Abstract
To examine the flexibility of rRNA operons with respect to fundamental organization, transcription, processing, and assembly of ribosomes, operon variations were introduced by a plasmid into an Escherichia coli strain that has deletions of all chromosomal copies of rRNA genes. In the reconstructed operons, a Salmonella intervening sequence (IVS) from 23S helix 45 was introduced into the E. coli 23S gene at the same position. Three different constructs of the E. coli 16S gene were then placed wholly within the IVS sequence, and the 16S gene was deleted from its normal position. The resulting plasmids thus had the normal operon promoters and the leader region followed by the 5′ one-third of the 23S gene, the entire 16S gene within the IVS, the last two-thirds of the 23S gene, and the normal end of the operon. The three constructs differed in the amount of 16S leader and spacer regions they contained. Only two of the three constructs, those with redundant leader and spacer antiterminator signals, resulted in viable cultures of the rrn deletion strain. Electron micrographs of the variant operon suggest that the 23S rRNA is made in two separate parts which then must form subassemblies before assembling into a functional 50S subunit. Cells containing only the reshuffled genes were debilitated in their growth properties and ribosome contents. The fact that such out of the ordinary manipulation of rRNA sequences in E. coli is possible paves the way for detailed analysis of ribosome assembly and evolution.
According to the “RNA world” theory of ribosome evolution, RNA sequences were first able to promote peptide bond formation and were later incorporated into more efficient RNA-protein complexes (24). Although rRNA sequences have not been precisely conserved throughout evolution, the basic structural features of the ribosome are highly conserved in all kingdoms (18). The structure and function of ribosomes are rapidly becoming clearer, particularly given recent advances in solving the crystal structure of bacterial ribosomes (6, 32, 44). Processing and assembly of transcripts into mature rRNA species are less well described, with assembly being a key feature of ribosome biogenesis that is still very poorly understood (41).
Particularly in prokaryotes, the three rRNA genes often lie within ribosomal DNA (rDNA) operons in the order small-subunit rRNA, large-subunit rRNA, and 5S rRNA. However, this organization can vary. In the bacterium Thermus thermophilus, the 16S gene is separated from and transcribed independently of the 23S and 5S genes (21). The 5S genes are separated from the 16S and 23S rRNA genes in Pirellula marina, and the three rRNAs are transcribed separately in Leptospira interrogans and Thermoplasma acidophilum (16, 28, 33). Many bacteria, such as Salmonella and Proteus, have intervening sequences (IVS) in the 23S gene, while others contain an IVS in the 16S gene (14, 37). Introns have been identified in the large rRNAs of two archaeal species, and a bacterial group I intron has been found in the 23S gene of Simkania negevensis (13, 27). In other organisms, ribosomal genes can be highly fragmented. For example, pieces of the rRNA genes of mitochondrial genomes in green algae are encoded in separate regions of the mitochondrial genome. Details describing how ribosomes are made from multiple pieces of rRNA are lacking (31, 34).
Initial processing of rRNA in Escherichia coli is carried out by the endonuclease RNase III, which separates the 16S, 23S, and 5S rRNAs and any tRNAs that are encoded in the operon. The cleavage reactions occur quite rapidly and prior to complete transcription of the operon (2, 15, 23). Additional enzymes generate the 5′ and 3′ ends of the mature rRNA species and can substitute for RNase III in a mutant background lacking this enzyme (11, 22, 25, 26, 35, 43). IVS elements in rRNA genes are interesting because they alter rRNA primary structure. Such elements are cotranscribed as part of the rRNA precursor and are removed by RNase III without religation of the resultant fragments (9, 10). Presumably, the structural integrity of the mature RNA molecules in ribosomal subunits is maintained by secondary and tertiary structure, as well as by cooperation with ribosomal proteins, resulting in functional ribosomes. IVS elements are mostly found in two sites of eubacterial 23S rRNA, at nucleotides 533 to 560 (helix 25) and 1164 to 1185 (helix 45) (9, 29). These preferred positions might reflect selection against the presence of IVS elements at rRNA functional sites, ribosomal protein binding sites, or sites where fragmentation of the RNA molecule would cause instability of the 50S subunit. Interestingly, in an RNase III mutant strain, uncleaved IVS elements are phenotypically silent, as their presence does not affect growth or incorporation of the longer 23S rRNA into fully functional ribosomes (19).
In the study presented here, we addressed the question of whether or not the rrn genes of E. coli could be disrupted and if the disrupting element could be expressed by placing a plasmid-borne, rearranged E. coli rRNA operon into an E. coli strain whose only rRNA was encoded by this plasmid. The altered operon had the 16S rRNA gene situated wholly within a 23S gene in an IVS element in helix 45. This placement of the 16S gene within the IVS element in the 23S gene led to the separation of the 5′ 40% of the 23S gene from the remaining 60% by approximately 2,000 nucleotides (Fig. 1). E. coli cells containing only this rearranged operon were viable but had greatly increased doubling times and aberrant ribosome assembly. Our results show that a substantial alteration of the usual evolutionary scheme for rRNA gene arrangement in E. coli is possible, suggesting that cells possess the ability to cope with quite different arrangements of rRNA segments. However, it was also clear that the cells paid a high price in physiological efficiency in order to cope with these rearrangements.
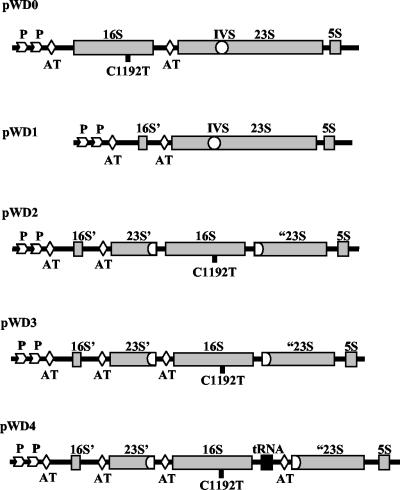
FIG. 1.
