extracellular accumulation of galactose (40). Moreover, S. salivarius cells growing on lactose contain large amounts of HPr(Ser-P)(His∼P) during the exponential growth phase (22). The purpose of the present study was to determine whether S. salivarius LacS is controlled by phosphorylation and whether the doubly phosphorylated form of HPr, in addition to HPr(His∼P), can serve as a phosphate donor.
MATERIALS AND METHODS
Strains and growth conditions.
The strains and plasmids used in the present study are listed in Table 1. S. salivarius was grown at 37°C as described previously (3). Escherichia coli strains were grown with aeration at 37°C in Luria-Bertani medium. When necessary, 20 μg of tetracycline/ml, 50 μg of ampicillin/ml, and/or 30 μg of kanamycin/ml was added. Generation times were determined as described previously (22). To determine sugar utilization by growing cells, cells were grown in tubes containing 10 ml of medium, and 0.2-ml samples were removed at intervals, heated at 100°C for 10 min to stop metabolism, centrifuged to remove cells, and stored at −20°C until sugar assays were conducted.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant genotype and/or characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| S. salivarius | ||
| ATCC 25975 | Wild-type, Lac+ Glu+ Gal+ | I. R. Hamilton, University of Manitoba |
| G71 | El-negative mutant derived from S. salivarius ATCC 25975 | 9 |
| E. coli | ||
| BL21 (DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| LMG194 | F− ΔlacX74 galE thi rpsL ΔphoA (PvuII) Δara714 leu::Tn10 | Invitrogen |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU rpsL endA1 nupG | Invitrogen |
| Plasmids | ||
| pET-28a(+) | Expression vector | Novagen |
| pET-29a(+) | Expression vector | Novagen |
| pET-19b | Expression vector | Novagen |
| pBAD/His | Expression vector | Invitrogen |
| pCR-Blunt | Cloning vector | Invitrogen |
| pETI-16 | Contains the ptsI gene of S. salivarius cloned into pET-28a(+) | This work |
| pLacSIIA | Contains the last 519 nucleotides of lacS from S. salivarius (which code for the IIA domain), cloned into pET29a(+) | This work |
| pLacSIIAH552R | Contains the last 519 nucleotides of lacS from S. salivarius (which code for the IIA domain), with a mutation replacing LacS H552 by R, cloned into pET29a(+) | This work |
| pTML2 | Contains the manL gene of S. salivarius cloned into pET19b | This work |
| pDR3 | Contains the manH gene of S. salivarius cloned into pET19b | This work |
| pHPW18 | Contains the ptsH gene of S. salivarius cloned into pBAD | 8 |
| pHPK229 | Contains the hprK gene of S. salivarius cloned into pCR-Blunt | 3 |
DNA purification and manipulations.
Chromosomal DNA was isolated from streptococci as described by Gauthier et al. (9). Unless otherwise mentioned, DNA manipulations were performed by standard procedures (1). E. coli BL21(DE3) cells were made competent and transformed with plasmid DNA by electroporation (30). DNA fragments used for sequencing and subcloning were recovered from agarose gels by using a QIAquick purification kit (Qiagen). Unless otherwise specified, the PCRs were performed by using a DNA Thermal Cycler 480 (Perkin-Elmer) in a total volume of 100 μl containing 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 1 μg of DNA, 0.2 μM concentrations of primers, and 100 μM concentrations (each) of the four deoxynucleotide triphosphates. The reactions were carried out for 30 cycles in the presence of 1 U of Vent DNA polymerase (Promega) with the following temperature-time profile: 94°C for 1 min, 54°C for 1 min, and 72°C for 40 s.
Gene cloning.
S. salivarius ptsI, the gene coding for EI of the PTS, was PCR amplified by using the forward primer ptsI69-N and the reverse primer ptsI1804R-X (Table 2). The amplicon was cloned into the overexpression plasmid pET-28a(+), adding a His6 tag and cleaving a thrombin site at the N terminus of EI to give plasmid pETI-16. The portion of S. salivarius lacS coding for IIALacS was PCR amplified by using the forward primer IIA173 and the reverse primer IIA173R. Primer IIA173 covered positions 1,373 to 1,405 relative to the adenine of the ATG initiation codon of S. salivarius lacS and primer IIA173R covered positions 2,951 to 2,979, including the first eight nucleotides of lacZ (40). The amplified DNA fragment comprised a region of lacS encoding the entire IIA domain of LacS, as well as 37 amino acids upstream from the IIA domain. The amplicon was cloned into the overexpression plasmid pET-29a(+) (Novagen), adding two amino acids (LE) and a His6 tag at the C terminus of IIALacS to give plasmid pLacSIIA. Replacement of IIALacS His552 by Arg was carried out by PCR with pLacSIIA as a template and the QuickChange site-directed mutagenesis kit (Stratagene). The PCR mixture contained 10 ng of pLacSIIA, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphate, 125 ng of the oligonucleotide primers IIA-H91R-F and IIA-H91R-R, and 2.5 U of Pfu Turbo DNA polymerase (Promega). After a 30-s incubation at 95°C, the amplification reaction was carried out for 16 cycles, each with a 30-s denaturing step at 95°C, a 1-min annealing step at 54°C, and a 12-min extension step at 68°C. After digestion with DpnI and transformation of E. coli XL1-Blue with the resulting mixture, we obtained plasmid pLacSIIAH552R, which bore the same DNA fragment as pLacSIIA, with a two-nucleotide substitution that replaced His552 with Arg. S. salivarius manL, which codes for IIABLMan, was PCR amplified with the forward primer manL44 and the reverse primer manL1041R. S. salivarius manH, which codes for IIABHMan, was PCR amplified with the forward primer manH53F and the reverse primer manH1071R. The amplicons were cloned into the overexpression plasmid pET-19b (Novagen), adding an enterokinase site and a His10 tag at the N termini of IIABLMan and IIABHMan, to yield plasmids pTML2 and pDR3, respectively.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′→3′)a |
|---|---|
| ptsI69-N | CCGTAAGCATATGACAGAAATGCTTAAA |
| pts1804R-X | GGCCTCGAGTTTTTAAATGATTAGTCCCTAC |
| IIA173 | TGAAGTACATATGGAATTGGAACATCGCTTTA |
| IIA173R | GTTCATCTCGAGTTCTCCTTTTTTGAAG |
| IIA-H91R-F | TTGTTCTTATCCGAGTTGGTATCGGAACAGTTAA |
| IIA-H91R-R | TTAACTGTTCCGATACCAACTCGGATAAGAACAA |
| manL44 | GGAGAACACATATGGGTATCGGTATTAT |
| manL1041R | ACTAATGGATCCGAATGAAGGTTATTGA |
| manH53F | GGAAGAACACATATGGGTATCGGTATTAT |
| manH1071R | AACTCATTTATGCATCCTCGAGAATTA |
Underlining indicates nucleotides participating in restriction sites. The nucleotides in boldface indicate the codon replacing His by Arg in IIALacS.
