Abstract
Objective
To evaluate the tumor response and patient survival rate following transcatheter arterial chemoembolization (TACE) in recurrent hepatocellular carcinoma (r-HCC) after living donor liver transplantation (LDLT).
Materials and Methods
Twenty-eight patients with r-HCC underwent one or more cycles of TACE after LDLT (mean, 2.5 cycles). After a mixture of iodized oil and anti-cancer drugs was injected via the arteries feeding the tumors, these vessels were embolized with a gelatin sponge. Tumor response was determined by follow-up CT imaging on all patients four weeks after each TACE procedure. Patient survival was calculated using the Kaplan-Meier survival curve.
Results
After TACE, targeted tumor reduced in size by 25% or more in 19 of the 28 study patients (67.9%). However, intrahepatic recurrence or extrahepatic metastasis occurred in 21 of the 28 patients (75.0%) during the 3-month follow-up period and in 26 of the 28 patients (92.9%) during the 6-month period following TACE. Extrahepatic metastasis was noted in 18 of the 28 patients (64.3%). The 1-, 3- and 5-year survival rates following TACE were 47.9, 6.0 and 0%, respectively, with a mean survival of nine months in all patients. There were no significant complications related to TACE.
Conclusion
TACE produces an effective tumor response for targeted r-HCC after LDLT. However, the survival rate of patients with r-HCC after LDLT is poor due to extrahepatic metastasis and intrahepatic recurrence.
Keywords: Hepatocellular carcinoma, Liver transplantation, Chemoembolization
One million patients worldwide suffer from hepatocellular carcinoma (HCC) each year, accounting for approximately one million deaths annually (1). In recent years, liver transplantation has become an excellent treatment option for HCC patients because the procedure is able to cure the tumor as well as the underlying cirrhosis. Furthermore, in an effort to bridge the gap between the available organ supply and demand, living donor liver transplantation (LDLT) has become an acceptable, alternative treatment option for patients with HCC (2).
However, significant HCC recurrence ranges from 15% to 69%, which is considered treatment failure, in different series (3-6). The poor actuarial recurrence rates reported with LDLT likely reflect the selection of patients with more advanced HCC stages, rather than the hypothetical risk of recurrence related to neoplastic cell stimulation by hepatic growth factors (7, 8). Surprisingly little is known about the natural history, the therapeutic effect or the outcome of patients with cancer recurrence after liver transplantation (9-11). This article describes the tumor response and survival rate of patients after transcatheter arterial chemoembolization (TACE) treatment of recurrent HCC (r-HCC) after LDLT.
MATERIALS AND METHODS
Patients
Between August 1992 and June 2005, 1,008 LDLTs were performed and followed in our institution; patients who died within three months of the procedure were excluded from this study. Pathologic examination of extracted cirrhotic liver revealed HCCs in 252 patients (25.0%). During the follow-up period after LDLT, 42 of these 252 patients (16.7%) were diagnosed with recurrent HCC in the transplanted liver as well as in extrahepatic organs such as lung or bone.
TACE was indicated after LDLT for r-HCC with intrahepatic or extrahepatic locations with feeding arteries from the celiac trunk or right inferior phrenic artery or intercostal artery. Contraindications were Child-Pugh class C liver profile (n = 8), hyperbilirubinemia > 3 ng/ml (n = 6), and complete obstruction of the main portal vein (n = 3). TACE was performed in 28 of the 42 patients with r-HCC. All 28 patients had unresectable r-HCC (n = 26) or refused repeat surgery for resection of r-HCC (n = 2). Written informed consent was obtained from the patients and their family members. The patient demographics for those included in the study are summarized in Table 1. There were two female patients and 26 male patients, with a mean age of 53.4 years (range, 38-65 years). LDLT was performed in 28 patients as left lobe (n = 6), right lobe (n = 18), and dual LDLT from different donors (n = 4). Radiological diagnostic imaging studies and elevation of the alpha fetoprotein (AFP) values were used to establish the r-HCC diagnosis; diagnostic imaging studies included ultrasound (US), computed tomography (CT), and angiography. CT of the chest, abdomen, and pelvis was performed in each patient before TACE treatment to detect extrahepatic disease.