Diagram of the constructed rrn operons. pWD0, pKK3535 derivative with a C1192T transition in the 16S gene and an insertion of the IVS element from the S. enterica serovar Typhimurium 23S rDNA gene into helix 45; pWD1, deletion of 1,500 bp of the 1,542-bp 16S gene and most of tRNAGlu2 by use of StuI and XbaI restriction sites; pWD2, a PCR-generated copy of the 16S gene from pWD0, designed with artificial AatII sticky ends, was inserted into the Salmonella IVS at the unique AatII site in pWD1. pWD3 and pWD4 were constructed similarly to pWD2 except that the 5′ and 3′ ends of the PCR fragments differed (see Materials and Methods for details). pWD3 had additional 5′ leader sequences, and pWD4 had tRNAGlu2 and an additional rrn AT sequence from the intergenic spacer region of the rrnB operon. 16S’, 42-bp fragment left after deletion of the 16S gene; 23S’ and “23S, proximal and distal parts of the 23S gene before and after the IVS insertion, respectively.
MATERIALS AND METHODS
Bacterial strains.
Strains TA527 and TA531 were described previously. They are referred to as Δ7 strains because all seven chromosomal copies of rRNA operons have deletions and the cell's only copy of rRNA is plasmid encoded (3, 4). TZ131 was derived from TA531 by replacing ptRNA66 with ptRNA67. ptRNA67 has the gene encoding tRNAglu2 inserted into ptRNA66. TZ136 was derived from TZ131 by replacing pHK-rrnC+ with prrnC-sacB. TZ221 and TZ230 were TZ131 derivatives bearing pWD3 and pWD4 instead of prrnC-sacB, respectively. HP115 was a gift of K. Sanderson and contains rnc::Tn10 (tetracycline resistance). This strain was used as a source of a mutant rnc allele (30). TZ221 was transduced to rnc::Tn10, resulting in strain TZ225. AQ8809 polA zih::Tn10 was used as a source of the polA mutation and was a gift of T. Kogoma. TZ249 was derived from TA527 by transduction to polA zih::Tn10 using P1 phage and selection for tetracycline resistance. In the resulting strain, ptRNA67 is no longer present as a plasmid and the strain is spectinomycin sensitive. The polA mutation affects replication of pACYC184-based plasmids as well as ColE1 replicon plasmids, so the crucial parts of ptRNA67 were expected to be recombined onto the chromosome (20). The polA strain TZ249 transformed with pWD3 was designated TZ254. This strain has the pWD3 plasmid recombined into the chromosome.
Plasmid constructions.
The pWD plasmids were derived from a plasmid, pKK3535, carrying a wild-type rrnB operon by several steps (7) (Fig. 1). First, the 106-bp IVS in helix 45 of the Salmonella enterica serovar Typhimurium 23S rRNA was subcloned from pSH3 into the 23S gene of rrnB in plasmid pKK3535 (8, 9). Next, the spectinomycin-resistant allele (C1192T) of the 16S rRNA gene was constructed to eliminate an AatII restriction endonuclease site at position 1192. This plasmid was designated pWD0. A 1,678-bp fragment, from the StuI site in the 16S gene to the XbaI site within the tRNAGlu gene, was removed from pWD0, giving pWD1. The rrn operon promoter and the leader region were intact, but only 42 bp of the 16S gene remained in this construct. Next, AatII ends were used to insert a PCR-generated 16S fragment from pWD0 into the Salmonella IVS at the unique AatII site in pWD1, resulting in pWD2. In pWD2, the 16S PCR fragment started at nucleotide −101 with respect to the mature 5′ end of 16S, which is after the leader rrn antiterminator (AT) sequence. It was also missing the RNase III recognition site in the leader region. The fragment ended 95 nucleotides after the 3′ end of the 16S gene. In pWD3, the 16S fragment started at nucleotide −157, which is before the AT sequence, and the 3′ end was the same as in pWD2. In pWD4, the 5′ end of the 16S gene was the same as in pWD3, but the 3′ end was located after tRNAGlu2 and the AT sequence that was in the spacer region. The primers used to produce the three different 16S gene variants were as follows: for pWD2, ec11 (5′-GATACGGATCCTTGACGTCGCAAGACG) (positions −101 to 75, counting from +1 of 16S) and ec12 (5′-ATTCTCTTCGACGTCACTCCCAA) (positions 1614 to 1636); for pWD3, ec13 (5′-AGCGAGACGTCACTGCTCTTTAACA) (positions −157 to 133) and ec12; and for pWD4, ec13 and ec14 (5′- TTGAGAGACGTCCGAACAACTCT) (positions 1911 to 1933). Each primer included an AatII restriction site (underlined) that was used for cloning. The composition of each of the PCR-generated 16S gene insertions was verified by direct sequencing.
prrnB is a shorter version of pKK3535. It is missing the tet gene completely plus the flanking sequences of the cloned 7.5-kb rrn-containing fragment (7).
prrnC-sacB is a pSC101 derivative bearing the E. coli rrnC operon and a Km-sacB cassette. It was made from pHK-rrnC+ (3). The 4.7-kb BamHI Km-sacB cassette from pBIP3 was subcloned into a BamHI site in pHK-rrnC+. The sacB gene allows selection against this plasmid, as the enzyme produces a lethal compound in cells grown on media containing 5% sucrose at 30°C (36). p23S is a pHK-rrnC+ derivative containing a deletion of a StuI-XbaI fragment encompassing most of the 16S gene and part of the spacer tRNAGlu2.
