Abstract
Gastrointestinal (GI) fistulas are frequently very serious complications that are associated with high morbidity and mortality. GI fistulas can cause a wide array of pathophysiological effects by allowing abnormal diversion of the GI contents, including digestive fluid, water, electrolytes, and nutrients, from either one intestine to another or from the intestine to the skin. As an alternative to surgery, recent technical advances in interventional radiology and percutaneous techniques have been shown as advantageous to lower the morbidity and mortality rate, and allow for superior accessibility to the fistulous tracts via the use of fistulography. In addition, new interventional management techniques continue to emerge. We describe the clinical and imaging features of GI fistulas and outline the interventional management of GI fistulas.
Keywords: Gastrointestinal tract, interventional procedure; Fistula, gastrointestinal; Abdomen, abscess; Abscess, percutaneous drainage;
The majority of gastrointestinal (GI) fistulas (approximately 75-85%) occur as a complication of recent abdominal surgery and are caused by a variety of factors including incorrect placement of drainage, an improper surgical technique, infection or failure of anastomosis. Only a small percentage of GI fistulas occur as a complication of inflammatory bowel disease, neoplasia, trauma or radiation therapy (1, 2).
Gastrointestinal fistulas originate from an orifice in the intestinal wall or the biliary or pancreatic ducts and lead to the skin or other internal spaces by abnormal abdominal communications. The fistulas drain a mixture of GI contents, purulent fluid and/or necrotic matter (1, 2). The fistulas cause a wide range of deleterious effects in patients including pain, complicated wound care, diminished self-image and self-esteem, reduced quality of life and a delayed return to social and work activities. In addition, patients experience increased anxiety about future operative procedures and possible death. There is a major impact of GI fistulas on the morbidity and mortality of patients, as well as on the health care costs for diagnosis and treatment.
Until recently, radiological studies were limited to the identification of the morbid anatomy. However, since McLean et al. (3) first reported their experience in radiological percutaneous management of high-output postoperative enterocutaneous fistulas, technical advances in interventional radiology have made it possible to perform selective catheterization of the most tortuous tracts and allow effluent drainage (4). In addition, new interventional management techniques continue to emerge, and a great potential exists for the development of novel devices for fistula closure. We feel that there will be continued future advances in interventional management techniques. In this review, we describe the clinical and imaging features of GI fistulas and outline the interventional management of GI fistulas with a review of the recent literature.
Clinical Features of GI Fistulas
Various systems have been used to classify GI fistulas; however, the three features that have been most widely used to classify fistulas are anatomical classification, output volume and etiological parameters (1, 2, 4). Each of these features has specific implications for the likelihood of spontaneous closure, prognosis, operative timing and non-operative care planning. These classifications are often used in combination with each other to achieve an integrated understanding of the fistula and its potential impact on the patient.
Anatomical classification of fistulas is divided into internal and external fistulas. External fistulas (Fig. 1) are pathological communications between any portion of the gastrointestinal tract and the skin, and represent the most common type of postoperative fistula. Internal fistulas (Fig. 2) are a connection between the gastrointestinal tract and another internal organ, the peritoneal space, retroperitoneum or the thorax (the pleural space or the mediastinum) (1).
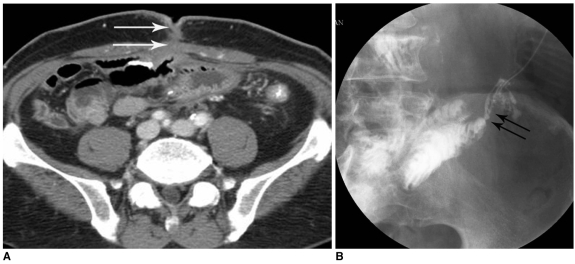
Fig. 1.
External fistula after right hemicolectomy due to colon cancer.
A. Axial CT image shows jejuno-cutaneous fistula in lower abdomen (arrows).
B. Fistulogram using iodinated water-soluble contrast media shows direct communication into jejunal loops (arrows).
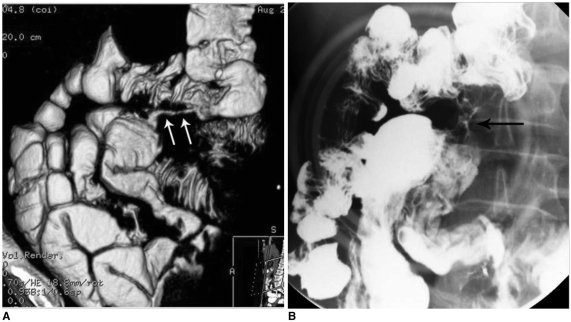
Fig. 2.
