Abstract
Primary hepatic carcinosarcoma is a rare tumor comprised of a mixture of carcinomatous and sarcomatous elements. Less than 20 adequately documented cases have been reported, however the imaging features of two cases were briefly described. We present here a case of carcinosarcoma of the liver in a 46-year-old woman, which was confirmed based on pathology. Imaging showed a large mass with large necrotic portions, small cystic portions, calcifications and bone formations.
Keywords: Liver neoplasms; Liver neoplasms, CT
Carcinosarcoma is a rare tumor comprised of a mixture of carcinomatous and sarcomatous elements that may occur in any organ (1). Primary hepatic carcinosarcoma is rare, with less than 20 adequately documented cases reported (1-9). However, the imaging features of two of these cases were briefly described (2, 3). We present here a case of carcinosarcoma of the liver with cholangiocarcinomatous elements and osteosarcomatous and chondrosarcomatous elements.
CASE REPORT
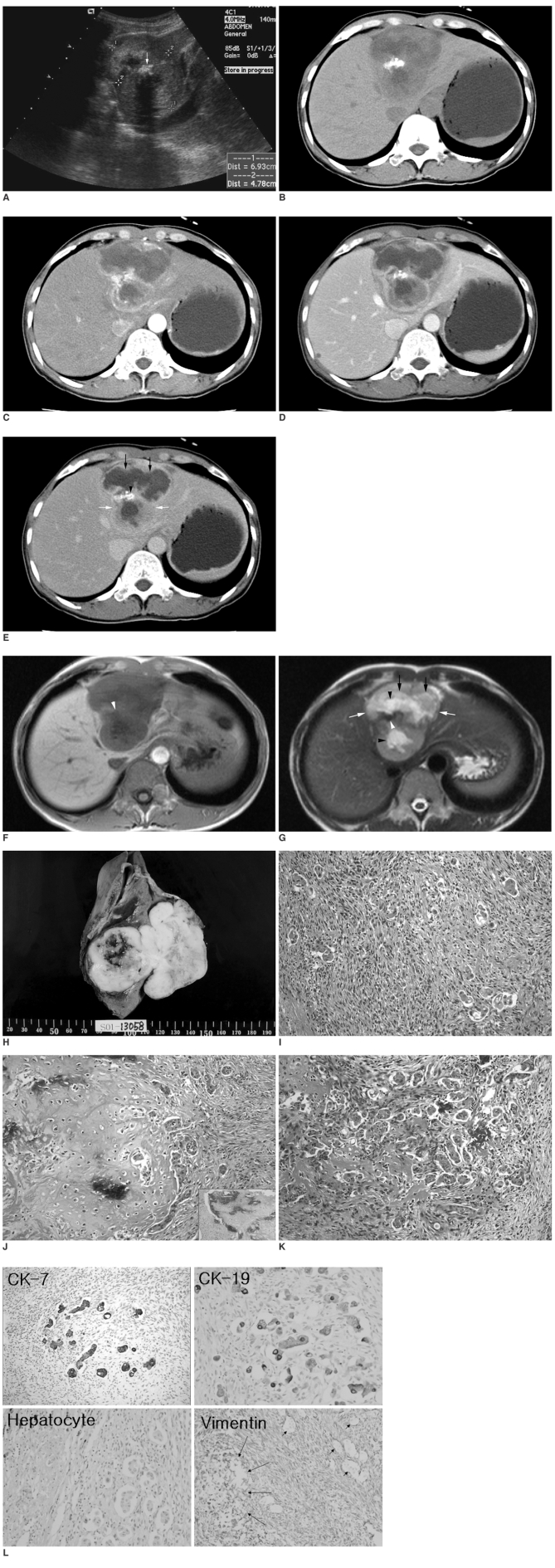
A 46-year-old woman was referred after having general weakness for one month. Biochemical indices of liver function were normal. The tumor markers revealed the following: alpha-fetoprotein level, 2.1 ng/mL (< 9.6 ng/mL); carcinoembryonic antigen level, 1.5 ng/mL (< 5.0 ng/mL); serum carbohydrate antigen 19-9 level, 13.2 U/mL (< 39 U/mL). Serum markers for hepatitis B and C were negative. Ultrasonography showed a lobulate heterogeneous echogenic solid mass with a highly echogenic lesion with posterior acoustic shadowing within the mass in the left lobe of the liver (Fig. 1A). Computed tomographic analysis of the mass revealed peripheral enhancing viable portions, large internal non-enhancing necrotic portions, and a dense radiopaque lesion (Figs. 1B-E). The enhancing portion of this mass was hypoattenuating in the precontrast scan (Fig. 1B), hyperattenuating in the arterial phase (Fig. 1C), hypoattenuating in the portal phase (Fig. 1D), and isoattenuating in the equilibrium phase (Fig. 1E). Upon magnetic resonance imaging, this mass was observed to be hypointense on the T1-weighted image (Fig. 1F) and hyperintense on the T2-weighted image (Fig. 1G). On the T2-weighted images, peripheral viable portions were slightly hyperintense, internal necrotic portions were moderately hyperintense, and small central cystic portions were very hyperintense. Calcification and ossification had low or dark signal intensity on the T1- and T2-weighted images (Figs. 1F, G).
