Abstract
The crystal structure of Bacillus subtilis α-amylase, in complex with the pseudotetrasaccharide inhibitor acarbose, revealed an hexasaccharide in the active site as a result of transglycosylation. After comparison with the known structure of the catalytic-site mutant complexed with the native substrate maltopentaose, it is suggested that the present structure represents a mimic intermediate in the initial stage of the catalytic process.
α-Amylase (α-1,4-glucan-4-glucanohydrolase, EC 3.2.1.1) catalyzes the hydrolysis of α-d-(1,4)-glucosidic linkages in starch, glycogen, and various malto-oligosaccharides, releasing α-anomeric products. α-Amylase has been studied extensively from various aspects, including structure and function, secretion, and industrial application. α-Amylase is also the most widely studied member of the glycosyl hydrolase family 13, and details of this family may be found at the website http://afmb.cnrs-mrs.fr/∼cazy/CAZY/index.html (6). α-Amylase has two aspartic residues and one glutamic acid residue that are conserved among species and are presumed to be the catalytic residues. Specifically, we showed previously by site-directed mutagenesis that substitution of any of these residues in Bacillus subtilis α-amylase (BSUA) causes an almost complete loss of catalytic activity, with substrate-binding ability being retained (20, 21). We also determined the X-ray structure of one of the catalytic-site mutants of BSUA complexed with a natural substrate, maltopentaose (G5), which revealed the roles of the catalytic residues (9).
Acarbose is a pseudotetrasaccharide inhibitor of the α-amylase family, in which the cyclitol group resembles the presumed oxocarbenium ion-like transition state expected for glycoside hydrolysis (23). The structures of the complexes with acarbose-derived oligosaccharides have recently become available for many α-amylases. They include pig pancreatic amylase I (16, 17), pig pancreatic amylase II (10, 13), Aspergillus oryzae α-amylase (4), barley α-amylase (11), Bacillus circulans cyclodextrin glycosyltransferase (14, 18, 19), Pseudoalteromonas haloplanctis α-amylase (1), and Bacillus stearothermophilus “maltogenic” α-amylase (15). Also, amylomaltase from Thermus aquaticus in complex with acarbose has been reported, and amylomaltase is a member of the α-amylase family (7). These studies have revealed the overall fold, substrate-binding structure, and the roles of the catalytic residues. Despite differences in their amino acid sequences, they have similar three-dimensional structures with three domains: domain A consisting of a central (β/α)8 barrel flanking the active site, domain B overlaying the active site from one side, and domain C consisting of a β-structure with a Greek key motif. Acarbose derivatives have been found bound to the active site in a similar manner in all of these α-amylases, suggesting a similar catalytic mechanism.
In previous studies (9, 20, 21), we created three catalytic-site mutants of BSUA by site-directed mutagenesis: DN176 (Asp176→Asn), EQ208 (Glu208→Gln), and DN269 (Asp269→Asn). Interestingly, the affinity of EQ208 for G5 was greatly increased over that of the wild-type enzyme, while that for acarbose was greatly decreased (20, 21). This property of EQ208 allowed us to crystallize a complex of EQ208 with G5 (EQ208/G5) suitable for X-ray diffraction, which revealed information on the interaction with the natural substrate at the atomic level (9). In the present study, we report on the X-ray structure of BSUA complexed with acarbose (BSUA/acarbose) and compare the structure with that of EQ208/G5. Crystallographic studies of such a series of wild-type and mutant α-amylases, including the complexes with substrates or inhibitors, should provide insight into the catalytic mechanisms involved.
Enzyme preparation and crystallization.
The N42 form of BSUA protein (22) was expressed using the B. subtilis expression system and the protein purified from the culture medium, as described previously (21). The BSUA in this study was a site-directed mutant (Asn356→Gln), which had been prepared for a different purpose, and the activity and protein stability of this BSUA showed no differences from those of the wild type. Acarbose was a generous gift of Bayer AG (Wuppertal, Germany).