Plasmid exchange.
Plasmids pWD2, pWD3, and pWD4 were used to attempt to make strains that carried only mutant copies of the rRNA genes. Strain TZ136, a Δ7 strain with deletions of all chromosomal rRNA operons, contained prrnC-sacB, which conferred resistance to kanamycin, and was used as the recipient. TZ136 was transformed with pWD2, pWD3, or pWD4, and transformants were selected for resistance to ampicillin. Resulting colonies were picked and grown to saturation without kanamycin. The cultures were then spread on plates containing 5% sucrose and incubated at 30°C for 2 days. Colonies growing on the sucrose plates were screened for kanamycin sensitivity. Ampicillin-resistant, kanamycin-sensitive colonies were used for further analysis. Judging by yields, the plasmid copy numbers were similar to that of pKK3535, about 14 copies per cell. The DNA arrangements in the plasmids were verified by restriction analysis and sequencing of the inserted rRNA genes.
Ribosome isolation and fractionation.
Forty-five milliliters of cells was grown at 37°C in Luria-Bertani broth to an optical density at 430 nm (OD430) of 0.8, harvested, rapidly chilled, and resuspended in 150 μl of lysozyme solution (20% sucrose, 25 mM Tris [pH 8.0], 60 mM KCl, 10 mM MgCl2, and 150 μg of lysozyme per ml) (17). Samples were then subjected to four freeze-thaw cycles and placed on ice. Ninety microliters of 5% Brij, 150 μl of 1% deoxycholate, 37 μl of 0.1 M MgSO4, and 30 μl of 1-mg/ml DNase were then added. Cell debris was removed by centrifugation (10 min, 12,000 rpm in an SW28 rotor at 0°C). Cell lysates were loaded onto linear 10 to 40% (wt/vol) sucrose gradients in 25 mM Tris (pH 8.0)-50 mM KCl-10 mM MgCl2-1 mM dithiothreitol and centrifuged for 14 h at 17,000 rpm in an SW28 rotor (Beckman) at 4°C. The OD260 was measured to generate the resulting profiles of ribosomes and ribosomal subunits. Ribosomal proteins from 30S and 50S peaks were examined by using SDS-PAGE gels.
RNA extraction.
Total RNA from Luria-Bertani liquid cultures was extracted by using an RNeasy Mini kit (Qiagen Inc., Santa Clarita, Calif.).
EM.
For electron microscopy (EM), the strains were grown to mid-log phase in Luria-Bertani medium, harvested, lysed, and centrifuged onto carbon-coated electron microscope grids as described by French and Miller (15).
RESULTS
To approach an understanding of ribosome structure, assembly, and evolution, we tested the ability of E. coli to assemble functional ribosomes from the products of rRNA genes that had major rearrangements of their basic elements. As a first step, we separated the 23S rDNA sequence at helix 45 by insertion of a Salmonella IVS sequence from the same region. This IVS insertion should result in the production of discrete pieces of 23S rRNA when the IVS is processed out (Fig. 1). We then inserted the entire 16S gene into the IVS element at a unique restriction site. RNase III processing of both the 16S rRNA and the IVS element should assure separation of the two parts of 23S rRNA. Plasmids containing several variations of these rearrangements were then tested for the ability to replace a resident plasmid carrying a wild-type rRNA operon in a strain with deletions of all chromosomal rRNA operons. Resulting viable strains having only these rearranged rDNA genes were examined with respect to their growth properties, ribosome profiles, and (by EM) in vivo rRNA transcription patterns.
Isolation of strains containing only rearranged and fragmented rRNA genes.
We used a derivative of an E. coli strain developed in our laboratory that has deletions of all seven chromosomal rRNA operons (Δ7 strain) (3). This strain has only plasmid-encoded rRNA operons, allowing the production of a single species of ribosomes in the cell. Replacement of the resident plasmid with one carrying rearranged ribosomal genes permitted screening for those rearranged rRNA genes that were able to support cell growth and survival. The resident plasmid also carried a counter-selectable marker, the sacB gene, whose expression kills host cells in the presence of sucrose and thus provides a powerful selection method for plasmid replacement (36). Using this system, we were able to displace the resident prrnC-sacB plasmid in the Δ7 strain with rearranged plasmids pWD3 and pWD4 (Fig. 1). Plasmid pWD2 was not exchanged. In pWD2, a PCR fragment containing the gene for 16S rRNA (primer ec11; see Materials and Methods) lacked the leader antiterminator sequence and did not have the proximal part of the sequence that forms a stem structure recognized by RNase III (see Discussion).
Growth properties of cells containing rearranged and fragmented rRNA genes.
The Δ7 strains carrying plasmids pWD3 and pWD4 as the sole source of rRNAs had dramatically increased doubling times when compared to the same strain containing a wild-type prrnB plasmid (Table 1). The doubling time for the Δ7 strain bearing prrnB (48 min) was approximately 2.5- and 5-fold faster than those of the strains carrying pWD3 (116 min) and pWD4 (250 min), respectively.
TABLE 1.
Doubling times of rrn deletion strainsa
| Strain | Doubling time (min) |
|---|---|
| Δ6 | 62 ± 3 |
| Δ6(pWD3) | 72 ± 4 |
| Δ7(prrnB) | 48 ± 2 |
| Δ7(pWD3) | 116 ± 5 |
| Δ7(pWD3) rnc | 115 ± 5 |
| Δ7(pWD3 + p23S) | 96 ± 4 |
| Δ7(pWD4) | 250 ± 8 |
Overnight liquid cultures were diluted to OD600 values of 0.05 to 0.1 in fresh Luria-Bertani broth. They were then grown with shaking at 37°C, and OD600 values were measured to follow growth of the cultures. Data shown are averages ± standard deviations for at least three measurements.