Protein purification.
EI and HPr were purified from S. salivarius as described previously (39). S. salivarius HPrK/P, the enzyme that phosphorylates HPr on Ser46 at the expense of ATP, was purified without a His tag from E. coli bearing pHPK229 as previously reported (3). His6-HPr was purified from E. coli LMG194 bearing pHPW18 (8). EI-, IIALacS-, IIALacSH552R-, IIABLMan-, and IIABHMan-overproducing strains were generated by transforming E. coli BL21(DE3) with the plasmids pETI-16, pLacSIIA, pLacSIIAH552R, pTML2, and pDR3, respectively. Cells were grown in 500 ml of medium to the late exponential phase, and expression was induced with IPTG (isopropyl-β-d-thiogalactopyranoside) according to the manufacturer's instructions (Novagen). Preparation of the cell extracts and purification of the recombinant proteins on an Ni-nitrilotriacetic acid (NTA) Superflow column were performed as described previously (8). His6-EI was further purified on a Superdex 200 HR column (Pharmacia), S. salivarius IIALacS and IIALacSH552R were further purified by chromatography on a MonoQ HR 5/5 column (Pharmacia), and His10-IIABLMan and His10-IIABHMan were further purified by chromatography on a Superdex 75HR column (Pharmacia). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses showed that the EI and the IIALacS proteins were >99% pure, whereas His10-IIABLMan and His10-IIABHMan were >97% pure.
Synthesis of His6-HPr(Ser-P).
The synthesis of His6-HPr(Ser-P) was carried out by using purified S. salivarius HPrK/P (5 μg) and His6-HPr (500 μg), which were incubated for 60 min at 37°C in 50 mM Tris-HCl (pH 7.5) containing 2 mM ATP and 5 mM MgCl2. The reaction product [His6-HPr(Ser-P)] was purified on an Ni-NTA column and by size exclusion chromatography on a Superdex 75HR 10/30 column (Pharmacia) equilibrated with 10 mM potassium phosphate (pH 7.5) containing 100 mM NaCl. The purity of the His6-HPr(Ser-P) was verified by PAGE under native conditions (28).
Phosphorylation of His6-IIALacS by His6-HPr(His∼32P) and His6-HPr(Ser-P)(His∼32P).
[32P]PEP was prepared according to the method of Mattoo and Waygood (18) by using purified PEP carboxykinase from E. coli K-12 HFr 3000, which was kindly provided by A. H. Goldie (University of Saskatchewan). Phosphorylation of His6-IIALacS by His6-HPr(His∼P) was carried out in 50 mM Tris-acetate (pH 7.5) containing 1 mM dithiothreitol (DTT), 1 mM MgCl2, 0.8 μM His6-EI, 24 μM His6-HPr, 5.8 μM His6-IIALacS or His6-IIALacSH552R, and 1 mM [32P]PEP (30 μCi/μmol). The mixture was incubated at 10°C for 2 min. Samples were withdrawn at intervals, and the reaction was stopped by adding an equal volume of a solution containing 180 mM Tris-HCl (pH 6.8), 200 mM SDS, 30% glycerol, 2 M β-mercaptoethanol, and 0.003% bromophenol blue (stop solution). The proteins were separated by SDS-PAGE and revealed by autoradiography as described previously (28). His6-HPr(Ser-P)(His∼32P) was synthesized in 50 mM Tris-acetate (pH 7.5) containing 4 mM DTT, 4 mM MgCl2, 1.5 μM His6-EI, and 40 μM His6-HPr(Ser-P). After the mixture was incubated at 37°C for 10 min, 1 mM [32P]PEP (30 μCi/μmol) was added, and the solution was incubated at 37°C for an additional 45 min. Analysis by SDS-PAGE revealed that 50% of the His6-HPr(Ser-P) was transformed into His6-HPr(Ser-P)(His∼P) under these conditions. The solution was then incubated at 10°C and His6-IIALacS or His6-IIALacSH552R was added to a final concentration of 5.8 μM. The reaction products were analyzed as described for the phosphorylation of His6-IIALacS by His6-HPr(His∼P).
Phosphorylation of His6-IIALacS by His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P).
IIABLMan and IIABHMan are PTS proteins phosphorylated on His residues by HPr(His∼P) (20). His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P) were synthesized by using 10 mM HEPES (pH 7.5) containing 5 mM MgCl2, 0.6 μM EI, 16 μM HPr, 9 μM His10-IIABLMan or His10-IIABHMan, and 1 mM [32P]PEP (180 μCi/μmol). The mixture was incubated at room temperature for 10 min, after which His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P) were isolated by chromatography on a 200-μl Ni-NTA Superflow column as described above. His6-IIALacS was phosphorylated by His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P) in 10 mM HEPES (pH 7.5) containing 5 mM MgCl2, 2.8 μM His10-IIABLMan(His∼32P) or His10-IIABHMan(His∼32P), and 5.8 μM His6-IIALacS in a total volume of 30 μl. The mixture was incubated at room temperature for 10 min, and the reaction was stopped by adding 15 μl of the stop solution described above. The proteins were separated by SDS-PAGE and revealed by autoradiography (28).
Dephosphorylation of His6-IIALacS(His∼32P) by His6-HPr and His6-HPr(Ser-P).