Internal fistula (ileo-colic fistula) in patient with Crohn's disease.
A. Three-dimensional reconstruction abdomen CT image shows ileo-colic fistula (arrows).
B. In small bowel study, fistula tract between ileum and transverse colon is seen (arrow).
Classification of fistulas by output volume can be divided into high output and low output fistulas. High output fistulas (Fig. 3) drain between 300 and 4,000 ml per day, and usually arise from a lesion located between the inferior third of the esophagus and the ligament of Treitz. Low-output fistulas (Fig. 4) that drain less than 100 ml per day generally arise from the ileum or colon, except in the case of intestinal malabsorption (4). Recently, the output of pancreatic and intestinal fistulas has been characterized as either high or low output according to the volume of discharge over a 24-hour period (1, 2). Etiologic classification is determined with respect to the underlying disease.
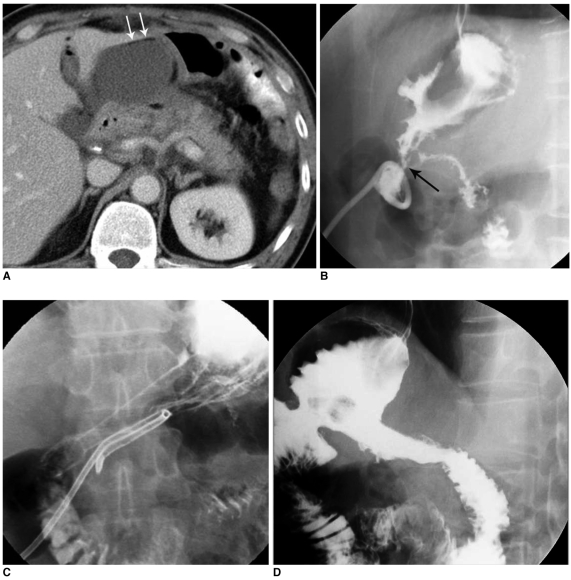
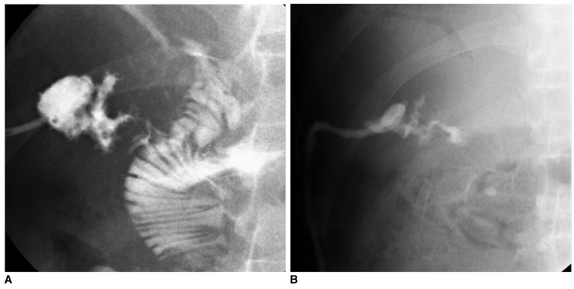
Fig. 3.
High-output fistula after Billroth I operation due to stomach cancer.
A. Abdomen CT image shows abnormal loculated fluid collection with scanty air-bubbles (arrows) adjacent to gastroduodenal anastomotic site.
B. Fistulogram after insertion of drainage tube shows fistula tract (arrow) from intra-abdominal abscess pocket to remnant of stomach via anastomosis site and stenosis at anastomosis site.
C. Upper GI series obtained immediately after placement of covered Nitinol stent at gastroduodenal anastomosis site shows fully expanded stent with good passage of contrast media without visible fistula tract.
D. Follow-up upper GI series taken three months after stent placement shows properly located stent with excellent patency.