If a shortage of 50S subunits led to the very slow growth rates observed, then providing an intact 23S rRNA gene in the Δ7(pWD3) strain should result in faster growth rates. To test this idea, we inserted a compatible plasmid containing an intact 23S gene expressed from the rRNA P1P2 promoters into the Δ7(pWD3) strain. The doubling time of this Δ7(pWD3 + p23S) strain was in fact decreased, but only to 96 min. Possible explanations for this incomplete complementation are that the pSC101 derivative used for cloning the p23S gene had three- to fourfold fewer copies than the pBR322-derived pWD3 or that 23S rRNA is not the growth-limiting factor.
To determine if the plasmids were deleterious to cell growth even in the presence of wild-type ribosomes, we transformed a Δ6 strain containing one natural rRNA operon on the chromosome with pWD3. The Δ6 strain carrying pWD3 had a prolonged doubling time of 72 compared to 62 min for the Δ6 strain (Table 1), suggesting that products from the plasmid interfere with normal ribosome assembly.
The major early processing event in rRNA maturation is cleavage by RNase III, encoded by the rnc gene (25) (Fig. 1). We investigated the effect of an rnc mutation on the doubling time of the Δ7(pWD3) strain. Our logic was that a delay in rRNA processing would place an additional burden on this strain. However, the absence of RNase III did not affect the growth rate of Δ7(pWD3) (Table 1).
Because ribosome assembly is known to be sensitive to temperature (12, 42), we also tested the ability of Δ7 strains carrying pWD3 and pWD4 to grow at 20°C. The Δ7 strain containing prrnB grew at 20°C, but neither strain carrying the rearranged plasmids could grow at this low temperature (data not shown).
rRNA and ribosome subunit analyses.
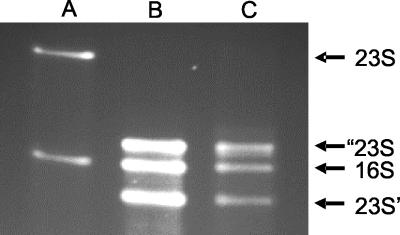
Helix 45 is located between positions 1164 and 1185 of E. coli 23S rRNA. In S. enterica serovar Typhimurium, six of the seven rRNA operons have a 106-base IVS inserted at helix 45. RNase III cleavage of the IVS-containing transcript leaves a shortened helix with nonreligated ends. Thus, when rRNA from S. enterica serovar Typhimurium is examined on gels, fragments of 1,170 and 1,738 bases replace the usual band corresponding to 23S rRNA (9, 29). Plasmids pWD3 and pWD4 have the Salmonella IVS in helix 45 as well as the entire 16S gene inserted into a unique AatII site in the IVS. Therefore, the 23S rRNA sequence should be divided into two fragments in strains containing these two plasmids as the only source of 23S rRNA. Figure 2 shows total RNA extracted from strains Δ7(prrnB), Δ7(pWD3), and Δ7(pWD4). Lanes containing rRNA isolated from pWD3- and pWD4-carrying strains show that 23S rRNA was resolved into two bands of the predicted sizes, whereas rRNA from Δ7(prrnB) showed the expected intact 23S rRNA.
FIG. 2.
Agarose gel of rRNA from a Δ7 strain with rrn plasmids prrnB (lane A), pWD3 (lane B), and pWD4 (lane C). In panels B and C, the 23S rRNA is fragmented into pieces of 1,170 (23S’) and 1,738 (“23S) nucleotides, corresponding to the positions of the IVS element in helix 45.
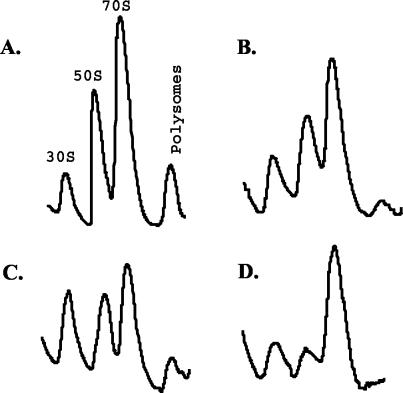
Ribosomes and ribosomal subunits have characteristic migrations in sucrose gradients. Normally, three distinct peaks correspond to 30S and 50S subunits and to 70S mature ribosomes. The Δ7(prrnB) strain showed a normal distribution of subunits, with 70S ribosomes corresponding to 60% of the total (Table 2). The 30S/50S ratio was 0.38 and was used as a baseline for comparison for the other strains. A decreased pool of 70S ribosomes was found in strains containing plasmids pWD3 and pWD4 (Table 2 and Fig. 3). The pool of 50S subunits also declined, increasing the 30S/50S ratio from 0.38 in the wild type to 0.60 for pWD3 and 1.07 for pWD4. To check that the 30S peak was not artificially augmented by 50S subassembly particles, we examined r-protein profiles for each peak. The 30S peak contained only small-subunit proteins (data not shown). We also introduced an rnc mutation into the Δ7(pWD3) strain. Our reasoning was that the lack of RNase III might alter the 30S/50S subunit ratio by delaying processing and, therefore, the assembly of the 30S and 50S subunits. Indeed, the Δ7(pWD3) rnc strain showed a decrease in the relative proportion of 50S subunits (30S/50S = 1.00). However, the percentage of 70S ribosomes remained at 60% in the Δ7(pWD3) rnc strain (Table 2) and likely accounts for the lack of a different growth rate for this strain when compared to that for the wild-type rnc+ allele in the Δ7(pWD3) strain (Table 1).