Fig. 1.
A. Longitudinal ultrasonography through the left liver showed a lobulate heterogeneous echogenic solid mass (cursors) with a highly echogenic lesion (arrow) that had posterior acoustic shadowing within it.
B-E. Computed tomographic scan of this mass revealed peripheral enhancing viable portions (white arrows), large internal non-enhancing necrotic portions (black arrows), and a dense radiopaque lesion (arrowhead). The enhancing portion of this mass is hypoattenuating in precontrast scan (B), hyperattenuating in arterial phase (C), hypoattenuating in portal phase (D), and isoattenuating in equilibrium phase (E).
F-G. Upon magnetic resonance imaging this mass is hypointense on the T1-weighted image (F) and hyperintense on the T2-weighted image (G). On the T2-weighted images, peripheral viable portions (white arrows) are slightly hyperintense, necrotic portions (black arrows) are moderately hyperintense, and small cystic portions (black arrowheads) are very hyperintense. Calcification and ossification have low or dark signal intensity (white arrowheads) on T1- and T2-weighted images.
H. A section of gross specimen showing a well-demarcated, dumbbell-shaped, multilobulate, grayish white, solid and firm mass with a central area of necrosis and hemorrhage, measuring 8.0 cm in its greatest dimension.
I. The tumor mass was composed of diffusely proliferating spindle-shaped atypical cells and scattered epithelial cell clusters (Hematoxylin & Eosin staining, × 200).
J. Large atypical pyknotic cells embedded in a hyaline chondroid matrix were detected mixed with adjacent anaplastic tubular epithelial clusters and anaplastic spindle cells (Hematoxylin & Eosin staining, × 200) (Inset). Calcification within the cartilage component was occasionally detected (Hematoxylin & Eosin staining, × 100).
K. Transitional features of atypical spindle and epithelial cells are seen with an irregular anastomosing reddish osteoid matrix (Hematoxylin & Eosin staining, × 200).
L. The epithelial component was positive for cytokeratin 7 and 19 (cholangiocytic differentiation markers), but negative for hepatocyte (hepatocytic differentiation marker). Atypical spindle cells and cartilaginous components (large arrow) were all positive for vimentin (mesenchymal differentiation marker), however the scattered epithelial clusters were negative for vimentin (small arrows).
A provisional diagnosis of primary pleomorphic mesenchymal sarcoma was made and no preoperative treatments were performed (e.g. transcatheter arterial chemoembolization or hepatic arterial infusion therapy of chemotherapeutic agents). The patient underwent a left hemihepatectomy with the expectation of achieving a curative resection. No extrahepatic disease was seen during the laparotomy.