Table 1.
Demographic Data of Study Patients Prior to LDLT
Note.-LDLT = living donor liver tranplantation; TACE = transcatheter arterial chemoembolization; RFA = radiofrequency ablation; PEI = percutaneous ethanol injection
*Omental seeding was diagnosed only in pathology.
Tumors exceeded the Milan criteria in the explanted liver in 153 of the 252 patients (60.7%) diagnosed with HCC before LDLT and in 29 of the 42 patients (69.0%) with recurrent HCC (12). Tumors in the explanted liver of 19 of the 28 study patients (67.9%) also exceeded the Milan criteria.
Patterns of Recurrent Hepatoccellular Carcinoma
The characteristics of r-HCC are summarized in Table 2. The median time from liver transplantation to detection of r-HCC was 15 months (range, 2-57 months). TACE was performed within one year, between one and three years, between two and three years, or more than three years after LDLT in 16 (57.1%), seven (25.0%), four (14.3%), or one (3.6%) patient, respectively. Nine of 26 patients with intrahepatic recurrence had solitary nodular r-HCC (Fig. 1), while the remaining 17 patients had multiple r-HCC (Fig. 2). Eighteen study patients (64.3%) were initially diagnosed with r-HCC extrahepatic metastasis, of which lung metastasis was the most common followed by bone and peritoneal metastases (Table 2). AFP values were greater than 400 ng/ml in 18 of 28 patients (64.3%).
Table 2.
Characteristics of r-HCC after LDLT
Note.-r-HCC = recurrent hepatocellular carcinoma; LDLT = living donor liver tranplantation
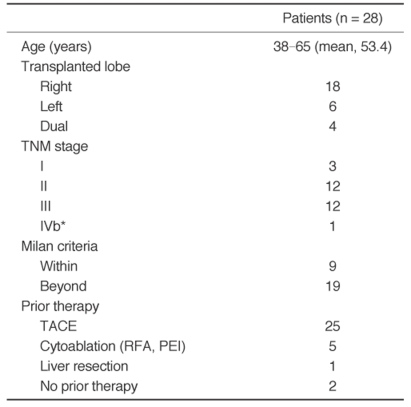
Fig. 1.
A.Contrast-enhanced arterial-phase axial CT image of the liver 17 months after living donor liver transplantation shows the single arterial enhancing mass (arrow) in the posterior portion of the transplanted liver.
B.Arteriogram from the celiac trunk (late arterial phase) shows an area of tumor blush in the transplanted liver (arrow).
C.Arteriogram from the celiac trunk immediately after embolization shows that the tumor blush has disappeared.
D.Contrast-enhanced arterial-phase axial CT image of the liver one month after transcatheter arterial chemoembolization reveals complete iodized oil accumulation (arrow) in the tumor. We interpreted this patient as having CR.
E.Contrast-enhanced arterial-phase axial CT image of the liver 22 months after transcatheter arterial chemoembolization shows a decrease in the size of the recurrent hepatocellular carcinoma with iodized oil accumulation.
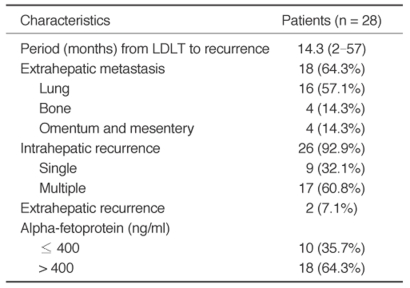
Fig. 2.
A.Contrast-enhanced delayed-phase axial CT image of a transplanted liver six months after living donor liver transplantation, with the left lobe showing multiple recurrent hepatocellular carcinomas in the entire transplanted liver; however, blood flow is preserved in the portal vein.
B.Arteriogram from the celiac trunk (late arterial phase) shows multiple hypervascular tumors in the entire transplanted liver.