TABLE 2.
Expression of plasmid-encoded rRNAa
| Strain | Amt (%) of rRNA
|
|||
|---|---|---|---|---|
| 30S | 50S | 70S | 30S/50S ratio | |
| Δ7(prrnB) | 11 ± 1 | 29 ± 3 | 60 ± 5 | 0.38 |
| Δ7(pWD3) | 18 ± 1 | 30 ± 2 | 52 ± 3 | 0.60 |
| Δ7(pWD4) | 31 ± 1 | 29 ± 1 | 40 ± 1 | 1.07 |
| Δ7(pWD3) rnc | 20 ± 2 | 20 ± 1 | 60 ± 2 | 1.00 |
The relative cellular content of ribosomes was analyzed on linear 10 to 40% sucrose gradients. The percentage of each species was calculated as well as the 30S/50S ratio. Data shown are averages ± standard deviations for three separate determinations.
FIG. 3.
Sucrose gradient profiles of 30S and 50S subunits, 70S ribosomes, and polysomes from Δ7 strains with various plasmids as indicated. Lysates from the Δ7 strains were centrifuged through a 10 to 40% sucrose gradient. Shown are absorbency profiles at OD260 versus location in the collection tube. (A) Δ7(prrnB) (wild type). (B) Δ7(pWD3). (C) Δ7(pWD4). (D) Δ7(pWD3) rnc.
We conclude from the above analyses that the Δ7 strain carrying either pWD3 or pWD4 contained only fragmented 23S rRNA and dramatically fewer 50S subunits than Δ7(prrnB).
EM analysis.
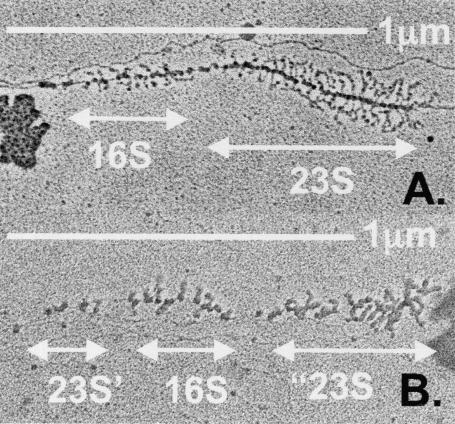
We visualized the transcription and processing patterns of the rearranged operons by EM. Representative pictures of transcription from a putative rearranged rRNA operon from a Δ7 strain in which the pWD3 plasmid was integrated into the chromosome and from a wild-type operon are shown in Fig. 4 (see Materials and Methods for details of deletion strain construction). Wild-type rRNA operons have a well-described double Christmas tree structure resulting from transcription and processing of newly synthesized 16S rRNA followed by transcription and processing of 23S rRNA and are very easy to identify in electron micrographs of chromosomal spreads (French and Miller [15]). When compared to wild-type operons, distinct triple Christmas tree transcription patterns were observed for the mutant strain. The DNA lengths measured were consistent with sizes of 1,170 bp for the 5′ region of 23S rRNA, ∼1,800 bp for 16S rRNA plus spacer, and ∼1,850 bp for the 3′ region of 23S plus 5S rRNA. However, exactly which processing events generated the observed transcription pattern is not clear (see Discussion).
FIG. 4.
Electron micrographs of transcribed wild-type operon (A) and a putative “weird” ribosomal operon (B). Bars, 1 μm. Note that the pictures have different magnifications. Arrows indicate our interpretation of transcribed 16S, 23S, 23S’, and “23S rRNA sequences. The 16S transcript in panel B appears to be degraded from the 5′ end, a result seen for eukaryotic rRNA transcripts when normal processing events are disrupted (S. French, unpublished data).
DISCUSSION
Although the 23S rRNA gene sequences in E. coli are normally contiguous, this bacterium has enough flexibility in its ribosomal assembly mechanism to deal with a 23S rRNA gene in which two segments are separated by almost 2,000 transcribed bases. Because the cells were viable when possessing only such separated 23S rRNA pieces, the parts must have folded properly, assembled with ribosomal proteins, and associated with one another and with 30S subunits to form functional ribosomes. However, the 2.5- to 5-fold increases in doubling times of the Δ7 strains containing these rearranged rrn-encoding plasmids indicate that the cells were, in fact, exceedingly stressed by having to cope with the restructured rrn operon. The usual rrn gene order in E. coli presumably facilitates coordinated control of rRNAs for both subunits as well as the coupling of transcription, rRNA processing, and assembly.