Crystallization trials of the BSUA/acarbose complex were performed by using the hanging drop vapor diffusion method. A mixture consisting of BSUA (20 mg/ml) in 10 mM Tris-HCl (pH 7.2) buffer containing 3.5 mM CaCl2 and 0.26% acarbose was incubated at room temperature for 30 min. A 2-μl droplet of this solution was mixed with an equal volume of reservoir solution containing 10% polyethylene glycol 3350 and equilibrated with reservoir solution at 20°C. Crystals grew as rods after about 3 weeks. During further refinement of the crystallization conditions, the largest crystals obtained had dimensions of about 0.2 by 0.2 by 1.0 mm when the mixture contained 13 mg of BSUA per ml and 0.32% acarbose.
Data collection and model building.
X-ray diffraction data were collected at 100 K using imaging plate detector R-AXIS IV++, with copper anode X-ray generator (Rigaku UltraX 18). The collected data were processed with the program CRYSTALCLEAR (Rigaku). The native intensity data set at 2.3-Å resolution contained a total of 109,248 observations, which reduced to 27,358 unique reflections with 96.8% completeness and a merging R factor of 14.0% on intensities. The crystals belonged to the orthorhombic space group P212121 with the following cell constants: a = 70.1 Å, b = 73.9 Å, and c = 115.5 Å; the crystals contained one molecule in the asymmetric unit (Table 1). These parameters and intensity profile indicate that the crystal is isomorphous with that of the complex with EQ208/G5.
TABLE 1.
Crystal parameters and refinement statistics
| Crystal parameter or refinement statistic | Valuea |
|---|---|
| Data collection | |
| Space group | P212121 |
| Cell dimensions | |
| a (Å) | 70.1 |
| b (Å) | 73.9 |
| c (Å) | 115.5 |
| Solvent content (%) | 58 |
| Resolution range (Å) | 44.6-2.3 (2.38-2.3) |
| Measured reflections | 109,248 (10,555) |
| Unique reflections | 27,358 (2,678) |
| Completeness (%) | 96.8 (98.4) |
| Rmergeb | 0.140 (0.246) |
| I/σ(I) | 4.6 (2.4) |
| Refinement | |
| Refinement range (Å) | 20-2.3 (2.38-2.3) |
| No. of reflections in refinements | 26,306 (2,560) |
| R factor (%)c | 20.1 (24.5) |
| Rfree factor (%)c | 25.9 (29.4) |
| No. of all nonhydrogen atoms | 3,808 |
| No. of protein nonhydrogen atoms | 3,303 |
| No. of acarbose nonhydrogen atoms | 65 |
| No. of calcium ions | 3 |
| No. of water molecules | 437 |
| Average B factor (Å2) | 21.6 |
| Protein atoms (Å2) | 20.7 |
| Acarbose atoms (Å2) | 23.2 |
| Calcium ions (Å2) | 18.0 |
| Water molecules (Å2) | 28.1 |
| r.m.s. deviations | |
| Bond lengths (Å) | 0.006 |
| Bond angles (°) | 1.2 |
Values in parentheses are for the highest-resolution shell.
Rmerge = Σ|I − 〈I〉|/ΣI, where I is the observed intensity and 〈I〉 is the average intensity for replicated data.
R factor is defined as R = Σ||Fobs| − |Fcalc||/Σ|Fobs|, where Fobs is the observed F and Fcalc is the calculated F. Rfree factor was calculated using 10% of the unique reflections.
The atomic coordinates of the EQ208/G5 complex from the Protein Data Bank with the identification code of 1BAG were used as the starting model. Manual model building was conducted with the QUANTA program (Molecular Simulations, Inc.). The structure was refined by simulated annealing with the CNS program (3). Of the observed reflections, 10% were randomly removed for the cross-validation (2). After rigid body and simulated annealing refinement, continuous density corresponding to a hexasaccharide was obtained.
Topology and parameter files for acarbose were generated by using program PRODRG (24), according to the acarbose model in crystal complex with PPAI (17) and PPAII (10). Several cycles of refinement and manual rebuilding led to the model, in which the final R value was 20.1% (Rfree, 25.9%). Details of data collection and refinement are listed in Table 1. The program PROCHECK (12) was used to evaluate the structure.
Overall structure.
The model of the BSUA/acarbose complex refined at a 2.3-Å resolution was made up of 422 amino acid residues (N-terminal residues 1 to 3 and C-terminal residues 426 to 436 were excluded from the model due to possible disorder), one acarbose-derived molecule, three Ca2+ ions, and 437 water molecules. Acarbose was bound in a deep pocket (active center) on the C-terminal side of the (β/α)8 barrel. The overall structure of BSUA in complex with acarbose was essentially the same as that in complex with G5, with a root mean square (r.m.s.) difference of 0.28 Å for all Cα atom pairs.