His6-IIALacS(His∼32P) was synthesized by using 50 mM Tris-acetate (pH 7.5) containing 1 mM DTT, 2 mM MgCl2, 1.7 μM EI, and 18 μM HPr in a total volume of 270 μl. After a 10-min incubation at 37°C, [32P]PEP was added to the mixture at a final concentration of 1 mM (30 μCi/μmol), and the solution was incubated at 37°C for an additional 25 min. His6-IIALacS was then added to the solution to a final concentration of 15 μM, and the incubation was extended for another 25 min to allow the synthesis of His6-IIALacS(His∼32P). Phosphorylated His6-IIALacS was purified on a 260-μl Ni-NTA column equilibrated with 50 mM potassium phosphate (pH 7.0). The column was first washed with 1.4 ml of 50 mM potassium phosphate (pH 7.0), and the His6-IIALacS(His∼32P) was eluted with the same buffer containing 300 mM imidazole. Analysis by SDS-PAGE revealed that the preparation was devoid of EI and HPr. The dephosphorylation of His6-IIALacS(His∼32P) by HPr and HPr(Ser-P) was carried out in 50 mM Tris-acetate (pH 7.5) containing 1 mM DTT, 2 mM MgCl2, and either 20 μM HPr or HPr(Ser-P) in a total volume of 15 μl. After the mixture was incubated for 10 min at 37°C, His6-IIALacS(His∼32P) was added to a final concentration of 2 μM, and the incubation was extended for 5 min. The reaction was stopped by the addition of an equal volume of the stop solution described above. The proteins were separated by SDS-PAGE and revealed as described above.
Sugar and protein assays.
Glucose concentrations were measured by using a peroxidase-glucose oxidase assay (Sigma). Galactose was determined by using a peroxidase-galactose oxidase assay (2). Lactose was assayed by measuring the concentration of glucose or galactose in samples both before and after hydrolysis with β-galactosidase for 1 h at 37°C in 233 mM citrate buffer (pH 6.6) containing 60 mM MgSO4 and 0.05 U of β-galactosidase (Worthington)/μl. Protein concentrations were measured by using the method of Peterson (21) with bovine serum albumin as the standard.
RESULTS
Growth of S. salivarius on lactose and galactose.
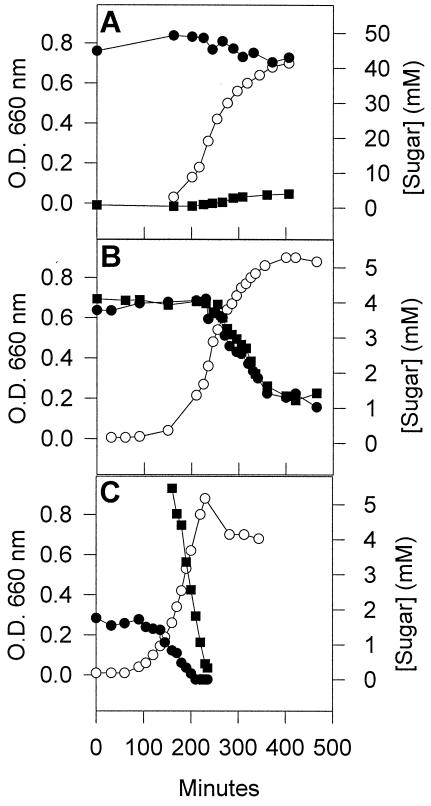
The growth of S. salivarius in M17 medium supplemented with 4.4 mM lactose does not result in the accumulation of galactose in the medium (40). To determine whether the type of culture medium and the extracellular lactose concentration influences the release of galactose by S. salivarius, we looked for an accumulation of galactose in the medium during growth in M17 (Fig. 1A) and TYE (not shown) media containing 50 to 60 mM lactose. S. salivarius did not release galactose during growth in either M17 or TYE. When S. salivarius was grown in a medium containing a mixture of lactose and galactose, the two sugars were consumed concomitantly and at the same rate (Fig. 1B). When galactose was provided to cells growing on lactose, the galactose was immediately taken up and did not interfere with lactose uptake (Fig. 1C). Thus, unlike Gal− and Gal+ strains of S. thermophilus (15, 34), S. salivarius readily metabolized galactose, even in the presence of lactose.
FIG. 1.
Growth of S. salivarius on lactose and in a mixture of lactose and galactose. (A) Cells were grown at 37°C in M17 medium containing 50 mM lactose. Symbols: ○, growth; • and ▪, concentrations of lactose and galactose, respectively, in the medium. (B) S. salivarius was grown in a medium containing 5 mM lactose and 5 mM galactose. The symbols are as indicated in panel A. (C) An overnight culture of S. salivarius was used to inoculate a medium containing ca. 2 mM lactose. When the culture reached mid-log phase, the medium was supplemented with 5 mM galactose. The symbols are as indicated in panel A.
Amino acid sequence comparison of S. salivarius and S. thermophilus IIALacS.
The expulsion of galactose by S. thermophilus is mediated by LacS and is stimulated when the IIA domain (IIALacS), which is located at the C-terminal end of the protein, is phosphorylated (11, 23-24). We recently cloned and sequenced the gene coding for S. salivarius LacS (40). A comparison of the translated amino acid sequence of the C-terminal of S. salivarius LacS with the same sequence from various strains of S. thermophilus (LMG18311, A147, and SMQ-301) revealed that S. salivarius LacS possessed a C-terminal IIA-like domain that shared >97% identity with S. thermophilus orthologues. S. salivarius IIALacS possessed the His residue (His552) that is phosphorylated by HPr(His∼P) in S. thermophilus (23, 24). However, the amino acid sequence of S. salivarius IIALacS differed from the sequence of S. thermophilus IIALacS at three positions: Ile532 was replaced by Val, Asn561 was replaced by Lys, and Lys616 was replaced by Glu. To determine whether these changes prevented phosphorylation of His552, we overproduced S. salivarius His6-IIALacS in E. coli, purified it, and carried out phosphorylation tests with His6-HPr(His∼P) and His6-HPr(Ser-P)(His∼P).
Phosphorylation of S. salivarius His6-IIALacS by His6-HPr(His∼P).