The failure of our initial plasmid construct, pWD2, to displace the prrnC-sacB plasmid in the Δ7 strain was unexpected. The difference between pWD2 and pWD3 is a 64-bp sequence that includes the leader AT sequence and the 5′ section of the 16S RNase III processing site. We thought that both features would be redundant in pWD2 (Fig. 1). Leader AT sequences at the operon promoter region were still present in pWD2, and we predicted that the RNase III cleavage site in the IVS would act as efficiently as the 16S RNase III processing site. Since RNase III was not essential for survival of Δ7(pWD3), we have concluded that the presence or absence of the leader 5′ part of the 16S RNase III processing site was not the reason for the failure of pWD2 to displace prrnC-sacB in the Δ7 strain. However, this conclusion suggests that leader AT sequences must be adjacent to the 16S gene for successful replacement. The constructs that were successful in the replacement of prrnC-sacB each contained three AT sequences prior to the 16S coding sequence, with a 16S proximal AT sequence seemingly the key to being able to replace prrnC-sacB. The rrn AT sequences catalyze the modification of RNA polymerase such that it transcribes the rrn operons at an increased rate and is resistant to Rho-dependent terminators (1, 45). Perhaps an antiterminated RNA polymerase transcription complex that has transcribed 1,200 bases of 23S rRNA no longer is associated with all of the required factors necessary to efficiently transcribe the nested 16S gene (40). In addition to transcription antitermination, studies of mutations in the leader sequence upstream of the 16S gene suggest that the entire leader region plays an important role in rRNA transcription and assembly (38, 39). Another mystery is why cells containing pWD4 had such an aggravated increase in doubling time (250 min versus 115 min for pWD3). Inclusion of the spacer region tRNA gene and a fourth AT sequence had an unanticipated deleterious effect on cell growth. It was interesting, however, that a plasmid so debilitated in producing a required cellular component or that made toxic subcomponents could still replace the resident prrnC-sacB plasmid. This result demonstrates the efficiency of sacB counterselection (36).
Cold sensitivity is a hallmark of ribosome assembly defects and has been noted in many previous studies (12, 42). Thus, the cold-sensitive phenotype of the Δ7(pWD3) and Δ7(pWD4) strains was not surprising, particularly since the six domains of the 23S rRNA are intertwined in the 50S subunit, unlike the 30S subunit, in which the three domains (5′, center, and 3′) are located in separate regions of the subunit (body, platform, and head) (5).
Why did the Δ7 strain containing either pWD3 or pWD4 have such a dramatic increase in doubling time? The ribosome profiles showed a disproportionate increase in the 30S/50S ratio, suggesting that although assembly and association of the individually transcribed regions of 23S are possible, the process is not efficient. Degradation of the 23S rRNA pieces, inability to stably associate with crucial r-proteins, and haphazard linking of the 5′ 23S transcript with the 3′ part would all lead to a serious deficiency in forming a 50S subunit. Previous studies with rrn operon deletion strains demonstrated that cells unable to make sufficient ribosomes grow slowly (4). Our observation that adding a third plasmid containing a 23S-encoding gene to Δ7(pWD3) decreased the doubling time from 115 to 96 min is consistent with the conclusion that a lack of sufficient 50S subunits caused the slow growth rate. However, because the doubling time was not restored to that of Δ7(prrnB) (48 min), it is also possible that the fragments of 23S rRNA led to increased degradation of all 23S rRNA sequences in the cell, including those encoded by the intact plasmid. Excess 23S rRNA throws off regulation of ribosomal proteins from the polycistronic mRNA, many of which are proteins for both the large and small subunits (46). Competition for crucial ribosomal proteins leading to slow or incorrectly folded subunits and interference with normal 30S function are other factors that would result in slower growth rates. Alternatively, as we have suggested above, the lower plasmid copy number of our p23S construct may have resulted in an inadequate number of 50S subunits in the cell. However, the finding that pWD3 also slowed the growth of a Δ6 strain strongly favors the conclusion that the 23S fragments are deleterious to the cell. Taken together, our results suggest that the inability to assemble the needed number of 50S subunits led to the slower growth rate for cells containing pWD3 or pWD4 and that this lack of functional 50S particles was exacerbated in pWD4. These observations provide fertile ground for future studies of rRNA processing and assembly.
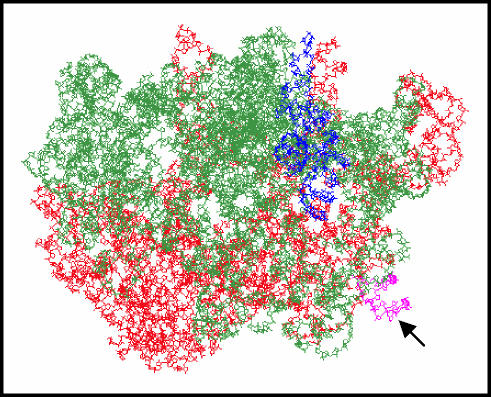
Chromosomal spreads of cells containing pWD3 integrated into the chromosome unexpectedly revealed triple Christmas tree structures of lengths that correspond to the split and rearranged operon encoded by pWD3. This result was unexpected because there were no recognizable processing sites on the 5′ and 3′ sides of the split 23S gene pieces. The structures seen in the EM pictures imply that the 16S rRNA is processed out of the chimeric transcript during synthesis and that the 23S rRNA with an internal 16S rRNA sequence never exists as an intact species in the cell. Although it is not clear from structural models how the two putative pieces of the 50S subunit might interact to form an active subunit, perhaps r-protein interactions and RNA-RNA helical interactions allow association and stabilization of an active 50S particle. The IVS insertion in helix 45 does occur between two major 23S subassembly domains (5), making plausible the idea that a functional 50S subunit could result from the products seen in the EM pictures. Models of ribosome crystal structure indicate that helix 45, containing the IVS, falls into an outcropping on the 50S subunit that would be easily accessible to processing by RNase III (44) (Fig. 5 shows particulars of the modeling).
FIG. 5.
Three-dimensional modeling of 23S rRNA. Red is used to label the proximal 1,170 nucleotides of 23S rRNA, green shows the distal 1,738 nucleotides of 23S rRNA, and purple shows 5S rRNA. Pink coloring and the arrow identify helix 45, which harbors the IVS element from S. enterica serovar Typhimurium in pWD3 and pWD4. The subunit interface is located on the opposite side of the 50S molecule as shown. The three-dimensional structure presented is for Thermus thermophilus (44) (PDB accession number 1GIY).