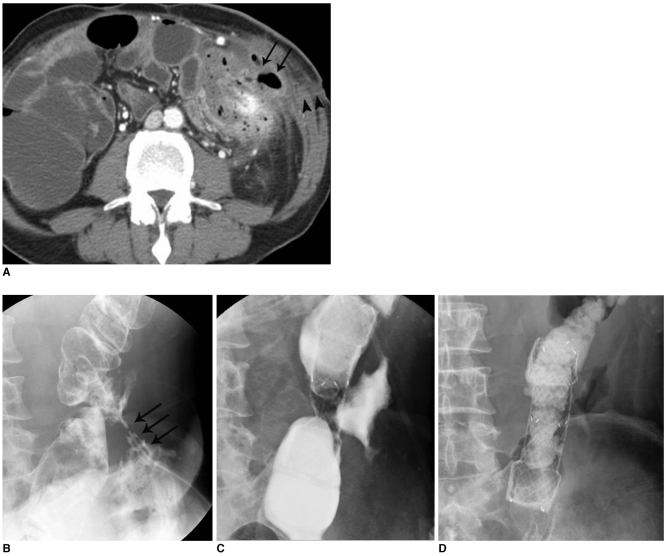
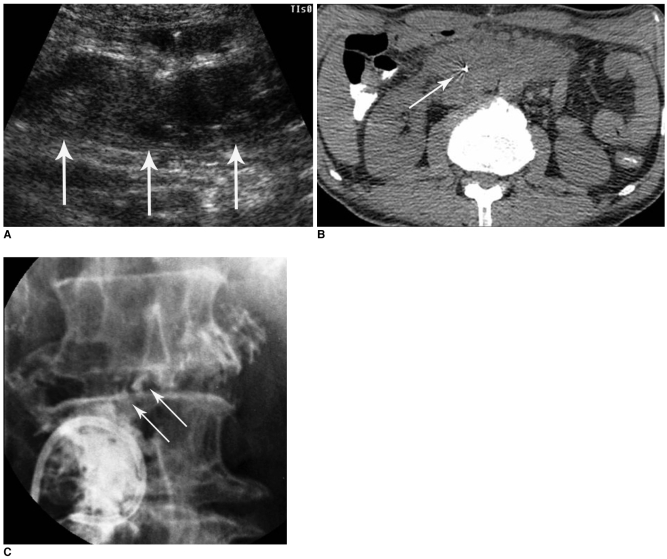
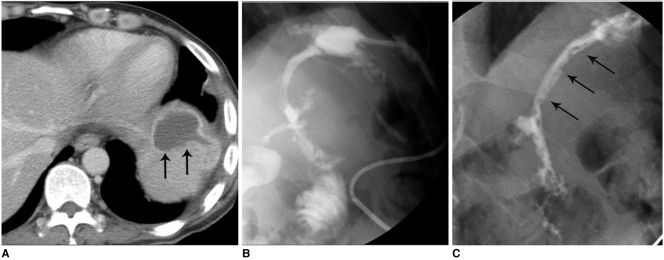
Fig. 4.
Low-output, colo-cutaneous fistula with anastomotic stricture in patient with descending colon segmental resection due to trauma.
A. Abdominal CT image shows small air-containing abscess pocket (arrows) and subcutaneous fistula tract formation (arrowheads).
B. Initial fistulogram shows colo-cutaneous fistula (arrows) with anastomotic stricture at descending colon.
C. Placement of covered metallic stent at descending colon anastomosis site.
D. Follow-up image obtained after placement of covered metallic stent shows fully expanded stent with good passage of contrast media without visible fistula tract.
Fistula closure is considered as spontaneous if no radiological or surgical intervention is required, although artificial nutrition and drug therapy may have been administered. Anatomical factors that may adversely affect spontaneous fistula closure include complete disruption, a lateral (side) fistula, large adjacent bowel abscess, distal obstruction, a fistula tract less than 2 cm, an enteral defect larger than 1 cm, and gastric, lateral duodenal, ligament of Treitz and ileal fistula sites. Other factors include cancer, chemotherapy, radiation, underlying inflammatory bowel disease, uncontrolled sepsis, infected fistula fluid, hypoproteinemia, large and early leakage of the anastomosis, diabetes, corticosteroid use and renal failure (1, 2, 5).
Imaging Modalities of GI Fistulas
It is vital to identify the source and route of the fistula tract in addition to the etiological features that may influence patient outcome such as the presence of obstructions, abscesses, or pancreatic pseudocysts. Comprehensive determination of the fistula anatomy is usually obtained through radiological investigation, utilization of a plain radiograph of the abdomen, contrast studies (barium and/or water-soluble contrast medium fistulogram), ultrasonography (US), a computed tomography (CT) scan, magnetic resonance imaging (MRI) or a radionuclide study (1-4).
Before performing contrast studies, a plain radiograph of the abdomen helps locate surgical anastomosis clips, drains that have been left in place, and opacities or lucencies outside the alimentary tract that may indicate propagation of an abscess. In general, barium is considered the contrast medium of choice to utilize due to its ability to reveal mucosal surfaces and remain undiluted; it can directly show the fistulous tract as well (Figs. 2, 3). However, extravasated barium may induce an acute inflammatory reaction in the thoracic or peritoneal cavity and therefore an alternative-iodinated water-soluble medium-should be used when perforation of the esophagus, stomach, small bowel or colon is suspected (Figs. 1, 4).