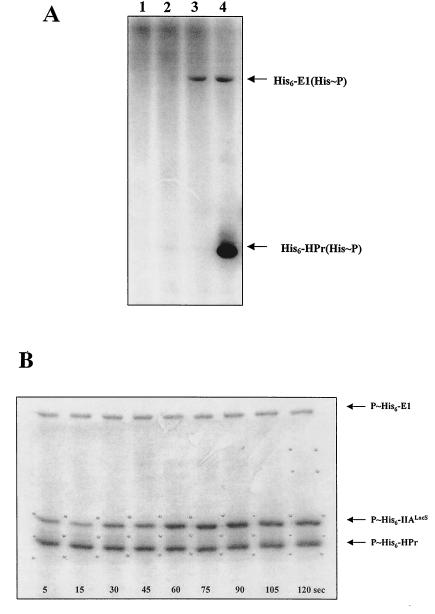
The 3′ end of S. salivarius lacS, which codes for IIALacS, was expressed in E. coli BL21(DE3) as described in Materials and Methods. The purified protein migrated electrophoretically as a protein with a molecular mass of ∼21,000 Da, which was close to the molecular mass calculated from the translated amino acid sequence (19,755 Da). The purified protein was used to test the phosphorylation of His552 by S. salivarius His6-HPr(His∼P). Incubating His6-EI with [32P]PEP resulted in the autophosphorylation of the recombinant enzyme (Fig. 2A, lane 3), a phenomenon that was not observed with His6-IIALacS or His6-HPr (Fig. 2A, lanes 1 and 2). Incubating His6-EI and His6-IIALacS with labeled PEP resulted in the phosphorylation of single protein corresponding to EI (not shown), whereas incubating His6-EI and His6-HPr with labeled PEP resulted in the phosphorylation of both proteins (Fig. 2A, lane 4). These results indicated that (i) the His-tag added to recombinant EI and HPr did not interfere with their capacity to receive and transfer a phosphate group and (ii) His6-IIALacS could not be phosphorylated at the expense of PEP or His6-EI(His∼P). We then incubated His6-EI, His6-HPr, His6-IIALacS, and [32P]PEP together, removed samples at intervals, and analyzed the products by SDS-PAGE (Fig. 2B). The results clearly indicated that His6-IIALacS was phosphorylated and that the amount of phosphorylated protein increased over time. When His6-IIALacSH552R was used instead of His6-IIALacS, no phosphorylated His6-IIALacSH552R was detected on the autoradiogram (not shown), suggesting that His6-HPr(His∼P) phosphorylated the His552 of S. salivarius LacS.
FIG. 2.
PEP-dependent phosphorylation of His6-IIALacS by His6-HPr(His∼P). The reactions were carried out in 50 mM Tris-acetate (pH 7.5) containing 1 mM DTT, 1 mM MgCl2, 0.8 μM His6-EI, 24 μM His6-HPr, 5.8 μM His6-IIALacS, and 1 mM [32P]PEP (30 μCi/μmol). The reactions were stopped by adding an equal volume of a solution containing 180 mM Tris-HCl (pH 6.8), 200 mM SDS, 30% glycerol, 2 M β-mercaptoethanol, and 0.003% bromophenol blue. Proteins were separated by SDS-PAGE, and phosphoproteins were revealed by autoradiography. (A) Lanes: 1, control experiment conducted without EI and HPr; 2, control experiment conducted without EI and IIALacS; 3, control experiment conducted without HPr and IIALacS; 4, control experiment conducted without IIALacS. (B) PEP-dependent phosphorylation experiment conducted in a medium containing EI, HPr, and IIALacS. Samples were withdrawn at the intervals indicated on the autoradiogram.
Phosphorylation of S. salivarius His6-IIALacS by His6-HPr(Ser-P)(His∼P).
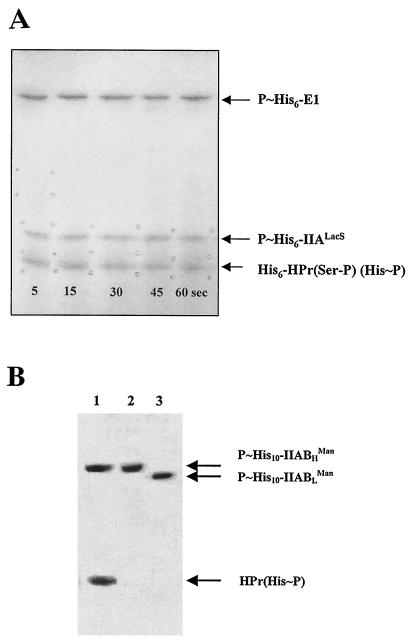
To determine whether His6-IIALacS could be phosphorylated by His6-HPr(Ser-P)(His∼P), His6-HPr(Ser-P) was first synthesized as described in Materials and Methods. The purity of His6-HPr(Ser-P) was verified by native PAGE and silver nitrate staining. The results (data not shown) indicated that the preparation was free of His6-HPr and HPrK/P. The absence of free His6-HPr in the preparation was also demonstrated by incubating the purified preparation of His6-HPr(Ser-P) with His6-EI and [32P]PEP and detecting the reaction products by autoradiography after separation by native PAGE. Only His6-EI(His∼P) and His6-HPr(Ser-P)(His∼P) were detected (data not shown). Analyses conducted with unlabeled PEP indicated that ca. 50% of the His6-HPr(Ser-P) in the medium was transformed into His6-HPr(Ser-P)(His∼P) under the experimental conditions used. We thus incubated His6-EI, His6-HPr(Ser-P), and [32P]PEP together to synthesize His6-HPr(Ser-P)(His∼32P). Since only half of the His6-HPr(Ser-P) was transformed into the doubly phosphorylated form under these conditions, we increased the concentration of His6-HPr(Ser-P) twofold in the reaction medium to obtain a concentration of HPr(Ser-P)(His∼P) similar to the concentration of HPr(His∼P) (24 μM) that was used in the IIALacS phosphorylation experiments. His6-IIALacS was then added to the reaction mixture. The results presented in Fig. 3A univocally indicate that His6-IIALacS was phosphorylated by His6-HPr(Ser-P)(His∼P). No further increase in the amounts of His6-IIALacS(His∼P) was observed after 5 s, suggesting that the transfer of a phosphate group from HPr(Ser-P)(His∼P) to IIALacS is a rapid process. The mutated protein His6-IIALacSH552R was not phosphorylated by His6-HPr(Ser-P)(His∼P) (not shown), suggesting that His6-HPr(Ser-P)(His∼P) and His6-HPr(His∼P) phosphorylated His6-IIALacS on the same residue.
