Abstract
Objective
To evaluate the diagnostic accuracy of the use of an ultrasonography (US)-guided vacuum-assisted biopsy for microcalcifications of breast lesions and to evaluate the efficacy of the use of US-guided vacuum-assisted biopsy with long-term follow-up results.
Materials and Methods
US-guided vacuum-assisted biopsy cases of breast lesions that were performed between 2002 and 2006 for microcalcifications were retrospectively reviewed. A total of 62 breast lesions were identified where further pathological confirmation was obtained or where at least two years of mammography follow-up was obtained. These lesions were divided into the benign and malignant lesions (benign and malignant group) and were divided into underestimated group and not-underestimated lesions (underestimated and not-underestimated group) according to the diagnosis after a vacuum-assisted biopsy. The total number of specimens that contained microcalcifications was analyzed and the total number of microcalcification flecks as depicted on specimen mammography was analyzed to determine if there was any statistical difference between the groups.
Results
There were no false negative cases after more than two years of follow-up. Twenty-nine lesions were diagnosed as malignant (two invasive carcinomas and 27 carcinoma in situ lesions). Two of the 27 carcinoma in situ lesions were upgraded to invasive cancers after surgery. Among three patients diagnosed with atypical ductal hyperplasia, the diagnosis was upgraded to a ductal carcinoma in situ after surgery in one patient. There was no statistically significant difference in the number of specimens with microcalcifications and the total number of microcalcification flecks between the benign group and malignant group of patients and between the underestimated group and not-underestimated group of patients.
Conclusion
US-guided vacuum-assisted biopsy can be an effective alternative to stereotactic-guided vacuum-assisted biopsy in cases where microcalcifications are visible with the use of high-resolution US.
Keywords: Breast, US; Breast, Biopsy; Breast, Calcification
An image-guided percutaneous biopsy is currently recognized as a reliable alternative to an excisional biopsy for the histopathological diagnosis of breast lesions (1-3). Moreover, vacuum-assisted devices provide larger core samples and allow more contiguous sampling than the use of automated guns, potentially leading to more complete sampling of the targeted lesions and reducing the chances of sampling error (4). For the guiding method, ultrasonography (US) guidance may be preferable in lesions that are amenable to biopsy with either stereotactic or US guidance as US guidance offers a number of advantages over the use of stereotactic guidance (5, 6). Although US guidance has some critical limitations in biopsies for a mammographic abnormality such as the presence of microcalcifications without a mass, there are conditions when a percutaneous stereotactic biopsy is not available or not accessible. For example, for lesions in thin breast tissue, too close to the chest wall or in the breast tissue of axillary tail, the procedure could or should be converted to a US-guided procedure if possible (7). Following a review of the published literature, there has been no study on the efficacy of US-guided vacuum-assisted biopsy (VAB) for microcalcifications. This study was designed to evaluate the diagnostic accuracy of US-guided VAB (US-VAB) for microcalcifications in breast lesions and its efficacy with long-term follow-up results.
MATERIALS AND METHODS
Our institutional review board approved this research study, and waived the requirement for informed consent, as the study was retrospective.
Study Population
Between February 2002 and February 2006, 834 percutaneous US-VABs of consecutive breast lesions in patients were performed in our institution using the Mammotome system (Biopsys/Ethicon Endo-Surgery, Cincinnati, OH). We reviewed the clinical and imaging findings of 837 consecutive lesions and 736 lesions from patients that underwent a VAB for mass or architectural distortion were excluded from the study. After a review of follow-up imaging and the pathological findings of the remaining 101 lesions, we further excluded 39 lesions from patients who did not receive follow-up for at least two years nor underwent further pathological confirmation of the lesions. The remaining 62 lesions from patients who had undergone a VAB due to the presence of microcalcifications as depicted on mammography and then had received at least two years follow-up or underwent excision for pathological confirmation of the lesions were included in this study. Among the patients with the 62 lesions, three patients underwent a VAB for each breast as they presented with microcalcifications in both breasts. Finally, 62 lesions of 59 patients were included in this study.
The mean age of the 59 patients was 46.7 years (age range, 27 to 70 years). The patients underwent mammography for various reasons. Three patients had a nipple discharge and another three patients complained of the presence of palpable lesions. One patient had an incidental finding of increased flurodeoxyglucose (FDG) uptake on the breast on a PET scan. Fifty-two patients had mammography performed for routine breast cancer screening.