C.Contrast-enhanced arterial-phase axial CT image of the transplanted liver one month after transcatheter arterial chemoembolization shows progression of the recurrent hepatocellular carcinomas in both size and number. Some nodules show partial accumulation of iodized oil. We interpreted this patient as having progressive disease.
Transcatheter Arterial Chemoembolization
The tumor size and number, the feeding arteries, and the degree of portal venous thrombosis were evaluated by celiac and superior mesenteric angiography. TACE was performed after a 3 Fr microcatheter (MicroFerret, Cook Inc, Bloomington, IN) was advanced into the feeding artery. In the first procedure, cisplatin (Cisplan, Dong-A, Seosan, Korea) was infused into the hepatic artery for 15 minutes without embolic particle administration. A mixture of iodized oil (Lipiodol, Laboratoire Guerbet, Cedex, France) and cisplatin was then injected into the feeding arteries followed by embolization with gelatin sponge (Spongostan, Pharmacia and Upjohn, Kalamazoo, MI); the infused dose of cisplatin was 2 mg/Kg of the patient's weight. The injected dose of iodized oil depended on the tumor size, ranging from 1 to 10 ml (mean: 4.6 ml), and the gelatin sponge consisted of cubes measuring 1-2 mm.
Transcatheter arterial chemoembolization was performed in a lobar artery or a segmental artery in 26 patients with intrahepatic recurrence. TACE was performed in the left gastric artery and in the right inferior phrenic and right intercostal arteries in two patients with extrahepatic recurrence. One of these two patients had an extrahepatic r-HCC attached to the posterior portion of the transplanted liver that was supplied by right inferior phrenic and right intercostal arteries (Fig. 3). The other patient had r-HCC in the posterior portion of the left lateral segment of the transplanted liver that was supplied by left gastric artery. The included patients underwent 1-5 cycles of TACE (mean, 2.5 cycles per patient) during the follow-up period.
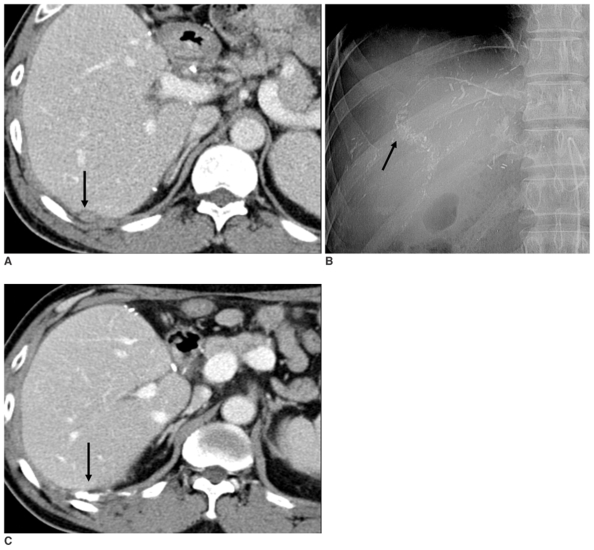
Fig. 3.
A.Contrast-enhanced delayed-phase axial CT image of the liver after living donor liver transplantation reveals a small nodule in the extrahepatic space of the transplanted liver (arrow). This patient had an elevated alpha fetoprotein level at that time.
B.Digital image of the intercostal and right inferior phrenic arteries after transcatheter arterial chemoembolization shows subtle accumulation of iodized oil (arrow).
C.Contrast-enhanced arterial-phase axial CT image of a transplanted liver one month after transcatheter arterial chemoembolization shows completed accumulation of iodized oil in the nodule (arrow). This patient's alpha fetoprotein level decreased after transcatheter arterial chemoembolization.
Follow-up
Tumor response was evaluated based on CT studies performed 1-3 months after TACE and was classified into five grades as follows: complete response (CR), total disappearance of the tumor (Figs. 1, 3); partial response (PR), reduction of 50% or more in maximum tumor size on CT images; minimal response (MR), reduction of 25-50%; stable disease (SD), change of < 25% in tumor size; and progressive disease (PD), increase of 25% or more in tumor size (Fig. 2) (13). Tumor size was measured by electronic calipers as the maximum perpendicular diameters of each tumor (a, b). The following formula was used to calculate the change in total tumor size:
Percentage change in tumor size = 100 * Σ(a*b-a'*b')/Σ(a*b)
where a*b represents the product of the longest perpendicular diameters of each tumor before treatment, and a'*b' is the product of the tumor diameters after treatment.