Oligosaccharide binding.
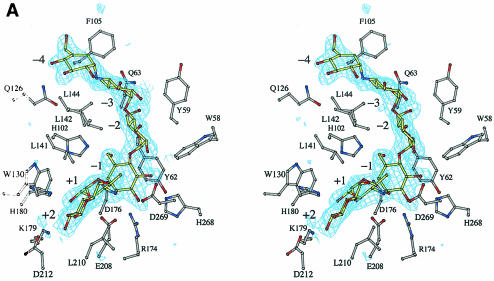
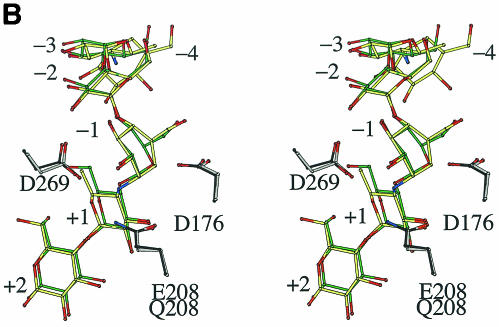
Continuous density corresponding to six saccharide units in the active site was clearly observed in a Fobs − Fcalc omit electron density map (Fig. 1A). Although acarbose is a tetrasaccharide, hexasaccharide was observed probably at a higher degree of polymerization. Such acarbose-derived oligosaccharides are frequently observed in the structures of acarbose complexes of family 13 enzymes (4, 10, 11, 13, 16, 17, 18, 19), because the enzyme is able to transglycosylate or condense molecules to make a longer and hence tighter-binding inhibitor. The acarbose-derived hexasaccharide binds in subsites −4 to +2 in a manner identical to that previously described (10, 17). Subsites −4 and −1 contain cyclitol units. The subsite −4 in α-amylases has been known (1, 5, 10, 17). Indeed, this cyclitol unit was exposed to the solvent region, and the hydroxyl groups interacted with water molecules via hydrogen bonds (Fig. 2). Figure 1B shows a structural comparison between acarbose-derived hexasaccharide and G5. The cyclitol unit bound to subsite −1 assumed a half-chair conformation, so that the C-5 atom was displaced from the corresponding atom position of the chair form observed in G5. This difference at subsite −1 confirms that when BSUA binds its substrate, the sugar in the catalytic subsite −1 is not distorted. The remaining ring atom positions superimposed well, allowing for no significant change in both glycosidic bond directions from C-1 and C-4 to the neighboring saccharides. It is worth noting that the overall structures of acarbose-derived hexasaccharide and G5 are very similar to each other when bound to the enzyme.
FIG. 1.
Stereoviews of acarbose bound to BSUA. (A) A Fobs− Fcalc omit electron density map for acarbose contoured at 3.0 σ using 2.3-Å resolution refined phases. The saccharide units are numbered so that the scissile bond is between −1 and +1. (B) Superposition of acarbose and G5 bound to the active site. The atoms are colored as follows: carbon atoms are gray (BSUA), yellow (acarbose), dark gray (EQ208), and green (G5); oxygen atoms are red, and nitrogen atoms are blue. The figure was generated with BOBSCRIPT (8, 12).
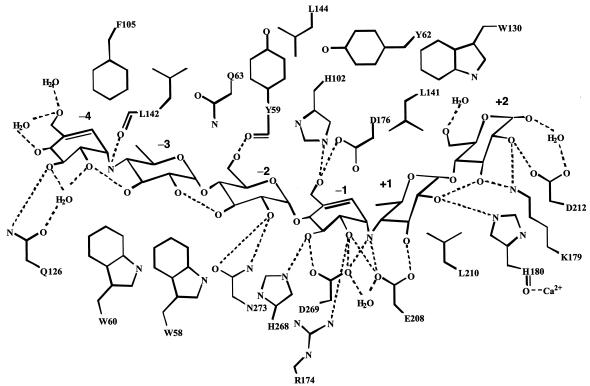
FIG. 2.
