Abstract
A 36-year-old man was diagnosed with a right temporal lobe grade II cerebral arteriovenous malformation (cAVM) and was treated with radiosurgery. At nine months after the cAVM radiosurgery, the patient began to develop bilateral focal narrowing at the M1 segments of the bilateral middle cerebral arteries. The narrowing progressively deteriorated as was demonstrated on longitudinal serial follow-up MR imaging. X-ray angiography performed at 51 months after radiosurgery confirmed that the cAVM was cured and a diagnosis of moyamoya disease. To the best of our knowledge, this is the first case of cAVM-associated moyamoya disease that developed after radiosurgery. Given the chronological sequence of disease development and radiation dose distribution of radiosurgery, it is proposed that humoral or unknown predisposing factors, rather than direct radiation effects, are the cause of moyamoya disease associated with cAVM.
Keywords: Cerebral arteriovenous, malformation; Moyamoya disease; Radiosurgery
Although a relatively uncommon vascular disorder, a cerebral arteriovenous malformation (cAVM) is the major cause of hemorrhagic stroke in young and middle-aged patients, second only to the rupture of arterial aneurysms. A variety of cAVM related dysplastic phenomena affecting the feeding arteries and draining veins, including flow-related aneurysms, arterial stenosis and occlusion as well as and venous stenosis, stricture and occlusion, have been reported (1, 2). Among these features, an association of a cAVM and moyamoya disease has been rarely observed. Moreover, the longitudinally sequential development of a cAVM and moyamoya disease association is also extremely rare.
Gamma-knife radiosurgery (GKRS) is an established treatment alternative for cAVM with clearly defined benefits and risks. The risks include adverse radiation effects and a risk of intracranial hemorrhage that develops during the latency period after GKRS (3). We present here a case of moyamoya disease that developed after cAVM radiosurgery. To the best of our knowledge and from a literature review, this is the first report of cAVM-associated moyamoya disease developed after radiosurgery.
CASE REPORT
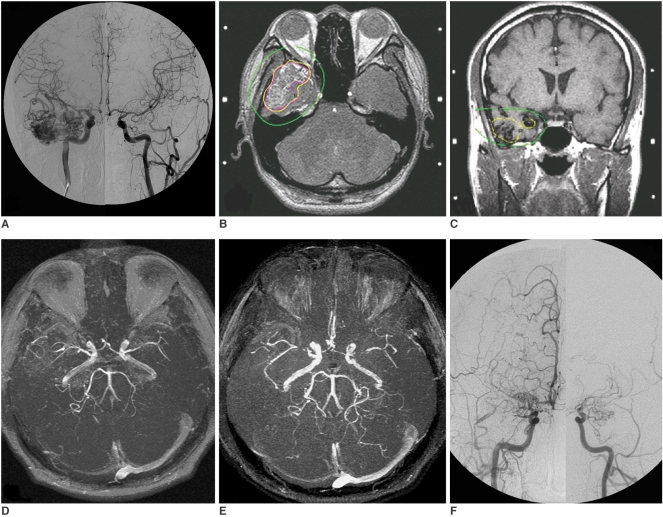
A 36-year-old man suffered from intermittent episodes of dizziness and complex visual hallucinations for 2-3 years of irregular duration and interval. Due to a newly developed numbness sensation over the right temporal region for one month, the patient arrived at our hospital and asked for assistance. No loss of consciousness was found for each attack and the patient was neurologically intact. The cerebral angiography (Fig. 1A) showed a Spetzler grade II cAVM located at the right anterior temporal lobe that received an arterial supply mainly from the anterior temporal branches of the right middle cerebral artery (MCA) and the right posterior cerebral artery (PCA) and showed early draining mainly into the right superficial middle cerebral vein. No focal stenosis of the intracrainal vessels was found at the initial presentation. The patient underwent GKRS with a maximum target dose of 31.25 Gy and a minimum dose of 17.5 Gy to the periphery of the cAVM nidus at a 56% isodose level of the maximum target dose (average dose of 23.6 Gy). The cAVM nidus volume was estimated as 20.7 ml. The supraclinoid segment of the right internal carotid artery and proximal MCA and anterior cerebral artery (ACA) received as dose less than 6.25 Gy (20% isodose level) (Figs. 1B, C). The arteries on the contra-lateral side received far less than this radiation dose.