The resected left lobe of liver measured 19.0 × 12.0 × 10.0 cm and weighed 433.0 gm. There was a large exophytic tumor mass elevating falciform ligament. The cut surface showed a well-demarcated, dumbbell-shaped, multilobulate, grayish white, solid and firm mass, measuring 8.0 cm at its greatest dimension. An area of hemorrhage and necrosis was detected in the central portion of the intrahepatic tumor mass. The central area of the extrahepatic fungating tumor mass also showed necrosis and myxoid microcystic change. The peripheral portion of both the intra- and extrahepatic tumor mass had a pale tan, solid and firm appearance (Fig. 1H). The surrounding parenchyma showed no cirrhotic change.
The major component of the viable tumor was the sarcomatous component, which was mostly characterized by a diffuse proliferation of spindle-shaped atypical cells. A few epithelial cell clusters were scattered in a background of spindle cells (Fig. 1I). Chondrosarcomatous change and large atypical pyknotic cells embedded in a hyaline chondroid matrix were observed together with adjacent anaplastic tubular epithelial clusters and anaplastic spindle cells. Highly atypical spindle and epithelial cells were intermingled with an irregular anastomosing reddish osteoid matrix. Chondrosarcomatous or osteosarcomatous changes showed focal calcification or bone formation (Fig. 1J). Transitional features from poorly differentiated carcinoma to sarcomatous cells were seen in a limited area (Fig. 1K). Surrounding non-tumorous liver parenchyma showed non-cirrhotic change.
Most spindle cells, including multinuclear giant cells, were positively stained by vimentin, however the scattered epithelial clusters were negative for vimentin. These epithelial cells were strongly positive for cytokeratin 7 and 19 as well as pan-cytokeratin and epithelial membrane antigen (EMA). However, hepatocyte antibody was not stained in any epithelial tumor cells. Transitional features of carcinomatous elements and sarcomatous elements were present in a limited area, and these were positive for both vimentin and cytokeratin (Fig. 1L).
One year later, the patient had a local recurrence with abdominal wall invasion, which was followed by death 10 months later due to disseminated malignancy.
DISCUSSION
Carcinosarcoma has numerous synonyms, including spindle cell carcinoma, pseudosarcoma, polypoid carcinoma, sarcomatoid carcinoma, and spindle cell variants of other usual carcinomas. Confusing terminology used to describe carcinosarcoma of the liver has caused uncertainty regarding the characteristics of these tumors. However, the World Health Organization (WHO) has defined carcinosarcomas as tumors containing both carcinomatous (either hepatocellular or cholangiocellular) and sarcomatous elements, including malignant mixed tumors (4). If the sarcomatous area with malignant epithelial components were composed of variable malignant mesenchymal components, such as chondrosarcoma or osteosarcoma, it would be considered a carcinosarcoma. However, if the sarcomatous area were composed of only malignant spindle cells, and if these areas of malignant spindle cells were shown to have epithelial characteristics based on both immunohistochemical stains and electron microscopic findings, the tumor mass would be diagnosed as sarcomatoid carcinoma or spindle cell carcinoma. In addition, if hepatic differentiation of tumor cells were demonstrated by immunohistochemical staining, it would be categorized as sarcomatoid hepatocellular carcinoma. Furthermore, if cholangiocytic differentiation of tumor cells were detected, the tumor would be classified as a sarcomatoid cholangiocarcinoma.
In this case, histologically, the carcinomatous elements were cholangiocarcinoma and the sarcomatous elements were osteosarcoma and chondrosarcoma. A tumor metastasizing to the liver from other organs was excluded based on findings from the operation. Therefore, according to the WHO definition, we consider our case to be a carcinosarcoma arising in the liver. In our case, transitional areas were observed between the carcinomatous and sarcomatous elements. Immunohistochemically, cells from the carcinomatous element expressed cytokeratin but not vimentin, whereas cells from the sarcomatous elements showed the opposite staining pattern. Cells from transitional areas expressed both cytokeratin and vimentin. These tumors could be distinguished from collision tumors and carcinoma with foci of spindled epithelial cells based on the presence of transitional areas and by the immunohistochemical findings, respectively (5, 6).