Schematic drawing of the hydrogen-bonding network between BSUA and acarbose. Water molecules mediating hydrogen bonds and hydrophobic residues interacting with acarbose are also shown.
Biochemical data show that acarbose inhibits the activity of BSUA quite effectively: the concentration needed for 50% inhibition is 0.2 or 1.6 μM, depending on whether the enzyme is preincubated with acarbose (21). Indeed, as expected, acarbose was tightly bound in the active center (Fig. 2). A hydrogen-bonding scheme between protein and ligand in the BSUA/acarbose complex was almost the same as that in the EQ208/G5 complex. G5 also binds to EQ208 tightly with a Kd value of 7.0 μM (20). The carboxyl oxygen of the catalytic residue Glu208 hydrogen bonded to the glycosidic nitrogen between the −1 and +1 subsites, which corresponded to a scissile bond. In the case of the EQ208/G5 complex, the amide nitrogen of Gln208 hydrogen bonded to the glycosidic oxygen at the corresponding site of G5.
The most interesting finding of the present study is that the structure of the transition state analogue acarbose is very similar to that of the native substrate G5 when bound to the enzyme (Fig. 1B). One particular difference is in the C-5 atom position of the saccharide in the −1 subsite, as mentioned above. The C-5 atom positions of G5 and acarbose ligands are related by a flip between the chair and half-chair forms. Thus, the present results suggest that, at the initial stage of the catalytic process, a transition from the chair to half-chair form of the glucose unit occurs at the −1 subsite immediately after the protonation to the glycosidic oxygen of the scissile bond (14). The present structure of the BSUA/acarbose complex is probably a mimic intermediate in this process.
Coordinates.
The coordinates and structure factors are deposited in Protein Data Bank as 1UA7.
REFERENCES
- 1.Aghajari, N., M. Roth, and R. Haser. 2002. Crystallographic evidence of a transglycosylation reaction: ternary complexes of a psychrophilic α-amylase. Biochemistry 41:4273-4280. [DOI] [PubMed] [Google Scholar]
- 2.Brünger, A. T. 1992. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355:472-475. [DOI] [PubMed] [Google Scholar]
- 3.Brünger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gros, R. W. Grosse-Kunstleve, J.-S. Jiang, J. Kuszewski, N. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. Sect. D 54:905-921. [DOI] [PubMed] [Google Scholar]
- 4.Brzozowski, A. M., and G. J. Davies. 1997. Structure of the Aspergillus oryzae α-amylase complexed with the inhibitor acarbose at 2.0 Å resolution. Biochemistry 36:10837-10845. [DOI] [PubMed] [Google Scholar]
- 5.Brzozowski, A. M., D. M. Lawson, J. P. Turkenburg, H. Bisgaard-Frantzen, A. Svendsen, T. V. Borchert, Z. Dauter, K. S. Wilson, and G. J. Davies. 2000. Structural analysis of a chimeric bacterial α-amylase. High-resolution analysis of native and ligand complexes. Biochemistry 39:9099-9107. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho, P. M., and B. Henrissat. 1999. Carbohydrate-active enzymes: an integrated database approach, p. 3-12. In H. J. Gilbert, G. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 7.Dauter, Z., M. Dauter, A. M. Brzozowski, S. Christensen, T. V. Borchert, L. Beier, K. S. Wilson, and G. J. Davies. 1999. X-ray structure of Novamyl, the five-domain “maltogenic” α-amylase from Bacillus stearothermophilus: maltose and acarbose complexes at 1.7 Å resolution. Biochemistry 38:8385-8392. [DOI] [PubMed] [Google Scholar]
- 8.Esnouf, R. M. 1997. An extensively modified version of Molscript that includes greatly enhanced coloring capabilities. J. Mol. Graph. 15:132-134. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto, Z., K. Takase, N. Doui, M. Momma, T. Matsumoto, and H. Mizuno. 1998. Crystal structure of a catalytic-site mutant α-amylase from Bacillus subtilis complexed with maltopentaose. J. Mol. Biol. 277:393-407. [DOI] [PubMed] [Google Scholar]