Imaging Evaluation
Mammography was performed with dedicated equipment (DMR; General Electric Medical Systems, Milwaukee, WI) until April 2005 and a Lorad/Hologic Selenia Full Field Digital Mammography System (Lorad/Hologic, Danbury, CT) from May 2005 onwards. Standard craniocaudal and mediolateral oblique views were routinely obtained and additional mammographic views were obtained as needed. All of the cases underwent mammography. Digital mammography was performed for 29 lesions and film mammography was performed for 33 lesions.
Ultrasonography was performed using a high-resolution US unit (HDI 5000, Philips-Advanced Technology Laboratories, Bothell, WA) with 12-MHz linear array transducers. Prior to a VAB, lesions were assigned to the final assessment categories of the Breast Imaging Reporting and Data System (BI-RADS) based on the findings of mammography and sonography (8) and data was entered prospectively into a database using a computerized spreadsheet (Excel, Microsoft, Redmond, WA).
The microcalcification management protocol in our institution is the following. A US-guided automated core-needle is used for microcalcifications with an associated mass lesion. A US-VAB is used for microcalcifications visible by US but without an associated mass lesion. An excisional biopsy is used after mammography-guided needle localization for microcalcifications that cannot be delineated by US. Except in cases where a patient or clinician requested a different procedure, all of the lesions included in this study were managed according the above protocol.
US-Guided Vacuum-Assisted Biopsy and Management
After a meticulous US examination, a BB marker was placed on the skin above suspicious microcalcifications and the patient underwent mammography to assure that the calcification noted on US was consistent with the suspicious calcification depicted on mammography. The VAB procedure was performed by one of two board-certificated radiologists with four and 10 years experience in breast imaging, respectively. After administration of local anesthesia, an 11-gauge probe was inserted into the breast through a small skin incision and was guided into the biopsy position under direct ultrasound visualization (HDI 5000). Multiple core samples were taken until the suspected microcalcifications were invisible on US. After the VAB, specimen mammography was taken in every case to ensure that the aimed microcalcifications were retrieved. All of the specimens with calcification and without calcification were sent for a pathological examination separately.
In cases where the pathological diagnosis was malignancy or borderline lesions such as atypical ductal hyperplasia (ADH), surgical resection was recommended. In the case of a benign finding where calcification was confirmed by specimen mammography with concordant imaging-pathological diagnosis, follow-up mammography in six months, 12 months and 24 months intervals were recommended. When the imaging-histological diagnosis was discordant or the histological diagnosis was benign but without calcification as depicted on specimen mammography, excision under mammographic localization was recommended.
Follow-Up and Data Analysis
The clinical, pathological and imaging findings of the 62 lesions, including the results of subsequent excisions and follow-up imaging studies, were reviewed. For four lesions, mammography films were not available and we reviewed the original mammography report instead. With respect to the follow-up data, we evaluated whether there was histological underestimation or a false-negative result at follow-up. Histological underestimation included a VAB-diagnosed ductal carcinoma in situ (DCIS) that was later revealed as an invasive carcinoma and a VAB-diagnosed ADH that was later revealed as an DCIS or invasive cancer by surgery. The underestimation rate was determined from dividing the number of lesions with underestimation in the final diagnosis by the total number of lesions with ADH or DCIS as determined after a VAB. A false negative was defined as the final diagnosis being malignancy with a benign diagnosis as determined by a VAB. The false negative rate was determined by dividing the number of lesions with a false negative result in the final diagnosis by the total number of VABs performed.
We reviewed the specimen mammography findings and assessed the average number of specimen samples with its range and standard deviation. We also estimated the number of specimens containing microcalcifications out of the total number of specimens and the number of microcalcifications to examine the efficacy of the use of US-VAB to retrieve a targeted microcalcification. When the total number of microcalcifications was greater then 100, it was referred to as 'innumerable'. We analyzed the number of microcalcifications and the number of the core specimens containing microcalcifications to determine if there was any statistically significant difference between lesions identified as benign and malignant. The Chi-squared or Fisher's exact tests for nonparametric variables and the t-test for parametric inference were performed. Statistical analysis was performed with SAS software (SAS system for Windows, version 9.0 SAS Institute, Cary, NC).
RESULTS
Mammography
All the patients were seen with a heterogeneously dense fibroglandular background on mammography. The BIRADS category of each patient is shown in Table 1. The extent of microcalcifications was assessed by measuring the longest diameter of the microcalcification distribution on routine mammography. The average diameter was 2.8 cm with a range from 0.5 cm to 8.6 cm and a standard deviation of 2.0 cm.