Changes in AFP values one month after TACE were also evaluated in patients with abnormal AFP values at the time of TACE.
Statistical Analysis
The estimated survival rates were calculated using the Kaplan-Meier method.
RESULTS
Targeted lesion tumor response to TACE.
The tumor response of the targeted lesion to TACE was summarized in Table 3. Iodized oil accumulated in the main tumor in all patients, but complete response corresponding to total disappearance of the tumor was only observed in three patients on follow-up CT (Figs. 1, 3). Although complete or partial response of the targeted lesions to TACE was observed in 14 of 28 patients (50.0%), 21 of these 28 patients (75.0%) had another r-HCC during the 3-month follow-up period, and 26 of the 28 patients (92.9%) manifested another r-HCC in an intrahepatic or extrahepatic location during the 6-month follow-up period. Six of 28 patients experienced progressive disease (increased tumor size) despite TACE treatment. Two of three patients still living at the time of writing have been in complete remission for 22 and eight months, respectively, since the initial TACE therapy (Figs. 1, 3).
Table 3.
Targeted Lesion Tumor Response to TACE
The AFP levels of eight of 18 patients with abnormal baseline AFP levels (> 400 ng/ml) had decreased one month after TACE. Ten of the 18 patients with elevated AFP levels had another intrahepatic recurrence or progressive extrahepatic recurrence.
Overall, the TACE procedure was well tolerated by all patients, and no major complications developed during follow-up period. Five patients (17.9%) experienced some immediate side effects after TACE, including transient nausea, vomiting, diarrhea, hypertension, tachycardia, and right upper quadrant pain, but these effects resolved within a few days of the procedure.
Survival
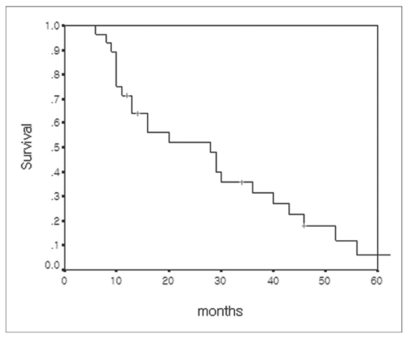
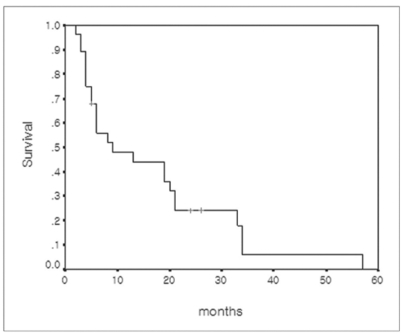
Survival was measured from the date of LDLT, r-HCC diagnosis after LDLT, or TACE treatment until death or until December 2005. At the time of this writing, 25 patients have died and three are still living. The causes of deaths were pneumonia and sepsis (n = 18), hepatic failure (n = 5), and organ failure due to extensive metastasis (n = 2). The actual 1-, 3-, and 5-year survival rates after LDLT were 71.4%, 31.5%, and 6.0%, respectively (Fig. 4). The estimated median survival after LDLT in the included patients was 28 months (95% CI, 12.4-43.5 months). The actual 1-, 3-, and 5-year survival rates after TACE for r-HCC were 47.9%, 6.0%, and 0%, respectively (Fig. 5). The estimated median survival after TACE for r-HCC was nine months.
Fig. 4.
Overall cumulative survival curve of 28 patients with recurrent hepatocellular carcinoma calculated from the time of living donor liver transplantation.
Fig. 5.
Cumulative survival curve of 28 patients with recurrent hepatocellular carcinoma calculated from the time of recurrent hepatocellular carcinoma diagnosis.