FIG. 3.
Phosphorylation of His6-IIALacS by His6-HPr(Ser-P)(His∼P) and by His10-IIABLMan(His∼P) and His10-IIABHMan(His∼P). (A) The synthesis of His6-HPr(Ser-P)(His∼32P) is described in Materials and Methods. Phosphorylation of 5.8 μM His6-IIALacS by ca. 20 μM His6-HPr(Ser-P)(His∼32P) was conducted at 10°C. Samples were withdrawn at intervals, proteins were separated by SDS-PAGE, and phosphoproteins were revealed by autoradiography. (B) His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P) were synthesized and purified as described in Materials and Methods. Phosphorylation of His6-IIALacS by His10-IIABLMan(His∼32P) and His10-IIABHMan(His∼32P) was carried out in 10 mM HEPES (pH 7.5), containing 5 mM MgCl2, either 2.8 μM His10-IIABLMan(His∼32P) or His10-IIABHMan(His∼32P), and 5.8 μM His6-IIALacS in a total volume of 30 μl. The proteins were separated by SDS-PAGE and revealed by autoradiography. Lanes: 1, phosphorylated products resulting from the incubation of His10-IIABHMan(His∼32P) with His6-HPr; 2, phosphorylated products resulting from the incubation of His10-IIABHMan(His∼32P) with His6-IIALacS; 3, phosphorylated products resulting from the incubation of His10-IIABLMan(His∼32P) with His6-IIALacS.
His6-IIALacS could not be phosphorylated by His10-IIABLMan(His∼P) or His10-IIABHMan(His∼P).
In a previous study, we demonstrated that S. salivarius P∼IIABLMan and P∼IIABHMan can transfer their phosphate groups to each other and possibly to other proteins (20). Circumstantial evidence also suggests that these PTS proteins control sugar metabolism by a mechanism that has yet to be characterized (5, 26, 38). We thus looked at whether these proteins could phosphorylate His6-IIALacS. Purified His10-IIABMan(His∼P) proteins were first incubated with free HPr to determine whether the His tag interfered with their phosphotransfer capacity. As shown in Fig. 3B (lane 1), His10-IIABHMan(His∼P) could readily transfer its phosphate group to HPr. Identical results were obtained with His10-IIABLMan(His∼P) (not shown). The results shown in Fig. 3B (lanes 2 and 3) indicate that His10-IIABLMan(His∼P) and His10-IIABHMan(His∼P) were unable to phosphorylate His6-IIALacS.
Dephosphorylation of His6-IIALacS(His∼P) by HPr and HPr(Ser-P).
To determine whether HPr(His∼P) and HPr(Ser-P)(His∼P) reversibly phosphorylated IIALacS, we incubated purified His6-IIALacS(His∼32P) with HPr and HPr(Ser-P) under the conditions described in Materials and Methods and looked for the synthesis of HPr(His∼32P) and HPr(Ser-P)(His∼32P). As illustrated in Fig. 4, both HPr (lane 3) and HPr(Ser-P) (lane 2) could be phosphorylated by purified His6-IIALacS(His∼32P).
FIG.4.
Dephosphorylation of His6-IIALacS(His∼32P) by HPr(Ser-P) and HPr. The synthesis and purification of His6-IIALacS(His∼32P) is described in Materials and Methods. His6-IIALacS(His∼32P) was dephosphorylated by HPr and HPr(Ser-P) in 50 mM Tris-acetate (pH 7.5) containing 1 mM DTT, 2 mM MgCl2, and 20 μM HPr or HPr(Ser-P) in a total volume of 15 μl. After the mixture was incubated for 10 min at 37°C, His6-IIALacS(His∼32P) was added to a final concentration of 2 μM, and the incubation was extended for 5 min. The proteins were separated by SDS-PAGE and revealed by autoradiography. Lanes: 1, purified His6-IIALacS(His∼32P); 2, phosphorylated products resulting from the incubation of His6-IIALacS(His∼32P) with HPr(Ser-P); 3, phosphorylated products resulting from the incubation of His6-IIALacS(His∼32P) with HPr.
Effect of LacS phosphorylation on the growth of S. salivarius on lactose and galactose.
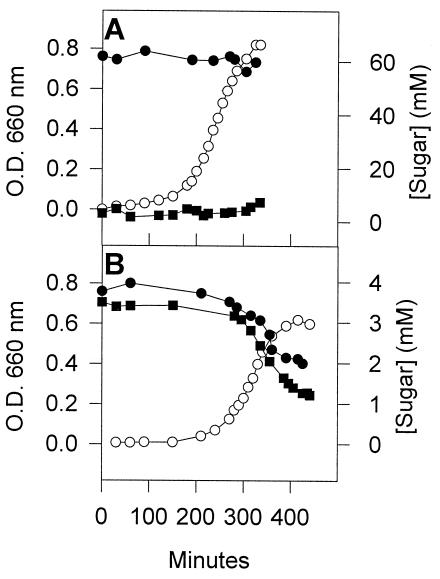
To determine whether LacS phosphorylation influences the growth of S. salivarius on lactose and galactose, two LacS substrates (13), we compared the growth properties of the wild-type and strain G71, an EI-negative mutant derived from S. salivarius ATCC 25975 (9). Since this mutant does not produce EI, growing cells do not contain HPr(His∼P) or HPr(Ser-P)(His∼P), which impedes LacS phosphorylation. The generation times of the wild-type and mutant strains on lactose and galactose are listed in Table 3. Strain G71 grew as well as the parental strain on 5.8 and 29 mM lactose. Moreover, like the wild-type strain (Fig. 1) (40), G71 did not release galactose into the external medium during growth on 5.8 mM (results not shown) and 58 mM lactose (Fig. 5A). Growth of the mutant strain was slightly reduced on galactose, with a generation time ∼1.2-fold longer than that of the wild-type. The growth rates of the wild-type and mutant strains in a medium containing lactose and galactose also differed slightly. The growth of the wild-type strain under these conditions was as rapid as it was in a medium containing only lactose or galactose (about 27 min), whereas the doubling time of the mutant strain in a lactose-galactose mixture was 37 min, which corresponded to the generation time observed on galactose. Despite this difference in generation times, sugars were consumed concomitantly by both strains during growth under these conditions (Fig. 1B and 5B).