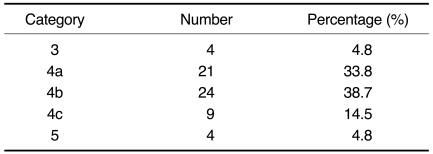
Table 1.
BIRADS Category of 62 Lesions with Microcalcifications
Note.-BIRADS = Breast Imaging Reporting and Data System
Pathological Diagnosis and Follow-Up
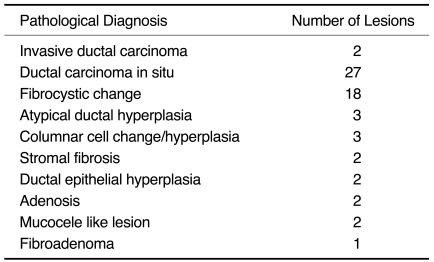
Twenty-nine out of 62 lesions were identified as a breast malignancy (Fig. 1). Twenty-seven lesions were identified as DCIS by VAB and two lesions were identified as invasive ductal carcinoma (IDC). The remaining 33 lesions were benign (Table 2). Three of the patients had ADH.
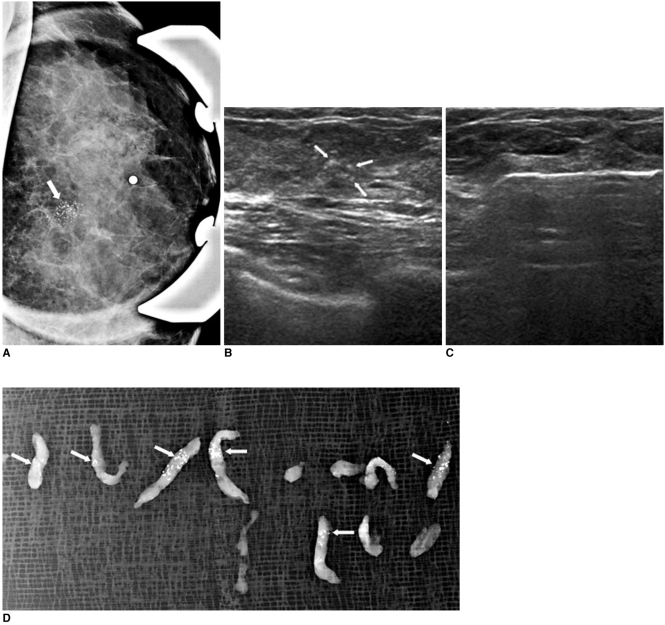
Fig. 1.
44-year-old female who underwent mammography for routine breast screening.
A. Magnification view of left breast reveals suspicious clustered and pleomorphic microcalcification (white arrow).
B. Careful US study of left breast shows microcalcification of suspicion (white arrows) in left upper central aspect.
C. Patient underwent 11 gauge US-guided vacuam-assisted biopsy targeted at microcalcification.
D. Specimen mammography shows that aimed microcalcification is retrieved (white arrows). Patient was diagnosed with ductal carcinoma in situ and underwent further partial mastectomy, which also revealed presence of ductal carcinoma in situ.
Table 2.
Pathology from VAB Specimens
Note.-VAB = vacuum-assisted biopsy
Patients with the 29 malignant lesions underwent a mastectomy or breast conservation surgery. The pathological results after surgery revealed the presence of an IDC for two lesions of a VAB-diagnosed DCIS. The underestimation rate was 7.4%. One patient who was diagnosed with a DCIS showed no residual cancer after surgery and all of the other patients had a residual malignancy as identified in the pathological specimens. Nine lesions had residual microcalcifications that were confirmed in the pathological specimens.
The three patients who were diagnosed with ADH underwent excision for further pathological confirmation. One of the lesions from the patients was discovered to have a DCIS component. The remaining two patients were diagnosed with ADH with no residual atypia. The ADH underestimation rate was 33.3%.
Five other lesions from patients diagnosed with benign disease by VAB underwent excision. For two lesions, the patients wanted the lesions excised, as the lesions were palpable although they were imaging-histologically concordant. Another three lesions showed an increased number of microcalcifications on follow-up mammography. The follow-up period was 10 months, 23 months and 25 months, respectively with an average of 19.3 months. The lesions were excised after mammographically guided localization; all of the lesions were finally identified with a fibrocystic change with microcalcifications.