The implication of our results is that pieces of 23S rRNA in E. coli folded, associated with one another and with r-proteins, and subsequently linked together in vivo to form a particle capable of participating in protein synthesis. This result is reminiscent of what must happen in the mitochondria of some lower eukaryotes and in organisms with multiple internally transcribed spacers in rRNA transcripts that are processed out during maturation of the large-subunit rRNA (18). Thus, the E. coli system we have described should be useful for further studies of ribosome assembly and evolution.
Acknowledgments
We are especially grateful to Al Dahlberg for his enthusiasm and suggestions on this project and constructive comments on the manuscript and to members of his lab for help with the sucrose gradients. We particularly thank Linc Sonenshein, Murray Schnare, Charley Yanofsky, and Jill Thompson for their comments and critical reading of the manuscript.
This work was supported by NIH grant R01 GM24751 to C.L.S. S.L.F. was supported by NIH grant R01 GM63952 to Ann Beyer.
REFERENCES
- 1.Albrechtsen, B., C. L. Squires, S. Li, and C. Squires. 1990. Antitermination of characterized transcriptional terminators by the Escherichia coli rrnG leader region. J. Mol. Biol. 213:123-134. [DOI] [PubMed] [Google Scholar]
- 2.Apirion, D., and P. Gegenheimer. 1984. Molecular biology of RNA processing in prokaryotic cells, p. 36-52. In D. Apirion (ed.), Processing of RNA. CRC Press, Inc., Boca Raton, Fla.
- 3.Asai, T., D. Zaporojets, C. Squires, and C. L. Squires. 1999. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc. Natl. Acad. Sci. USA 96:1971-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai, T., C. Condon, J. Voulgaris, D. Zaporojets, B. Shen, M. Al-Omar, C. Squires, and C. L. Squires. 1999. Construction and initial characterization of Escherichia coli strains with few or no intact chromosomal rRNA operons. J. Bacteriol. 181:3803-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ban, N., P. Nissen, J. Hansen, P. B. Moore, and T. A. Steitz. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289:905-920. [DOI] [PubMed] [Google Scholar]
- 6.Brodersen, D. E., W. M. Clemons, Jr., A. P. Carter, B. T. Wimberly, and V. Ramakrishnan. 2002. Crystal structure of the 30S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16S RNA. J. Mol. Biol. 316:725-768. [DOI] [PubMed] [Google Scholar]
- 7.Brosius, J., T. Dull, D. D. Sleeter, and H. F. Noller. 1981. Gene organization and primary structure of a ribosomal RNA operon from E. coli. J. Mol. Biol. 148:107-127. [DOI] [PubMed] [Google Scholar]
- 8.Brosius, J., T. J. Dull, and H. F. Noller. 1980. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 77:201-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgin, A. B., K. Parodos, D. Lane, and N. Pace. 1990. The excision of intervening sequences from Salmonella 23S ribosomal RNA. Cell 60:405-414. [DOI] [PubMed] [Google Scholar]
- 10.Conrad, C., R. Rauhut, and G. Klug. 1998. Different cleavage specificities of RNase III from Rhodobacter capsulatus and Escherichia coli. Nucleic Acids Res. 26:4446-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlberg, A. E., H. Tokimatsu, M. Zahalak, F. Reynolds, P. Calvert, and A. B. Rabson. 1977. Processing of the 17S precursor ribosomal RNA, p. 509-517. In J. H. Vogel (ed.), Nucleic acid protein recognition. Academic Press, Inc., New York, N.Y.
- 12.Dammel, C. S., and H. F. Noller. 1993. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 7:660-670. [DOI] [PubMed] [Google Scholar]
- 13.Everett, K. D., S. Kahane, R. M. Bush, and M. G. Friedman. 1999. An unspliced group I intron in 23S rRNA links Chlamydiales, chloroplasts, and mitochondria. J. Bacteriol. 181:4734-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evguenieva-Hackenberg, E., and G. Klug. 2000. RNase III processing of intervening sequences found in helix 9 of 23S rRNA in the alpha subclass of proteobacteria. J. Bacteriol. 182:4719-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French, S. L., and O. L. Miller, Jr. 1989. Transcription mapping of the Escherichia coli chromosome by electron microscopy. J. Bacteriol. 171:4207-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukunaga, M., and I. Mifuchi. 1989. Unique organization of Leptospira interrogans rRNA genes. J. Bacteriol. 171:5763-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godson, G. N., and R. L. Sinsheimer. 1967. Lysis of Escherichia coli with a neutral detergent. Biochim. Biophys. Acta 149:476-488. [DOI] [PubMed] [Google Scholar]
- 18.Gray, M. W., and M. N. Schnare. 1996. Evolution of rRNA gene organization, p. 49-69. In R. A. Zimmermann and A. E. Dahlberg (ed.), Ribosomal RNA: structure, evolution, processing and function in protein biosynthesis. CRC Press, Boca Raton, Fla.