The histogenesis of carcinosarcomas of the liver has been the subject of debate, however. Fayyazi et al. speculated that this tumor originates from a single totipotential stem cell that differentiates in separate epithelial and mesenchymal directions (7). However, recently, the most likely explanation for the pathogenesis of carcinosarcoma is that a carcinoma undergoes metaplastic transformation and thereby develops a sarcomatous component. This has been described as a conversion tumor (2).
The outcome of patients with primary hepatic sarcoma depends primarily on tumor histology and the ability to achieve complete tumor resection. The prognosis for patients with primary hepatic carcinosarcoma is worse than for the majority of patients with hepatic sarcoma, however this excludes angiosarcoma. Early tumor stages are usually associated with improved survival because surgical resection is a treatment option. Detection and removal of this type of tumor during the early stages of its development was the only strategy to improve survival in view of the aggressive characteristics and poor prognosis observed in our patient (8, 9).
The presence of dense radiopaque lesions within the mass suggested calcifications or bone formations, however, no imaging modality could differentiate calcifications from ossifications. Malignant hepatic tumors have a wide spectrum of calcification patterns, varying from fine granular, single or multiple punctuate calcifications to coarse and conglomerate calcifications (10). However, if dense rocky calcifications or bone formations are seen within the mass, this finding may suggest chondrosarcomatous or osteosarcomatous components.
In conclusion, the imaging findings of hepatic carcinosarcoma presented in this report showed a large mass with large necrotic portions, small cystic portions, calcifications and bone formations. Although, from a clinical standpoint, establishing an accurate preoperative diagnosis is difficult, carcinosarcoma should be included in the differential diagnosis of a large necrotic hepatic mass, especially if rocky calcifications or bone formations are seen.
References
- 1.Freeman AJ, Bullpitt P, Keogh GW. Primary hepatic carcinosarcoma. ANZ J Surg. 2004;74:1021–1023. doi: 10.1111/j.1445-1433.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- 2.Sumiyoshi S, Kikuyama M, Matsubayashi Y, Kageyama F, Ide Y, Kobayashi Y, et al. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809–812. doi: 10.3748/wjg.v13.i5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rummeny E, Weissleder R, Stark DD, Saini S, Compton CC, Bennett W, et al. Primary liver tumors: diagnosis by MR imaging. AJR Am J Roentgenol. 1989;152:63–72. doi: 10.2214/ajr.152.1.63. [DOI] [PubMed] [Google Scholar]
- 4.Nomura K, Aizawa S, Ushigome S. Carcinosarcoma of the liver. Arch Pathol Lab Med. 2000;124:888–890. doi: 10.5858/2000-124-0888-COTL. [DOI] [PubMed] [Google Scholar]
- 5.Wang XW, Liang P, Li HY. Primary hepatic carcinosarcoma: a case report. Chin Med J. 2004;117:1586–1587. [PubMed] [Google Scholar]
- 6.Leger-Ravet MB, Borgonovo G, Amato A, Lemaigre G, Franco D. Carcinosarcoma of the liver with mesenchymal differentiation: a case report. Hepatogastroenterology. 1996;43:255–259. [PubMed] [Google Scholar]
- 7.Fayyazi A, Nolte W, Oestmann JW, Sattler B, Ramadori G, Radzun HJ. Carcinosarcoma of the liver. Histopathology. 1998;32:385–387. doi: 10.1046/j.1365-2559.1998.0401j.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcez-Silva MH, Gonzalez AM, Moura RA, Linhares MM, Lanzoni VP, Trivino T. Carcinosarcoma of the liver: A case report. Transplant Proc. 2006;38:1918–1919. doi: 10.1016/j.transproceed.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 9.Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, et al. Management of primary liver sarcomas. Cancer. 2007;109:1391–1396. doi: 10.1002/cncr.22530. [DOI] [PubMed] [Google Scholar]
- 10.Stoupis C, Taylor HM, Paley MR, Buetow PC, Marre S, Baer HU, et al. The Rocky liver: radiologic-pathologic correlation of calcified hepatic masses. RadioGraphics. 1998;18:675–685. doi: 10.1148/radiographics.18.3.9599391. [DOI] [PubMed] [Google Scholar]