DISCUSSION
Systemic chemotherapy, surgical resection and nonsurgical treatments, such as TACE and ablation, are performed to treat r-HCC after LDLT (10). Few previous studies have evaluated the tumor responses to various treatment modalities in r-HCC after liver transplantation. In particular, to our knowledge, there were no reports regarding TACE treatment of r-HCC after LDLT. However, the targeted lesion tumor response in the present study was the same or more favorable compared with other studies on HCC before transplantation. In many previous studies, TACE achieved a partial response or better in 15%-55% of patients with HCC before transplantation (14-17). Ebied et al. studied 72 patients with biopsy-proven, unresectable HCC and focused on 186 individual tumor masses. The patients were classified as responders or non-responders based on CT evidence of altered tumor size and tumor necrosis. Thirty-eight patients (47.2%) were responders and 34 were nonresponders. In the present study, 28 patients with r-HCC underwent TACE. A favorable tumor response in the targeted lesion (partial response or better) was observed in 50% of patients after TACE.
Nevertheless, favorable tumor response in the targeted lesion did not reflect favorable survival, and the survival rate in the present study was similar to other reports. Recurrence of HCC after liver transplantation clearly has a major impact on outcome. The five-year survival was reported to be significantly lower for patients with recurrence (22%) than for patients without recurrence (64%) (p < 0.0001) (18); the median survival was around nine months after the diagnosis of recurrence. Shorter time to recurrence and the presence of bone metastases were the main factors significantly associated with poorer prognosis once recurrence manifested (18). In another series, however, 18 of 57 patients with r-HCC underwent potentially curative treatments such as surgical resection or radiofrequency ablation. The five-year post-transplant survival among these patients was 47% (10). This is consistent with the 57% survival at four years reported elsewhere for similar patients (9). However, it is impossible to determine from these studies whether surgical treatment of recurrence prolongs survival or whether patients who are amenable to surgical treatment are a more favorable group. In the present study, 26 of 28 patients had unresectable r-HCC. The mean survival periods were 28 months overall and nine months after diagnosis of r-HCC. And the actual 1-, 3-, and 5-year survival rates after LDLT were 71.4%, 31.5%, and 6.0%, respectively, in this study. These mean survival values were similar to or lower than those of the studies mentioned previously. We believe that this resulted from the inclusion of patients with advanced and unresectable r-HCC at the time of r-HCC diagnosis, as mentioned above.
The small benefit to survival afforded by TACE, in spite of favorable tumor response to targeted r-HCC, may be caused by the aggressiveness of the tumor, which is indicated by the high rate of extrahepatic recurrence and rapid tumor recurrence in the remaining liver after initial TACE treatment. Early reports suggested that the course of recurrent HCC after transplant is more aggressive than that of recurrence after hepatic resection, presumably due to immune suppression (19). This has not been borne out in subsequent studies, however (20). Nevertheless, the drugs used to prevent transplant rejection have a variety of effects that go beyond immune suppression (10).
In this study, extrahepatic metastasis were observed in 18 of 28 (64.3%) included patients. In another study, 48 of 57 patients (84.2%) with r-HCC after liver transplantation had extrahepatic metastases (18). These patterns of metastases in patients with r-HCC after liver transplantation differ from those in patients with primary HCC without liver transplantation. In one study, for example 65 patients (13.5%) had extrahepatic metastases among 482 patients who were diagnosed with HCC (14). There is no standard treatment for extrahepatic metastases, and few previous reports exist regarding its treatment (21). In the study by Chung et al., 68 HCC patients with major portal vein thrombosis (n = 47) or distant metastasis (n = 27) were randomly allocated to groups with or without intra-arterial Cisplatin infusion. The group receiving intra-arterial Cisplatin infusion had a significantly higher 1-year survival rate than the other group (22). We also performed intra-arterial Cisplatin infusion before embolization with iodized oil and gelatin sponge to treat intrahepatic r-HCC as well as extrahepatic metastasis.