TABLE 3.
Generation times of S. salivarius ATCC 25975 and mutant G71
| Sugar | Concn (mM) | Mean generation timea (min) ± SE
|
|
|---|---|---|---|
| Parental strain | Mutant G71 | ||
| Lactose | 5.8 | 29 ± 1 | 28 ± 1 |
| Lactose | 29 | 28 ± 2 | 30 ± 2 |
| Galactose | 11 | 30 ± 1 | 36 ± 2 |
| Galactose | 55 | 32 ± 1 | 40 ± 3 |
Growth was monitored by determining the optical density at 660 nm. Generation times were calculated for cultures in the exponential growth phase by plotting the logarithm of the optical density at 660 nm as a function of time. Values represent the means of eight separate experiments.
FIG. 5.
Growth of mutant G71on lactose and in a mixture of lactose and galactose. (A) Cells were grown at 37°C in M17 medium containing 58 mM lactose. Symbols: ○, growth; • and ▪, concentrations of lactose and galactose, respectively, in the medium. (B) Mutant G71 was grown in a medium containing 4 mM lactose and 4 mM galactose. The symbols are as indicated in panel A.
DISCUSSION
Both S. salivarius and S. thermophilus, two closely phylogenetically related bacteria, take up lactose via a non-PTS transporter called LacS and hydrolyze intracellular lactose into glucose and galactose via a β-galactosidase (13, 14, 40). S. thermophilus LacS is a member of a subgroup of the galactoside-pentose-hexuronide family of transporters. These proteins have a C-terminal domain that shares significant amino acid sequence identity with PTS IIA domains, which are phosphorylated on a histidine residue by HPr(His∼P) (25). Studies of lactose transport by S. thermophilus have provided compelling evidence that phosphorylation of LacS at His552 by HPr(His∼P) stimulates lactose-galactose exchange by LacS (11, 12, 17, 41), resulting in galactose accumulation in the medium during growth on lactose. S. salivarius LacS shares 95% identity with the LacS from S. thermophilus SMQ-301 over the total length of the protein (40). The results reported here showed that S. salivarius LacS possessed a IIA domain with high levels of identity with IIALacS from various S. thermophilus strains. However, unlike S. thermophilus, S. salivarius did not accumulate galactose into the medium during growth on lactose and readily metabolized galactose, even in the presence of lactose (40) (Fig. 1). This raised the question of whether S. salivarius LacS was phosphorylated and, if so, what was the effect on growth at the expense of lactose and galactose.
Phosphorylation experiments conducted in vitro with purified proteins univocally showed that S. salivarius IIALacS could be reversibly phosphorylated by HPr(His∼P) on residue His552. S. salivarius cells contain considerable amounts of HPr(His∼P) under conditions of limited growth and during the stationary growth phase, whereas this form of HPr is barely detectable in rapidly growing cells (32, 37). Thus, phosphorylation of LacS in vivo by HPr(His∼P) obviously occurs mainly under conditions of restricted growth. Does this mean that S. salivarius LacS is weakly or not phosphorylated in rapidly growing cells? S. salivarius synthesizes at least two IIAB PTS proteins, IIABLMan and IIABHMan, which catalyze interpeptide phosphotransfer and possibly phosphorylate other cellular proteins (20). Moreover, during the exponential phase of growth, S. salivarius synthesizes large amounts of the doubly phosphorylated form of HPr, HPr(Ser-P)(His∼P), which accounts for approximately half of total cellular HPr (10, 22, 37). We thus looked at whether these proteins could phosphorylate IIALacS. Our results indicated that neither P∼IIABLMan nor P∼IIABHMan was able to transfer a phosphate group to LacS. However, the doubly phosphorylated form of HPr could readily phosphorylate LacS on His552. These results suggest that a high proportion of LacS is in a phosphorylated state in rapidly growing S. salivarius cells.
It has been frequently reported that phosphorylation of HPr at Ser46 prevents phosphorylation at His15 and, conversely, phosphorylation at His15 impedes phosphorylation at Ser46. Based on this observation and other biochemical studies, it is assumed that the phosphorylation of HPr at Ser46 by HPrK/P serves to reduce sugar uptake by the PTS (29). The fact, however, that growing streptococci contain considerable amounts of HPr(Ser-P)(His∼P) suggests that phosphorylation of HPr at His15 or Ser46 does not prevent the synthesis of the doubly phosphorylated form of HPr and that the synthesis of HPr(Ser-P) does not interfere with the uptake of PTS sugars in streptococci (36). Our findings that HPr(Ser-P)(His∼P) could be readily synthesized in vitro and was able to reversibly transfer a phosphate group to a IIA domain strengthen the view that the phosphorylation of HPr at Ser46 does not reduce PTS sugar transports in streptococci.
Since slowly and rapidly growing S. salivarius cells contain large amounts of a form of HPr that is able to phosphorylate LacS, the S. salivarius lactose permease most likely remains in a phosphorylated form. This contrasts with the results obtained from studies carried out with S. thermophilus, indicating that only 30% of LacS is phosphorylated in exponentially growing cells, whereas about two-thirds of the transporters are phosphorylated in early or late exponential cells (12). However, the results obtained with S. thermophilus cannot be compared to those from S. salivarius for at least two reasons. First, HPr(Ser-P)(His∼P) in growing S. thermophilus cells does not exceed 5% of the total HPr (12), which is nearly 10 times lower than the levels in S. salivarius. Second, the levels of phosphorylated LacS in S. thermophilus were determined by using a strain in which the chromosomal lacS was deleted and which contained a plasmid bearing a copy of lacS under the control of its own promoter. Consequently, LacS levels during the exponential growth phase are 40-fold higher in the transformed strain than in the wild-type strain. This difference drops to sevenfold in early- and late-exponential-phase cells. Since the amounts of HPr should be the same in the engineered and wild-type strains and do not change as a function of the growth phase (12), the ratios of HPr(Ser-P)(His∼P)/LacS and HPr(His∼P)/LacS in the engineered strain differ considerably from those in the wild-type cells. Thus, the amount of phosphorylated LacS in the engineered strain is likely different from that in the wild-type strain.