In addition to the five lesions that underwent excision with a pathological diagnosis of benign disease other than ADH after a VAB, the remaining 25 lesions had long-term follow-up results of more than two years. On follow-up mammography, there were no residual microcalcifications seen in four lesions and the remaining 21 lesions showed residual microcalcifications. There were no additional cases of malignancy after more than two years of follow-up. The false negative rate was 0%.
Specimen Mammography
We were able to examine specimen mammography in all 58 lesions except for four patients where mammography films were not available. The average number of VAB specimens per lesion was 9.6 specimens, ranging from three to 19 specimens with a standard deviation of 3.2. The number of specimens with microcalcifications ranged from 0 to 16, with an average of 4.5 specimens with microcalcifications seen on specimen mammography. The percentage of the number of specimens with microcalcifications above the total was 46.9%. The average number of microcalcifications was 43.8 countable microcalcification flecks ranging from 0 to over 100. It was possible to validate microcalcifications on specimen mammography (Fig. 1) in all cases except for two lesions. One lesion was identified with DCIS after VAB and though we could not validate the presence of microcalcifications on specimen mammography, pathology revealed a DCIS with microcalcifications. The patient underwent a mastectomy and the final diagnosis was DCIS. The other lesion was from a patient who had a probably benign lesion with microcalcifications in the right breast and a lesion with low suspicious segmental microcalcifications in the left breast. US-VAB was planned for the low suspicious lesion in the left breast but the patient also wanted confirmation of the probably benign lesion in the right breast. After VAB of both lesions, specimen radiography showed microcalcifications in the left breast while there were no microcalcifications in the right breast. Both lesions were identified as a fibrocystic change but there was no documentation of microcalcifications in the right breast on the pathology report. This patient has received follow-up without further pathological confirmation for more than four years and there has been no significant interval change.
The number of underestimated lesions (n = 3) was too small to statistically compare to the number of notunderestimated lesions (n = 59) and the total number of specimens, the number of specimens containing microcalcifications and the number of microcalcifications are shown in Table 3.
Table 3.
Number of Specimens According to Estimation
There was no significant statistical difference in the total number of specimens, the number of specimens containing microcalcifications and the number of microcalcifications between benign lesions and malignant lesions (Table 4).
Table 4.
Number of Specimens According to Diagnosis
DISCUSSION
The increasing use of mammographic screening has led to the detection of smaller, earlier-stage malignancies, which most often present as microcalcifications (9, 10). Microcalcifications detected on mammography represent a confounding situation as although the finding is the most common mammographic finding of DCIS (10), at the same time it is a very common finding in aging women. The majority of lesions of screening-provoked surgical biopsies that are ultimately identified as benign are due to calcifications (11). For decades, the use of the needle-localized biopsy was accepted as the standard choice for a biopsy of calcification detected on mammography (12, 13). However, as the imaging equipment has evolved and the biopsy skills have accumulated, the use of the image-guided percutaneous biopsy became an another alternative (14). Moreover, when the patient condition is not adequate to undergo general anesthesia or when there is no stereotactic biopsy unit available, as in our institution, the use of US VAB can be helpful if the microcalcifications can be delineated by US (7). In addition, US guidance offers a number of advantages over the use of stereotactic guidance. The patient is in supine position rather than a prone position. Moreover, there is no need for compression. There is no radiation hazard. The image acquisition is in real-time. Furthermore, the equipment is more affordable and readily available.
A shortcoming of US is that microcalcifications are not depicted as clearly as in mammography. However, as the high-resolution US equipment has evolved, the chance of finding a microcalcification of question by careful US examination has also increased (15). We were able to confirm retrieved microcalcifications by specimen mammography in 96.8% of cases, which is within the range of 95% to 100% that was previously reported by the use of successful stereotactic guided VAB (ST-VAB) for microcalcifications (2, 16-18). Though there were two lesions without microcalcifications seen on specimen mammography, there was no case of missed diagnosis of cancer in this study. The reported rate of a missed diagnosis of cancer after an ST-VAB is from 0% to 3% (19). However, as microcalcifications are not well visualized on US as in mammography, to apply US-VAB in cases of microcalcifications, a meticulous US examination by an experienced operator is mandatory and the insertion of a retrievable wire can be also helpful (20) to locate a microcalcification of question. The underestimation rate for a carcinoma was 7.4% in the current study with two of the DCIS lesions identified as an IDC and one of the DCIS lesions identified as a DCIS with microinvasion. In past studies, the underestimation rate of the use of an ST-VAB for a carcinoma was reported from 18% to 20% (16, 21).