- 19.Gregory, S. T., M. O'Connor, and A. E. Dahlberg. 1996. Functional Escherichia coli 23S rRNAs containing processed and unprocessed intervening sequences from Salmonella typhimurium. Nucleic Acids Res. 24:4918-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grindley, N. D. F., and W. S. Kelley. 1976. Effects of different alleles of the E. coli K12 polA gene on the replication of non-transferring plasmids. Mol. Gen. Genet. 143:311-318. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann, R. K., and V. A. Erdmann. 1989. Thermus thermophilus 16S rRNA is transcribed from an isolated transcription unit. J. Bacteriol. 171:2933-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes, F., and M. Vasseur. 1976. Processing of the 17S Escherichia coli precursor RNA in the 27S pre-ribosomal particle. Eur. J. Biochem. 61:433-442. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann, S., and O. L. Miller, Jr. 1977. Visualization of ribosomal ribonucleic acid synthesis in a ribonuclease III-deficient strain of Escherichia coli. J. Bacteriol. 132:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffares, D. C., A. M. Poole, and D. J. Penny. 1998. Relics from the RNA world. Mol. Evol. 46:18-36. [DOI] [PubMed] [Google Scholar]
- 25.Kindler, P., T. U. Keil, and P. H. Hofschneider. 1973. Isolation and characterization of a ribonuclease 3 deficient mutant of Escherichia coli. Mol. Gen. Genet. 126:53-59. [DOI] [PubMed] [Google Scholar]
- 26.King, T., and D. Schlessinger. 1987. Processing of RNA transcripts, p. 703-717. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella typhimurium. Cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 27.Kjems, J., and R. A. Garrett. 1991. Ribosomal RNA introns in archaea and evidence for RNA conformational changes associated with splicing. Proc. Natl. Acad. Sci. USA 88:439-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liezack, W., and E. Stackebrandt. 1989. Evidence for unlinked rrn operons in the planctomycete Pirellula marina. J. Bacteriol. 171:5025-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattatall, N. R., and K. E. Sanderson. 1996. Salmonella enterica serovar Typhimurium LT2 possesses three distinct 23S rRNA intervening sequences. J. Bacteriol. 178:2272-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattatall, N. R., and K. E. Sanderson. 1998. RNase III deficient Salmonella typhimurium LT2 contains intervening sequences (IVSs) in its 23S rRNA. FEMS Microbiol. Lett. 159:179-185. [DOI] [PubMed] [Google Scholar]
- 31.Nedelcu, A. M., R. W. Lee, C. Lemieux, M. W. Gray, and G. Burger. 2000. The complete mitochondrial DNA sequence of Scenedesmus obliquus reflects an intermediate stage in the evolution of the green algal mitochondrial genome. Genome Res. 10:819-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramakrishnan, V. 2002. Ribosome structure and the mechanism of translation. Cell 108:557-572. [DOI] [PubMed] [Google Scholar]
- 33.Ree, H. K., and R. A. Zimmermann. 1990. Organization and expression of the 16S, 23S and 5S ribosomal RNA genes from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 18:4471-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnare, M. N., J. R. Cook, and M. W. Gray. 1990. Fourteen internal transcribed spacers in the circular ribosomal DNA of Euglena gracilis. J. Mol. Biol. 215:85-91. [DOI] [PubMed] [Google Scholar]
- 35.Sirdeshmukh, R., and D. Schlessinger. 1985. Ordered processing of Escherichia coli 23S rRNA in vitro. Nucleic Acids Res. 13:5041-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slater, S., and R. Maurer. 1993. Simple phagemid-based system for generating allele replacements in Escherichia coli. J. Bacteriol. 175:4260-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Springer, N., W. Ludwig, R. Amann, H. J. Schmidt, H. D. Gortz, and K. H. Schleifer. 1993. Occurrence of fragmented 16S rRNA in an obligate bacterial endosymbiont of Paramecium caudatum. Proc. Natl. Acad. Sci. USA 90:9892-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theissen, G., S. E. Behrens, and R. Wagner. 1990. Functional importance of the Escherichia coli ribosomal RNA leader box A sequence for post-transcriptional events. Mol. Microbiol. 10:1667-1678. [DOI] [PubMed] [Google Scholar]
- 39.Theissen, G., L. Thelen, and R. Wagner. 1993. Some base substitutions in the leader of an Escherichia coli ribosomal RNA operon affect the structure and function of ribosomes. Evidence for a transient scaffold function of the rRNA leader. J. Mol. Biol. 233:203-218. [DOI] [PubMed] [Google Scholar]
- 40.Torres, M., C. Condon, J. M. Balada, C. Squires, and C. L. Squires. 2001. Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J. 20:3811-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traub, P., and M. Nomura. 1968. Structure and function of Escherichia coli ribosomes. I. Partial fractionation of the functionally active ribosomal proteins and reconstitution of artificial subribosomal particles. J. Mol. Biol. 34:575-593. [DOI] [PubMed] [Google Scholar]
- 42.Triman, K., E. Becker, C. Dammel, J. Katz, H. Mori, S. Douthwaite, C. Yapijakis, S. Yoast, and H. F. Noller. 1989. Isolation of temperature-sensitive mutants of 16S rRNA in Escherichia coli. J. Mol. Biol. 209:645-653. [DOI] [PubMed] [Google Scholar]
- 43.Wachi, M., G. Umitsuki, M. Shimizu, A. Takada, and K. Nagai. 1999. Escherichia coli cafA gene encodes a novel RNase, designated as RNase G, involved in processing of the 5′ end of 16S rRNA. Biochem. Biophys. Res. Commun. 259:483-488. [DOI] [PubMed] [Google Scholar]
- 44.Yusupov, M. M., G. Z. Yusupova, A. Baucom, K. Lieberman, T. N. Earnest, J. H. Cate, and H. F. Noller. 2001. Crystal structure of the ribosome at 5.5 A resolution. Science 292:883-896. [DOI] [PubMed] [Google Scholar]
- 45.Zellars, M., and C. L. Squires. 1999. Antiterminator-dependent modulation of transcription elongation rates by NusB and NusG. Mol. Microbiol. 32:1296-1304. [DOI] [PubMed] [Google Scholar]
- 46.Zengel, J. M., and L. Lindahl. 1994. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog. Nucleic Acids Res. Mol. Biol. 47:331-370. [DOI] [PubMed] [Google Scholar]