To determine the effect of S. salivarius LacS phosphorylation on the ability of cells to metabolize lactose and galactose, we studied the growth of strain G71, an EI-negative mutant derived from S. salivarius ATCC 25975 (9). Since this strain does not synthesize HPr(His∼P) or HPr(Ser-P)(His∼P), LacS should remain permanently unphosphorylated. Our results revealed that the wild-type and mutant strains had identical generation times on lactose and that neither expelled galactose during growth on lactose. Moreover, both strains metabolized lactose and galactose concomitantly when grown in a medium containing both sugars. These results suggested that S. salivarius LacS phosphorylation was not involved in the rate of growth on lactose, did not promote a discernible LacS-mediated lactose-galactose exchange, and did not change the ability of the transporter to transport galactose and lactose at the same time. We did observe, however, that the mutant strain grew slightly more slowly than the wild-type strain on galactose. This effect may result from the absence of LacS phosphorylation but may also result from other cellular perturbations caused by a modification in the relative proportion of the different forms of HPr in the EI-negative mutant. For instance, change in the intracellular amount of HPr(Ser-P) could affect transcription of genes under the control of the complex CcpA-HPr(Ser-P) (7, 13, 29) and the activity of permeases controlled by HPr(Ser-P) (29, 42). Although there is no direct evidence that S. salivarius LacS is regulated by HPr(Ser-P), it was demonstrated that the I47T substitution in S. salivarius HPr inhibits the preferential metabolism of glucose and fructose over lactose (10), indicating that somehow HPr is involved in the regulation of LacS. Moreover, a CcpA binding site (cre sequence) has been identified in the promoter region of the S. salivarius gal operon (40), and reduction in the levels of intracellular HPr by a factor of 3 interferes with expression of the gal operon (33). Lastly, we cannot rule out that S. salivarius possesses a second, as-yet-unidentified galactose transporter that would allow growth of mutant G71 on galactose. Thus, the small increase in the generation time on galactose observed with mutant G71 may result from several factors.
In conclusion, S. salivarius LacS could be readily phosphorylated on His552 by HPr(His∼P), which is abundant in cells under conditions of energy privation, and by HPr(Ser-P)(His∼P), which is synthesized in large amounts when energy sources are plentiful. The role of this phosphorylation remains unclear but did not seem to be related to galactose-lactose exchange and did not affect growth on lactose.
Acknowledgments
This study received financial support from the following organizations: Action Concertée FCAR-NOVALAIT-MAPAQ, Action Concertée FQRNT-NOVALAIT-MAPAQ-Agriculture Canada, Fonds Québecois de la Recherche sur la Nature et les Technologies, and the Canadian Institutes of Health Research.
We thank Sédé Alodéhou for providing plasmid pTML2, Israël Casabon for helping with the HPr(Ser-P) synthesis, and Gene Bourgeau for providing editorial assistance.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1997. Current protocols in molecular biology. Greene Publishing and Wiley Interscience, New York, N.Y.
- 2.Avigad, G., D. Amaral, C. Ascension, and B. L. Horecker. 1962. The d-galactose oxydase of Polyporus circinatus. J. Biol. Chem. 237:2736-2743. [PubMed] [Google Scholar]
- 3.Brochu, D., and C. Vadeboncoeur. 1999. The HPr(Ser) kinase of Streptococcus salivarius: purification, properties, and cloning of the hprK gene. J. Bacteriol. 181:709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson, J. 2000. Growth and nutrition as ecological factors, p. 67-130. In H. K. Kuramitsu and R. P. Ellen (ed.), Oral bacterial ecology: the molecular basis. Horizon Scientific Press, Wymondham, United Kingdom.
- 5.Chaillou, S., P. W. Postma, and P. H. Pouwels. 2001. Contribution of the phosphoenolpyruvate:mannose phosphotransferase system to carbon catabolite repression in Lactobacillus pentosus. Microbiology 147:671-679. [DOI] [PubMed] [Google Scholar]
- 6.Deutscher, J., A. Galinier, and I. Martin-Verstraete. 2002. Carbohydrate uptake and metabolism, p. 129-150. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 7.Deutscher, J., E. Küster, U. Bergstedt, V. Charrier, and W. Hillen. 1995. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to catabolite repression in gram-positive bacteria. Mol. Microbiol. 15:1049-1053. [DOI] [PubMed] [Google Scholar]
- 8.Frey, N., S. Nessler, S. Fieulaine, K. Vaillancourt, M. Frenette, and C. Vadeboncoeur. 2003. The HPr(Ser) kinase of Streptococcus salivarius re-examined: a hexameric bifunctional enzyme controlled by glycolytic intermediates and inorganic phosphate. FEMS Microbiol. Lett. 224:67-72. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier, L., S. Thomas, G. Gagnon, M. Frenette, L. Trahan, and C. Vadeboncoeur. 1994. Positive selection for resistance to 2-deoxyglucose gives rise, in Streptococcus salivarius, to seven classes of pleiotropic mutants, including ptsH and ptsI, missense mutants. Mol. Microbiol. 13:1101-1109. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier, M., D. Brochu, L. D. Eltis, S. Thomas, and C. Vadeboncoeur. 1997. Replacement of isoleucine-47 by threonine in the HPr protein of Streptococcus salivarius abrogates the preferential metabolism of glucose and fructose over lactose and melibiose but does not prevent phosphorylation of HPr on serine 46. Mol. Microbiol. 25:695-705. [DOI] [PubMed] [Google Scholar]
- 11.Gunnewijk, M. G. W., and B. Poolman. 2000. HPr(His∼P)-mediated phosphorylation differently affects counterflow and proton motive force-driven uptake via the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34080-34085. [DOI] [PubMed] [Google Scholar]
- 12.Gunnewijk, M. G. W., and B. Poolman. 2000. Phosphorylation state of HPr determines the level of expression and the extent of phosphorylation of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 275:34073-34079. [DOI] [PubMed] [Google Scholar]
- 13.Gunnewijk, M. G. W., P. T. C. van den Bogaard, L. M. Veenhoff, E. H. Heuberger, W. M. de Vos, M. Kleerebezem, O. P. Kuipers, and B. Poolman. 2001. Hierarchical control versus autoregulation of carbohydrate utilization in bacteria. J. Mol. Microbiol. Biotechnol. 3:401-413. [PubMed] [Google Scholar]