In the case of an ADH identified after a VAB, surgical excision was recommended, as over 50% of ADH lesions are known to have a carcinoma at the time of excision (21, 22). The underestimation rate for an ADH was 33.3%, one cancer out of three ADH lesions in the current study, which is within the recently reported range of 7% to 35% after an ST-VAB.
There was no statistical difference in the number of the specimen cores with microcalcifications and the total number of microcalcifications between the malignant lesions and benign lesions in this study. This result suggests that the efficacy of a US-VAB for microcalcifications is independent of the nature of the lesion as being malignant or benign.
In the case of a stereotactic-automated core-needle-biopsy, at least five or more flecks of calcium or three or more cores containing calcium are necessary to be present to ensure adequate sampling of microcalcifications with increasing retrieval of calcification that is associated with increasing sensitivity (23). In this study, the average number of specimens with microcalcifications was 4.5 specimens, ranging from 0 to 16 specimens with a mean number of 43.8 calcification flecks and there was no statistically significant difference between benign lesions and the malignant lesions. This finding is concordant with a previous report with the use of automated core needle biopsies. The amount of microcalcification retrieval after a VAB in this study was higher than that required for an automated core-needle-biopsy, which may explain why there was no statistically significant difference between the malignant lesions and benign lesions. To the best of our knowledge, there has been no previous report of an adequate amount of microcalcification retrieval after a US-VAB. However, it has been reported that in the case of an ST-VAB, to yield 92% of diagnostic accuracy, 12 specimens were necessary and we obtained an average of 9.6 specimens in this study.
This study only included patients with lesions that received follow-up for at least two years or patients that underwent surgery for further confirmation. A previous study on the use of an ST-VAB for breast microcalcifications has suggested that a 6-month follow-up might already provide evidence that a VAB finding is representative (19). In the case of US-VAB for microcalcifications, given the limitations of the use of US for visualization of microcalcifications, long term follow up with mammography longer than six months should be necessary. In the case of a core needle biopsy, a two-year-follow-up is generally accepted to make a confident diagnosis of benign disease (19) and hence a follow-up period of more than two years can ensure a confident diagnosis for a US-VAB of breast microcalcifications.
There are some limitations to this study. First, only microcalcifications visible on linear probe US were included in this study and thus the study does not reflect all of the microcalcifications, including microcalcifications not delineated by US. Second, patients without a follow-up period longer than two years were excluded in this study and there could be a possible missed diagnosis in these patients. Also, we set a standard follow-up period of at least two years, which may be insufficient to diagnose a lesion with microcalcifications as benign and a further follow-up would be necessary.
In conclusion, the use of a US-VAB can be an effective alternative to the use of an ST-VAB in cases where microcalcifications are visible on high-resolution US.
References
- 1.Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193:359–364. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 2.Meyer JE, Smith DN, Lester SC, Kaelin C, DiPiro PJ, Denison CM, et al. Large-core needle biopsy of nonpalpable breast lesions. JAMA. 1999;281:1638–1641. doi: 10.1001/jama.281.17.1638. [DOI] [PubMed] [Google Scholar]
- 3.Brenner RJ, Bassett LW, Fajardo LL, Dershaw DD, Evans WP, 3rd, Hunt R, et al. Stereotactic core-needle breast biopsy: a multi-institutional prospective trial. Radiology. 2001;218:866–872. doi: 10.1148/radiology.218.3.r01mr44866. [DOI] [PubMed] [Google Scholar]
- 4.Philpotts LE, Hooley RJ, Lee CH. Comparison of automated versus vacuum-assisted biopsy methods for sonographically guided core biopsy of the breast. AJR Am J Roentgenol. 2003;180:347–351. doi: 10.2214/ajr.180.2.1800347. [DOI] [PubMed] [Google Scholar]