Fig. 1.
Moyamoya disease and concurrent cerebral arteriovenous malformation in 36-year-man.
A. Composite anteroposterior (AP) view of bilateral carotid angiogram shows right temporal cerebral rteriovenous malformation.
B, C. Stereotactic MRI with dose plan show that 17.5 Gy (yellow isodose line) is prescribed to perihphery of cerebral arteriovenous malformation nidus (magenta line). Supraclinoid segment of right internal carotid arteries and proximal middle cerebral artery received dose of 6.25 Gy (green isodose line).
D, E. Nine months (D) and 34 months (E) after gamma-knife surgery. Time-of-light (TOF) MR angiograms show progressive focal stenosis at bilateral middle cerebral artery.
F. At 51 months after gamma-knife radiosurgery, bilateral carotid angiogram confirms cure of cerebral arteriovenous malformation and occlusion of bilateral supraclinoid internal carotid arteries, proximal middle cerebral artery and anterior cerebral artery with moyamoya collateral vessels.
Continuous regression of the cAVM was observed on serial follow-up brain MRI. Clinically the frequency of seizure attacks decreased. However, focal narrowing at the M1 segments of the bilateral MCA was found on follow-up brain MR angiograms obtained at nine months and 34 months after radiosurgery (Figs. 1D, E) with progressive deterioration. The patient suffered from near-fainting and slurred speech at 51 months after GKRS. Acute infarcts at the right corona radiatae were found on brain MRI. A cerebral angiogram (Fig. 1F) confirmed cure of the right anterior temporal cAVM and the presence of moyamoya disease with occlusion of the bilateral supraclinoid internal carotid arteries (ICA), bilateral M1 segments of the MCA and bilateral A1 segments of the ACA and collateral vessels in the bilateral basal ganglia.
DISCUSSION
To the best of our knowledge and from a review of the clinical literature, only 14 cases (mean age, 34 years; age range, 11-50 years; male to female ratio 1:1) of combined cAVM and bilateral moyamoya disease has been reported (1, 4-9). Most of the cases (12 cases) had both neurovasuclar anomalies at the first presentation (1, 4-7). Another two cases had de novo development of a cAVM at a nine years and four years follow-up of moyamoya disease (8, 9). In two large retrospective reviews of occlusive vascular disease associated with cAVMs, twenty cases of cAVM with occlusion or focal stenosis of the major feeding arteries of a cAVM were reported, respectively accounting for 3% and 1.3% in each patient group (1, 2). Nine out of the 20 cases had a unilateral supraclinoid ICA occlusion with a variety of moyamoya collaterals.
According to the reports mentioned above, two plausible hypotheses were proposed to describe the pathogenesis and relationship between an associated cAVM and moyamoya disease. First, it was proposed that the intimal layers of feeding arteries proliferated to accommodate the high-flow stress that stemmed from a cAVM. The inherited protective mechanism eventually resulted in total occlusion of the supraclinoid ICA and the accompanying changes in moyamoya collateral vessels (1, 7). It was also speculated that the blood demand of brain tissue in moyamoya disease might reactivate the pre-quiescent angiogenesis process and finally contribute to a de novo anomalous arterio-venous connection (8, 9). For both hypotheses, angiogenetic factors might play an important role. One may speculate that the radiation delivered in GKRS might induce the development of moyamoya disease. However, as shown in Figures 1B and C, the occluded right supraclinoid ICA, ACA and MCA received approximately a dose of 6.25 Gy during GKRS and the arteries in the contra-lateral side received far less than this dose. It is reasonable to speculate that the humoral angiogenetic factors or unknown predisposing factors from the irradiated cAVM may have been involved in the final development of moyamoya disease in this patient. Apart from the two cases of acquired unilateral moyamoya syndrome after cAVM radiosurgery that were presented at the 1997 Annual Meeting of the Congress of Neurological Surgeons, this is the first report of cAVM-associated moyamoya disease that developed after radiosurgery (10).