A fistulogram is best performed by injecting contrast agent directly into the cutaneous opening in order to demonstrate the main axis and to avoid false passage into the secondary subcutaneous tracts at the time of cannulation (Fig. 1). Following complete visualization of the tract, further investigation to delineate associated pockets and cavities may be safely performed using angiographic catheters and guide wires under fluoroscopic guidance (3). A water-soluble contrast agent is slowly injected and spot radiographs of several projections are obtained to delineate the anatomy of the tracts.
US is portable, rapid, free of ionizing radiation, and inexpensive to perform. The diagnostic accuracy for the identification of an abscess cavity compares favorably to CT. US is used as the first screening modality in most patients (Figs. 5, 6). However, patients who are obese, have an ileus or extensive surgical wounds may be difficult to examine.
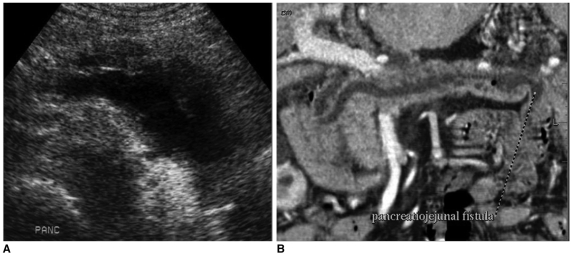
Fig. 5.
Pancreatico-jejunal fistula in malignant intramural papillary mucinous tumor patient.
A. Pancreas shows diffuse pancreatic duct dilatation on US.
B. Follow-up three-dimensional reconstruction CT image shows pancreatico-jejunal fistula. Pancreatic duct dilatation is somewhat improved due to this fistula.
Fig. 6.
Large abscess formation in mesenteric root after Billroth II operation due to stomach cancer.
A. US image shows heterogenous echoic fluid collection (arrows) in mesenteric root.
B, C. CT guided 21-gauge needle puncture (B, arrow) and fluoroscopy-guide 10 Fr drainage catheter insertion (C). In C, fistulous tract to third portion of duodenum is observed (arrows).
CT provides more standardized information, independent of the skill of the operator or the body habitus of the patient. The use of CT may not always visualize a fistula, though certain CT findings may suggest the need for a contrast study in order to provide a definitive diagnosis. Additionally, in patients with known enteric fistulas, CT imaging may disclose other ancillary extraluminal abnormalities or complications, such as abscess formation, peritonitis, or lymphadnopathy that a contrast study alone might not reveal.
Interventional Management of GI Fistulas
Most GI fistulas create abscess cavities in the intra-abdominal space, regardless of classification. The treatment of patients with significant gastrointestinal fistulas is generally a two-staged process, with initial management efforts centered on controlling bowel effluent, clearing associated abscesses and improving the nutritional status of the patient, with an ultimate therapeutic goal of closing the enteric fistula (6). Percutaneous, radiological catheter drainage is an effective and safe method for the treatment of abdominal abscesses associated with fistulas and can substitute for surgery (Fig. 7). A high rate of successful percutaneous drainage has been reported (1-4, 6), with several investigators documenting a closure rate ranging from 57% to 88% following percutaneous abscess drainage.
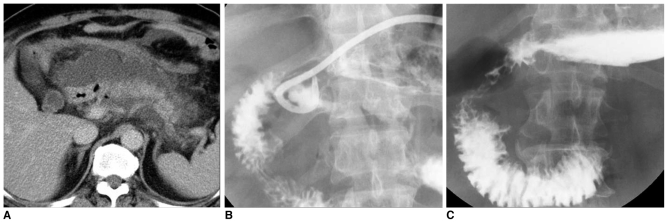
Fig. 7.
Percutaneous management of high-output fistulas.
A. After Billroth I operation, abdominal CT image shows abnormal fluid collection at anastomotic site and peri-pancreatic portion.
B. After drainage of large abscesses, significant decrease in fluid collection is seen. Communication to duodenal bulb is noted.
C. Two month follow-up upper GI series shows disappearance of fistulous tract and abscess.
Prior to the initiation of a radiological intervention, a consultation with the referring physician and a careful review of previous radiological examinations provides the interventional radiologist with a satisfactory understanding of the clinical problem. The crucial questions are the following: what is the underlying disease, what type of surgical anastomosis was performed, what is the supposed origin of the fistula, is the fistula a high-output or a low-output fistula, what does the lost fluid consist of and were surgical sump drains left and where do they lead (4).