- 5.Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002;40:483–500. doi: 10.1016/s0033-8389(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis GA, Moran RE, Fajardo LL, Mugler JP, Christopher JM, Harvey JA. MRI-guided needle localization: technique. Semin Ultrasound CT MR. 2000;21:337–350. doi: 10.1016/s0887-2171(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 7.Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: How to reduce them. Radiographics. 2007;27:79–94. doi: 10.1148/rg.271065029. [DOI] [PubMed] [Google Scholar]
- 8.American College of Radiology. Breast imaging reporting and data system (BI-RADS) 4th ed. Reston: Va: America College of Radiology; 2003. [Google Scholar]
- 9.Cady B, Stone MD, Schuler JG, Thakur R, Wanner MA, Lavin PT. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131:301–308. doi: 10.1001/archsurg.1996.01430150079015. [DOI] [PubMed] [Google Scholar]
- 10.Holland R, Hendriks JH, Vebeek AL, Mravunac M, Schuurmans Stekhoven JH. Extent, distribution, and mammographic/histological correlations of breast ductal carcinoma in situ. Lancet. 1990;335:519–522. doi: 10.1016/0140-6736(90)90747-s. [DOI] [PubMed] [Google Scholar]
- 11.Spencer NJ, Evans AJ, Galea M, Sibbering DM, Yeoman LJ, Pinder SE, et al. Pathological-radiological correlations in benign lesions excised during a breast screening programme. Clin Radiol. 1994;49:853–856. doi: 10.1016/s0009-9260(05)82874-0. [DOI] [PubMed] [Google Scholar]
- 12.Jackman RJ, Marzoni FA., Jr Needle-localized breast biopsy: why do we fail? Radiology. 1997;204:677–684. doi: 10.1148/radiology.204.3.9280243. [DOI] [PubMed] [Google Scholar]
- 13.Head JF, Haynes AE, Elliott MC, Elliott RL. Stereotaxic localization and core needle biopsy of nonpalpable breast lesions: two-year follow-up of a prospective study. Am Surg. 1996;62:1018–1023. [PubMed] [Google Scholar]
- 14.Elvecrog EL, Lechner MC, Nelson MT. Nonpalpable breast lesions: correlation of stereotaxic large-core needle biopsy and surgical biopsy results. Radiology. 1993;188:453–455. doi: 10.1148/radiology.188.2.8327696. [DOI] [PubMed] [Google Scholar]
- 15.Soo MS, Baker JA, Rosen EL. Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003;180:941–948. doi: 10.2214/ajr.180.4.1800941. [DOI] [PubMed] [Google Scholar]
- 16.Cangiarella J, Waisman J, Symmans WF, Gross J, Cohen JM, Wu H, et al. Mammotome core biopsy for mammary microcalcification: analysis of 160 biopsies from 142 women with surgical and radiologic follow-up. Cancer. 2001;91:173–177. doi: 10.1002/1097-0142(20010101)91:1<173::aid-cncr22>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Liberman L, Smolkin JH, Dershaw DD, Morris EA, Abramson AF, Rosen PP. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251–260. doi: 10.1148/radiology.208.1.9646821. [DOI] [PubMed] [Google Scholar]
- 18.Meyer JE, Smith DN, DiPiro PJ, Denison CM, Frenna TH, Harvey SC, et al. Stereotactic breast biopsy of clustered microcalcifications with a directional, vacuum-assisted device. Radiology. 1997;204:575–576. doi: 10.1148/radiology.204.2.9240556. [DOI] [PubMed] [Google Scholar]
- 19.Kettritz U, Rotter K, Schreer I, Murauer M, Schulz-Wendtland R, Peter D, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer. 2004;100:245–251. doi: 10.1002/cncr.11887. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Park HB, Ryu JW. Usefulness of ultrasound-guided mammotome biopsy for microcalcification. J Korean Radiol Soc. 2005;53:129–135. [Korean] [Google Scholar]
- 21.Jackman RJ, Burbank F, Parker SH, Evans WP, 3rd, Lechner MC, Richardson TR, et al. Atypical ductal hyperplasia diagnosed at stereotactic breast biopsy: Improved reliability with 14-gauge, directional, vacuum-assisted biopsy. Radiology. 1997;204:485–488. doi: 10.1148/radiology.204.2.9240540. [DOI] [PubMed] [Google Scholar]
- 22.Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. AJR Am J Roentgenol. 1995;164:1111–1113. doi: 10.2214/ajr.164.5.7717215. [DOI] [PubMed] [Google Scholar]
- 23.Bagnall MJ, Evans AJ, Wilson AR, Burrell H, Pinder SE, Ellis IO. When have mammographic calcifications been adequately sampled at needle core biopsy? Clin Radiol. 2000;55:548–553. doi: 10.1053/crad.1999.0483. [DOI] [PubMed] [Google Scholar]