- 14.Hamilton, I. R., and G. C. Y. Lo. 1978. Co-induction of β-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J. Bacteriol. 136:900-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutkins, R. W., and H. A. Morris. 1987. Carbohydrate metabolism by Streptococcus thermophilus: a review. J. Food Prot. 50:876-884. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura, Y., C.-G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406-408. [DOI] [PubMed] [Google Scholar]
- 17.Knol, J., L. M. Veenhoff, W. J. Liang, P. J. F. Henderson, G. Leblanc, and B. Poolman. 1996. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J. Biol. Chem. 271:15358-15366. [DOI] [PubMed] [Google Scholar]
- 18.Mattoo, R. L., and E. B. Waygood. 1983. An enzymatic method for [32P]-phosphoenolpyruvate synthesis. Anal. Biochem. 128:245-249. [DOI] [PubMed] [Google Scholar]
- 19.Pearce, C., G. H. Bowden, M. Evans, P. S. Fitzsimmons, J. Johnson, M. J. Sheridan, R. Wientzen, and M. F. Cole. 1995. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J. Med. Microbiol. 42:67-72. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier, M., L.-A. Lortie, M. Frenette, and C. Vadeboncoeur. 1998. The phosphoenolpyruvate:mannose phosphotransferase system of Streptococcus salivarius: functional and biochemical characterization of IIABLMan and IIABHMan. Biochemistry 37:1604-1612. [DOI] [PubMed] [Google Scholar]
- 21.Peterson, G. L. 1983. Determination of total protein. Methods Enzymol. 91:95-119. [DOI] [PubMed] [Google Scholar]
- 22.Plamondon, P., D. Brochu, S. Thomas, J. Fradette, L. Gauthier, K. Vaillancourt, N. Buckley, M. Frenette, and C. Vadeboncoeur. 1999. Phenotypic consequences resulting from a methionine-to-valine substitution at position 48 in the HPr protein of Streptococcus salivarius. J. Bacteriol. 181:6914-6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poolman, B., R. Modderman, and J. Reizer. 1992. Lactose transport system of Streptococcus thermophilus: the role of histidine residues. J. Biol. Chem. 267:9150-9157. [PubMed] [Google Scholar]
- 24.Poolman, B., J. Knol, B. Mollet, B. Nieuwenhuis, and G. Sulter. 1995. Regulation of bacterial sugar-H+ symport by phosphoenolpyvuvate-dependent enzyme I/HPr-mediated phosphorylation. Proc. Natl. Acad. Sci. USA 92:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poolman, B., J. Knol, C. van der Does, P. J. F. Henderson, W.-J. Liang, G. Leblanc, T. Pourcher, and I. Mus-Veteau. 1996. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol. Microbiol. 19:911-922. [DOI] [PubMed] [Google Scholar]
- 26.Posthuma, C. C., R. Bader, R. Engelmann, P. W. Postma, W. Hengstenberg, and P. H. Pouwels. 2002. Expression of the xylulose 5-phosphate phosphoketolase gene, xpkA, from Lactobacillus pentosus MD363 is induced by sugars that are fermented via the phosphoketolase pathway and is repressed by glucose mediated by CcpA and the mannose phosphoenolpyruvate phosphotransferase system. Appl. Environ. Microbiol. 68:831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotranferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robitaille, D., L. Gauthier, and C. Vadeboncoeur. 1991. The presence of two forms of the phosphocarrier protein HPr of the phosphoenolpyruvate:sugar phosphotransferase system in streptococci. Biochimie 73:573-581. [DOI] [PubMed] [Google Scholar]
- 29.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in gram-positive bacteria. Microbiology 142:217-230. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Stülke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD: a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28:865-874. [DOI] [PubMed] [Google Scholar]
- 32.Thevenot, T., D. Brochu, C. Vadeboncoeur, and I. R. Hamilton. 1995. Regulation of ATP-dependent P-(Ser)-HPr formation in Streptococcus mutans and Streptococcus salivarius. J. Bacteriol. 177:2751-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, S., D. Brochu, and C. Vadeboncoeur. 2001. Diversity of Streptococcus salivarius ptsH mutants that can be isolated in the presence of 2-deoxyglucose and galactose and characterization of two mutants synthesizing reduced levels of HPr, a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 183:5145-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas, T. D., and V. L. Crow. 1984. Selection of galactose-fermenting Streptococcus thermophilus in lactose-limited chemostat cultures. Appl. Environ. Microbiol. 48:186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson, J. 1987. Sugar transport in the lactic acid bacteria, p. 13-38. In J. Reizer and A. Peterkofsky (ed.), Sugar transport and metabolism in gram-positive bacteria. Ellis Horwood, Ltd., London, England.
- 36.Vadeboncoeur, C. 1995. HPr: Heteromorphous protein. Res. Microbiol. 146:525-530. [DOI] [PubMed] [Google Scholar]
- 37.Vadeboncoeur, C., D. Brochu, and J. Reizer. 1991. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system in growing cells of oral streptococci. Anal. Biochem. 196:24-30. [DOI] [PubMed] [Google Scholar]
- 38.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 39.Vadeboncoeur, C., M. Proulx, and L. Trahan. 1983. Purification of proteins similar to HPr and enzyme I from the oral bacterium Streptococcus salivarius: biochemical and immunochemical properties. Can. J. Microbiol. 29:1694-1705. [DOI] [PubMed] [Google Scholar]
- 40.Vaillancourt, K., S. Moineau, M. Frenette, C. Lessard, and C. Vadeboncoeur. 2002. Galactose and lactose genes from the galactose-positive bacterium Streptococcus salivarius and the phylogenetically related galactose-negative bacterium Streptococcus thermophilus: organization, sequence, transcription, and activity of the gal gene products. J. Bacteriol. 184:785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenhoff, L. M., and B. Poolman. 1999. Substrate recognition at the cytoplasmic and extracellular binding site of the lactose transport protein of Streptococcus thermophilus. J. Biol. Chem. 274:33244-33250. [DOI] [PubMed] [Google Scholar]
- 42.Ye, J.-J., and M. H. Saier, Jr. 1995. Cooperative binding of lactose and HPr(Ser-P) to the lactose:H+ symport permease of Lactobacillus brevis. Proc. Natl. Acad. Sci. USA 92:417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]