There are limitations of the present study. Most of the included patients had extrahepatic recurrence as well as intrahepatic recurrence, and systemic chemotherapy was not controlled. A controlled study is needed for further evaluation of TACE with regard to its effect on the survival of patients with r-HCC after LDLT.
In conclusion, TACE appears to produce an effective tumor response in targeted r-HCC after LDLT. However, survival of patients with r-HCC after LDLT is poor due to extrahepatic metastasis and additional intrahepatic recurrence.
Acknowledgement
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2007.
Abbreviations
- TACE
transcatheter arterial chemoembolization
- r-HCC
recurrent hepatocellular carcinoma
- LDLT
living donor liver transplantation
- AFP
alpha fetoprotein
- US
ultrasound
- CT
computed tomography
- CR
complete response
- PR
partial response
- MR
minimal response
- SD
stable disease
- PD
progressive disease
References
- 1.Kaihara S, Kiuchi T, Ueda M, Oike F, Fujimoto Y, Ogawa K, et al. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75:S37–S40. doi: 10.1097/01.TP.0000047029.02806.16. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 3.O'Grady JG, Polson RJ, Rolles K, Calne RY, Williams R. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg. 1988;207:373–379. doi: 10.1097/00000658-198804000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–285. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 5.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan KC, Rela M, Ryder SD, Rizzi PM, Karani J, Portmann B, et al. Experience of orthotopic liver transplantation and hepatic resection for hepatocellular carcinoma of less than 8 cm in patients with cirrhosis. Br J Surg. 1995;82:253–256. doi: 10.1002/bjs.1800820239. [DOI] [PubMed] [Google Scholar]
- 7.Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–459. doi: 10.1097/01.sla.0000137129.98894.42. discussion 459-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gondolesi GE, Roayaie S, Munoz L, Kim-Schluger L, Schiano T, Fishbein TM, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142–149. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, et al. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998;5:29–34. doi: 10.1007/pl00009947. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M, Roayaie S, Llovet J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J Hepatol. 2005;43:584–589. doi: 10.1016/j.jhep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Margarit C, Charco R, Hidalgo E, Allende H, Castells L, Bilbao I. Liver transplantation for malignant diseases: selection and pattern of recurrence. World J Surg. 2002;26:257–263. doi: 10.1007/s00268-001-0214-1. [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 13.Atiq OT, Kemeny N, Niedzwiecki D, Botet J. Treatment of unresectable primary liver cancer with intrahepatic fluorodeoxyuridine and mitomycin C through an implantable pump. Cancer. 1992;69:920–924. doi: 10.1002/1097-0142(19920215)69:4<920::aid-cncr2820690414>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Ebied OM, Federle MP, Carr BI, Pealer KM, Li W, Amesur N, et al. Evaluation of responses to chemoembolization in patients with unresectable hepatocellular carcinoma. Cancer. 2003;97:1042–1050. doi: 10.1002/cncr.11111. [DOI] [PubMed] [Google Scholar]
- 18.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095–2100. doi: 10.1002/1097-0142(19911115)68:10<2095::aid-cncr2820681002>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steininger R, Herbst F, Fugger R, Muhlbacher F, Fritsch A. Immunosuppression does not enhance tumor growth after orthotopic liver transplantation for hepatoma. Transplant Proc. 1992;24:2690–2692. [PubMed] [Google Scholar]
- 21.Ishii H, Furuse J, Kinoshita T, Konishi M, Nakagohri T, Takahashi S, et al. Extrahepatic spread from hepatocellular carcinoma: who are candidates for aggressive anti-cancer treatment? Jpn J Clin Oncol. 2004;34:733–739. doi: 10.1093/jjco/hyh135. [DOI] [PubMed] [Google Scholar]
- 22.Chung YH, Song IH, Song BC, Lee GC, Koh MS, Yoon HK, et al. Combined therapy consisting of intraarterial cisplatin infusion and systemic interferon-alpha for hepatocellular carcinoma patients with major portal vein thrombosis or distant metastasis. Cancer. 2000;88:1986–1991. [PubMed] [Google Scholar]