- 10.Gilles, C., J. P. Astier, G. Marchis-Mouren, C. Cambillau, and F. Payan. 1996. Crystal structure of pig pancreatic α-amylase isoenzyme II, in complex with the carbohydrate inhibitor acarbose. Eur. J. Biochem. 238:561-569. [DOI] [PubMed] [Google Scholar]
- 11.Kadziola, A., M. Søgaard, B. Svensson, and R. Haser. 1998. Molecular structure of a barley α-amylase-inhibitor complex: implications for starch binding and catalysis. J. Mol. Biol. 278:205-217. [DOI] [PubMed] [Google Scholar]
- 12.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemistrial quality of protein structures. J. Appl. Crystallogr. 26:289-291. [Google Scholar]
- 13.Machius, M., L. Vertesy, R. Huber, and G. Wiegand. 1996. Carbohydrate and protein-based inhibitors of porcine pancreatic α-amylase: structure analysis and comparison of their binding characteristics. J. Mol. Biol. 260:409-421. [DOI] [PubMed] [Google Scholar]
- 14.Mosi, R., H. Sham, J. C. Uitdehaag, R. Ruiterkamp, B. W. Dijkstra, and S. G. Withers. 1998. Reassessment of acarbose as a transition state analogue inhibitor of cyclodextrin glycosyltransferase. Biochemistry 37:17192-17198. [DOI] [PubMed] [Google Scholar]
- 15.Przylas, I., Y. Terada, K. Fujii, T. Takaha, W. Saenger, and N. Sträter. 2000. X-ray structure of acarbose bound to amylomaltase from Thermus aquaticus: implications for the synthesis of large cyclic glucans. Eur. J. Biochem. 267:6903-6913. [DOI] [PubMed] [Google Scholar]
- 16.Qian, M., R. Haser, G. Buisson, E. Dúee, and F. Payan. 1994. The active center of a mammalian α-amylase. Structure of the complex of a pancreatic α-amylase with a carbohydrate inhibitor refined to 2.2-Å resolution. Biochemistry 33:6284-6294. [DOI] [PubMed] [Google Scholar]
- 17.Qian, M., V. Nahoum, J. Bonicel, H. Bischoff, B. Henrissat, and F. Payan. 2001. Enzyme-catalyzed condensation reaction in a mammalian α-amylase. High-resolution structural analysis of an enzyme-inhibitor complex. Biochemistry 40:7700-7709. [DOI] [PubMed] [Google Scholar]
- 18.Strokopytov, B., R. M. Knegtel, D. Penninga, H. J. Rozeboom, K. H. Kalk, L. Dijkhuizen, and B. W. Dijkstra. 1996. Structure of cyclodextrin glycosyltransferase complexed with a maltononaose inhibitor at 2.6 Å resolution. Implications for product specificity. Biochemistry 35:4241-4249. [DOI] [PubMed] [Google Scholar]
- 19.Strokopytov, B., D. Penninga, H. J. Rozeboom, K. H. Kalk, L. Dijkhuizen, and B. W. Dijkstra. 1995. X-ray structure of cyclodextrin glycosyltransferase complexed with acarbose. Implications for the catalytic mechanism of glycosidases. Biochemistry 34:2234-2240. [DOI] [PubMed] [Google Scholar]
- 20.Takase, K. 1992. Interaction of catalytic-site mutants of Bacillus subtilis α-amylase with substrates and acarbose. Biochim. Biophys. Acta 1122:278-282. [DOI] [PubMed] [Google Scholar]
- 21.Takase, K., T. Matsumoto, H. Mizuno, and K. Yamane. 1992. Site-directed mutagenesis of active site residues in Bacillus subtilis α-amylase. Biochim. Biophys. Acta 1120:281-288. [DOI] [PubMed] [Google Scholar]
- 22.Takase, K., H. Mizuno, and K. Yamane. 1988. NH2-terminal processing of Bacillus subtilis α-amylase. J. Biol. Chem. 263:11548-11553. [PubMed] [Google Scholar]
- 23.Truscheit, E., W. Frommer, B. Junge, L. Müller, D. D. Schmidt, and W. Wingender. 1981. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew. Chem. Int. 20:744-761. [Google Scholar]
- 24.van Aalten, D. M. F., R. Bywater, J. B. C. Findlay, M. Hendlich, R. W. W. Hooft, and G. Vriend. 1996. PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J. Comput. Aided Mol. Des. 10:255-262. [DOI] [PubMed] [Google Scholar]