When the decision has been made to percutaneously drain an abscess, coagulation factors and times should be checked. Coverage with broad-spectrum antibiotics should be initiated before a procedure and the antibiotics altered, if appropriate, based on Gram staining and cultures; however, the risks and benefits should be weighed cautiously. No patient is too severely ill to undergo percutaneous catheter drainage of an abscess.
Percutaneous needle puncture for the treatment of fluid collection or abscess cavity can be performed under US, CT or fluoroscopic guidance (Fig. 6). The puncture route should be meticulously planned, and the shortest pathway chosen from the cross-sectional images. There should be no intervening viscera, bowel, or vital structures such as blood vessels in the path of the needle. After diagnostic aspiration, 5-10 ml of contrast medium is injected to outline the cavity. Under fluoroscopic observation, injected contrast medium will define the size and configuration of the abscess and any septations or will demonstrate any fistulous communication with the adjacent bowel. However, simple drainage procedures can be performed without fluoroscopic guidance. After final placement of the catheter, contrast medium is injected to ensure that all catheter side-holes are within the cavity and are draining properly. Otherwise, side-holes within the tract can cause leakage of purulent material and the tract may not heal. A transgluteal, transvaginal (Fig. 8) or transrectal route can be used for deep-seated pelvic abscesses.
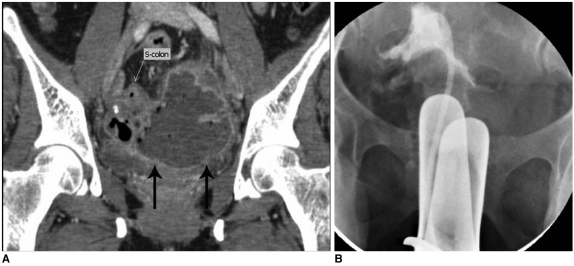
Fig. 8.
Transvaginal approach for pelvic abscess drainage.
A. 9×5 cm sized abscess cavity (arrows) formation in pelvic cavity following hysterectomy. Fistula tract between sigmoid colon and abscess is observed.
B. 8.5 Fr drainage catheter insertion was performed by fluoroscopic guided transvaginal approaches.
Occlusion of the catheter by debris can usually be resolved by saline irrigation or reaming the tube with a guidewire. Ability to remove only a small portion of the irrigant indicates either 1) debris has partially occluded the catheter and has produced a one-way valve obstruction; 2) there is a complex compartment within the cavity, and fluid flows away into a more deeply seated cavity; 3) there is an internal enteric fistula; or 4) the contents are semisolid, and the irrigant is spreading within the interstices of the necrotic debris (7). The criteria for catheter removal are 1) volume of daily drainage < 10 mL; 2) improvement in the patient symptoms (e.g. no fever); 3) minimal residual cavity; and 4) nonpurulent drained fluid. It is critical to recognize the clinical signs of infection to determine if the catheter should be removed. Usually, patients with bowel leaks or fistulas require a longer duration of catheter drainage because of persistent flow through the fistula and the relatively larger amounts of fluid present.
Levin tube or jejunal feeding tube insertion, under fluoroscopic guidance, is effective for decompression of bowel loops and enteral feeding. Fluoroscopic or US-guided central venous catheter insertion has also been performed by interventional radiologists to provide total parenteral nutrition in high-risk patients. Moreover, we have experienced many successful cases of radiological percutaneous gastrostomy or enterostomy for diversion or decompression of the GI contents.
Over the past decade, various alternative methods have been developed using interventional techniques such as interventional stenting (8, 9) and interventional gluing with fibrin or histoacryl (10). We successfully used a covered metallic stent to treat a postoperative fistula with anastomotic stricture in a patient who underwent a Billroth I operation due to advanced gastric cancer (Fig. 3). We have also successfully treated a colo-cutaneous fistula with anastomotic stricture in a patient with a descending colon segmental resection using a covered stent (Fig. 4).
In specific cases, when fistulous tracts have communicated with the biliary tree, transhepatic biliary drainage may be necessary. We have treated a patient with a fistulous tract located between a hepatic abscess and the jejunum. In this case, the fistulous tract was successfully treated by percutaneous drainage of the hepatic abscess (Fig. 9).
Fig. 9.
Fistula located between hepatic abscess and jejunum.
A. Tubogram after 8.5 Fr drainage catheter insertion into hepatic abscess shows fistula tract between abscess and jejunum.