Successful staged bypass surgery and radiosurgery for patients with concurrent cAVM and moyamoya disease was reported by Seol et al. (6). Our reported patient developed moyamoya disease after cAVM GKRS. It seems simplified that these two anomalies could be managed in two separate stages. However, the question about which disorder should be treated first for patients with a concurrent cAVM and moyamoya disease might be answered by the following hypothesis. Radiosurgery mitigates the hypoperfusion in the adjacent brain tissues of cAVM by the steady and progressive obliteration of cAVM nidus while avoiding the risk of sudden interruption of the flimsy moyamoya vessels. However, hemorrhagic events and ischemic changes in the time window between the administration of both therapies and before complete nidus obliteration of a cAVM are possible.
In summary, we have presented the first case of bilateral moyamoya disease developing after cAVM radiosurgery. Based on the chronological sequence of disease development and the distribution of the radiation dose in radiosurgery, it is proposed that humoral angiogenetic factors or unknown predisposing factors, rather than direct radiation effects, are the cause of moyamoya disease associated with a cAVM.
References
- 1.Mawad ME, Hilal SK, Michelsen WJ, Stein B, Ganti SR. Occlusive vascular disease associated with cerebral arteriovenous malformations. Radiology. 1984;153:401–408. doi: 10.1148/radiology.153.2.6484172. [DOI] [PubMed] [Google Scholar]
- 2.Enam SA, Malik GM. Association of cerebral arteriovenous malformations and spontaneous occlusion of major feeding arteries: clinical and therapeutic implications. Neurosurgery. 1999;45:1105–1111. doi: 10.1097/00006123-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Söderman M, Guo WY, Karlsson B, Pelz DM, Ulfarsson E, Andersson T. Neurovascular radiosurgery. Interventional Neuroradiology. 2006;12:189–202. doi: 10.1177/159101990601200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima T, Nakayama N, Furuichi M, Kokuzawa J, Murukawa T, Sakai N. Arteriovenous malformation in association with moyamoya disease Report of two cases. Neurosurg Focus. 1998;5:E6. doi: 10.3171/foc.1998.5.5.9. [DOI] [PubMed] [Google Scholar]
- 5.Halatsch ME, Rustenbeck HH, Jansen J. Progression of arteriovenous malformation in moyamoya syndrome. Acta Neurochir (Wien) 1997;139:82–85. doi: 10.1007/BF01850873. [DOI] [PubMed] [Google Scholar]
- 6.Seol HJ, Kim DG, Oh CW, Han DH. Radiosurgical treatment of a cerebral arteriovenous malformation in a patient with moyamoya disease: case report. Neurosurgery. 2002;51:478–482. [PubMed] [Google Scholar]
- 7.Montanera W, Marotta TR, terBrugge KG, Lasjaunias P, Willinsky R, Wallace MC. Cerebral arteriovenous malformations associated with moyamoya phenomenon. AJNR Am J Neuroradiol. 1990;11:1153–1156. [PMC free article] [PubMed] [Google Scholar]
- 8.Schmit BP, Burrows PE, Kuban K, Goumnerova L, Scott RM. Acquired cerebral arteriovenous malformation in a child with moyamoya disease: case report. J Neurosurg. 1996;84:677–680. doi: 10.3171/jns.1996.84.4.0677. [DOI] [PubMed] [Google Scholar]
- 9.Fuse T, Takagi T, Fukushima T, Hashimoto N, Yamada K. Arteriovenous malformation associated with moyamoya disease. Childs Nerv Syst. 1996;12:404–408. doi: 10.1007/BF00395095. [DOI] [PubMed] [Google Scholar]
- 10.Pincus DW, Choudhri T, Feldstein NA, Sisti MB, Stein BM. Moyamoya syndrome following stereotactic radiosurgery for AVM (abstract of oral presentation in the 1997 Annual Meeting of the Congress of Neurological Surgeons) Neurosurgery. 1997;4:731. [Google Scholar]