B. Follow-up tubogram shows decreased abscess size and disappearance of fistula tract to jejunum.
Rabago et al. (10) recently reported the effective treatment of postoperative fistulas resistant to conservative treatment using biological fibrin glue. Similarly, we successfully managed a post-traumatic jejuno-cutaneous fistula using fibrin glue (Fig. 10). The simple blockage of a fistula tract with biological fibrin glue has a limited role; however, in some cases, it can be an effective treatment.
Fig. 10.
Jejuno-cutaneous fistula after traffic accident.
A. Abdomen CT image shows abscess pocket of approximately 3.9 cm in left upper quadrant (arrows).
B. Fistulogram shows jejuno-cutaneous fistula and focal abscess pocket in left upper quadrant.
C. Follow-up fistulogram after blockage of fistula tract (arrows) with fibrin glue shows no more visible fistula tracts to jejunal loops.
As long as a fistula tract is continually bathed in a combination of gastric fluid, bile, and pancreatic secretions, the tissues remain inflamed and necrotic areas cannot heal. When drainage is externally controlled, a mature fibrous tract can form and the fistula will close spontaneously when the drainage tube is removed (3). If outpatient care of the catheter is anticipated, the patient or responsible caregiver must thoroughly understand the management of the tube and need to be taught to recognize possible complications. In addition, care must be taken by members of the interventional radiology service (both the physicians and nurses) to explain these points, as well as how to quickly contact service members should any problem arise. In-depth consultation with the clinical service regarding drainage feasibility and overall goals prior to percutaneous treatment is essential.
CONCLUSION
Interventional management of GI fistulas is a valuable non-surgical therapy for seriously ill patients and is a comprehensive treatment option that includes percutaneous drainage and several other interventional procedures. However, interventional management is not easy and numerous manipulations and controls are required over many days. Furthermore, not all patients will respond to interventional treatment alone. For these patients, a combination of various methods for controlling intestinal output and decreasing the amount of infection will increase the chances of a successful outcome.
References
- 1.Falconi M, Pederzoli P. The relevance of gastrointestinal fistulae in clinical practice: a review. Gut. 2001;49(Suppl 4):iv2–iv10. doi: 10.1136/gut.49.suppl_4.iv2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Pinto I, Gonzalez EM. Optimising the treatment of upper gastrointestinal fistulae. Gut. 2001;49(Suppl 4):iv22–iv31. doi: 10.1136/gut.49.suppl_4.iv21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLean GK, Mackie JA, Freiman DB, Ring EJ. Enterocutaneous fistulae: interventional radiologic management. AJR Am J Roentgenol. 1982;138:615–619. doi: 10.2214/ajr.138.4.615. [DOI] [PubMed] [Google Scholar]
- 4.Boverie JH, Remont A. Percutaneous management of fistulas in the digestive tract. In: Dondelinger RF, Rossi P, Kurdziel JC, Wallace S, editors. Interventional radiology. Stuttgart: Thieme; 1990. pp. 746–753. [Google Scholar]
- 5.Berry SM, Fischer JE. Classification and pathophysiology of enterocutaneous fistulas. Surg Clin North Am. 1996;76:1009–1018. doi: 10.1016/s0039-6109(05)70495-3. [DOI] [PubMed] [Google Scholar]
- 6.LaBerge JM, Kerlan RK, Jr, Gordon RL, Ring EJ. Nonoperative treatment of enteric fistulas: results in 53 patients. J Vasc Interv Radiol. 1992;3:353–357. doi: 10.1016/s1051-0443(92)72043-0. [DOI] [PubMed] [Google Scholar]
- 7.Han JK. Percutaneous abdominal abscess drainage. In: Han MC, Park JH, editors. Interventional radiology. Seoul: Ilchokak; 1999. pp. 707–714. [Google Scholar]
- 8.Grunshaw ND, Ball CS. Palliative treatment of an enterorectal fistula with a covered metallic stent. Cardiovasc Intervent Radiol. 2001;24:438–440. doi: 10.1007/s00270-001-0056-0. [DOI] [PubMed] [Google Scholar]
- 9.Kang YJ, Oh JH, Yoon Y, Kim EJ, Ryu KN, Lim JW, et al. Covered metallic stent placement in the treatment of postoperative fistula resistant to conservative management after Billroth I operation. Cardiovasc Intervent Radiol. 2005;28:90–92. doi: 10.1007/s00270-004-0103-8. [DOI] [PubMed] [Google Scholar]
- 10.Rabago LR, Ventosa N, Castro JL, Marco J, Herrera N, Gea F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy. 2002;34:632–638. doi: 10.1055/s-2002-33237. [DOI] [PubMed] [Google Scholar]